Applicability Domain of the Sens-Is In Vitro Assay for Testing the Skin Sensitization Potential of Rheology-Modifying Polymers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sens-Is Assay
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | Three Dimensional |
| ACD | Allergic Contact Dermatitis |
| AOP | Adverse Outcome Pathway |
| ARE | Antioxidant/electrophile Response |
| C1 to C6 | Synthetic polymers |
| Ca2+ | Calcium cation |
| CaCl2 | Calcium Chloride |
| cDNA | complementary Deoxyribonucleic Acid |
| Cl− | Chlorine anion |
| Da | Dalton |
| DMSO | DiMethylvitro SulfOxide |
| DPG | Dipropylene Glycol |
| ECHA | European Chemicals Agency |
| HRIPT | Human Repeated Insult Patch Test |
| INCI | International Nomenclature Cosmetic Ingredient |
| KE | Key Events |
| N1 to N6 | Natural polymers (Composition in Table 1) |
| Na+ | Sodium cation |
| NaCl | Sodium Chloride |
| NMR | Nuclear Magnetic Resonance |
| OECD | Organisation for Economic Co-operation and Development |
| PBS | Phosphate Buffered Saline |
| PR | Positive Reference |
| R1 to R3 | References of natural polymers (Composition in Table 1) |
| RT-PCR | Reverse Transcriptase—Polymerase Chain Reaction |
| SOP | Standard Operating Protocol |
| TNBS | 2,4,6-trinitrobenzene sulfonic acid |
| UVCB | Unknown or Variable composition, Complex reaction products or Biological materials |
Appendix A
| Polymer | Dose | Viscosity * in Demineralized Water (in mPa·s) | Viscosity * in Demineralized Water + 0.6% NaCl (in mPa·s) | Viscosity * in Demineralized Water + 1% NaCl (in mPa·s) | Trend **: Viscosity Evolution After NaCl Addition |
|---|---|---|---|---|---|
| C1 | 2% | 79,000 | <50 | <50 | ➘ Decrease |
| C2 | 2% | 155,000 | 10,000 | 4500 | ➘ Decrease |
| C3 | 2% | 90,000 | <50 | <50 | ➘ Decrease |
| C4 | 2% | 52,000 | <50 | <50 | ➘ Decrease |
| C5 | 2% | 90,000 | 500 | <50 | ➘ Decrease |
| N1 | 1% | 4700 | Not done | 4500 | ➞ Constant |
| N2 | 1% | 5000 | Not done | 21,000 | ➚ Increase + |
| 2% | 11,000 | Not done | 62,000 | ➚ Increase + | |
| N2’ | 1% | 2900 | Not done | 11,000 | ➚ Increase |
| 2% | 13,000 | Not done | 40,000 | ➚ Increase | |
| N3 | 1% | <50 | Not done | <50 | ➞ Constant |
| N4 | 1% | 24,000 | Not done | 17,000 | ➞ Constant |
| N5 | 1% | 44,000 | Not done | 41,000 | ➞ Constant |
| N6 | 1% | Oil rheology modifier—Not soluble in water, no effect of salts | |||
| R1 | 1% | 14,500 | Not done | 18,000 | ➞ Constant |
| R2 | 1% | 8000 | Not done | 8500 | ➞ Constant |
References
- Murphy, P.B.; Atwater, A.R.; Mueller, M. Allergic Contact Dermatitis. [Updated 2023 Jul 13]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK532866/ (accessed on 12 May 2025).
- Lampel, H.P.; Powell, H.B. Occupational and Hand Dermatitis: A Practical Approach. Clin. Rev. Allergy Immunol. 2019, 56, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Alinaghi, F.; Bennike, N.H.; Egeberg, A.; Thyssen, J.P.; Johansen, J.D. Prevalence of contact allergy in the general population: A systematic review and meta-analysis. Contact Dermat. 2019, 80, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Sukakul, T.; Chaweekulrat, P.; Limphoka, P.; Boonchai, W. Changing trends of contact allergens in Thailand: A 12-year retrospective study. Contact Derm. 2019, 81, 124–129. [Google Scholar] [CrossRef]
- OECD. The Adverse Outcome Pathway for Skin Sensitization Initiated by Covalent Binding to Proteins. Part 1: Scientific Evidence; OECD Environment Health and Safety Publication Series on Testing and Assessment; OECD: Paris, France, 2012. [Google Scholar]
- OECD. The Adverse Outcome Pathway for Skin Sensitization Initiated by Covalent Binding to Proteins. Part 2: Use of the Aop to Develop Chemical Categories and Integrated Assessment and Testing Approaches; OECD Environment Health and Safety Publication Series on Testing and Assessment; OECD: Paris, France, 2012. [Google Scholar]
- Gilmour, N.; Kern, P.S.; Alépée, N.; Boislève, F.; Bury, D.; Clouet, E.; Hirota, M.; Hoffmann, S.; Kühnl, J.; Lalko, J.F.; et al. Development of a next generation risk assessment framework for the evaluation of skin sensitisation of cosmetic ingredients. Regul. Toxicol. Pharmacol. 2020, 116, 104721. [Google Scholar] [CrossRef] [PubMed]
- Gądarowska, D.; Kalka, J.; Daniel-Wójcik, A.; Mrzyk, I. Alternative methods for skin-sensitization assessment. Toxics 2022, 10, 740. [Google Scholar] [CrossRef]
- Vandebriel, R.J.; Pennings, J.L.; Baken, K.A.; Pronk, T.E.; Boorsma, A.; Gottschalk, R.; Van Loveren, H. Keratinocyte gene expression profiles discriminate sensitizing and irritating compounds. Toxicol. Sci. 2010, 117, 81–89. [Google Scholar] [CrossRef]
- Cottrez, F.; Boitel, E.; Auriault, C.; Aeby, P.; Groux, H. Genes specifically modulated in sensitized skins allow the detection of sensitizers in a reconstructed human skin model. Development of the SENS-IS assay. Toxicol. In Vitro 2015, 9, 787–802. [Google Scholar] [CrossRef]
- Cottrez, F.; Boitel, E.; Ourlin, J.C.; Peiffer, J.L.; Fabre, I.; Henaoui, I.S.; Mari, B.; Vallauri, A.; Paquet, A.; Barbry, P.; et al. SENS-IS, a 3D reconstituted epidermis based model for quantifying chemical sensitization potency: Reproducibility and predictivity results from an inter-laboratory study. Toxicol. In Vitro 2016, 32, 248–260. [Google Scholar] [CrossRef]
- Na, M.; O’Brien, D.; Gerberick, G.F.; Kern, P.S.; Lavelle, M.; Lee, I.; Parakhia, R.; Ryan, C.; Api, A.M. Benchmarking performance of SENS-IS assay against weight of evidence skin sensitization potency categories. Regul. Toxicol. Pharmacol. 2022, 130, 105128. [Google Scholar] [CrossRef]
- Hoffmann, S.; Kleinstreuer, N.; Alépée, N.; Allen, D.; Api, A.M.; Ashikaga, T.; Clouet, E.; Cluzel, M.; Desprez, B.; Gellatly, N.; et al. Non-animal methods to predict skin sensitization (I): The Cosmetics Europe database. Crit. Rev. Toxicol. 2018, 48, 344–358. [Google Scholar] [CrossRef]
- OECD Public Consultation. First Commenting Round of the Working Party of the National Coordinators of the Test Guidelines Programme (WNT) on the Draft Test Guideline for the SENS-IS Assay. Draft Validation Report of the SENS-IS Assay, Submission period 25 July–13 December 2024, pp 24. oecd.org. Available online: https://www.oecd.org/en/events/public-consultations/2024/07/draft-test-guideline-for-the-sens-is-assay.html (accessed on 12 May 2025).
- Cottrez, F.; Boitel, E.; Berrada-Gomez, M.P.; Dalhuchyts, H.; Bidan, C.; Rattier, S.; Ferret, P.J.; Groux, H. In vitro measurement of skin sensitization hazard of mixtures and finished products: Results obtained with the SENS-IS assays. Toxicol. In Vitro 2020, 62, 104644. [Google Scholar] [CrossRef]
- Bergal, M.; Puginier, M.; Gerbeix, C.; Groux, H.; Roso, A.; Cottrez, F.; Milius, A. In vitro testing strategy for assessing the skin sensitizing potential of “difficult to test” cosmetic ingredients. Toxicol. In Vitro 2020, 65, 104781. [Google Scholar] [CrossRef] [PubMed]
- Puginier, M.; Roso, A.; Groux, H.; Gerbeix, C.; Cottrez, F. Strategy to avoid skin sensitization: Application to botanical cosmetic ingredients. Cosmetics 2022, 9, 40. [Google Scholar] [CrossRef]
- Kolle, S.N.; Flach, M.; Kleber, M.; Basketter, D.A.; Wareing, B.; Mehling, A.; Hareng, L.; Watzek, N.; Bade, S.; Funk-Weyer, D.; et al. Plant extracts, polymers and new approach methods: Practical experience with skin sensitization assessment. Regul. Toxicol. Pharmacol. 2023, 138, 105330. [Google Scholar] [CrossRef] [PubMed]
- Petry, T.; Bosch, A.; Koraïchi-Emeriau, F.; Eigler, D.; Germain, P.; Seidel, S. Assessment of the skin sensitisation hazard of functional polysiloxanes and silanes in the SENS-IS assay. Regul. Toxicol. Pharmacol. 2018, 98, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Pellevoisin, C.; Cottrez, F.; Johansson, J.; Pedersen, E.; Coleman, K.; Groux, H. Pre-validation of SENS-IS assay for in vitro skin sensitization of medical devices. Toxicol. In Vitro 2021, 71, 105068. [Google Scholar] [CrossRef]
- Guidance for Monomers and Polymers, European Chemicals Agency (ECHA). 2023. Available online: https://op.europa.eu/en/publication-detail/-/publication/9cc4e742-d42d-11ed-a05c-01aa75ed71a1/language-en (accessed on 19 May 2025).
- Cilurzo, F.; Vistoli, G.; Gennari, C.G.; Selmin, F.; Gardoni, F.; Franzè, S.; Campisi, M.; Minghetti, P. The role of the conformational profile of polysaccharides on skin penetration: The case of hyaluronan and its sulfates. Chem. Biodivers. 2014, 11, 551–561. [Google Scholar] [CrossRef]
- Alves, T.F.; Morsink, M.; Batain, F.; Chaud, M.V.; Almeida, T.; Fernandes, D.A.; da Silva, C.F.; Souto, E.B.; Severino, P. Applications of natural, semi-synthetic, and synthetic polymers in cosmetic formulations. Cosmetics 2020, 7, 75. [Google Scholar] [CrossRef]
- Lochhead, R.Y. The use of polymers in cosmetic products. In Cosmetic Science and Technology: Theoretical Principles and Applications, 1st ed.; Sakamoto, K., Lochhead, R.Y., Maibach, H.I., Yamashita, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 171–221. [Google Scholar]
- Bonacucina, G.; Cespi, M.; Palmieri, G.F. Characterization and stability of emulsion gels based on acrylamide/sodium acryloyldimethyl taurate copolymer. AAPS PharmSciTech 2009, 10, 368–375. [Google Scholar] [CrossRef]
- Medina-Torres, L.; Calderas, F.; Sanchez-Olivares, G.; Nuñez-Ramirez, D.-M. Rheology of Sodium Polyacrylate as an Emulsifier Employed in Cosmetic Emulsions. Ind. Eng. Chem. Res. 2014, 53, 18346–18351. [Google Scholar] [CrossRef]
- Kuchekar, S.; Bhise, K. Formulation and development of antipsoriatic herbal gelcream. J. Sci. Ind. Res. 2012, 71, 279–284. [Google Scholar]
- Ahmed, S.; Ali, A. (Eds.) Natural Gums: Extraction, Properties, and Applications; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Tafuro, G.; Costantini, A.; Baratto, G.; Francescato, S.; Semenzato, A. Evaluating natural alternatives to synthetic acrylic polymers: Rheological and texture analyses of polymeric water dispersions. ACS Omega 2020, 5, 15280–15289. [Google Scholar] [CrossRef]
- Sarraf, A.; Verton, E.; Addoun, N.; Boual, Z.; Ould El Hadj, M.D.; El Alaoui-Talibi, Z.; El Modafar, C.; Abdelkafi, S.; Fendri, I.; Delattre, C.; et al. Polysaccharides and derivatives from Africa to address and advance sustainable development and economic growth in the next decade. Appl. Sci. 2021, 11, 5243. [Google Scholar] [CrossRef]
- Picken, C.; Buensoz, O.; Price, P.; Fidge, C.; Points, L.; Shaver, M.P. Sustainable formulation polymers for home, beauty and personal care: Challenges and opportunities. Chem. Sci. 2023, 14, 12926–12940. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Kumari, S.; Singh, K.; Kushwaha, P. Influence of seasonal variation on chemical composition and nutritional profiles of macro-and microalgae. In Recent Advances in Micro and Macroalgal Processing: Food and Health Perspectives, 1st ed.; Rajauria, G., Yuan, Y.V., Eds.; John Wiley and Sons Ltd.: New York, NY, USA, 2021; pp. 1–71. [Google Scholar] [CrossRef]
- Vandanjon, L.; Burlot, A.S.; Zamanileha, E.F.; Douzenel, P.; Ravelonandro, P.H.; Bourgougnon, N.; Bedoux, G. The use of FTIR spectroscopy as a tool for the seasonal variation analysis and for the quality control of polysaccharides from seaweeds. Mar. Drugs 2023, 21, 482. [Google Scholar] [CrossRef]
- Hamouda, Y. Factors Affecting the Quality of Acacia Senegal Gums. Ph.D. Dissertation, University of Chester, Chester, UK, 2017. Available online: https://chesterrep.openrepository.com/handle/10034/620895 (accessed on 8 April 2025).
- Ibieta, G.; Bustos, A.S.; Ortiz-Sempértegui, J.; Linares-Pastén, J.A.; Peñarrieta, J.M. Molecular characterization of a galactomannan extracted from Tara (Caesalpinia spinosa) seeds. Sci. Rep. 2023, 13, 21893. [Google Scholar] [CrossRef]
- World Health Organization. Safety Evaluation of Certain Food Additives, WHO Food Additives Series: 42, Carrageenan (addendum). IPCS, International Programme on Chemical Safety, 1999. inchem.org. Available online: https://www.inchem.org/documents/jecfa/jecmono/v042je08.htm (accessed on 7 May 2025).
- Castillo, N.A.; Valdez, A.L.; Fariña, J.I. Microbial production of scleroglucan and downstream processing. Front. Microbiol. 2015, 6, 1106. [Google Scholar] [CrossRef]
- Shafie, M.H.; Kamal, M.L.; Zulkiflee, F.F.; Hasan, S.; Uyup, N.H.; Abdullah, S.; Hussin, N.A.M.; Tan, Y.C.; Zafarina, Z. Application of Carrageenan extract from red seaweed (Rhodophyta) in cosmetic products: A review. J. Indian Chem. Soc. 2022, 99, 100613. [Google Scholar] [CrossRef]
- Tafuro, G.; Costantini, A.; Baratto, G.; Francescato, S.; Busata, L.; Semenzato, A. Characterization of polysaccharidic associations for cosmetic use: Rheology and texture analysis. Cosmetics 2021, 8, 62. [Google Scholar] [CrossRef]
- López-Hortas, L.; Flórez-Fernández, N.; Torres, M.D.; Ferreira-Anta, T.; Casas, M.P.; Balboa, E.M.; Falqué, E.; Domínguez, H. Applying seaweed compounds in cosmetics, cosmeceuticals and nutricosmetics. Mar. Drugs 2021, 19, 552. [Google Scholar] [CrossRef]
- Dearman, R.J.; Betts, C.J.; Farr, C.; McLaughlin, J.; Berdasco, N.; Wiench, K.; Kimber, I. Comparative analysis of skin sensitization potency of acrylates (methyl acrylate, ethyl acrylate, butyl acrylate, and ethylhexyl acrylate) using the local lymph node assay. Contact Dermat. 2007, 57, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Candau, F. Polymerization in inverse emulsion and microemulsion. In An Introduction to Polymer Colloids; Candau, F., Ottewill, R.H., Eds.; Kluwer Academic: Dordrecht, The Netherlands, 1990; pp. 73–96. [Google Scholar]
- Gelman, R.A.; Harrington, J.C.; Vaynberg, K.A. Insight into the inversion mechanism of an inverse polymer emulsion. Langmuir 2008, 24, 12727–12729. [Google Scholar] [CrossRef]
- Bodoc, M.; Colas, A. Inverse emulsion polymers to improve naturality content of cosmetic formulations. In Proceedings of the Bordeaux Polymer Conference (BPC), Bordeaux, France, 13–16 June 2022; Poster Session. [Google Scholar]
- Fiume, M.M.; Heldreth, B.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; et al. Safety assessment of microbial polysaccharide gums as used in cosmetics. Int. J. Toxicol. 2016, 35, 5S–49S. [Google Scholar] [CrossRef] [PubMed]
- Fluhr, J.W.; Darlenski, R.; Angelova-Fischer, I.; Tsankov, N.; Basketter, D. Skin irritation and sensitization: Mechanisms and new approaches for risk assessment: 1. Skin irritation. Skin. Pharmacol. Physiol. 2008, 21, 124–135. [Google Scholar] [CrossRef]
- Puginier, M. (Seppic, Castres, Tarn, France). Clinical Trial Human Repeated Insult Patch Test (HRIPT). Marzulli & Maybach Method; OECD: Paris, France, 2015; Unpublished work. [Google Scholar]
- Puginier, M. (Seppic, Castres, Tarn, France). Clinical Trial Human Repeated Insult Patch Test (HRIPT). Marzulli & Maybach Method; OECD: Paris, France, 2023; Unpublished work. [Google Scholar]
- Puginier, M. (Seppic, Castres, Tarn, France). Clinical Trial Human Repeated Insult Patch Test (HRIPT). Marzulli & Maybach Method; OECD: Paris, France, 2017; Unpublished work. [Google Scholar]
- Puginier, M. (Seppic, Castres, Tarn, France). Clinical Trial Human Repeated Insult Patch Test (HRIPT). Marzulli & Maybach Method; OECD: Paris, France, 2020; Unpublished work. [Google Scholar]
- Johnson, W., Jr.; Heldreth, B. Final Report. Safety Assessment of Polysaccharide Gums as Used in Cosmetics. Unpublished Report Available from the Cosmetic Ingredient Review 2015 (CIR). 1620 L Street, N.W., Suite 1200, Washington, D.C. 20036, pp.1–86. Available online: https://www.cir-safety.org/sites/default/files/plpogu092015final.pdf (accessed on 8 April 2025).
- Puginier, M. (Seppic, Castres, Tarn, France). Clinical Trial Human Repeated Insult Patch Test (HRIPT). Marzulli & Maybach Method; OECD: Paris, France, 2019; Unpublished work. [Google Scholar]
- Puginier, M. (Seppic, Castres, Tarn, France). Clinical Trial Human Repeated Insult Patch Test (HRIPT). Marzulli & Maybach Method; OECD: Paris, France, 2024; Unpublished work. [Google Scholar]
- Richeux, F. (Phycher Bio Développement, Martillac, Gironde, France). OECD 406, Skin Sensitization, Guinea Pig Maximization Test Method; OECD: Paris, France, 2007; Unpublished work. [Google Scholar]
- Donny, E. (Envigo, Rossdorf, Germany). OECD 429, Skin Sensitization, Local Lymph Node Assay; OECD: Paris, France, 2008; Unpublished work. [Google Scholar]
- Washizaki, K.; Kanto, H.; Yazaki, S.; Ito, M. A case of allergic contact dermatitis to polyglyceryl laurate. Contact Dermat. 2008, 58, 187–188. [Google Scholar] [CrossRef] [PubMed]
- ISO 16128-1:2016; Guidelines on Technical Definitions and Criteria for Natural and Organic Cosmetic Ingredients and Products. Part 1: Definitions for ingredients. Edition 1. ISO International Organization for Standardization: Geneva, Switzerland, 2016. Available online: https://www.iso.org/standard/62503.html (accessed on 26 February 2025).
- ISO 16128-2:2017; Guidelines on Technical Definitions and Criteria for Natural and Organic Cosmetic Ingredients and Products. Part 2: Criteria for Ingredients and Products. Edition 1. ISO International Organization for Standardization: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/65197.html (accessed on 26 February 2025).
- Habibi, H.; Khosravi-Darani, K. Effective variables on production and structure of xanthan gum and its food applications: A review. Biocatal. Agric. Biotechnol. 2017, 10, 130–140. [Google Scholar] [CrossRef]
- Rosalam, S.; England, R. Review of xanthan gum production from unmodified starches by Xanthomonas comprestris sp. Enzyme Microb. Technol. 2006, 39, 197–207. [Google Scholar] [CrossRef]
- Stokke, B.T.; Christensen, B.E.; Smidsrod, O. Macromolecular properties of xanthan. In Polysaccharides: Structural Diversity and Functional Versatility; Dumitriu, S., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1998; pp. 433–472. [Google Scholar]
- Khan, N.H.; Darwis, Y.; Khiang, P.K. Preliminary investigation of in-vitro bioadhesive properties of selected natural gums. Arch. Pharm. Pract. 2015, 6, 3–10. [Google Scholar]
- Jadav, M.; Pooja, D.; Adams, D.J.; Kulhari, H. Advances in xanthan gum-based systems for the delivery of therapeutic agents. Pharmaceutics 2023, 15, 402. [Google Scholar] [CrossRef]
- Parente, M.E.; Ochoa Andrade, A.; Ares, G.; Russo, F.; Jiménez-Kairuz, Á. Bioadhesive hydrogels for cosmetic applications. Int. J. Cosmet. Sci. 2015, 37, 511–518. [Google Scholar] [CrossRef]
- Raschip, I.E.; Yakimets, I.; Martin, C.P.; Paes, S.S.; Vasile, C.; Mitchell, J.R. Effect of water content on thermal and dynamic mechanical properties of xanthan powder: A comparison between standard and novel techniques. Powder Technol. 2008, 182, 436–443. [Google Scholar] [CrossRef]
- Smith, I.H.; Symes, K.C.; Lawson, C.J.; Morris, E.R. Influence of the pyruvate content of xanthan on macromolecular association in solution. Int. J. Biol. Macromol. 1981, 3, 129–134. [Google Scholar] [CrossRef]
- Furtado, I.F.S.P.C.; Sydney, E.B.; Rodrigues, S.A.; Sydney, A.C.N. Xanthan gum: Applications, challenges, and advantages of this asset of biotechnological origin. Biotechnol. Res. Innov. J. 2022, 6, e202204. [Google Scholar] [CrossRef]
- Fjodorova, J.; Held, R.; Hublik, G.; Esteban Vazquez, J.M.; Walhorn, V.; Hellweg, T.; Anselmetti, D. Tuning xanthan viscosity by directed random coil-to-helix transition. Biomacromolecules 2022, 23, 4493–4503. [Google Scholar] [CrossRef] [PubMed]
- Mintel Global New Products Database, Ad Hoc Research from January 2019 to December 2024, Xanthan Gum as Included Ingredients, on Beauty and Personal Care Category and Across all Regions. Available online: https://www.mintel.com/products/gnpd/?_bt=652169658686&_bk=mintel%20gnpd&_bm=e&_bn=g&_bg=150165006391&utm_medium=cpc&utm_source=google&utm_content=Threepipe-GO19848722604~GO150165006391&gad_source=1&gad_campaignid=19848722604&gbraid=0AAAAAC3UrcBJLdBFqZXiklTJbGri42iMv&gclid=CjwKCAjwravBBhBjEiwAIr30VHHBnU2jCi1qH1_MhQvkRrLoFokKNmllneeLSdo6RN1kMs1iVIt9oRoCkQMQAvD_BwE (accessed on 15 January 2025).
- Aerts, O.; Clinck, B.; Schramme, M.; Lambert, J. Contact allergy caused by Tinosorb® M: Let us not forget about xanthan gum. Contact Dermat. 2014, 72, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Sivanantham, M.; Tata, B.V.R. Swelling/deswelling of polyacrylamide gels in aqueous NaCl solution: Light scattering and macroscopic swelling study. Pramana 2012, 79, 457–469. [Google Scholar] [CrossRef]
- Asyakina, L.; Dyshlyuk, L. Study of viscosity of aqueous solutions of natural polysaccharides. Sci. Evol. 2016, 1, 11–19. [Google Scholar] [CrossRef]


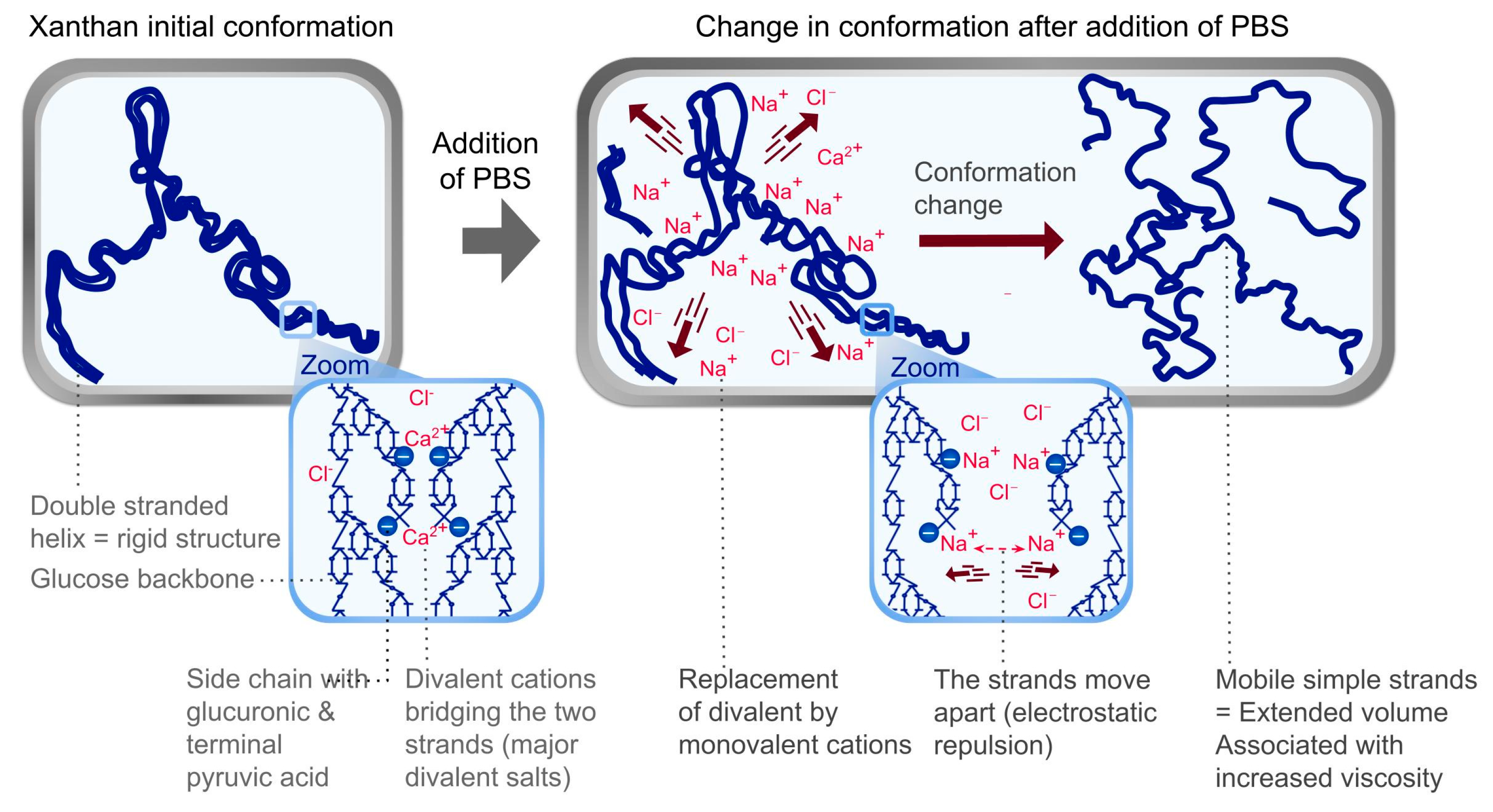
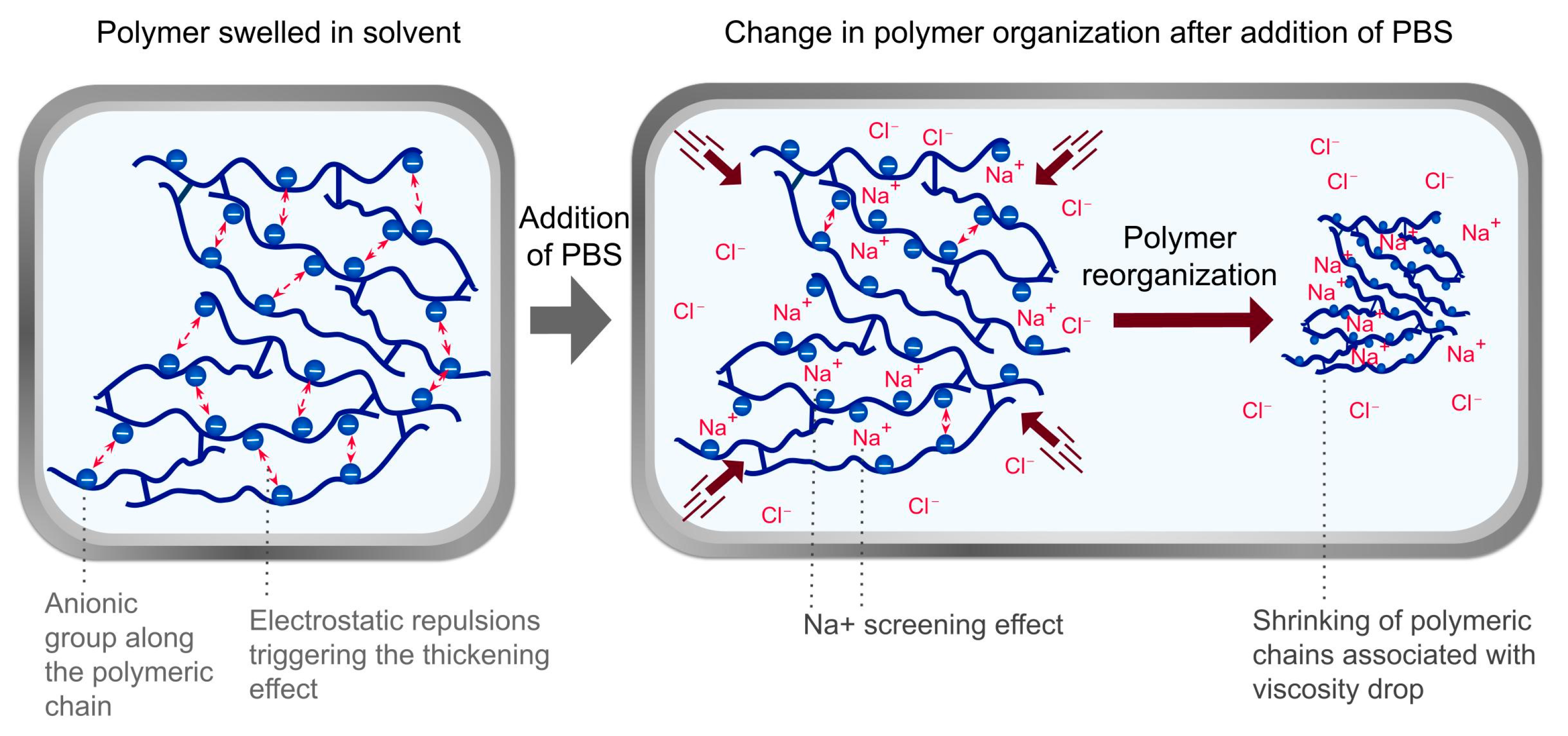
| Polymer Identification | Polymer IUPAC Name | Polymerization Excipient (INCI Name) | Production Method | Physical Form | Polymer Content (w/w %) |
|---|---|---|---|---|---|
| C1 (synthetic) | 1-Propanesulfonic acid, 2-methyl-2-[(1-oxo-2-propenyl)amino]-, monosodium salt, polymer with 2-propenamide | Inverse emulsion: -Water - Oil: Renewable Alkane - Inverter I1 *: Polyglyceryl-10 Laurate | Polymerization in inverse emulsion | Liquid | 35–40 |
| C2 (synthetic) | 1-Propanesulfonic acid, 2-methyl-2-[(1-oxo-2-propenyl)amino]-, monosodium salt, polymer with 2-propenamide | Inverse emulsion: -Water - Oil: Isoparaffin - Inverter: Laureth-7 | Polymerization in inverse emulsion | Liquid | 35–40 |
| C3 Experimental (synthetic) | 2-Propenoic acid, polymer with 2-methyl-2-[(1-oxo-2-propenyl)amino]-1-propanesulfonic acid, sodium salt | Inverse emulsion: -Water - Oil: Renewable alkane - Inverter I2 *: Polyglyceryl-10 laurate | Polymerization in inverse emulsion | Liquid | 35–40 |
| C4 (synthetic) | 2-Propenoic acid, polymer with 2-methyl-2-[(1-oxo-2-propenyl)amino]-1-propanesulfonic acid, sodium salt | Inverse emulsion: -Water - Oil: Renewable alkane - Inverter: Polyglyceryl-6 laurate | Polymerization in inverse emulsion | Liquid | 35–40 |
| C5 (synthetic) | 2-Propenoic acid, polymer with 2-methyl-2-[(1-oxo-2-propenyl)amino]-1-propanesulfonic acid monosodium salt, 2-propenamide and sodium 2-propenoate | Inverse emulsion: -Water - Oil: Hydrogenated Polyisobutene and Ethylhexyl Palmitate - Inverter I1 *: Polyglyceryl-10 Laurate | Polymerization in inverse emulsion | Liquid | 50–70 |
| N1 (natural) | (2R,3R,4S,5R,6S)-2-(hydroxymethyl)-6-[[(2R,3S,4R,5S,6R)-4,5,6-trihydroxy-3-[(2S,3S,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyoxan-2-yl]methoxy]oxane-3,4,5-triol | - | Extraction from seed of Caesalpinia spinosa tree | Powder | 100 |
| N2 ** (natural) | Not available INCI name: Xanthan gum | - | Biotechnology: fermentation of Xanthomonas Campestris | Powder | 100 |
| N2’ ** (natural) | Not available INCI name: Xanthan gum | - | Biotechnology: fermentation of Xanthomonas Campestris | Powder | 100 |
| N3 (natural) | (2R,3R,4S,5S,6R)-2-{[(2R,3S,4R,5R,6S)-6-{[(2R,3S,4R,5R,6R)-4,5-dihydroxy-6-{[(2R,3S,4R,5R,6S)-4,5,6-trihydroxy-2-(hydroxymethyl)oxan-3-yl]oxy}-3-{[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}oxan-2-yl]methoxy}-4,5-dihydroxy-2-(hydroxymethyl)oxan-3-yl]oxy}-6-(hydroxymethyl)oxane-3,4,5-triol | - | Extraction from babassu mesocarp | Powder | 100 |
| N4 (natural) | (2S,3S,4S,5S,6R)-2-[(2R,3S,4R,5R,6S)-6-[(2R,3S,4R,5S,6S)-4,5-dihydroxy-2-(hydroxymethyl)-6-[(2R,4R,5S,6R)-4,5,6-trihydroxy-2-(hydroxymethyl)oxan-3-yl]oxyoxan-3-yl]oxy-4,5-dihydroxy-2-(hydroxymethyl)oxan-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol | - | Extraction from roots of Amorphophallus konjac | Powder | 100 |
| N5 (natural) | (2S,3S,4S,5S,6R)-2-[(2R,3S,4R,5R,6S)-6-[(2R,3S,4R,5S,6S)-4,5-dihydroxy-2-(hydroxymethyl)-6-[(2R,4R,5S,6R)-4,5,6-trihydroxy-2-(hydroxymethyl)oxan-3-yl]oxyoxan-3-yl]oxy-4,5-dihydroxy-2-(hydroxymethyl)oxan-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol | - | Extraction from roots of Amorphophallus muelleri | Powder | 100 |
| N6 (natural) | 1,6,10-Dodecatriene, 7,11-dimethyl-3-methylene-, (6E)-, homopolymer, hydrogenated | Oil: Renewable alkane | Chemical transformation of sugar plant | Liquid | 30–50 |
| R1 (natural) | R1 (natural) [(2R,3R,4R,5R,6S)-4,5-dihydroxy-6-[[(1R,3R,4R,5S,8S)-3-[(2R,3S,4R,5R,6S)-5-hydroxy-2-(hydroxymethyl)-6-[[(1R,3S,4R,5S,8S)-3-hydroxy-4-sulfonatooxy-2,6-dioxabicyclo[3.2.1]octan-8-yl]oxy]-3-sulfonatooxyoxan-4-yl]oxy-4-sulfonatooxy-2,6-dioxabicyclo[3.2.1]oct | - | Extraction from red seaweeds | Powder | 100 |
| R2 (natural) | Not available (branched β-(1→3),(1→6)-D-glucan) | - | Biotechnology: fermentation of Sclerotium rolfsii | Powder | 100 |
| R3 (natural) | (2R,3S,4S,5R)-2-(hydroxymethyl)-6-[[(4R,5S)-4-hydroxy-3-methyl-2,6-dioxabicyclo[3.2.1]octan-8-yl]oxy]-4-methoxyoxane-3,5-diol | - | Extraction from red seaweeds | Powder | 100 |
| PR (synthetic) | 2-ethylhexyl prop-2-enoate | - | - | Powder | 100 |
| Conditions of Test | |||
|---|---|---|---|
| Polymer | Solvents | Concentrations | Results |
| C1 | PBS, DMSO, DPG | 100%; 50% | Non sensitizer |
| C2 | DMSO | 100%; 50% | Non sensitizer |
| C3 | DMSO | 100%; 50%; 10% | Very weak sensitizer |
| C4 | DMSO and PBS | 100%; 50%; 10% | Non sensitizer |
| C5 | DMSO | 100%; 50%; 10% | Non sensitizer |
| N1 | DMSO and PBS | 100% | Non sensitizer |
| N2 | DMSO and PBS | 100%; 50% | Very weak sensitizer |
| N2’ | DMSO | 100%; 50% | Non sensitizer |
| N3 | DMSO | 100%; 50%; 10% | Non sensitizer |
| N4 | DMSO | 100%; 50%; 10% | Non sensitizer |
| N5 | DMSO | 100%; 50%; 10% | Non sensitizer |
| N6 | Olive oil | 100%; 50%; 10% | Non sensitizer |
| R1 | DMSO and PBS | 100% | Non sensitizer |
| R2 | DMSO and PBS | 100% | Non sensitizer |
| R3 | DMSO and PBS | 100% | Non sensitizer |
| PR | Olive oil, DPG | 50%; 10% | Moderate sensitizer |
| Polymer | Sens-Is Assay Results | Skin Sensitization Conclusion (Safety Analysis and In Vivo Results *) |
|---|---|---|
| C1 | - | - a, c [47] |
| C2 | - | - a, c [48] |
| C3 | Very weak sensitizer | Not expected |
| C4 | - | - c [49] |
| C5 | - | - a, b, c [50] |
| N1 | - | - b |
| N2 | Very weak sensitizer | - a [51] |
| N2’ | - | - a [51] |
| N3 | - | - c [52] |
| N4 | - | - a [34] |
| N5 | - | - c [53] |
| N6 | - | - c [52] |
| R1 | - | - a [34] |
| R2 | - | - a [33] |
| R2 | - | - a [34] |
| PR | Moderate sensitizer | Moderate sensitizer b [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hochar, I.; Puginier, M.; Groux, H.; Guilbot, J.; Cottrez, F.; Roso, A. Applicability Domain of the Sens-Is In Vitro Assay for Testing the Skin Sensitization Potential of Rheology-Modifying Polymers. Polymers 2025, 17, 1408. https://doi.org/10.3390/polym17101408
Hochar I, Puginier M, Groux H, Guilbot J, Cottrez F, Roso A. Applicability Domain of the Sens-Is In Vitro Assay for Testing the Skin Sensitization Potential of Rheology-Modifying Polymers. Polymers. 2025; 17(10):1408. https://doi.org/10.3390/polym17101408
Chicago/Turabian StyleHochar, Isabelle, Mickaël Puginier, Hervé Groux, Jérôme Guilbot, Françoise Cottrez, and Alicia Roso. 2025. "Applicability Domain of the Sens-Is In Vitro Assay for Testing the Skin Sensitization Potential of Rheology-Modifying Polymers" Polymers 17, no. 10: 1408. https://doi.org/10.3390/polym17101408
APA StyleHochar, I., Puginier, M., Groux, H., Guilbot, J., Cottrez, F., & Roso, A. (2025). Applicability Domain of the Sens-Is In Vitro Assay for Testing the Skin Sensitization Potential of Rheology-Modifying Polymers. Polymers, 17(10), 1408. https://doi.org/10.3390/polym17101408







