Smart and UV-Resistant Edible Coating and Films Based on Alginate, Whey Protein, and Curcumin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Edible Films
2.3. Physical Appearance
2.4. Optical Microscopy
2.5. Mechanical Properties
2.6. Water Solubility Test
2.7. ATR-FTIR Spectroscopy
2.8. Color Measurement
2.9. Measurement of Water Contact Angle
2.10. Antioxidant Properties of the Films
2.11. UV-Blocking Efficiency Test
2.12. Water Vapor Transmission Rate
2.13. Application of Edible Film Packaging on Apple
2.14. Evaluation of pH Sensing Capability of the Films
3. Results and Discussion
3.1. Physical Appearance
3.2. Water Solubility and Swelling Test
3.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.4. Water Contact Angle
3.5. Antioxidant Properties of the Films
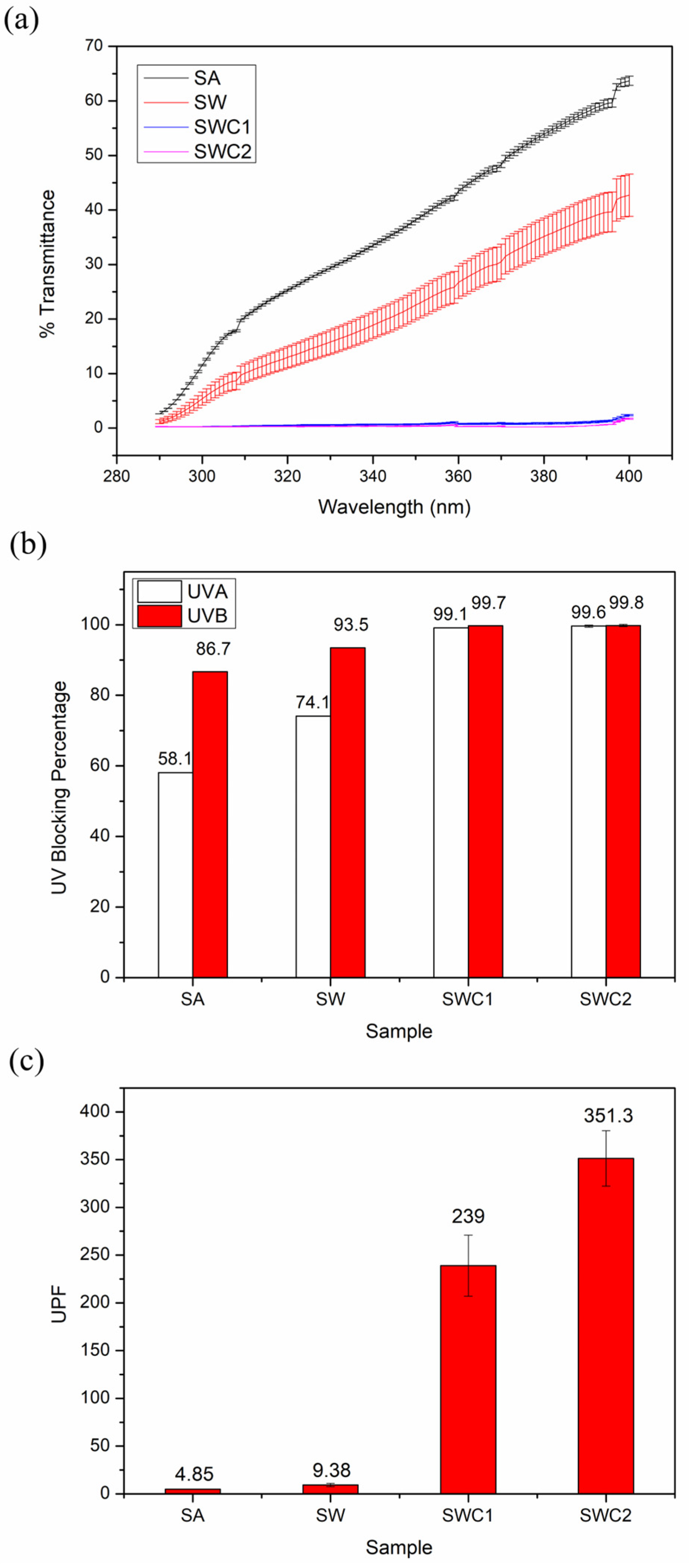
3.6. UV-Blocking Efficiency Test
3.7. Water Vapor Transmission
3.8. Mechanical Properties
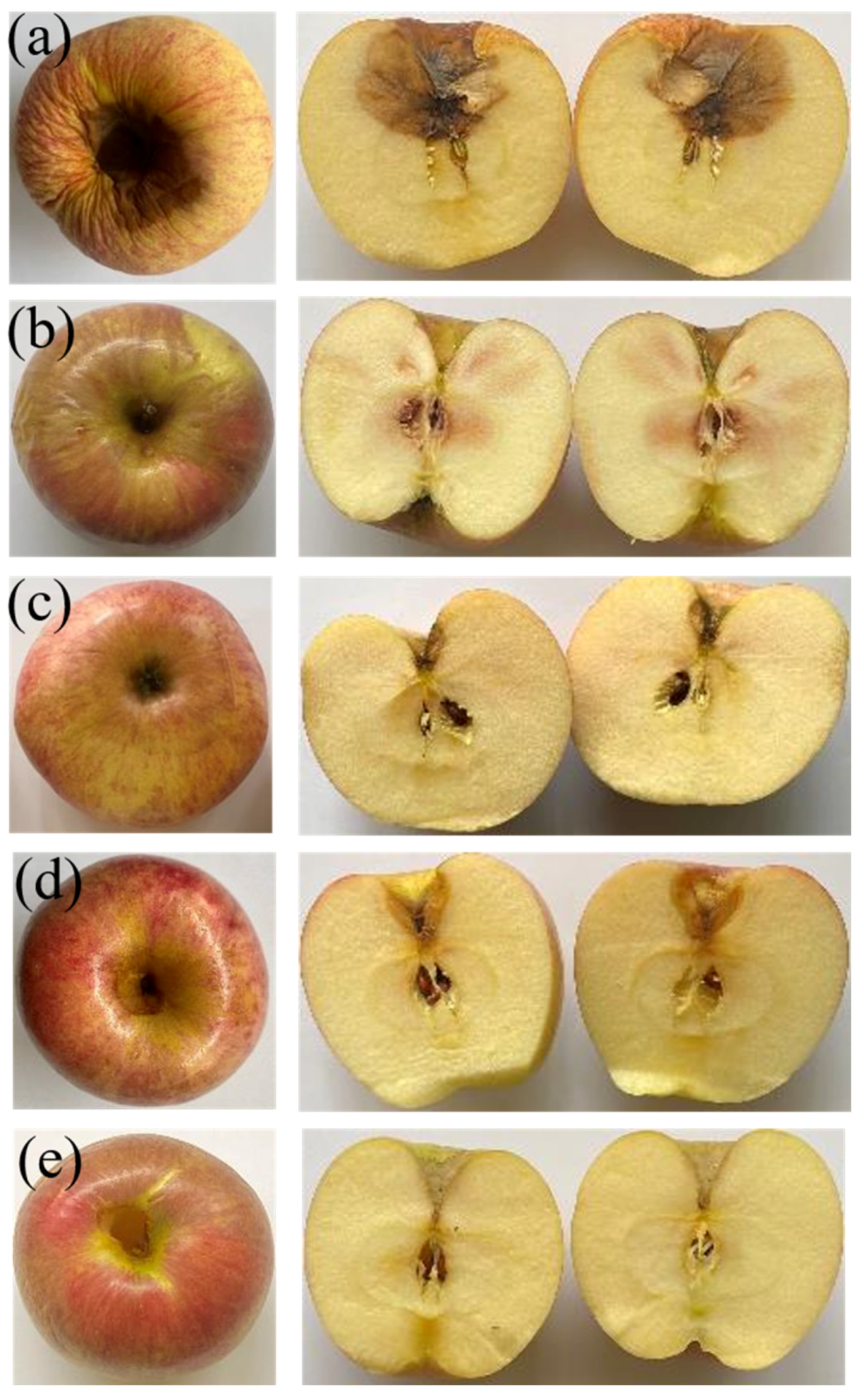
3.9. Application of Edible Film Packaging on Apple
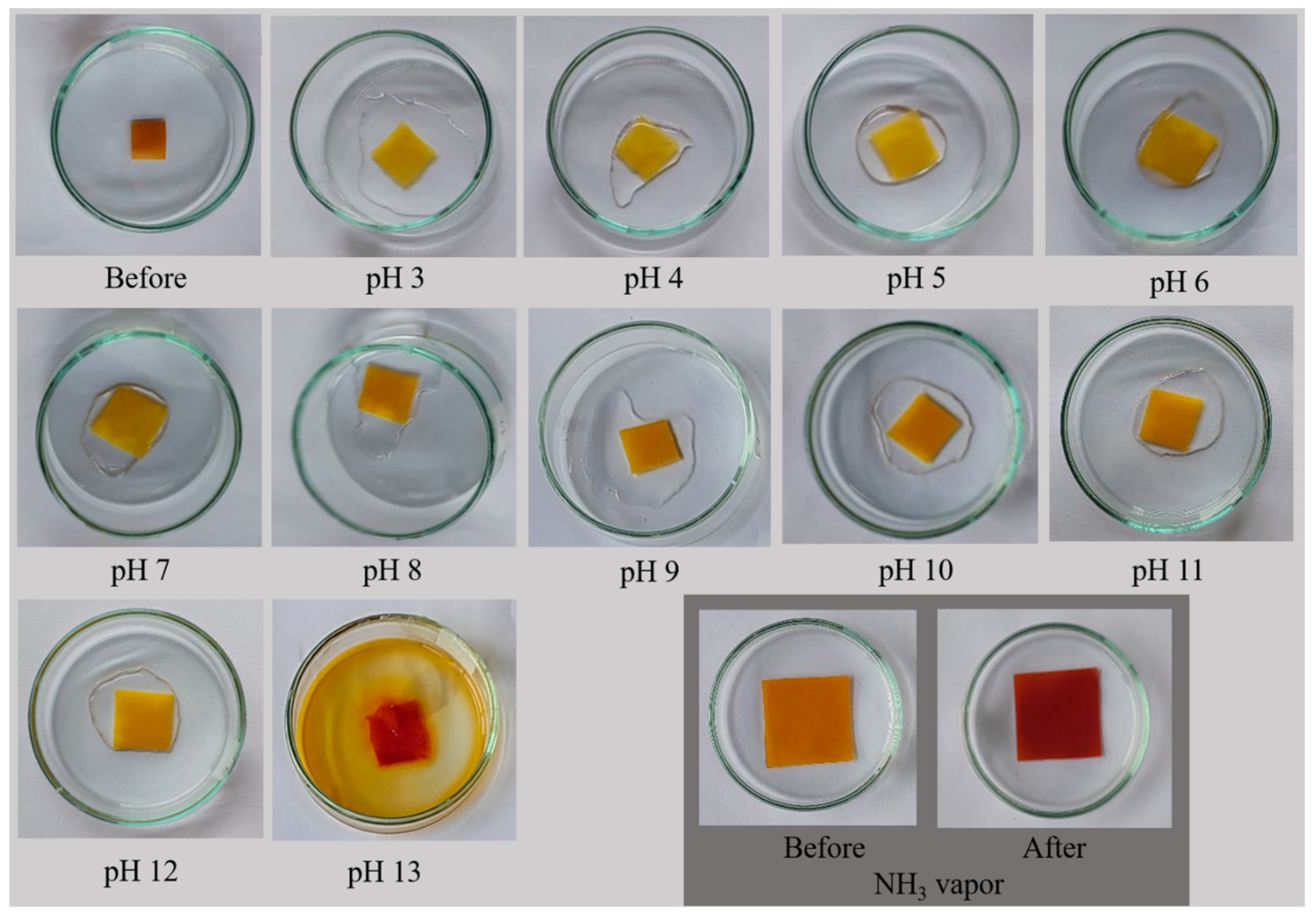
3.10. pH Sensitivity of the Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cofelice, M.; Lopez, F.; Cuomo, F. Quality Control of Fresh-Cut Apples After Coating Application. Foods 2019, 8, 189. [Google Scholar] [CrossRef] [PubMed]
- Senturk Parreidt, T.; Müller, K.; Schmid, M. Alginate-Based Edible Films and Coatings for Food Packaging Applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H. Edible Films and Coatings: A Review. In Innovations in Food Packaging, 2nd ed.; Han, J.H., Ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2014; Chapter 9; pp. 213–255. [Google Scholar] [CrossRef]
- Aayush, K.; McClements, D.J.; Sharma, S.; Sharma, R.; Singh, G.P.; Sharma, K.; Oberoi, K. Innovations in the Development and Application of Edible Coatings for Fresh and Minimally Processed Apple. Food Control 2022, 141, 109188. [Google Scholar] [CrossRef]
- Chen, S.; Brahma, S.; Mackay, J.; Cao, C.; Aliakbarian, B. The Role of Smart Packaging System in Food Supply Chain. J. Food Sci. 2020, 85, 517. [Google Scholar] [CrossRef]
- Boarca, B.; Lungu, I.; Holban, A.M. 3-Bioactive Packaging for Modern Beverage Industry. In Trends in Beverage Packaging; Grumezescu, A.M., Holban, A.M.M., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2019; Volume 16, pp. 51–71. [Google Scholar] [CrossRef]
- Han, B.; Chen, P.; Guo, J.; Yu, H.; Zhong, S.; Li, D.; Liu, C.; Feng, Z.; Jiang, B. A Novel Intelligent Indicator Film: Preparation, Characterization, and Application. Molecules 2023, 28, 3384. [Google Scholar] [CrossRef]
- Kurek, M.; Hlupić, L.; Ščetar, M.; Bosiljkov, T.; Galić, K. Comparison of Two pH Responsive Color Changing Bio-Based Films Containing Wasted Fruit Pomace as a Source of Colorants. J. Food Sci. 2019, 84, 2490. [Google Scholar] [CrossRef]
- Teixeira, R.F.; Balbinot Filho, C.A.; Borges, C.D. Essential Oils as Natural Antimicrobials for Application in Edible Coatings for Minimally Processed Apple and Melon: A Review on Antimicrobial Activity and Characteristics of Food Models. Food Packag. Shelf Life 2022, 31, 100781. [Google Scholar] [CrossRef]
- Valero, D.; Díaz-Mula, H.M.; Zapata, P.J.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Serrano, M. Effects of Alginate Edible Coating on Preserving Fruit Quality in Four Plum Cultivars During Postharvest Storage. Postharvest Biol. Technol. 2013, 77, 1–6. [Google Scholar] [CrossRef]
- Khan, U.M.; Selamoglu, Z. Nutritional and Medical Perspectives of Whey Protein: A Historical Overview. J. Pharm. Care 2019, 7, 112–117. [Google Scholar] [CrossRef]
- Chamchoy, K.; Thiangtrong, A.; Pisitsak, P.; Vanichvattanadecha, C. Magnetic Composite Sponges Based on Chitosan and Whey Protein Modified Magnetite Nanoparticles for Dye Removal from Water. J. Porous Mater. 2021, 29, 381–391. [Google Scholar] [CrossRef]
- Perez-Gago, M.B.; Serra, M.; Del Rio, M. Color Change of Fresh-Cut Apples Coated with Whey Protein Concentrate-Based Edible Coatings. Postharvest Biol. Technol. 2006, 39, 84–92. [Google Scholar] [CrossRef]
- Feng, Z.; Wu, G.; Liu, C.; Li, D.; Jiang, B.; Zhang, X. Edible Coating Based on Whey Protein Isolate Nanofibrils for Antioxidation and Inhibition of Product Browning. Food Hydrocoll. 2018, 79, 179–188. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the Golden Nutraceutical: Multitargeting for Multiple Chronic Diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef] [PubMed]
- Pisitsak, P.; Ruktanonchai, U. Preparation, Characterization, and In Vitro Evaluation of Antibacterial Sol–Gel Coated Cotton Textiles with Prolonged Release of Curcumin. Text. Res. J. 2015, 85, 949–959. [Google Scholar] [CrossRef]
- Yilmaz, A.; Bozkurt, F.; Cicek, P.K.; Dertli, E.; Durak, M.Z.; Yilmaz, M.T. A Novel Antifungal Surface-Coating Application to Limit Postharvest Decay on Coated Apples: Molecular, Thermal and Morphological Properties of Electrospun Zein–Nanofiber Mats Loaded with Curcumin. Innov. Food Sci. Emerg. Technol. 2016, 37, 74–83. [Google Scholar] [CrossRef]
- ASTM E313; Standard Practice for Calculating Yellowness and Whiteness Indices from Instrumentally Measured Color Coordinates. ASTM International: West Conshohocken, PA, USA, 2015.
- ASTM D1003-13; Standard Test Method for Haze and Luminous Transmittance of Transparent Plastics. ASTM International: West Conshohocken, PA, USA, 2013.
- Chitichotpanya, P.; Pisitsak, P.; Chitichotpanya, C. Sericin–Copper-Functionalized Silk Fabrics for Enhanced Ultraviolet Protection and Antibacterial Properties Using Response Surface Methodology. Text. Res. J. 2019, 89, 1166–1179. [Google Scholar] [CrossRef]
- AATCC TM183:2020; Transmittance or Blocking of Erythemally Weighted Ultraviolet Radiation through Fabrics. American Association of Textile Chemists and Colorists: Research Triangle, NC, USA, 2020.
- ASTM F1249; Standard Test Method for Water Vapor Transmission Rate through Plastic Film and Sheeting Using a Modulated Infrared Sensor. ASTM International: West Conshohocken, PA, USA, 2022.
- Lalnunthari, C.; Devi, L.M.; Badwaik, L.S. Extraction of Protein and Pectin from Pumpkin Industry By-Products and Their Utilization for Developing Edible Film. J. Food Sci. Technol. 2020, 57, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Chakravartula, S.S.N.; Soccio, M.; Lotti, N.; Balestra, F.; Dalla Rosa, M.; Siracusa, V. Characterization of Composite Edible Films Based on Pectin/Alginate/Whey Protein Concentrate. Materials 2019, 12, 2454. [Google Scholar] [CrossRef]
- Wu, H.; Lei, Y.; Lu, J.; Zhu, R.; Xiao, D.; Jiao, C.; Xia, R.; Zhang, Z.; Shen, G.; Liu, Y. Effect of Citric Acid Induced Crosslinking on the Structure and Properties of Potato Starch/Chitosan Composite Films. Food Hydrocoll. 2019, 97, 105208. [Google Scholar] [CrossRef]
- Wilpiszewska, K.; Antosik, A.K.; Zdanowicz, M. The Effect of Citric Acid on Physicochemical Properties of Hydrophilic Carboxymethyl Starch-Based Films. J. Polym. Environ. 2019, 27, 1379–1387. [Google Scholar] [CrossRef]
- Kurien, B.T.; Singh, A.; Matsumoto, H.; Scofield, R.H. Improving the Solubility and Pharmacological Efficacy of Curcumin by Heat Treatment. Assay Drug Dev. Technol. 2007, 5, 567–576. [Google Scholar] [CrossRef]
- Lai, W.-F. Design of Polymeric Films for Antioxidant Active Food Packaging. Int. J. Mol. Sci. 2021, 23, 12. [Google Scholar] [CrossRef] [PubMed]
- Ak, T.; Gülçin, I. Antioxidant and Radical Scavenging Properties of Curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- Ezati, P.; Khan, A.; Priyadarshi, R.; Bhattacharya, T.; Tammina, S.K.; Rhim, J.-W. Biopolymer-based UV Protection Functional Films for Food Packaging. Food Hydrocoll. 2023, 230, 108771. [Google Scholar] [CrossRef]
- Gorenšiek, M.; Sluga, F.; Urbas, R. Improving the Ultraviolet Protection Factor of Cotton Fabric. AATCC Rev. 2007, 7, 44–48. [Google Scholar]
- Chowdhury, S.; Teoh, Y.L.; Ong, K.M.; Zaidi, N.S.R.; Mah, S.-K. Poly(vinyl)alcohol Crosslinked Composite Packaging Film Containing Gold Nanoparticles on Shelf Life Extension of Banana. Food Packag. Shelf Life 2020, 24, 100463. [Google Scholar] [CrossRef]
- Júnior, L.M.; da Silva, R.G.; Vieira, R.P.; Alves, R.M.V. Water Vapor Sorption and Permeability of Sustainable Alginate/Collagen/SiO2 Composite Films. LWT 2021, 152, 112261. [Google Scholar] [CrossRef]
- bt Ibrahim, S.F.; Mohd Azam, N.A.N.; Mat Amin, K.A. Sodium alginate film: The Effect of Crosslinker on Physical and Mechanical Properties. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; p. 012063. [Google Scholar] [CrossRef]
- Baek, S.K.; Song, K.B. Characterization of Active Biodegradable Films Based on Proso Millet Starch and Curcumin. Starke 2019, 71, 1800174. [Google Scholar] [CrossRef]
- Rodrigues, C.; Souza, V.G.L.; Coelhoso, I.; Fernando, A.L. Bio-Based Sensors for Smart Food Packaging—Current Applications and Future Trends. Sensors 2021, 21, 2148. [Google Scholar] [CrossRef]
- Mondal, S.; Ghosh, S.; Moulik, S.P. Stability of Curcumin in Different Solvent and Solution Media: UV–Visible and Steady-State Fluorescence Spectral Study. Photochem. Photobiol. B Biol. 2016, 158, 212–218. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.-W. pH-responsive Pectin-based Multifunctional Films Incorporated with Curcumin and Sulfur Nanoparticles. Carbohydr. Polym. 2020, 230, 115638. [Google Scholar] [CrossRef]
- Taghinia, P.; Abdolshahi, A.; Sedaghati, S.; Shokrollahi, B. Smart Edible Films Based on Mucilage of Lallemantia Iberica Seed Incorporated with Curcumin for Freshness Monitoring. Food Sci. Nutr. 2021, 9, 1222–1231. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Zou, X.; Shi, J.; Zhai, X.; Liu, L.; Li, Z.; Holmes, M.; Gong, Y.; Povey, M. A Visual Indicator Based On Curcumin With High Stability For Monitoring The Freshness Of Freshwater Shrimp, Macrobrachium rosenbergii. J. Food Eng. 2021, 292, 110290. [Google Scholar] [CrossRef]
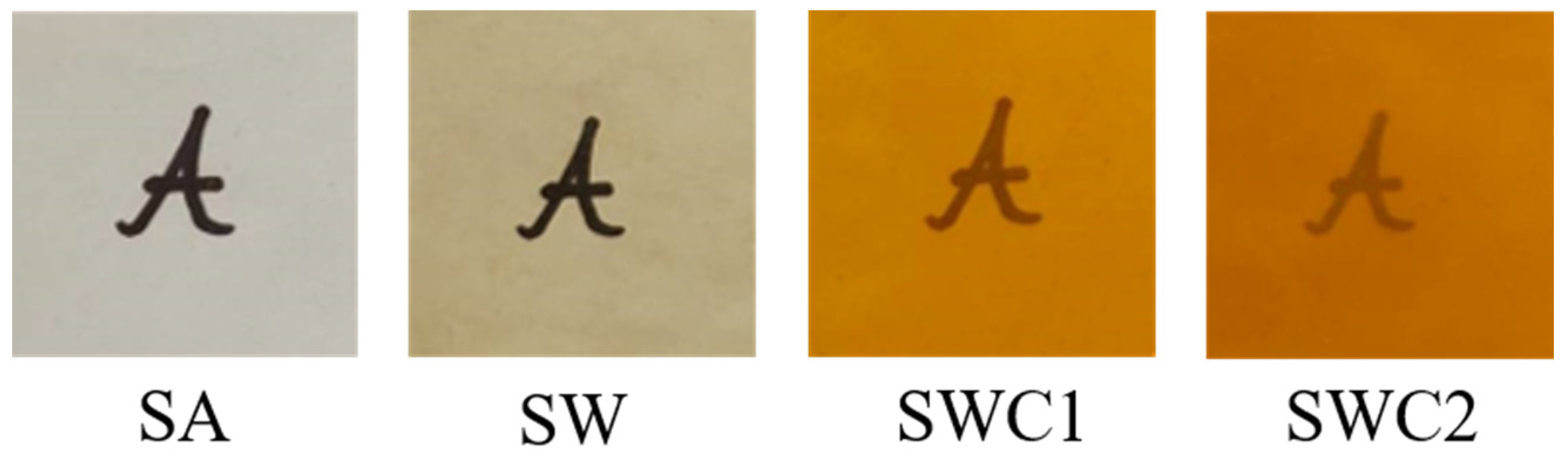
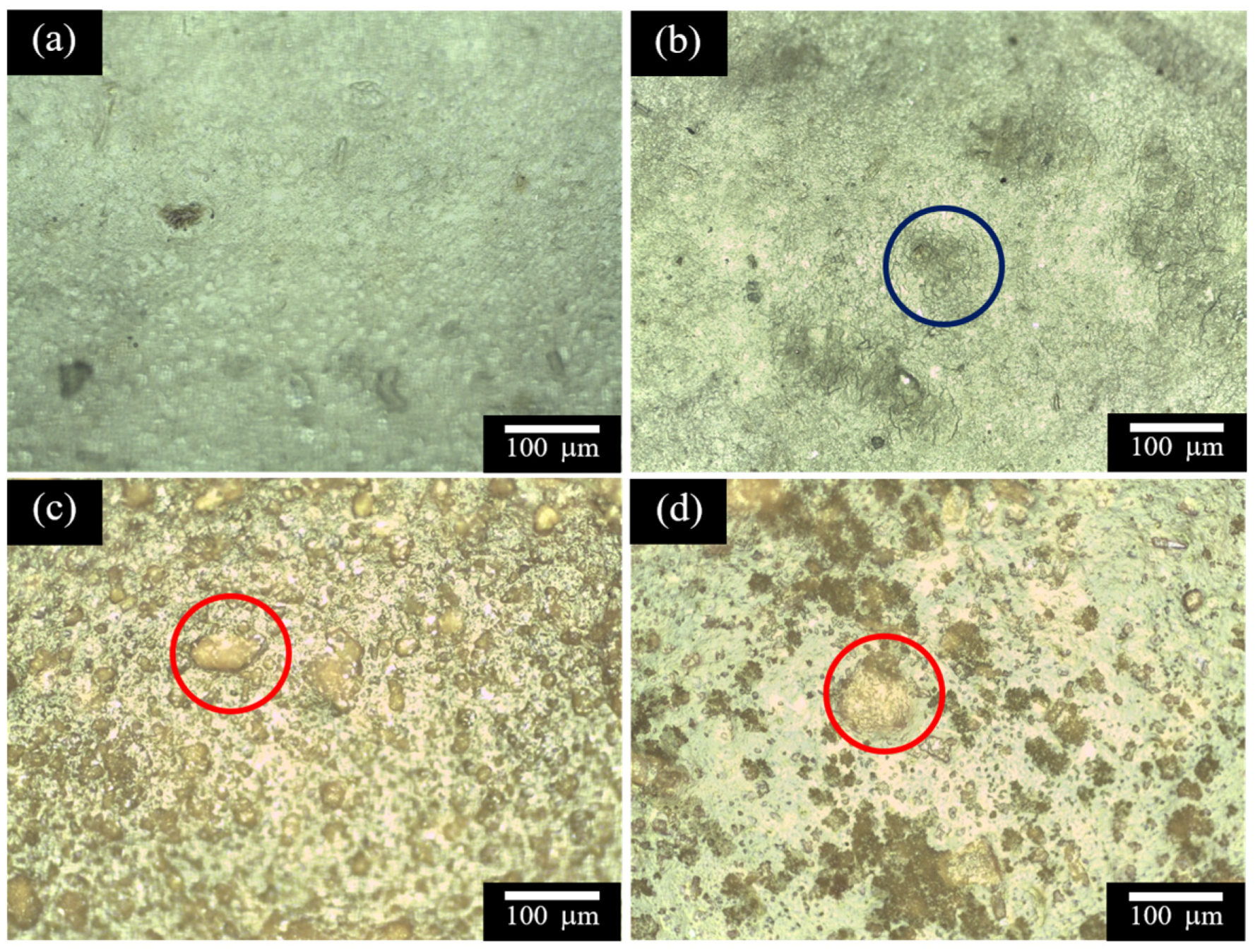
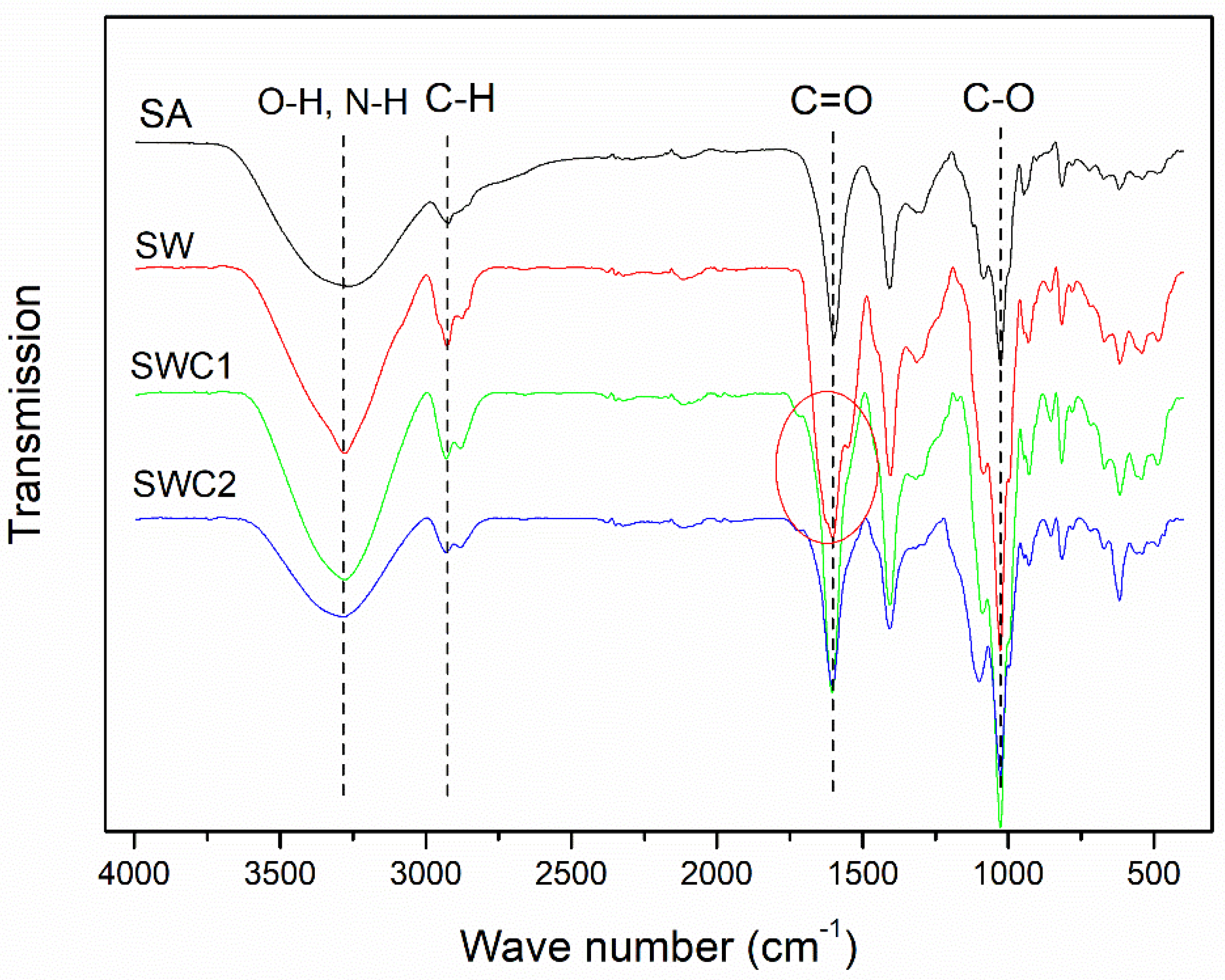
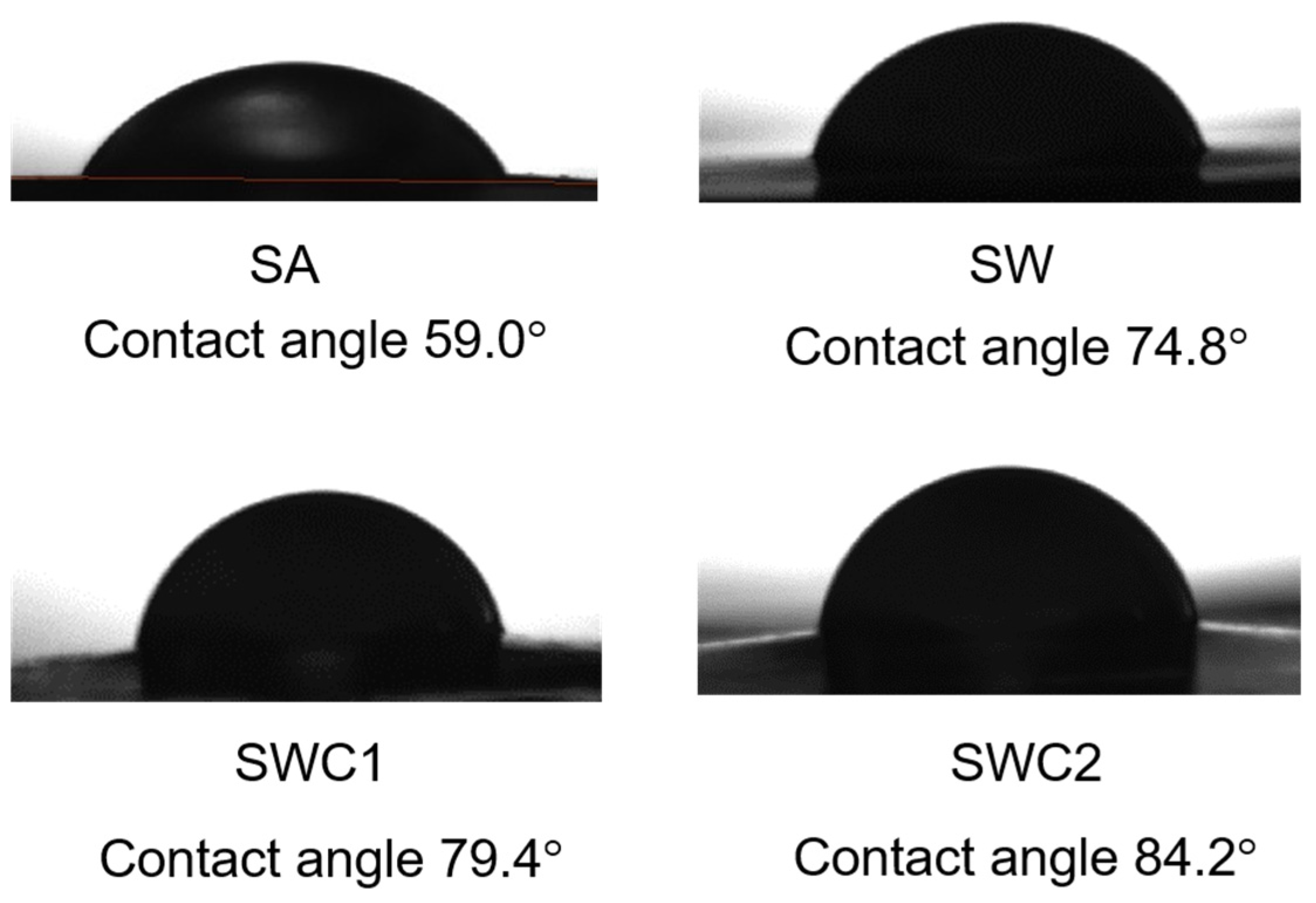

| Sample | L* | a* | b* | C* | ho | Yellowness Index | Haze (%) |
|---|---|---|---|---|---|---|---|
| SA | 91.55 ± 1.41 | −0.99 ± 0.25 | 16.27 ± 0.86 | 16.30 ± 0.86 | 93.49 ± 0.98 | 37.67 ± 0.20 | 18.03 ± 0.56 |
| SW | 92.85 ± 1.11 | −0.93 ± 0.13 | 15.51 ± 0.54 | 15.53 ± 0.55 | 93.43 ± 0.44 | 25.98 ± 0.14 | 50.01 ± 0.40 |
| SWC1 | 68.84 ± 1.13 | 14.34 ± 0.71 | 96.55 ± 0.59 | 97.61 ± 0.69 | 81.55 ± 0.36 | 127.74 ± 0.38 | 61.34 ± 0.42 |
| SWC2 | 51.52 ± 1.20 | 30.28 ± 0.67 | 86.40 ± 0.88 | 91.55 ± 0.99 | 70.69 ± 0.31 | 129.84 ± 0.50 | 63.67 ± 0.33 |
| Sample | Water Solubility (%) | Swelling Degree |
|---|---|---|
| SA | 75.55 ± 3.85 | 745.33 ± 6.11 |
| SW | 60.44 ± 1.84 | 887.47 ± 2.60 |
| SWC1 | 34.22 ± 1.17 | 1284.67 ± 2.60 |
| SWC2 | 16.67 ± 3.79 | 1526 ± 2.23 |
| Sample | AA (%) |
|---|---|
| SA | 12.54 ± 0.88 |
| SW | 19.65 ± 0.27 |
| SWC1 | 60.07 ± 0.07 |
| SWC2 | 82.39 ± 0.39 |
| Sample | WVTR (g·m−2·day−1) |
|---|---|
| SA | 569.44 ± 32.14 |
| SW | 533.75 ± 27.89 |
| SWC1 | 452.75 ± 9.47 |
| SWC2 | 454.49 ± 6.76 |
| Sample | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| SW | 37.25 ± 5.30 | 34.74 ± 7.99 |
| SWC1 | 18.64 ± 4.82 | 53.8 ± 3.46 |
| SWC2 | 17.39 ± 3.25 | 37.11 ± 3.82% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botalo, A.; Inprasit, T.; Ummartyotin, S.; Chainok, K.; Vatthanakul, S.; Pisitsak, P. Smart and UV-Resistant Edible Coating and Films Based on Alginate, Whey Protein, and Curcumin. Polymers 2024, 16, 447. https://doi.org/10.3390/polym16040447
Botalo A, Inprasit T, Ummartyotin S, Chainok K, Vatthanakul S, Pisitsak P. Smart and UV-Resistant Edible Coating and Films Based on Alginate, Whey Protein, and Curcumin. Polymers. 2024; 16(4):447. https://doi.org/10.3390/polym16040447
Chicago/Turabian StyleBotalo, Atcharaporn, Thitirat Inprasit, Sarute Ummartyotin, Kittipong Chainok, Suteera Vatthanakul, and Penwisa Pisitsak. 2024. "Smart and UV-Resistant Edible Coating and Films Based on Alginate, Whey Protein, and Curcumin" Polymers 16, no. 4: 447. https://doi.org/10.3390/polym16040447
APA StyleBotalo, A., Inprasit, T., Ummartyotin, S., Chainok, K., Vatthanakul, S., & Pisitsak, P. (2024). Smart and UV-Resistant Edible Coating and Films Based on Alginate, Whey Protein, and Curcumin. Polymers, 16(4), 447. https://doi.org/10.3390/polym16040447




_CHAINOK.jpg)


