Natural Polymers as Carriers for Encapsulation of Volatile Oils: Applications and Perspectives in Food Products
Abstract
1. Introduction
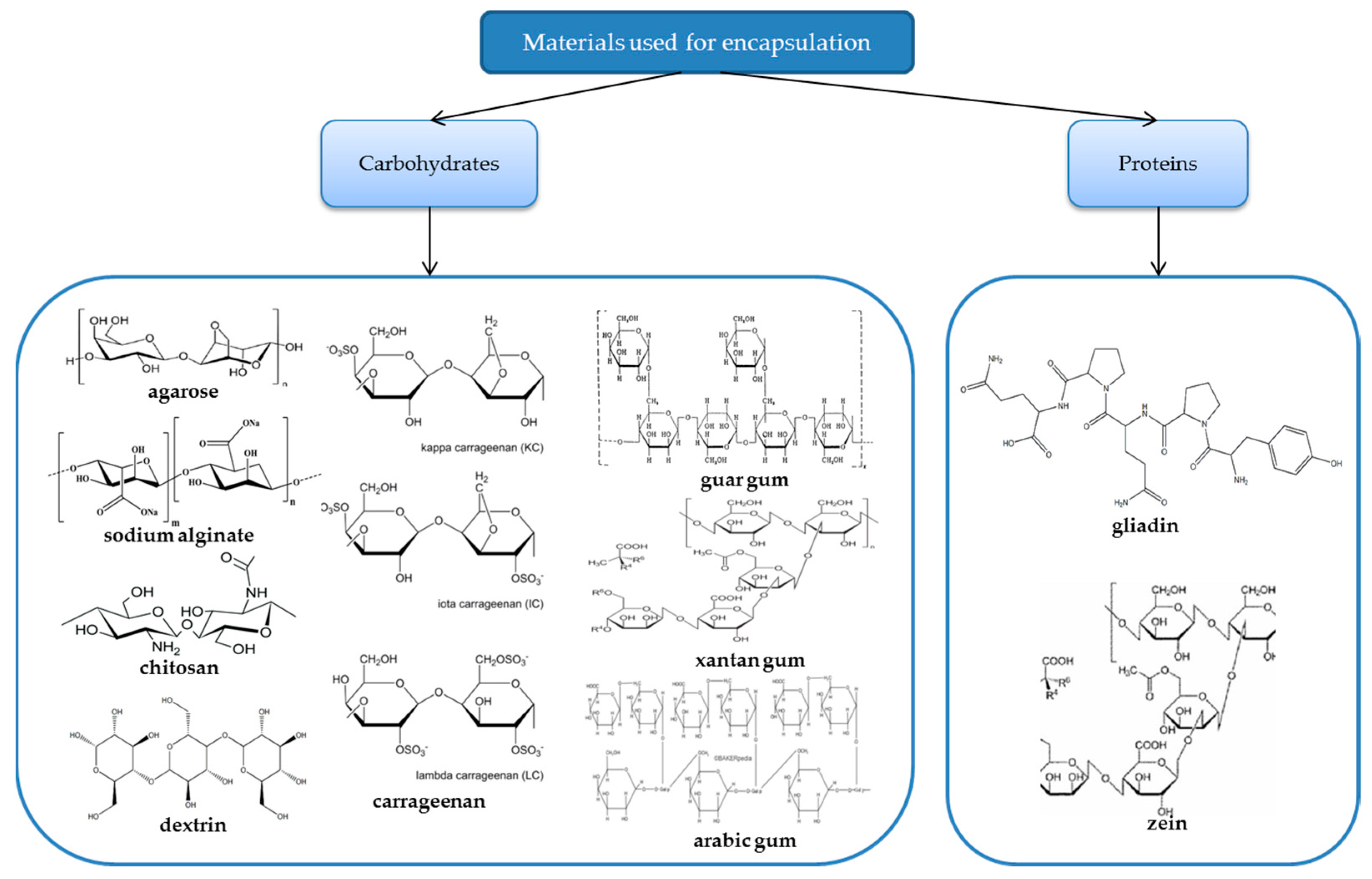
2. Natural Polymers Used for Encapsulation
2.1. Bibliographic Research Methodology
2.2. Agarose
2.3. Carrageenan
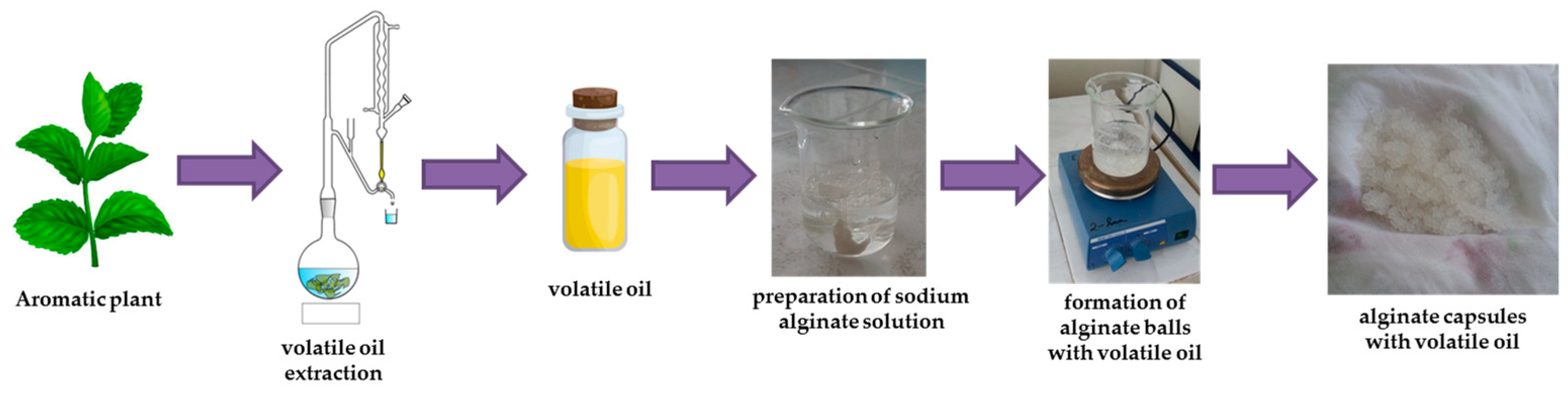
2.4. Alginate
2.5. Chitosan
2.6. Dextrin
2.7. Gum
2.8. Gliadin
2.9. Zein
3. Latest Advances in Food and Dairy Products with Encapsulated Volatile Oils
3.1. Bibliographic Research Methodology
3.2. Food Products with Encapsulated Volatile Oils
3.3. Dairy Products with Encapsulated Volatile Oils
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schlösser, I.; Prange, A. Antifungal Activity of Selected Natural Preservatives against Aspergillus westerdijkiae and Penicillium verrucosum and the Interactions of These Preservatives with Food Components. J. Food Prot. 2019, 82, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Tanu, B.; Harpreet, K. Benefits of essential oil: A review. J. Chem. Pharm. Res. 2016, 8, 143–149. [Google Scholar]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential oils: Sources of antimicrobials and food preservatives. Front. Microbiol. 2017, 7, 2161. [Google Scholar] [CrossRef] [PubMed]
- Whiley, H.; Gaskin, S.; Schroder, T.; Ross, K. Antifungal properties of essential oils for improvement of indoor air quality: A review. Rev. Environ. Health 2018, 33, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Coelho, J.P.; Cristino, A.F.; Matos, P.G.; Rauter, A.P.; Nobre, B.P.; Mendes, R.L.; Barroso, J.G.; Mainar, A.; Urieta, J.S.; Fareleira Palavra, J.M.N.A.; et al. Extraction of volatile oil from aromatic plants with supercritical carbon dioxide: Experiments and modeling. Molecules 2012, 17, 10550–10573. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.; Ramli, N.; Abd-Aziz, S.; Ibrahim, M.F. Comparison of hydro-distillation, hydro-distillation with enzyme-assisted and supercritical fluid for the extraction of essential oil from pineapple peels. 3 Biotech 2019, 9, 234. [Google Scholar] [CrossRef] [PubMed]
- Olalere, O.A.; Gan, C.Y.; Adeyi, O.; Taiwo, A.E.; Olaiya, F.G.; Adeyi, A.; Fawale, O.S. Upscalability and Techno-economic Perspectives of Nonconventional Essential Oils Extraction Techniques. Jundishapur J. Nat. Pharm. 2022, 17, e122792. [Google Scholar] [CrossRef]
- Arista, R.A.; Priosoeryanto, B.P.; Nurcholis, W. Profile Volatile Compounds in Essential Oils on Different Parts of Cardamom with Antioxidant Activity. Biointerface Res. Appl. Chem. 2023, 13, 328. [Google Scholar]
- Pande, K.R.; Preetha, R. Essential oil of fennel seeds as natural preservative in butter and its shelf life assessment. Asian J. Chem. 2017, 29, 711–714. [Google Scholar] [CrossRef]
- Aumeeruddy-Elalfi, Z.; Gurib-Fakim, A.; Mahomoodally, M.F. Kinetic studies of tyrosinase inhibitory activity of 19 essential oils extracted from endemic and exotic medicinal plants. S. Afr. J. Bot. 2016, 103, 89–94. [Google Scholar] [CrossRef]
- Dini, S.; Chen, Q.; Fatemi, F.; Asri, Y. Phytochemical and biological activities of some Iranian medicinal plants. Pharm. Biol. 2022, 60, 664–689. [Google Scholar] [CrossRef] [PubMed]
- Foodborne Diseases. Available online: https://www.who.int/health-topics/foodborne-diseases#tab=tab_1 (accessed on 16 March 2024).
- Fung, F.; Wang, H.S.; Menon, S. Food safety in the 21st century. Biomed. J. 2018, 41, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Alboofetileh, M.; Rezaei, M.; Hosseini, H.; Abdollahi, M. Antimicrobial activity of alginate/clay nanocomposite film enriched with essential oils against three common foodborne pathogens. Food Control 2014, 36, 1–7. [Google Scholar] [CrossRef]
- Lee, H.; Yoon, Y. Etiological Agents Implicated in Foodborne Illness World Wide. Food Sci. Anim. Resour. 2021, 41, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ehuwa, O.; Jaiswal, A.K.; Jaiswal, S. Salmonella, Food Safety and Food Handling Practices. Foods 2021, 10, 907. [Google Scholar] [CrossRef] [PubMed]
- Marasteanu, I.J.; Liggans, G.; Otto, J.; Lasher, A. Advancing Retail Food Policy Debates: Estimating the Risk of Contaminated Servings of Food Attributed to Employee Food Handling Practices in Retail Food Establishments. J. Food Prot. 2018, 81, 2034–2039. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Mallya, R.; Suvarna, V.; Khan, T.A.; Momin, M.; Omri, A. Nanoparticles—Attractive Carriers of Antimicrobial Essential Oils. Antibiotics 2022, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Abers, M.; Schroeder, S.; Goelz, L.; Sulser, A.; St. Rose, T.; Puchalski, K.; Langland, J. Antimicrobial activity of the volatile substances from essential oils. BMC Complement. Med. Ther. 2021, 21, 124. [Google Scholar] [CrossRef] [PubMed]
- Morais, V.P.; Fernandes, C.C.; Martins, C.H.G.; Crotti, A.E.M.; Miranda, M.L.D. Bioactive Hexane Extracts from Piper aduncum and Xylopia aromatica against Bacterial Strains which Cause Food Poisoning. Rev. Virtual Quim. 2023, 15, 1148–1153. [Google Scholar] [CrossRef]
- Julizan, N.; Ishmayana, S.; Zainuddin, A.; Van Hung, P.; Kurnia, D. Potential of Syzygnium polyanthum as Natural Food Preservative: A Review. Foods 2023, 12, 2275. [Google Scholar] [CrossRef]
- Tiţa, O.; Constantinescu, A.M.; Tiţa, M.A. Antioxidant and Antiseptic Properties of Volatile Oils from Different Medicinal Plants: A Review. Int. J. Pharmacogn. Chin. Med. 2019, 3, 000179. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Ben Halima, N.; Abdelkafi, S.; Hamdi, N. Essential oil from Artemisia phaeolepis: Chemical composition and antimicrobial activities. J. Oleo Sci. 2013, 62, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Clavijo-Romero, A.; Quintanilla-Carvajal, M.X.; Ruiz, Y. Stability and antimicrobial activity of eucalyptus essential oil emulsions. Food Sci. Technol. Int. 2019, 25, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Torres-Alvarez, C.; Núñez González, A.; Rodríguez, J.; Castillo, S.; Leos-Rivas, C.; Báez-González, J.G. Perfil químico, actividad antimicrobiana y antioxidante del aceite esencial de naranja y sus aceites concentrados. CyTA J. Food 2017, 15, 129–135. [Google Scholar]
- Djilani, A.; Dicko, A. The Therapeutic Benefits of Essential Oils. In Nutrition, Well-Being and Health; Bouayed, J., Bohn, T., Eds.; IntechOpen: London, UK, 2012; pp. 155–168. [Google Scholar]
- Blejan, E.I.; Popa, D.E.; Costea, T.; Cioacă, A.; Olariu, L.; Ghica, M.; Georgescu, M.; Stancov, G.; Arsene, A.L. The in vitro antimicrobial activity of some essential oils from aromatic plants. Farmacia 2021, 69, 290–298. [Google Scholar] [CrossRef]
- Dávila-Rodríguez, M.; López-Malo, A.; Palou, E.; Ramírez-Corona, N.; Jiménez-Munguía, M.T. Essential oils microemulsions prepared with high-frequency ultrasound: Physical properties and antimicrobial activity. J. Food Sci. Technol. 2020, 57, 4133–4142. [Google Scholar] [CrossRef] [PubMed]
- Özkinali, S.; Şener, N.; Gür, M.; Güney, K.; Olgun, Ç. Antimicrobial activity and chemical composition of coriander & galangal essential oil. Indian J. Pharm. Educ. Res. 2017, 51, S221–S224. [Google Scholar]
- Tanhaeian, A.; Sekhavati, M.H.; Moghaddam, M. Antimicrobial activity of some plant essential oils and an antimicrobial-peptide against some clinically isolated pathogens. Chem. Biol. Technol. Agric. 2020, 7, 13. [Google Scholar] [CrossRef]
- Angelini, P.; Pagiotti, R.; Menghini, A.; Vianello, B. Antimicrobial activities of various essential oils against foodborne pathogenic or spoilage moulds. Ann. Microbiol. 2006, 56, 65–69. [Google Scholar] [CrossRef]
- Reyes-Jurado, F.; López-Malo, A.; Palou, E. Antimicrobial activity of individual and combined essential oils against foodborne pathogenic bacteria. J. Food Prot. 2016, 79, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Matasyoh, J.; Maiyo, Z.K.; Raphael, N.; Chepkorir, R. Chemical composition and antimicrobial activity of essential oil of Coriandrum sativum. Food Chem. 2009, 113, 526–529. [Google Scholar] [CrossRef]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Antimicrobial Activity of Basil (Ocimum basilicum) Oil against Salmonella enteritidis In Vitro and in Food. Biosci. Biotechnol. Biochem. 2010, 74, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- de Boer, F.Y.; Imhof, A.; Velikov, K.P. Encapsulation of colorants by natural polymers for food applications. Color. Technol. 2019, 135, 183–194. [Google Scholar] [CrossRef]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural polymers for the microencapsulation of cells. J. R. Soc. Interface 2014, 11, 20140817. [Google Scholar] [CrossRef]
- Wang, L.; Yang, S.; Cao, J.; Zhao, S.; Wang, W. Microencapsulation of ginger volatile oil based on gelatin/sodium alginate polyelectrolyte complex. Chem. Pharm. Bull. 2016, 64, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Kokina, M.; Shamtsyan, M.; Georgescu, C.; Mironescu, M.; Nedovic, V. Essential oil/alginate microcapsules; obtaining and applying. Immunopathol. Persa 2019, 5, e04. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, T.; Chai, X.; Duan, X.; He, D.; Yu, H.; Liu, X.; Tao, Z. Encapsulation Efficiency and Functional Stability of Cinnamon Essential Oil in Modified β-cyclodextrins: In Vitro and In Silico Evidence. Foods 2023, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.P.; Shukla, T.; Dhote, V.K.; Mishra, D.K.; Maheshwari, R.; Tekade, R.K. Use of Polymers in Controlled Release of Active Agents. In Basic Fundamentals of Drug Delivery; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA; Elsevier: Cambridge, MA, USA, 2019; pp. 113–172. [Google Scholar]
- Alehosseini, A.; Gomez del Pulgar, E.M.; José Fabra, M.; Gómez-Mascaraque, L.G.; Benítez-Páez, A.; Sarabi-Jamab, M.; Ghorani, B.; Lopez-Rubio, A. Agarose-based freeze-dried capsules prepared by the oil-induced biphasic hydrogel particle formation approach for the protection of sensitive probiotic bacteria. Food Hydrocoll. 2019, 87, 487–496. [Google Scholar] [CrossRef]
- Xiang Ping, M.K.; Zhi, H.W.; Aziz, N.S.; Hadri, N.A.; Ghazalli, N.F.; Yusop, N. Optimization of agarose–alginate hydrogel bead components for encapsulation and transportation of stem cells. J. Taibah Univ. Med. Sci. 2023, 18, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Reys, L.L.; Silva, S.S.; Soares da Costa, D.; Rodrigues, L.C.; Reis, R.L.; Silva, T.H. Building Fucoidan/Agarose-Based Hydrogels as a Platform for the Development of Therapeutic Approaches against Diabetes. Molecules 2023, 28, 4523. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Mascaraque, L.G.; Lagarón, J.M.; López-Rubio, A. Electrosprayed gelatin submicroparticles as edible carriers for the encapsulation of polyphenols of interest in functional foods. Food Hydrocoll. 2015, 49, 42–52. [Google Scholar] [CrossRef]
- Postolovic, K.; Antoijevic, M.; Ljuji, B.; Gazdi, M. pH-Responsive Hydrogel Beads Based on Alginate, κ-Carrageenan and Poloxamer for Enhanced Curcumin, Natural Bioactive Compound, Encapsulation and Controlled Release Efficiency. Molecules 2022, 27, 4045. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.W.; Mirhosseini, H.; Taip, F.S.; Ling, T.C.; Nehdi, I.A.; Tan, C.P. Emulsion formulation optimization and characterization of spray-dried κ-carrageenan microparticles for the encapsulation of CoQ10. Food Sci. Biotechnol. 2016, 25, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, R.; Chen, L.; McClements, D.J. Encapsulation of lactase (β-galactosidase) into κ-carrageenan-based hydrogel beads: Impact of environmental conditions on enzyme activity. Food Chem. 2016, 200, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, D.; Yu, H.; Han, J.; Liu, W.; Qu, D. Encapsulation of Salmonella phage SL01 in alginate/carrageenan microcapsules as a delivery system and its application in vitro. Front. Microbiol. 2022, 13, 906103. [Google Scholar] [CrossRef] [PubMed]
- Polowsky, P.J.; Janaswamy, S. Hydrocolloid-based nutraceutical delivery systems: Effect of counter-ions on the encapsulation and release. Food Hydrocoll. 2015, 43, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Prasetyaningrum, A.; Wicaksono, B.S.; Hakiim, A.; Ashianti, A.D.; Manalu, S.F.C.; Rokhati, N.; Utomo, D.P.; Djaeni, M. Ultrasound-Assisted Encapsulation of Citronella Oil in Alginate/Carrageenan Beads: Characterization and Kinetic Models. Chem. Eng. 2023, 7, 10. [Google Scholar] [CrossRef]
- Weng, Y.; Yang, G.; Li, Y.; Xu, L.; Chen, X.; Song, H.; Zhao, C.-X. Alginate-based materials for enzyme encapsulation. Adv. Colloid Interface Sci. 2023, 318, 102957. [Google Scholar] [CrossRef] [PubMed]
- Ashimova, A.; Yegorov, S.; Negmetzhanov, B.; Hortelano, G. Cell Encapsulation within Alginate Microcapsules: Immunological Challenges and Outlook. Front. Bioeng. Biotechnol. 2019, 7, 380. [Google Scholar] [CrossRef] [PubMed]
- Riseh, R.S.; Skorik, Y.A.; Thakur, V.K.; Pour, M.M.; Tamanadar, E.; Noghabi, S.S. Encapsulation of plant biocontrol bacteria with alginate as a main polymer material. Int. J. Mol. Sci. 2021, 22, 11165. [Google Scholar] [CrossRef]
- Milivojević, M.; Popović, A.; Pajić-Lijaković, I.; Šoštarić, I.; Kolašinac, S.; Stevanović, Z.D. Alginate Gel-Based Carriers for Encapsulation of Carotenoids: On Challenges and Applications. Gels 2023, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Puertas-Bartolomé, M.; Mora-Boza, A.; García-Fernández, L. Emerging biofabrication techniques: A review on natural polymers for biomedical applications. Polymers 2021, 13, 1209. [Google Scholar] [CrossRef] [PubMed]
- Raza, Z.A.; Khalil, S.; Ayub, A.; Banat, I.M. Recent developments in chitosan encapsulation of various active ingredients for multifunctional applications. Carbohydr. Res. 2020, 492, 108004. [Google Scholar] [CrossRef] [PubMed]
- Abreu, F.O.M.S.; Oliveira, E.F.; Paula, H.C.B.; De Paula, R.C.M. Chitosan/cashew gum nanogels for essential oil encapsulation. Carbohydr. Polym. 2012, 89, 1277–1282. [Google Scholar] [CrossRef] [PubMed]
- Negi, A.; Kesari, K.K. Chitosan Nanoparticle Encapsulation of Antibacterial Essential Oils. Micromachines 2022, 13, 1265. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Kim, Y.; Kang, J.; Jeong, S.Y.; Yoo, H.S. Electrospun chitosan microspheres for complete encapsulation of anionic proteins: Controlling particle size and encapsulation efficiency. AAPS Pharm. Sci. Tech. 2013, 14, 794–801. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Detsi, A.; Kavetsou, E.; Kostopoulou, I.; Pitterou, I.; Pontillo, A.R.N.; Tzani, A.; Christodoulou, P.; Siliachli, A.; Zoumpoulakis, P. Nanosystems for the encapsulation of natural products: The case of chitosan biopolymer as a matrix. Pharmaceutics 2020, 12, 669. [Google Scholar] [CrossRef] [PubMed]
- Djaenudin; Budianto, E.; Saepudin, E.; Nasir, M. The encapsulation of Lactobacillus casei probiotic bacteria based on sodium alginate and chitosan. IOP Conf. Ser. Earth Environ. Sci. 2020, 483, 012043. [Google Scholar] [CrossRef]
- Suzery, M.; Hadiyanto, H.; Majid, D.; Setyawan, D.; Sutanto, H. Improvement of Stability and Antioxidant Activities by Using Phycocyanin-Chitosan Encapsulation Technique. IOP Conf. Ser. Earth Environ. Sci. 2016, 755, 1. [Google Scholar] [CrossRef]
- Yoplac, I.; Vargas, L.; Robert, P.; Hidalgo, A. Characterization and antimicrobial activity of microencapsulated citral with dextrin by spray drying. Heliyon 2021, 7, e06737. [Google Scholar] [CrossRef]
- Silva, D.M.; Nunes, C.; Pereira, I.; Moreira, A.S.P.; Domingues, M.R.M.; Coimbra, M.A.; Gama, F.M. Structural analysis of dextrins and characterization of dextrin-based biomedical hydrogels. Carbohydr. Polym. 2014, 114, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Todorović, A.; Šturm, L.; Salević-Jelić, A.; Lević, S.; Osojnik Črnivec, I.G.; Prislan, I.; Skrt, M.; Bjeković, A.; Ulrih, N.P.; Nedović, V. Encapsulation of Bilberry Extract with Maltodextrin and Gum Arabic by Freeze-Drying: Formulation, Characterisation, and Storage Stability. Processes 2022, 10, 1991. [Google Scholar] [CrossRef]
- Zabot, G.L.; Schaefer Rodrigues, F.; Polano Ody, L.; Vinícius Tres, M.; Herrera, E.; Palacin, H.; Córdova-Ramos, J.S.; Best, I.; Olivera-Montenegro, L. Encapsulation of Bioactive Compounds for Food and Agricultural Applications. Polymers 2022, 14, 4194. [Google Scholar] [CrossRef] [PubMed]
- Oliyaei, N.; Moosavi-Nasab, M.; Tamaddon, A.M.; Fazaeli, M. Double encapsulation of fucoxanthin using porous starch through sequential coating modification with maltodextrin and gum Arabic. Food Sci. Nutr. 2020, 8, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Gao, J.; Li, Y.; Liu, C.; Shi, J.; Ni, F.; Ren, G.; Xie, H. Complexation of β-lactoglobulin with gum arabic: Effect of heat treatment and enhanced encapsulation efficiency. Food Sci. Nutr. 2021, 9, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Narsaiah, K.; Jha, S.N.; Wilson, R.A.; Mandge, H.M.; Manikantan, M.R. Optimizing microencapsulation of nisin with sodium alginate and guar gum. J. Food Sci. Technol. 2014, 51, 4054–4059. [Google Scholar] [CrossRef] [PubMed]
- Tahmouzi, S.; Meftahizadeh, H.; Eyshi, S.; Mahmoudzadeh, A.; Alizadeh, B.; Mollakhalili-Meybodi, N.; Hatami, M. Application of guar (Cyamopsis tetragonoloba L.) gum in food technologies: A review of properties and mechanisms of action. Food Sci. Nutr. 2023, 11, 4869–4897. [Google Scholar] [CrossRef] [PubMed]
- Sharif, N.; Golmakani, M.T.; Niakousari, M.; Ghorani, B.; Lopez-Rubio, A. Food-grade gliadin microstructures obtained by electrohydrodynamic processing. Food Res. Int. 2019, 116, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wei, Z.; Li, X. Development, Characterization and Resveratrol Delivery of Hollow Gliadin Nanoparticles: Advantages over Solid Gliadin Nanoparticles. Foods 2023, 12, 2436. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wei, Z.; Wang, X.; Wang, Y.; Tang, Q.; Huang, Q.; Xue, C. Fabrication and characterization of core-shell gliadin/tremella polysaccharide nanoparticles for curcumin delivery: Encapsulation efficiency, physicochemical stability and bioaccessibility. Curr. Res. Food Sci. 2022, 5, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Peterson, S.C. Optimal conditions for the encapsulation of menthol into zein nanoparticles. LWT 2021, 144, 111213. [Google Scholar] [CrossRef]
- Yan, X.; Li, M.; Xu, X.; Liu, X.; Liu, F. Zein-based nano-delivery systems for encapsulation and protection of hydrophobic bioactives: A review. Front. Nutr. 2022, 9, 999373. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.A.; Orqueda, M.E.; Gómez-Mascaraque, L.G.; Isla, M.I.; López-Rubio, A. Crosslinked electrospun zein-based food packaging coatings containing bioactive chilto fruit extracts. Food Hydrocoll. 2019, 95, 496–505. [Google Scholar] [CrossRef]
- Rout, S.; Tambe, S.; Deshmukh, R.K.; Mali, S.; Cruz, J.; Srivastav, P.P.; Amin, P.D.; Gaikwad, K.K.; de Aguiar Andrade, E.H.; Santana de Oliveira, M. Recent trends in the application of essential oils: The next generation of food preservation and food packaging. Trends Food Sci. 2022, 129, 421–439. [Google Scholar] [CrossRef]
- Fadel, H.H.M.; Hassan, I.M.; Ibraheim, M.T.; Abd El Mageed, M.A.; Saad, R. Effect of using cinnamon oil encapsulated in maltodextrin as exogenous flavouring on flavour quality and stability of biscuits. J. Food Sci. Technol. 2019, 56, 4565–4574. [Google Scholar] [CrossRef]
- Hossain, F.; Follett, P.; Salmieri, S.; Dang Vu, K.; Fraschini, C.; Lacroixa, M. Antifungal activities of combined treatments of irradiation and essential oils (EOs) encapsulated chitosan nanocomposite films in in vitro and in situ conditions. Int. J. Food Microbiol. 2019, 295, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Nie, R.; Du, J.; Sun, H.; Liu, G. Effect of Nanoemulsion Containing Enterocin GR17 and Cinnamaldehyde on Microbiological, Physicochemical and Sensory Properties and Shelf Life of Liquid-Smoked Salmon Fillets. Food 2023, 12, 78. [Google Scholar] [CrossRef]
- Tița, O.; Constantinescu, M.A.; Tița, M.A.; Georgescu, C. Use of yoghurt enhanced with volatile plant oils encapsulated in sodium alginate to increase the human body’s immunity in the present fight against stress. Int. J. Environ. Res. Public Health 2020, 17, 7588. [Google Scholar] [CrossRef] [PubMed]
- Tița, M.A.; Constantinescu, M.A.; Tița, O.; Mathe, E.; Tamošaitienė, L.; Bradauskienė, V. Food Products with High Antioxidant and Antimicrobial Activities and Their Sensory Appreciation. Appl. Sci. 2022, 12, 790. [Google Scholar] [CrossRef]
- Tița, O.; Constantinescu, M.A.; Tița, M.A.; Opruța, T.I.; Dabija, A.; Georgescu, C. Valorization on the Antioxidant Potential of Volatile Oils of Lavandula angustifolia Mill., Mentha piperita L. and Foeniculum vulgare L. in the Production of Kefir. Appl. Sci. 2022, 12, 10287. [Google Scholar] [CrossRef]
- Tița, M.A.; Constantinescu, M.A.; Opruța, T.I.; Bătuşaru, C.; Rusu, L.; Tița, O. Kefir Enriched with Encapsulated Volatile Oils: Investigation of Antimicrobial Activity and Chemical Composition. Appl. Sci. 2023, 13, 2993. [Google Scholar] [CrossRef]
- Holgado, F.; García-Martínez, M.C.; Velasco, J.; Ruiz-Méndez, M.V.; Márquez-Ruiz, G. Microencapsulation of Conjugated Linoleic Acid (CLA)-Rich Oil with Skimmed Milk Components Protects against Polymerization. J. Am. Oil Chem. Soc. 2018, 95, 1399–1408. [Google Scholar] [CrossRef]
- Yakubu, H.G.; Ali, O.; Ilyés, I.; Vigyázó, D.; Bóta, B.; Bazar, G.; Tóth, T.; Szabó, A. Micro-Encapsulated Microalgae Oil Supplementation Has No Systematic Effect on the Odor of Vanilla Shake-Test of an Electronic Nose. Foods 2022, 11, 3452. [Google Scholar] [CrossRef] [PubMed]
- Terpou, A.; Nigam, P.S.; Bosnea, L.; Kanellaki, M. Evaluation of Chios mastic gum as antimicrobial agent and matrix forming material targeting probiotic cell encapsulation for functional fermented milk production. LWT Food Sci. Technol. 2018, 97, 109–116. [Google Scholar] [CrossRef]
- Radu Lupoae, S.D.; Mihalcea, L.; Aprodu, I.; Socaci, S.A.; Cotârlet, M.; Enachi, E.; Crăciunescu, O.; Barbu, V.; Oancea, A.; Dulf, F.V.; et al. Fostering Lavender as a Source for Valuable Bioactives for Food and Pharmaceutical Applications through Extraction and Microencapsulation. Molecules 2020, 25, 5001. [Google Scholar] [CrossRef] [PubMed]

| Plant Name | Scientific Name | Main Compounds | Reference |
|---|---|---|---|
| allspice | Pimenta dioica L. | eugenol, methyl eugenol, β-caryophyllene | [10] |
| caraway | Carum carvi L. | cumin aldehyde, γ-terpinene-7-al | [11] |
| cinnamon | Cinnamomum zeylanicum | (E)-cinnamaldehyde, benzaldehyde, (E)-cinnamyl acetate | [10] |
| cumin | Cuminum cyminum L. | trans-ocimene, cis-ocimene, γ-terpinene | [11] |
| ferulago | Ferulago angulata (Schlecht.) Boiss. | a-Pinen, z-β-ocimene, cis-β-ocimene | [11] |
| laurel | Laurus nobilis L. | 1,8-cineole, α-terpinyl acetate, methyl eugenol, sabinene, eugenol | [10] |
| lavender | Lavandula angustifolia L. | camphor, eucalyptol | [10] |
| lemon grass | Cymbopogon citratus | geranial, neral, myrcene | [10] |
| mandarin | Citrus reticulata | L-limonene, ϒ-terpene, β-felandren | [10] |
| mint | Mentha piperita L. | menthol, mentofuran, 1s-neomethyl acetate, menthone | [11] |
| pomelo | Citrus grandis L. | limonene, α-terpinene, α-pinen | [10] |
| rosemary | Rosmarinus officinalis L. | 1,8-cineole, camphor, α-pinen, limonene, camphene, linalool | [10] |
| sage | Salvia officinalis L. | β-Tujone, 1,8-cineole, camphor | [10,11] |
| yarrow | Achillea millefolium L. | limonene, a-pinen, borneol, thymol, carvacrol | [11] |
| Plant Name | Scientific Name | Used Part of the Plant | Antimicrobial Activity | Main Compounds | Reference |
|---|---|---|---|---|---|
| basil | Ocimum basilicum | whole plant | C. albicans, S. aureus | linalool | [18] |
| S. aureus, E. coli, C. albicans | 1,8-cineole, linalool, terpinen-4-ol, y-cadinol, T-cadinol, trans-α-bergamoten, eugenol, geraniol, germacren D | [27] | |||
| bergamot orange | Citrus bergamia | peel | Campylobacter jejuni, E. coli, L. monocytogenes, B. cereus, S. aureus | linalool, citral, linalyl acetate | [18] |
| black cumin | Bunium persicum | seed | L. monocytogenes, Listeria grayi, Aspergillus flavu | γ-terpinene, 1-felandrene, γ-terpene, couminaldehyde | [18] |
| chamomile | Matricaria chamomilla | fresh or dried flower heads | Leishmania amazonensis, E. coli, P. aeruginosa, B. subtilis, S. aureus, S. pyogenes, Schizosaccharomyces pombe, C. albicans, Candida tropicalis | α-bisabolol | [18] |
| cinnamon | Cinnamomum zeylanicum | peel | Borrelia burgdorferi, E. coli., S. aureus, P. aeruginosa | carvacrol | [18] |
| M. smegmatis, MRSA, S. aureus, S. epidermidis, S. pyogenes, B. bronchiseptica, P. aeruginosa, K. Pneumoniae, C. albicans | β-caryophyllene, trans-cinnamyl acetate, trans-cinnamaldehyde | [19] | |||
| Listeria monocytogenes, Escherichia coli | cinnamaldehyde, caryophyllene, Ccryophyllene oxide, α-caryophyllene, γ-cadinene | [28] | |||
| clove | Eugenia caryophyllata | flower buds | B. cereus, S. typhimurium, E. coli | eugenol, β-caryophyllene | [18] |
| Syzygium aromaticum | flower buds | E. coli, S. aureus, S. typhi, P. aeruginosa, B. cereus, L. monocytogenes | eugenol, eugenyl acetate | [18] | |
| MRSA, S. aureus, P. aeruginosa, M. smegmatis, S. pyogenes, B. bronchiseptica, K. pneumoniae, C. albicans | β-caryophyllene, eugenol, eugenyl acetate | [19] | |||
| coriander | Coriandrum sativum L. | seed | B. subtilis, C. albicans, E. faecalis, E. aerogenes, E. durans, E. faecium, E. coli, K. Pneumonia, L. monocytogenes, L. innocua, P. aeruginosa, P. fluorescens, S. enteritidis, S. infantis, S. kentucky, S. typhimurium, S. aureus, S. epidermidis | linalool, cis-ocimen, neryl acetate, y-terpinene | [29] |
| cumin | Cuminum cyminum L. | seed | Staphylococcus aureus, Klebsiella pneumoniae, Salmonella typhimurium, Pseudomonas aeruginosa, Salmonella enteritidis, Escherichia coli | a-thujene, α-pinen, sabinene, β-pinenene, β-mircene, α-phellandren, ∆-3-carene, α-terpinene, p-cymen, limonene, 1,8-cineole, β-phellandrene, γ-terpinene, α-terpinolen, terpinen-4-ol, α-terpineol, cumin aldehyde, safranal, cumin alcohol, β-caryophyllene, cis-β-farnesene, germacren-D, viridiflorol | [30] |
| fennel | Foeniculum vulgare | seed, leaves | S. aureus, E. coli, A. flavu | anethole | [18] |
| M. smegmatis, MRSA, S. aureus, S. epidermidis, S. pyogenes, P. aeruginosa, B. bronchiseptica, K. Pneumoniae, | limonen, α-pinen, trans-anethol | [19] | |||
| lavender | Lavandula angustifolia L. | aerial part | MRSA, S. aureus, E. coli | linalool, borneol, camphor | [18] |
| M. smegmatis, MRSA, S. aureus, S. epidermidis, S. pyogenes, B. bronchiseptica, K. Pneumoniae, C. albicans | lavandulyl acetate, linalyl acetate, linalool | [19] | |||
| lemon | Citrus limon | peel | M. smegmatis, MRSA, S. aureus, S. epidermidis, S. pyogenes, B. bronchiseptica, P. aeruginosa, K. Pneumoniae, C. albicans | γ-terpinen, β-pinen, limonen | [19] |
| lemon grass | Cymbopogon citratus | leaves | HSV-1, HSV-2, S. aureus, E. coli, Gaeumannomyces graminis | citral | [18] |
| M. smegmatis, MRSA, S. aureus, S. epidermidis, S. pyogenes, B. bronchiseptica, K. Pneumoniae, C. albicans | geraniol, neral, geranial | [19] | |||
| mandarin | Citrus reticulate | peel | S. aureus, E. coli, Penicillium italicum, Penicillium digitatum | limonen, γ-terpinen | [18] |
| mint | Mentha piperita L. | leaves | C. albicans, C. tropicalis, Pichia anomala, Saccharomycescerevisiae | menthol, menthone | [18] |
| M. smegmatis, MRSA, S. aureus, S. epidermidis, S. pyogenes, B. bronchiseptica, K. Pneumoniae, C. albicans | menthol acetate, menthone, menthol | [19] | |||
| Staphylococcus aureus, Klebsiella pneumoniae, Salmonella typhimurium, Pseudomonas aeruginosa, Salmonella enteritidis, Escherichia coli | a-thujene, α-pinene, sabinene, β-pinene, β-myrcene, α-terpinene, p-cymene, limonene, 1,8-cineole, cis-β-ocymene, γ-terpinene, cis-sabinene hydrate, α-terpinolen, linalool, menthone, izomentone, mentofuran, menthol, terpinen-4-ol, neo-menthol, α-terpineol, pulegone, piperiton, mentil acetate, β-borbonene, β-caryophyllene, trans-β-farnesen, germacrene-D, viridiflorol | [30] | |||
| oregano | Origanum vulgare | leaves | Trichophyton tonsurans, Trichophyton violaceum, Trichophyton floccosum, T. mentagrophytes | carvacrol, timol | [18] |
| S. aureus, E. coli, C. albicans | α-pinen, linalool, p-cymene, α-terpinene, γ-terpinene, α-tujen, β-caryophyllene, thymol, carvacrol | [27] | |||
| M. smegmatis, MRSA, S. aureus, S. epidermidis, S. pyogenes, B. bronchiseptica, P. aeruginosa, K. Pneumoniae, C. albicans | carvacrol | [19] | |||
| Listeria monocytogenes, Escherichia coli | β-cymen, linalool, carvacrol, γ-terpinene, caryophyllene | [28] | |||
| pomegranate | Punica granatum | seed | S. epidermidis | punicalagin, punicalin | [18] |
| rosemary | Rosmarinus officinalis L. | leaves | C. albicans, C. tropicalis | 1,8-cineole, camphor | [18] |
| M. smegmatis, MRSA, S. aureus, S. epidermidis, S. pyogenes, B. bronchiseptica, P. aeruginosa, K. Pneumoniae, C. albicans | α-pinen, camphor, 1,8-cineole | [19] | |||
| Listeria monocytogenes, Escherichia coli | 1R-α-pinen, eucalyptol, camphor, camphene, β-pinen, α-terpineol, bornyl acetate, caryophyllene | [28] | |||
| sage | Salvia officinalis L. | aerial part | Staphylococcus aureus, Klebsiella pneumoniae, Salmonella typhimurium, Pseudomonas aeruginosa, Salmonella enteritidis, Escherichia coli | cis-salvene, trans-salvene, tricyclene, a-thujene, α-pinene, camphene, sabinene, β-pinene, 1-octan-3-ol, p-myrcen, 3-octanol, α-phellandrene, α-terpinene, p-cymen, limonene, 1,8-cineole, cis-β-ocymene, γ-terpinene, cis-sabinene, α-terpinolen hydrate, linalool, α-thujonene, β-thujonene, trans-verbenol, camphor, borneol, δ-terpineol, α-terpineol, trans-sabinyl acetate, α-terpinyl acetate, β-caryophyllene, α-humulene, caryophyllene oxide, viridiflorol, β-selenene, humulene epoxide II | [30] |
| savory | Satureja hortensis | leaves | S. aureus, Corynebacterium glutamicum, P. aeruginosa and E. coli, C. albicans | carvacrol, timol | [18] |
| M. smegmatis, MRSA, S. aureus, S. epidermidis, S. pyogenes, B. bronchiseptica, P. aeruginosa, K. Pneumoniae, C. albicans | linalool, para-cimen, thymol, γ-terpinene | [19] | |||
| ylang-ylang | Cananga odorata | flower | Hepatitis B virus (HBV), Bacillus. subtilis, E. coli, S. typhi, Shigella shiga, Streptococcus-β- haemolyticus, A. flavu | linalool, β-caryophyllene | [18] |
| MRSA, M. smegmatis, S. epidermidis, S. pyogenes, B. bronchiseptica, K. Pneumoniae | linalool, geranyl acetate, trans-α-Farsen, β-caryophyllene, germacrene D | [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tița, O.; Constantinescu, M.A.; Rusu, L.; Tița, M.A. Natural Polymers as Carriers for Encapsulation of Volatile Oils: Applications and Perspectives in Food Products. Polymers 2024, 16, 1026. https://doi.org/10.3390/polym16081026
Tița O, Constantinescu MA, Rusu L, Tița MA. Natural Polymers as Carriers for Encapsulation of Volatile Oils: Applications and Perspectives in Food Products. Polymers. 2024; 16(8):1026. https://doi.org/10.3390/polym16081026
Chicago/Turabian StyleTița, Ovidiu, Maria Adelina Constantinescu, Lăcrămioara Rusu, and Mihaela Adriana Tița. 2024. "Natural Polymers as Carriers for Encapsulation of Volatile Oils: Applications and Perspectives in Food Products" Polymers 16, no. 8: 1026. https://doi.org/10.3390/polym16081026
APA StyleTița, O., Constantinescu, M. A., Rusu, L., & Tița, M. A. (2024). Natural Polymers as Carriers for Encapsulation of Volatile Oils: Applications and Perspectives in Food Products. Polymers, 16(8), 1026. https://doi.org/10.3390/polym16081026









