Investigation of Hybrid Electrodes of Polyaniline and Reduced Graphene Oxide with Bio-Waste-Derived Activated Carbon for Supercapacitor Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Apparatus
2.3. Preparation of Olive Stones (OS)
2.4. Synthesis of Reduced Graphene Oxide (rGO)
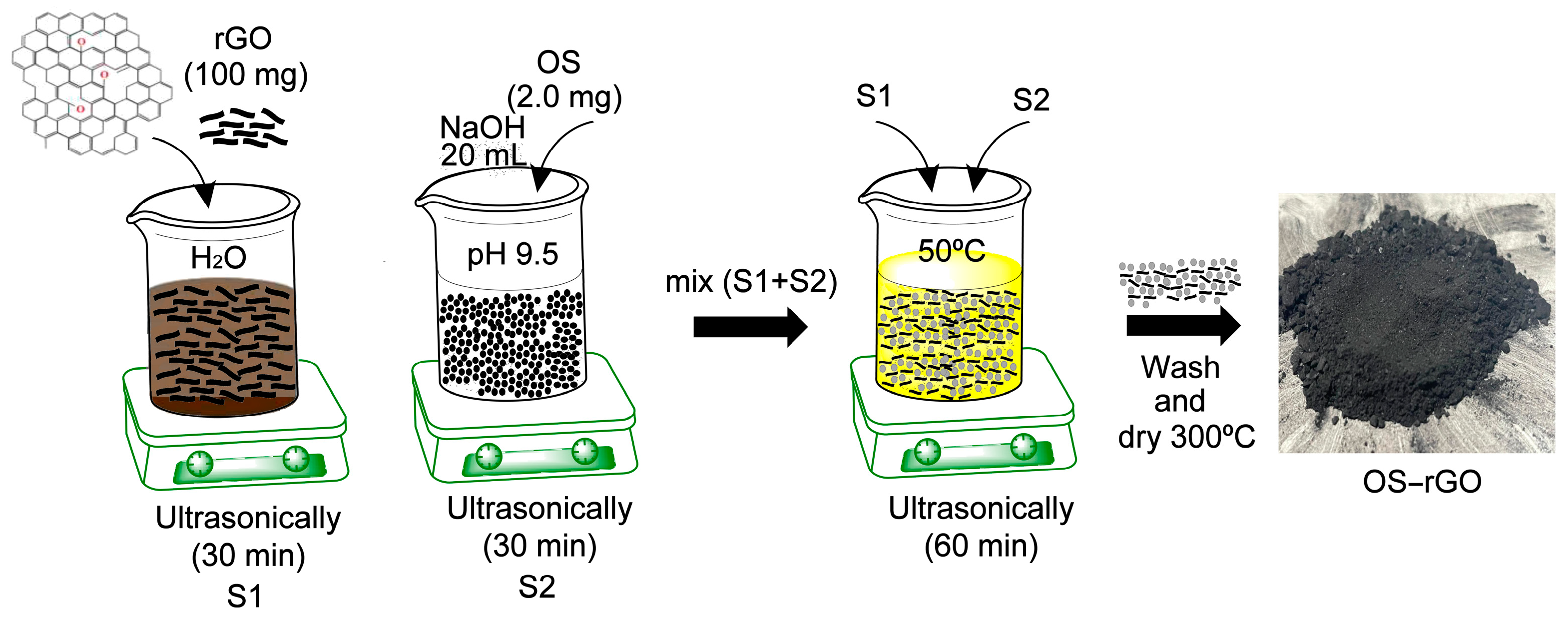
2.5. Preparation of OS−rGO
2.6. Synthesis of PAni@OS−rGO
2.7. Electrochemical Performance
3. Results
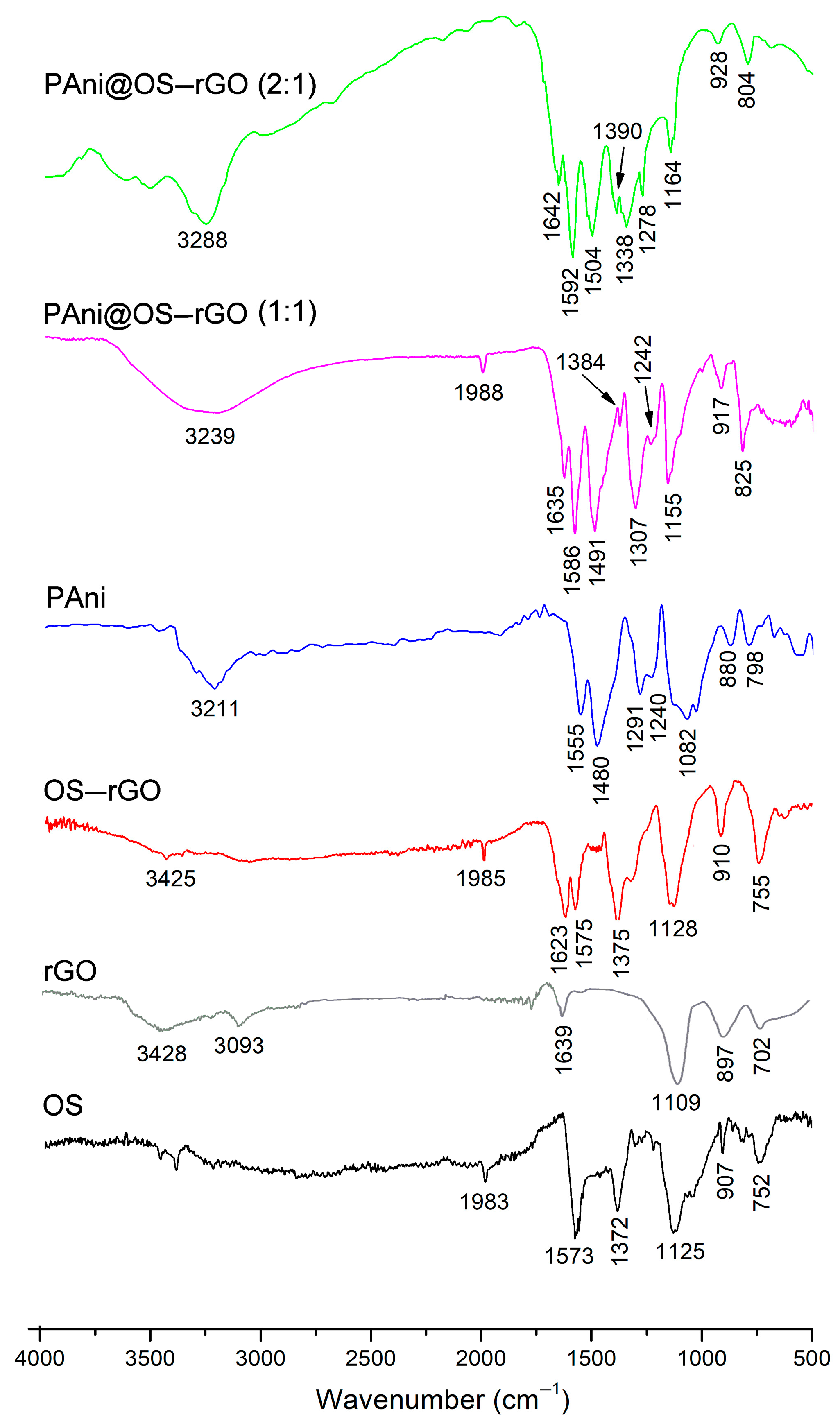
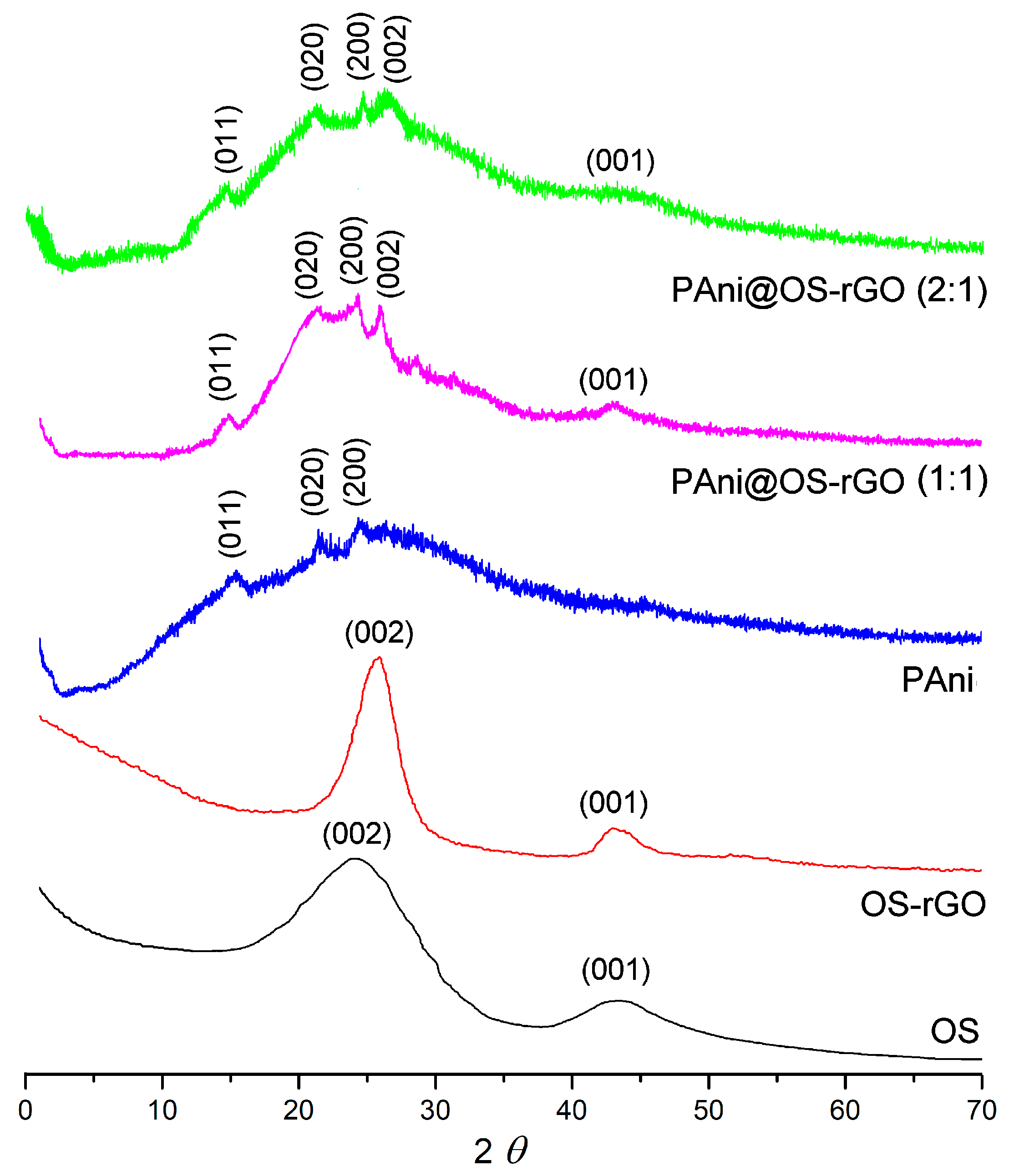
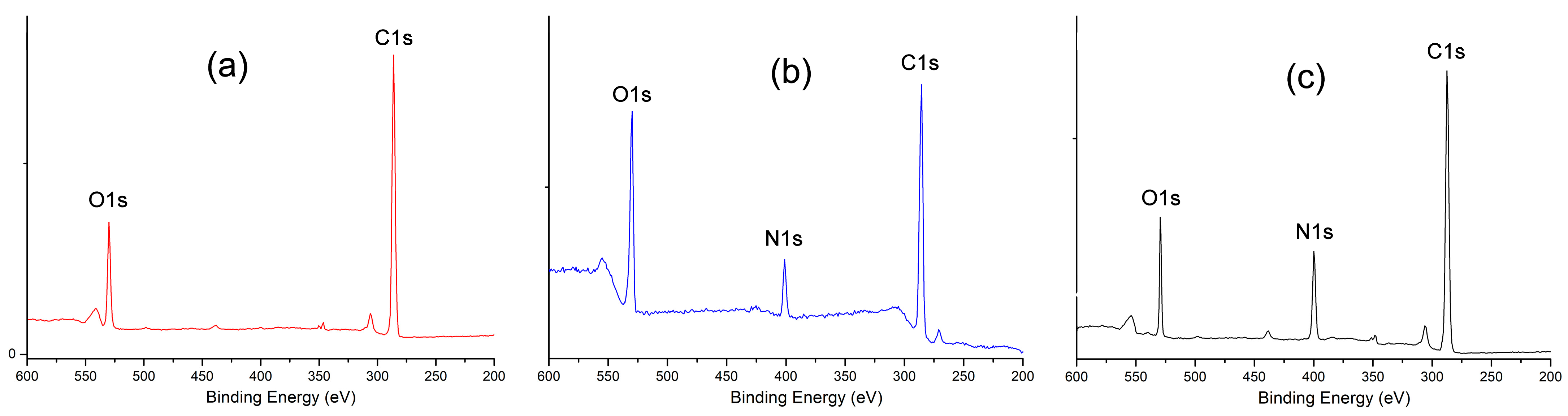
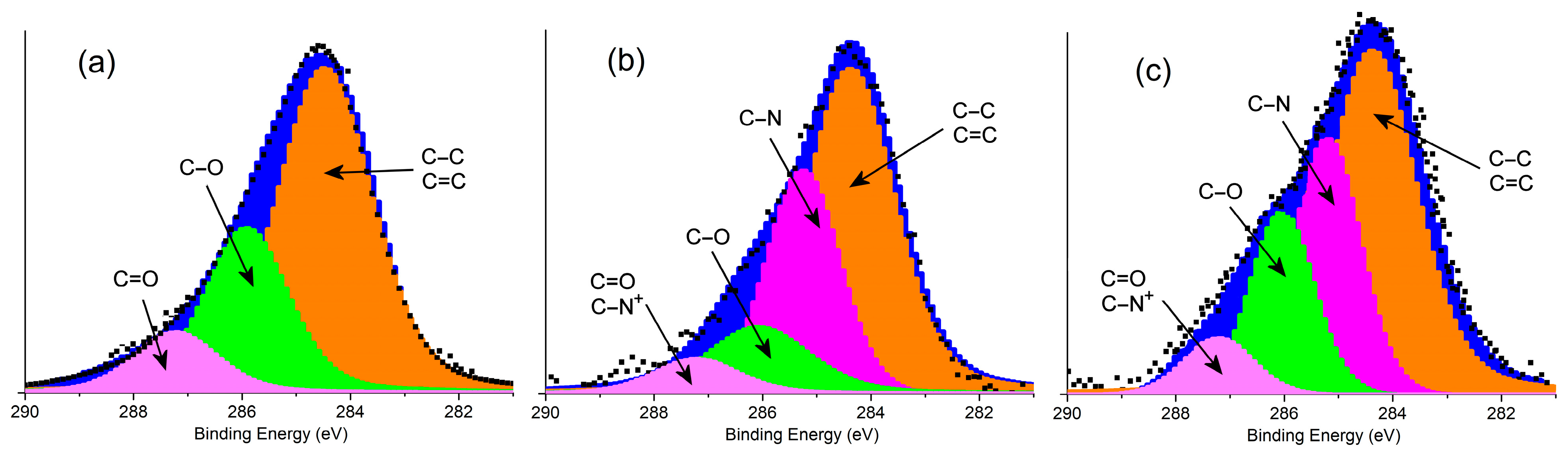
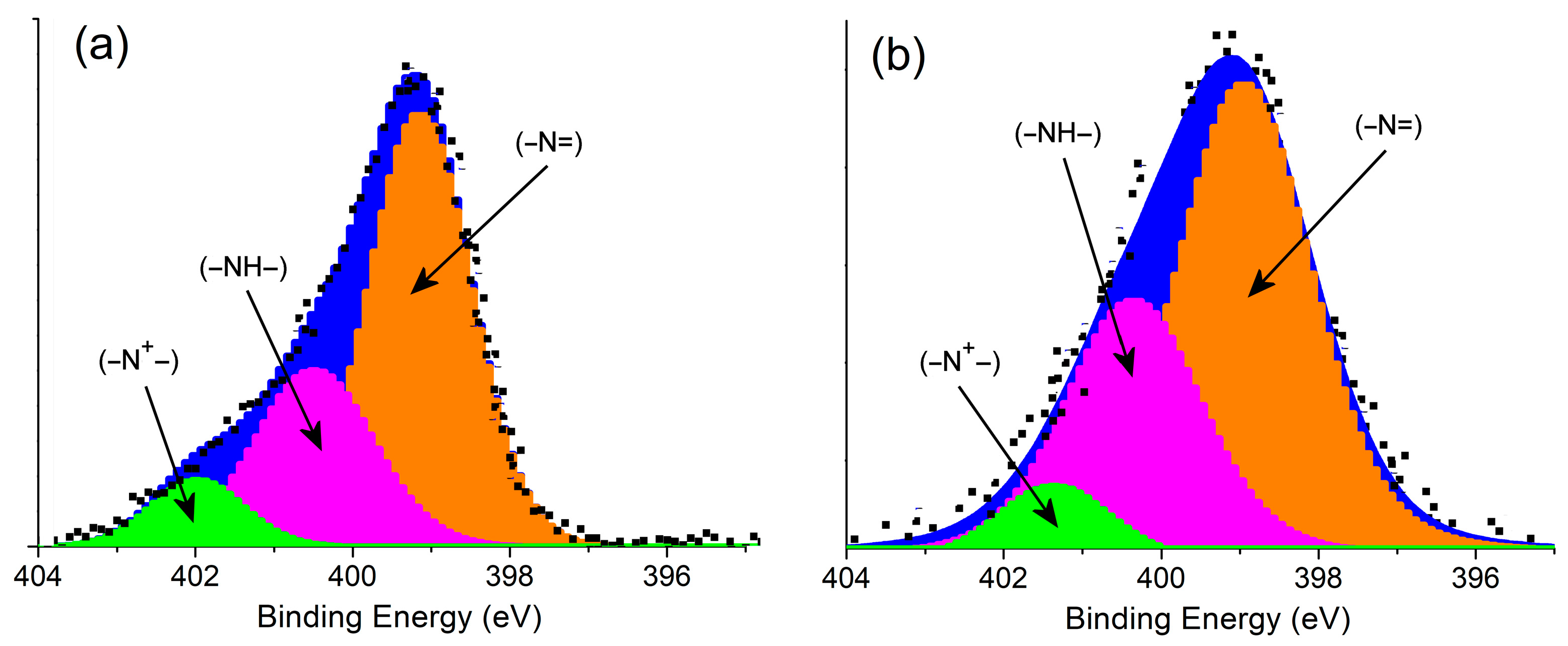
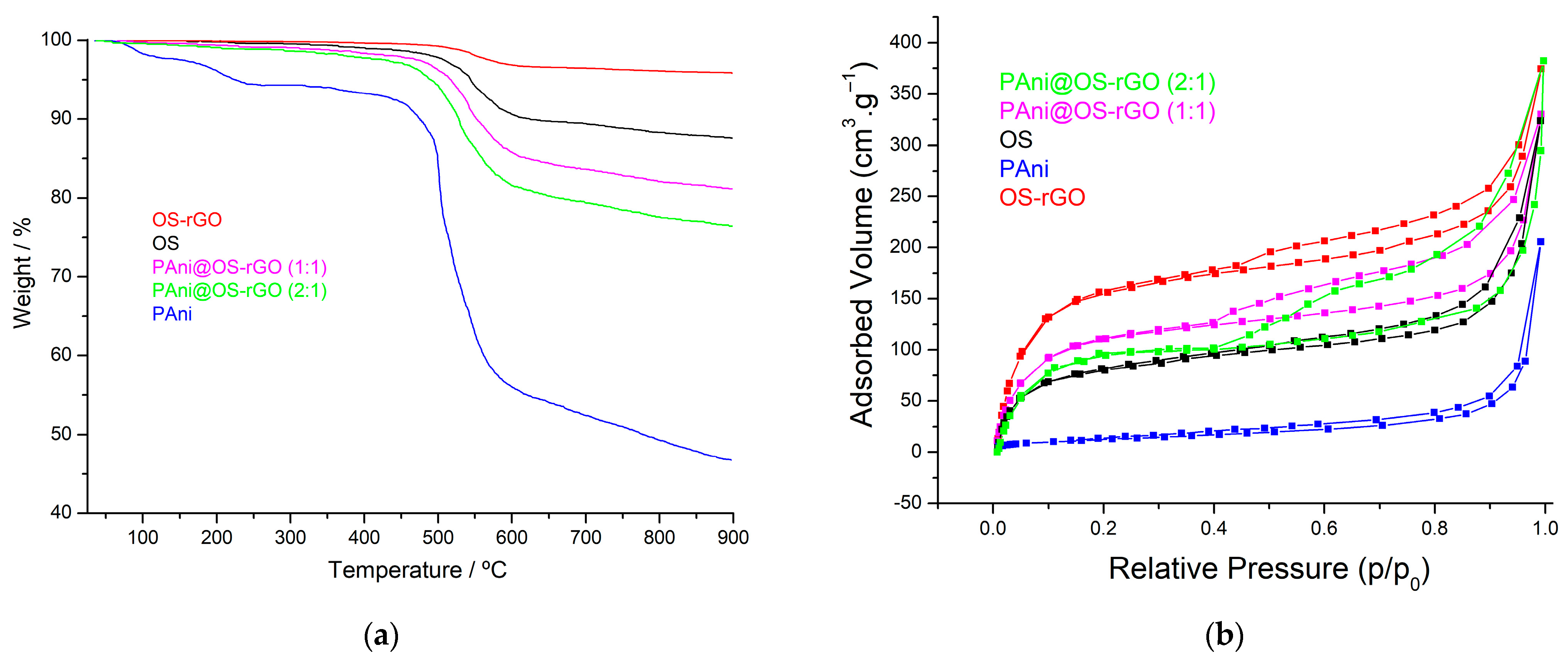
3.1. Characterization of Samples
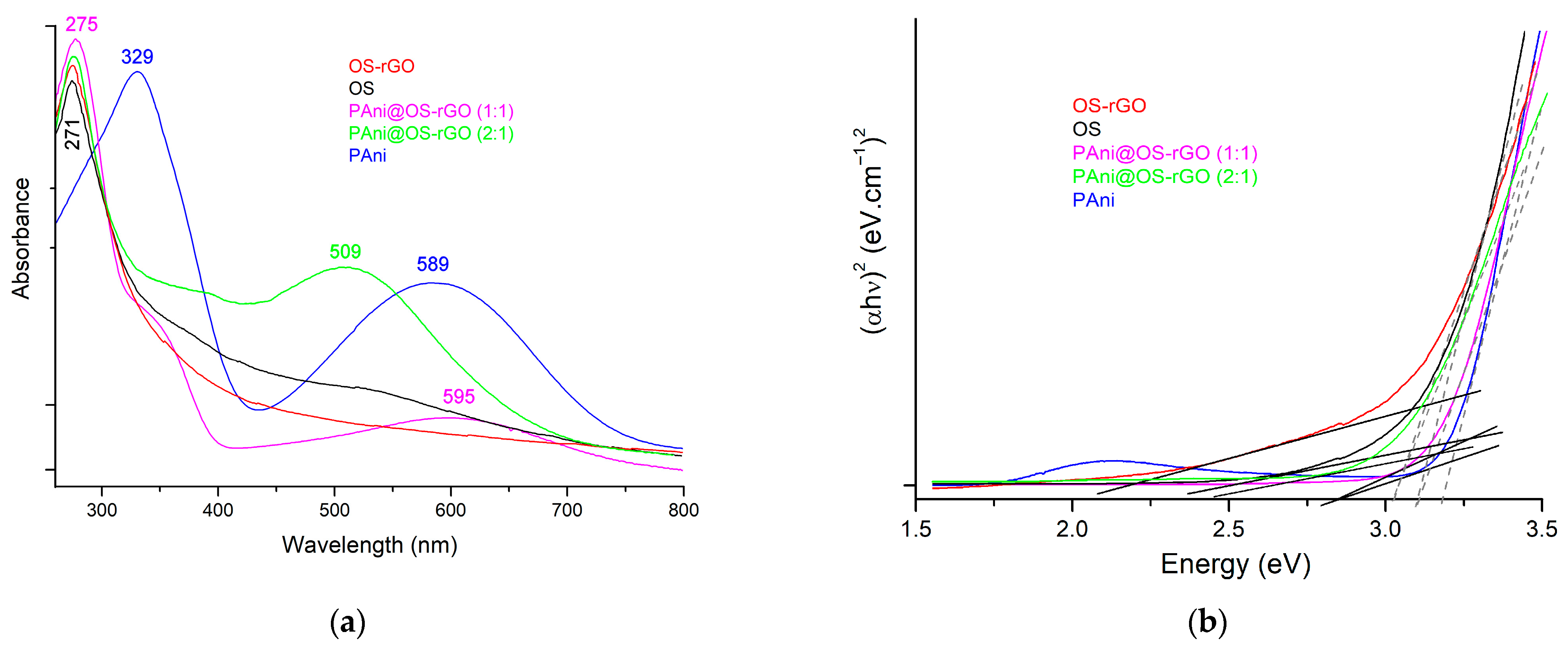
3.2. Optical Absorption Study
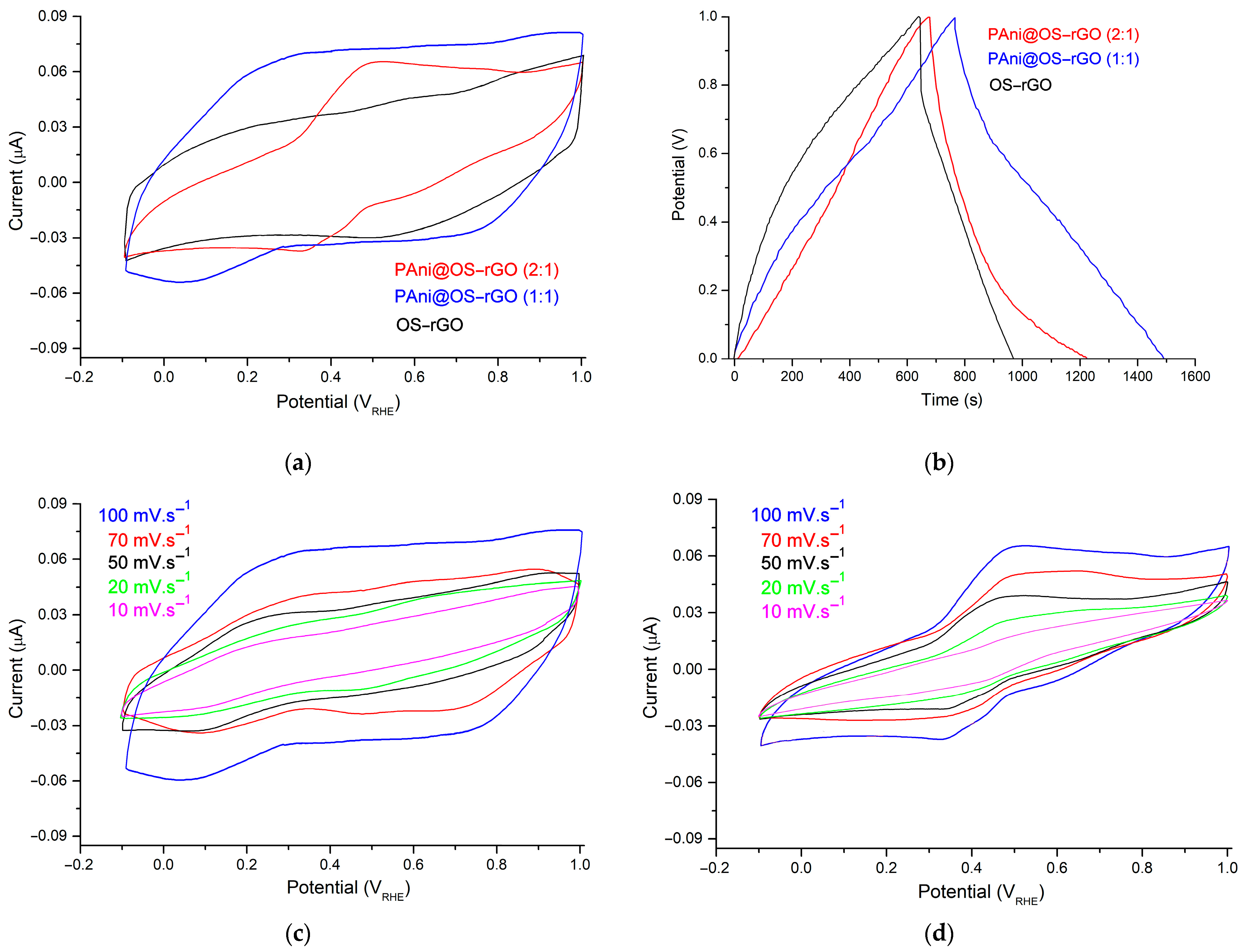
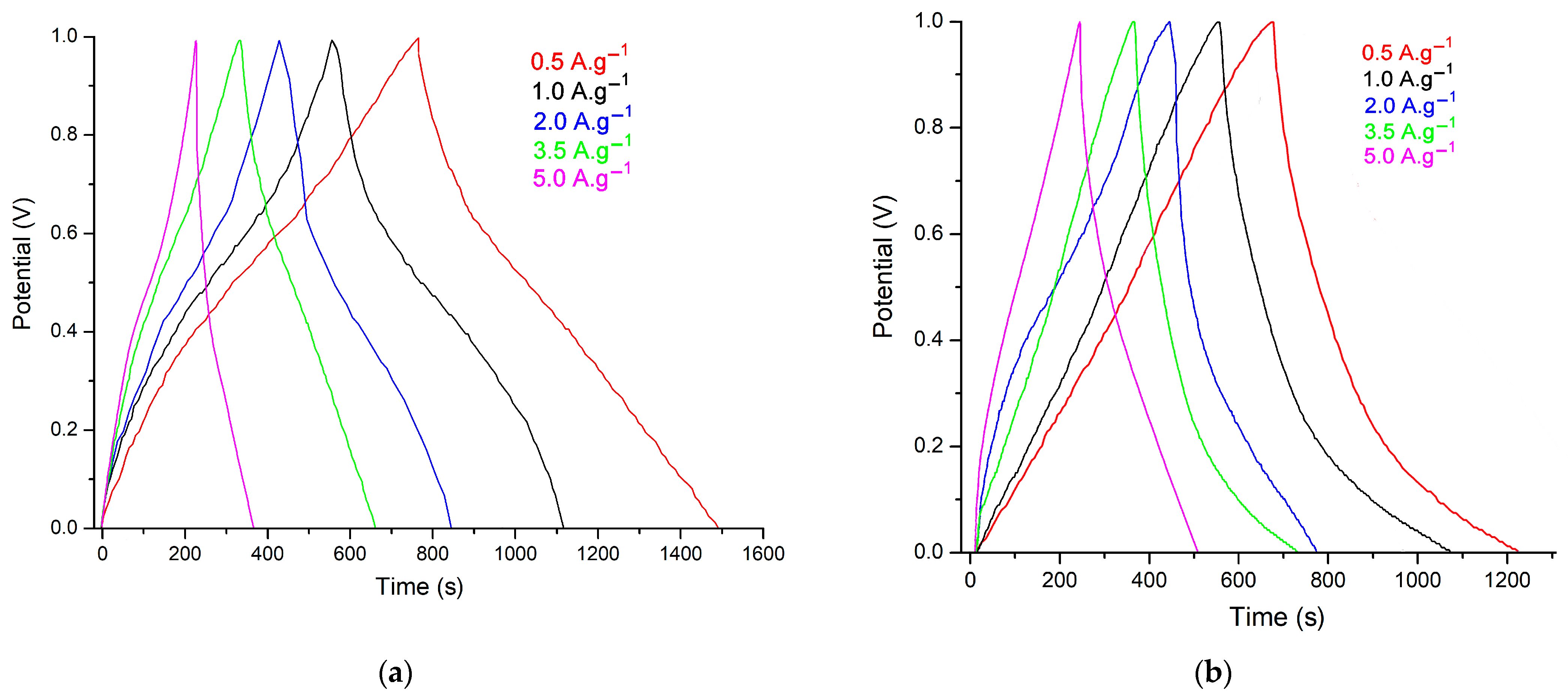
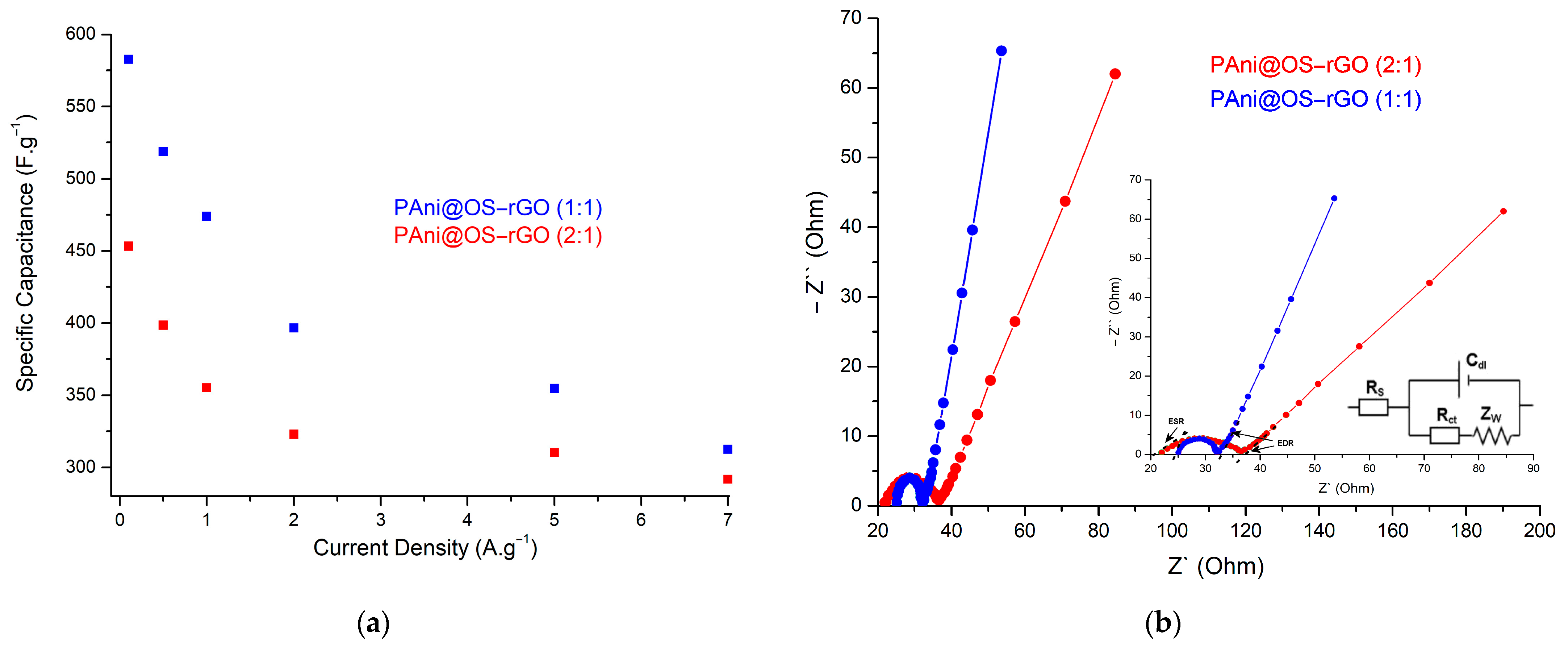
3.3. Electrochemical Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rastogi, R.P.; Pandey, A.; Larroche, C.; Madamwar, D. Algal Green Energy–R&D and technological perspectives for biodiesel production. Renew. Sust. Energy Rev. 2018, 82, 2946–2969. [Google Scholar] [CrossRef]
- Wu, W.; Huang, C.M.; Tsai, Y.H. Design and validation of a microalgae biorefinery using machine learning-assisted modeling of hydrothermal liquefaction. Algal Res. 2023, 74, 103230. [Google Scholar] [CrossRef]
- Hassan, A.M.; Asif, M.; Al-Mansur, M.A.; Uddin, M.R.; Alsufyani, S.J.; Yasmin, F.; Khandaker, M.U. Characterization of municipal solid waste for effective utilization as an alternative source for clean energy production. J. Radiat. Res. Appl. Sci. 2023, 16, 100683. [Google Scholar] [CrossRef]
- Bórawski, P.; Wyszomierski, R.; Bórawska, A.B.; Mickiewicz, B.; Kalinowska, B.; Dunn, J.W.; Rokicki, T. Development of Renewable Energy Sources in the European Union in the Context of Sustainable Development Policy. Energies 2022, 15, 1545. [Google Scholar] [CrossRef]
- Strielkowski, W.; Civín, L.; Tarkhanova, E.; Tvaronavičienė, M.; Petrenko, Y. Renewable Energy in the Sustainable Development of Electrical Power Sector: A Review. Energies 2021, 14, 8240. [Google Scholar] [CrossRef]
- Wang, F.X.; Wu, X.W.; Yuan, X.H.; Liu, Z.C.; Zhang, Y.; Fu, L.J.; Zhu, Y.S.; Zhou, Q.M.; Wu, Y.P.; Huang, W. Latest advances in supercapacitors: From new electrode materials to novel device designs. Chem. Soc. Rev. 2017, 46, 6816–6854. [Google Scholar] [CrossRef] [PubMed]
- Memou, C.H.; Bekhti, M.A.; Kiari, M.; Benyoucef, A.; Alelyani, M.; Alqahtani, M.S.; Alshihri, A.A.; Bakkour, Y. Fabrication and Characterization of a Poly(3,4-ethylenedioxythiophene)@Tungsten Trioxide–Graphene Oxide Hybrid Electrode Nanocomposite for Supercapacitor Applications. Nanomaterials 2023, 13, 2664. [Google Scholar] [CrossRef]
- Zenasni, M.; Belhadj, H.; Kiari, M.; Alelyani, M.; Alhailiy, A.B.; Benyoucef, A.; Bakkour, Y. Synthesis, characterization, and enhanced electrochemical behavior of polypyrrole doped ZrO2–ZnO electrode materials for supercapacitor applications. Front. Energy Res. 2023, 11, 1244699. [Google Scholar] [CrossRef]
- Boutaleb, N.; Dahou, F.Z.; Djelad, H.; Sabantina, L.; Moulefera, I.; Benyoucef, A. Facile Synthesis and Electrochemical Characterization of Polyaniline@TiO2-CuO Ternary Composite as Electrodes for Supercapacitor Applications. Polymers 2022, 14, 4562. [Google Scholar] [CrossRef]
- Sivakumar, P.; Kulandaivel, L.; Park, J.; Raj, C.J.; Savariraj, A.D.; Manikandan, R.; Rajendran, R.; Jung, H. Binary mixed metal oxide sphere-like structures for hybrid supercapacitor electrode with improved electrochemical properties. Surf. Interfaces 2023, 40, 103115. [Google Scholar] [CrossRef]
- Huang, J.; Xie, Y.; You, Y.; Yuan, J.; Xu, Q.; Xie, H.; Chen, Y. Rational Design of Electrode Materials for Advanced Supercapacitors: From Lab Research to Commercialization. Adv. Funct. Mater. 2023, 33, 2213095. [Google Scholar] [CrossRef]
- Aken, K.L.V.; Beidaghi, M.; Gogotsi, Y. Formulation of Ionic-Liquid Electrolyte to Expand the Voltage Window of Supercapacitors. Angew. Chem. Int. Ed. 2015, 54, 4806–4809. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yu, J.; Lu, Q.; Xiao, Z.; Ma, X.; Feng, Y. Preparation of Mn3O4 microspheres via glow discharge electrolysis plasma as a high-capacitance supercapacitor electrode material. J. Alloys Compd. 2022, 926, 166775. [Google Scholar] [CrossRef]
- Mamun, A.; García-Mateos, F.J.; Sabantina, L.; Klöcker, M.; Diestelhorst, E.; Ruiz-Rosas, R.; Rosas, J.M.; Rodríguez-Mirasol, J.; Blachowicz, T.; Cordero, T. Electrospinning of Magnetite–Polyacrylonitrile Composites for the Production of Oxygen Reduction Reaction Catalysts. Polymers 2023, 15, 4064. [Google Scholar] [CrossRef] [PubMed]
- Ammar, A.U.; Stan, M.; Popa, A.; Toloman, D.; Macavei, S.; Leostean, C.; Ciorita, A.; Erdem, E.; Rostas, A.M. All-in-one supercapacitor devices based on nanosized Mn4+-doped WO3. J. Energy Storage 2023, 72, 108599. [Google Scholar] [CrossRef]
- Shao, Y.L.; El-Kady, M.F.; Sun, J.Y.; Li, Y.G.; Zhang, Q.H.; Zhu, M.F.; Wang, H.Z.; Dunn, B.; Kaner, R.B. Design and Mechanisms of Asymmetric Supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef] [PubMed]
- Gogotsi, Y.; Penner, R.M. Energy Storage in Nanomaterials-Capacitive, Pseudocapacitive, or Battery-like? ACS Nano 2018, 12, 2081–2083. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.B.; Dannoun, E.M.A.; Abdullah, S.N.; Ghareeb, H.O.; Abdullah, R.M.; Abdalrahman, A.A.; Nofal, M.M.; Kakroo, S. The EDLC Energy Storage Device Based on a Natural Gelatin (NG) Biopolymer: Tuning the Capacitance through Plasticizer Variation. Polymers 2022, 14, 5044. [Google Scholar] [CrossRef]
- Liew, C.W.; Ramesh, S.; Arof, A.K. Investigation of ionic liquid-doped ion conducting polymer electrolytes for carbon-based electric double layer capacitors (EDLCs). Mater. Des. 2016, 92, 829–835. [Google Scholar] [CrossRef]
- Chang, P.; Yang, F.; Xie, Q.; Li, T.; Dong, J. 2D porous carbon nanosheet from sulfonated pitch-based graphene quantum dots for high volumetric performance EDLCs. J. Power Sources 2020, 479, 228825. [Google Scholar] [CrossRef]
- Choi, C.; Ashby, D.S.; Butts, D.M.; DeBlock, R.H.; Wei, Q.L.; Lau, J.; Dunn, B. Achieving high energy density and high power density with pseudocapacitive materials. Nat. Rev. Mater. 2020, 5, 5–19. [Google Scholar] [CrossRef]
- Belhadj, H.; Moulefera, I.; Sabantina, L.; Benyoucef, A. Effects of Incorporating Titanium Dioxide with Titanium Carbide on Hybrid Materials Reinforced with Polyaniline: Synthesis, Characterization, Electrochemical and Supercapacitive Properties. Fibers 2022, 10, 46. [Google Scholar] [CrossRef]
- Bonastre, J.; Molina, J.; Cases, F. Surface modification of jute fabrics by reduced graphene oxide-conducting polymer coatings for their application in low-cost and eco-friendly supercapacitors. J. Energy Storage 2023, 69, 107936. [Google Scholar] [CrossRef]
- Wang, D.W.; Li, F.; Zhao, J.; Ren, W.; Chen, Z.H.; Tan, J.; Wu, Z.S.; Gentle, I.; Lu, G.Q.; Cheng, H.M. Fabrication of Graphene/Polyaniline Composite Paper viaIn Situ Anodic Electropolymerization for High-Performance Flexible Electrode. ACS Nano 2009, 3, 1745–1752. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, L.L.; Zhao, X.S.; Wu, J. Graphene/Polyaniline Nanofiber Composites as Supercapacitor Electrodes. Chem. Mater. 2010, 22, 1392–1401. [Google Scholar] [CrossRef]
- Yu, D.; Li, J.; Jia, T.; Dong, B.; Han, Z.; Tian, W.; Jiang, R.; Lu, X.; Li, L. Electrochemical Synthesis of Functionalized Graphene/Polyaniline Composite Using Two Electrode Configuration for Supercapacitors. Nanomaterials 2023, 13, 3140. [Google Scholar] [CrossRef] [PubMed]
- Sundriyal, S.; Shrivastav, V.; Pham, H.D.; Mishra, S.; Deep, A.; Dubal, D.P. Advances in bio-waste derived activated carbon for supercapacitors: Trends, challenges and prospective. Resour. Conserv. Recycl. 2021, 169, 105548. [Google Scholar] [CrossRef]
- Demirbaş, A. Biomass resource facilities and biomass conversion processing for fuels and chemicals. Energy Convers. Manag. 2001, 42, 1357–1378. [Google Scholar] [CrossRef]
- Zhou, H.; Fan, T.; Zhang, D. Biotemplated materials for sustainable energy and environment: Current status and challenges. ChemSusChem 2011, 4, 1344–1387. [Google Scholar] [CrossRef]
- Jaouadi, M.; Marzouki, M.; Hamzaoui, A.H.; Ghodbane, O. Enhanced electrochemical performance of olive stones-derived activated carbon by silica coating for supercapacitor applications. J. Appl. Electrochem. 2022, 52, 125–137. [Google Scholar] [CrossRef]
- Hsiao, C.H.; Gupta, S.; Lee, C.Y.; Tai, N.H. Effects of physical and chemical activations on the performance of biochar applied in supercapacitors. Appl. Surf. Sci. 2023, 610, 155560. [Google Scholar] [CrossRef]
- El-Azazy, M.; Nabil, I.; Hassan, S.S.; El-Shafie, A.S. Adsorption Characteristics of Pristine and Magnetic Olive Stones Biochar with Respect to Clofazimine. Nanomaterials 2021, 11, 963. [Google Scholar] [CrossRef]
- Xing, L.L.; Huang, K.J.; Fang, L.X. Preparation of layered graphene and tungsten oxide hybrids for enhanced performance supercapacitors. Dalton Trans. 2016, 45, 17439–17446. [Google Scholar] [CrossRef]
- Wang, A.; Long, L.; Zhao, W.; Song, Y.; Humphrey, M.G.; Cifuentes, M.P.; Wu, X.; Fu, Y.; Zhang, D.; Li, X.; et al. Increased optical nonlinearities of graphene nanohybrids covalently functionalized by axially-coordinated porphyrins. Carbon 2013, 53, 327–338. [Google Scholar] [CrossRef]
- Zhou, P.; Le, Z.; Xie, Y.; Fang, J.; Xu, J. Studies on facile synthesis and properties of mesoporous CdS/TiO2 composite for photocatalysis applications. J. Alloys Compd. 2017, 692, 170–177. [Google Scholar] [CrossRef]
- Chatterjee, M.J.; Ghosh, A.; Mondal, A.; Banerjee, D. Polyaniline–single walled carbon nanotube composite–A photocatalyst to degrade rose bengal and methyl orange dyes under visible-light illumination. RSC Adv. 2017, 7, 36403–36415. [Google Scholar] [CrossRef]
- Naskar, A.; Khanal, R.; Choudhury, S. Role of Chemistry and Crystal Structure on the Electronic Defect States in Cs-Based Halide Perovskites. Materials 2021, 14, 1032. [Google Scholar] [CrossRef]
- Lin, L.; Zheng, Z.; Li, X.; Park, S.; Zhang, W.; Diao, G.; Piao, Y. Design strategy for porous carbon nanomaterials from rational utilization of natural rubber latex foam scraps. Ind. Crops Prod. 2023, 192, 116036. [Google Scholar] [CrossRef]
- Zhang, L.C.; He, Z.H.; Hou, J.F.; Gao, R.; Kong, L.B. Tuning pore structure of polymer-derived carbon materials for supercapacitor electrode materials using microscopic phase separation engineering. J. Energy Storage 2023, 73, 109028. [Google Scholar] [CrossRef]
- Xiong, C.; Li, B.; Duan, C.; Dai, L.; Nie, S.; Qin, C.; Xu, Y.; Ni, Y. Carbonized wood cell chamber-reduced graphene oxide@PVA flexible conductive material for supercapacitor, strain sensing and moisture-electric generation applications. Chem. Eng. J. 2021, 418, 129518. [Google Scholar] [CrossRef]
- Wang, H.; Li, M.; Gan, M.; Zhou, T.; Sun, X.; Dai, W.; Wang, H.; Wang, S. Design and assembly of reduced graphene oxide/polyaniline/urchin-like mesoporous TiO2 spheres ternary composite and its application in supercapacitors. Compos. Part B Eng. 2016, 92, 405–412. [Google Scholar] [CrossRef]
- Azizi, E.; Arjomandi, J.; Lee, J.Y. Reduced graphene Oxide/Poly(1,5 dihydroxynaphthalene)/TiO2 nanocomposite conducting polymer coated on gold as a supercapacitor electrode. Electrochim. Acta. 2019, 298, 726–734. [Google Scholar] [CrossRef]
- Liao, P.; Zeng, Y.; Qiu, Z.; Hao, S.; He, J.; Xu, H.; Chen, S. 3D Ti3C2TX@PANI-reduced graphene oxide hydrogel and defective reduced graphene oxide hydrogel as anode and cathode for high-energy asymmetric supercapacitor. J. Alloys Compd. 2023, 948, 169593. [Google Scholar] [CrossRef]
- Fan, H.; Zhao, N.; Wang, H.; Xu, J.; Pan, F. 3D conductive network-based free-standing PANI-RGO-MWNTs hybrid film for high-performance flexible supercapacitor. J. Mater. Chem. A 2014, 2, 12340–12347. [Google Scholar] [CrossRef]
- Xiong, C.; Li, T.; Zhu, Y.; Zhao, T.; Dang, A.; Li, H.; Ji, X.; Shang, Y.; Khan, M. Two-step approach of fabrication of interconnected nanoporous 3D reduced graphene oxide-carbon nanotube-polyaniline hybrid as a binder-free supercapacitor electrode. J. Alloys Compd. 2017, 695, 1248–1259. [Google Scholar] [CrossRef]
- Patil, P.H.; Kulkarni, V.V.; Dongale, T.D.; Jadhav, S.A. α-Manganese Dioxide (α-MnO2) Coated with Polyaniline (PANI) and Reduced Graphene Oxide (rGO)-Based Nanocomposite for Supercapacitor Application. J. Compos. Sci. 2023, 7, 167. [Google Scholar] [CrossRef]
- Liu, J.; Du, P.; Wang, Q.; Liu, D.; Liu, P. Mild synthesis of holey N-doped reduced graphene oxide and its double-edged effects in polyaniline hybrids for supercapacitor application. Electrochim. Acta. 2019, 305, 175–186. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.; Xin, S.; Yang, Z.; Li, Y.; Zhang, D.; Yao, P. Facile Synthesis of MoS2/Reduced Graphene Oxide@Polyaniline for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 21373–21380. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhou, J.; Gao, N.; Yin, Z.; Zhou, H.; Yang, X.; Kuang, Y. Synthesis of 3D reduced graphene oxide/unzipped carbon nanotubes/polyaniline composite for high-performance supercapacitors. Electrochim. Acta. 2018, 269, 649–656. [Google Scholar] [CrossRef]
- Hu, R.Y.; Liu, L.Y.; He, J.H.; Zhou, Y.; Wu, S.B.; Zheng, M.X.; Muslum Demir, M.; Ma, P.P. Preparation and electrochemical properties of bimetallic carbide Fe3Mo3C/Mo2C@carbon nanotubes as negative electrode material for supercapacitor. J. Energy Storage 2023, 72, 108656. [Google Scholar] [CrossRef]
- Liu, L.; Liu, G.; Wu, S.; He, J.; Zhou, Y.; Demir, M.; Huang, R.; Ruan, Z.; Jiang, G.; Ma, P. Fe-substituted SrCoO3 perovskites as electrode materials for wide temperature-tolerant supercapacitors. Ceram. Int. 2024, 50, 1970–1980. [Google Scholar] [CrossRef]
- Qiao, Y.; He, J.; Zhou, Y.; Wu, S.; Li, X.; Jiang, G.; Jiang, G.; Demir, M.; Ma, P. Flexible All-Solid-State Asymmetric Supercapacitors Based on PPy-Decorated SrFeO3−δ Perovskites on Carbon Cloth. ACS Appl. Mater. Interfaces 2023, 15, 52381–52391. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.H.; Ma, P.P.; Liu, G.F.; Qiao, Y.; Hu, R.Y.; Liu, L.Y.; Demir, M.; Jiang, G.H. Dual-Phase Coexistence Design and Advanced Electrochemical Performance of Cu2MoS4 Electrode Materials for Supercapacitor Application. Energy Fuels 2023, 37, 6158–6167. [Google Scholar] [CrossRef]
- Chang, T.W.; Lin, L.Y.; Peng, P.W.; Zhang, Y.X.; Huang, Y.Y. Enhanced electrocapacitive performance for the supercapacitor with tube-like polyaniline and graphene oxide composites. Electrochim. Acta. 2018, 259, 348–354. [Google Scholar] [CrossRef]
- Li, Z.F.; Zhang, H.; Liu, Q.; Liu, Y.; Stanciu, L.; Xie, J. Covalently-grafted polyaniline on graphene oxide sheets for high performance electrochemical supercapacitors. Carbon. 2014, 71, 257–267. [Google Scholar] [CrossRef]
- Wu, X.; Tang, L.; Zheng, S.; Huang, Y.; Yang, J.; Liu, Z.; Yang, W.; Yang, M. Hierarchical unidirectional graphene aerogel/polyaniline composite for high performance supercapacitors. J. Power Sources. 2018, 397, 189–195. [Google Scholar] [CrossRef]
- Zhang, Y.; Si, L.; Zhou, B.; Zhao, B.; Zhu, Y.; Zhu, L.; Jiang, X. Synthesis of novel graphene oxide/pristine graphene/polyaniline ternary composites and application to supercapacitor. Chem. Eng. J. 2016, 288, 689–700. [Google Scholar] [CrossRef]


| Electrode Materials | Specific Capacitance (F·g–1) | Energy Density (Wh·kg–1) | Power Density (W·kg–1) | Reference |
|---|---|---|---|---|
| PEDOT@WO3–GO | 478.3 | 54.2 | 971 | [7] |
| Graphene/Fe2O3 | 378.7 | 64.09 | 800.01 | [13] |
| CWCC-rGO@PVA | 288 | 36 | 3600 | [40] |
| rGO/PANI/urchin-like mesoporous TiO2 | 464 | 34 | 3720 | [41] |
| rGO/Poly(1,5 dihydroxynaphthalene)/TiO2 | 556 | // | // | [42] |
| Ti3C2TX@PANI-rGO | 617.84 | 33 | 503.42 | [43] |
| PANI–rGO–MWNTs | 498 | // | // | [44] |
| rGO-CNT-PANI | 741 | 92.4 | 6300 | [45] |
| α-MnO2/PANI/rGO | 661 | 11 | 1250 | [46] |
| N-doped rGO/PANI | 510 | 24.7 | 329.5 | [47] |
| rGO/MoS2/PANI | 160 | 22.3 | 5080 | [48] |
| rGO/UCNTs/PANI | 53 | 7.4 | 189 | [49] |
| Fe3Mo3C/Mo2C-800 | 202.3 | 39.9 | 1800 | [50] |
| SrCo0.9Fe0.1O3-δ | 1035.9 | 26.2 | 800 | [51] |
| PPy700@SFO@CC | 421 | 16.9 | 984 | [52] |
| Cu2MoS4 | 152.6 | 16.8 | 800 | [53] |
| PAni@OS–rGO (1:1) | 582.6 | 26.82 | 882 | This study |
| PAni@OS–rGO (2:1) | 453.3 | 20.55 | 522 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benchikh, I.; Ezzat, A.O.; Sabantina, L.; Benmimoun, Y.; Benyoucef, A. Investigation of Hybrid Electrodes of Polyaniline and Reduced Graphene Oxide with Bio-Waste-Derived Activated Carbon for Supercapacitor Applications. Polymers 2024, 16, 421. https://doi.org/10.3390/polym16030421
Benchikh I, Ezzat AO, Sabantina L, Benmimoun Y, Benyoucef A. Investigation of Hybrid Electrodes of Polyaniline and Reduced Graphene Oxide with Bio-Waste-Derived Activated Carbon for Supercapacitor Applications. Polymers. 2024; 16(3):421. https://doi.org/10.3390/polym16030421
Chicago/Turabian StyleBenchikh, Imen, Abdelrahman Osama Ezzat, Lilia Sabantina, Youcef Benmimoun, and Abdelghani Benyoucef. 2024. "Investigation of Hybrid Electrodes of Polyaniline and Reduced Graphene Oxide with Bio-Waste-Derived Activated Carbon for Supercapacitor Applications" Polymers 16, no. 3: 421. https://doi.org/10.3390/polym16030421
APA StyleBenchikh, I., Ezzat, A. O., Sabantina, L., Benmimoun, Y., & Benyoucef, A. (2024). Investigation of Hybrid Electrodes of Polyaniline and Reduced Graphene Oxide with Bio-Waste-Derived Activated Carbon for Supercapacitor Applications. Polymers, 16(3), 421. https://doi.org/10.3390/polym16030421








