Dielectric Characterization of Solutions of Galactomannan Extracted from Adenanthera pavonina L.: Effects of Purification and Ethanol Concentration
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation Methods
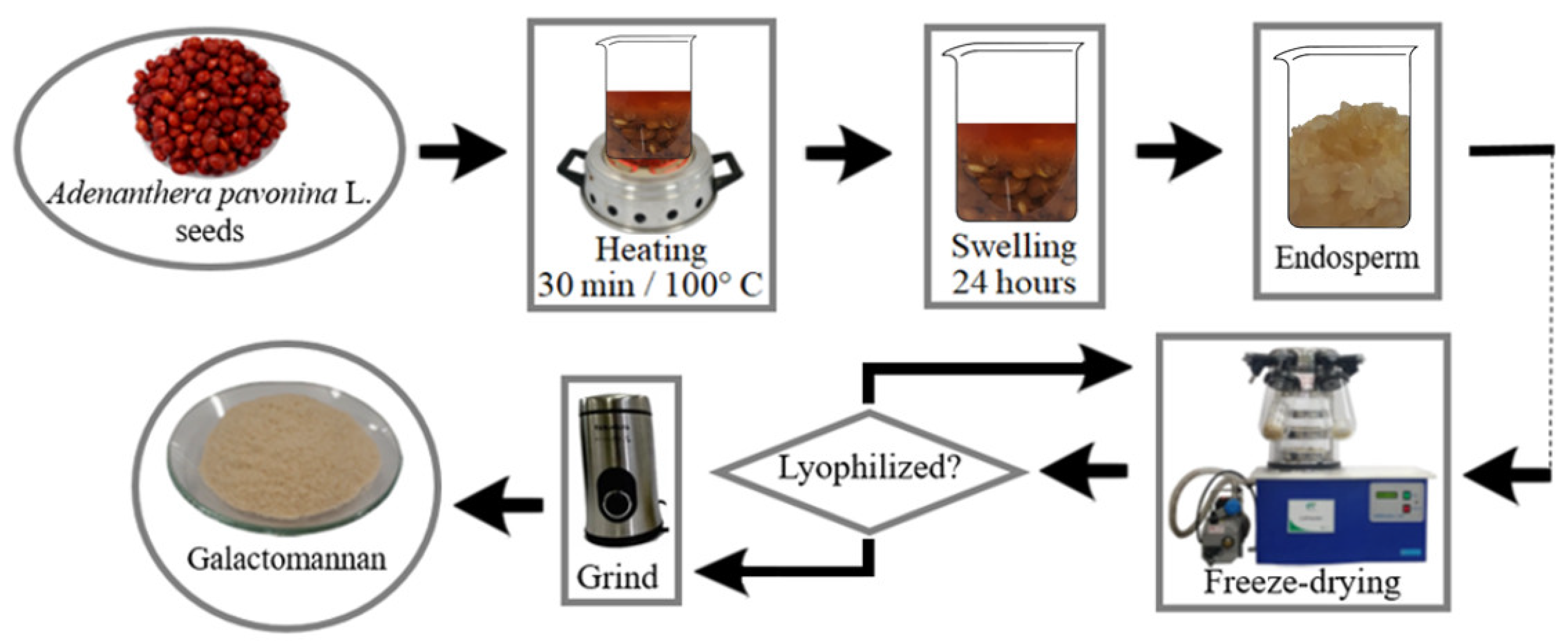
2.1.1. Extraction of Galactomannan from Adenanthera pavonina L.
2.1.2. Purification of Galactomannan from Adenanthera pavonina L. in Different Concentrations
2.1.3. Preparation of Galactomannan Solutions from Adenanthera pavonina L.
2.2. Characterization Methods
2.2.1. Infrared Spectroscopy
2.2.2. Electrical Measurements
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, C.; Li, S.; Han, M.; Chen, Z.; Zhou, W. In-Situ Dielectric Analysis on the Responses of Vegetable Cells to Freezing Treatment. Int. J. Refrig. 2020, 118, 384–391. [Google Scholar] [CrossRef]
- Ozturk, S.; Kong, F.; Trabelsi, S.; Singh, R.K. Dielectric Properties of Dried Vegetable Powders and Their Temperature Profile during Radio Frequency Heating. J. Food Eng. 2016, 169, 91–100. [Google Scholar] [CrossRef]
- Coronel, P.; Simunovic, J.; Sandeep, K.P.; Kumar, P. Dielectric Properties of Pumpable Food Materials at 915 MHz. Int. J. Food Prop. 2008, 11, 508–518. [Google Scholar] [CrossRef]
- Teseme, W.B.; Weldeselassie, H.W. Review on the study of dielectric properties of food materials. Am. J. Eng. Technol. Manag. 2020, 5, 76–83. [Google Scholar] [CrossRef]
- Naik, T.P.; Singh, I.; Sharma, A.K. Processing of polymer matrix composites using microwave energy: A review. Compos. Part. A 2022, 156, 106870. [Google Scholar] [CrossRef]
- Głowniak, S.; Szczęśniak, B.; Choma, J.; Jaroniec, M. Advances in microwave synthesis of nanoporous materials. Adv. Mater. 2021, 33, 2103477. [Google Scholar] [CrossRef]
- Jojo, S.; Mahendran, R. Radio frequency heating and its application in food processing: A review. Int. J. Curr. Agric. Res. 2013, 1, 42–46. [Google Scholar]
- Guo, W.; Liu, Y.; Zhu, X.; Wang, S. Temperature-Dependent Dielectric Properties of Honey Associated with Dielectric Heating. J. Food Eng. 2011, 102, 209–216. [Google Scholar] [CrossRef]
- Venkatesh, M.S.; Raghavan, G.S.V. An Overview of Microwave Processing and Dielectric Properties of Agri-Food Materials. Biosyst. Eng. 2004, 88, 1–18. [Google Scholar] [CrossRef]
- Piyasena, P.; Dussault, C.; Koutchma, T.; Ramaswamy, H.S.; Awuah, G.B. Radio Frequency Heating of Foods: Principles, Applications and Related Properties—A Review. Crit. Rev. Food Sci. Nutr. 2003, 43, 587–606. [Google Scholar] [CrossRef]
- Srivastava, M.; Kapoor, V.P. Seed Galactomannans: An Overview. Chem. Biodivers. 2005, 2, 295–317. [Google Scholar] [CrossRef] [PubMed]
- Boquet, M. Key Success Factors in the Semiconductor Industry: Comparative Analysis of the US, European and Chinese Semiconductor Industries. Louvain School of Management, Université Catholique de Louvain. Prom.: Defraigne, Jean-Christophe; Lejeune, Christophe. 2021. Available online: http://hdl.handle.net/2078.1/thesis:28300 (accessed on 23 October 2023).
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 6th ed.; Palgrave MacMillan: London, UK, 2013. [Google Scholar]
- Rashid, F.; Hussain, S.; Ahmed, Z. Extraction Purification and Characterization of Galactomannan from Fenugreek for Industrial Utilization. Carbohydr. Polym. 2018, 180, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Lavudi, H.N.; Kottapalli, S.; Goycoolea, F.M. Extraction and Physicochemical Characterization of Galactomannans from Dichrostachys Cinerea Seeds. Food Hydrocoll. 2018, 82, 451–456. [Google Scholar] [CrossRef]
- Milcovich, G.; Antunes, F.E.; Farra, R.; Grassi, G.; Grassi, M.; Asaro, F. Modulating carbohydrate-based hydrogels as viscoelastic lubricant substitute for articular cartilages. Int. J. Biol. Macromol. 2017, 102, 796–804. [Google Scholar] [CrossRef]
- Bento, J.F.; Mazzaro, I.; de Almeida Sliva, L.M.; de Azevedo Moreira, R.; Ferreira, M.L.C.; Reicher, F.; de Oliveira Petkowicz, C.L. Diverse Patterns of Cell Wall Mannan/Galactomannan Occurrence in Seeds of the Leguminosae. Carbohydr. Polym. 2013, 92, 192–199. [Google Scholar] [CrossRef]
- Buckeridge, M.S.; dos Santos, H.P.; Tiné, M.A.S. Mobilisation of Storage Cell Wall Polysaccharides in Seeds. Plant Physiol. Biochem. 2000, 38, 141–156. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S.; Khatkar, B.S. X-Ray Diffraction, IR Spectroscopy and Thermal Characterization of Partially Hydrolyzed Guar Gum. Int. J. Biol. Macromol. 2012, 50, 1035–1039. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Pinheiro, A.C.; Souza, B.W.; Lima, Á.M.; Ribeiro, C.; Miranda, C.; Teixeira, J.A.; Moreira, R.A.; Coimbra, M.A.; Gonçalves, M.P.; et al. Extraction, purification and characterization of galactomannans from non-traditional sources. Carbohydr. Polym. 2009, 75, 408–414. [Google Scholar] [CrossRef]
- Dea, I.C.; Morrison, A. Chemistry and interactions of seed galactomannans. Adv. Carbohydr. Chem. Biochem. 1975, 31, 241–312. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, Q.; Yang, M.; Tang, Y. A Ratiometric Fluorescent Biosensor Based on Conjugated Polymers for Sensitive Detection of Nitroreductase and Hypoxia Diagnosis in Tumor Cells. Sens. Actuators B 2020, 318, 128257. [Google Scholar] [CrossRef]
- Xu, Z.; Han, R.; Liu, N.; Gao, F.; Luo, X. Electrochemical Biosensors for the Detection of Carcinoembryonic Antigen with Low Fouling and High Sensitivity Based on Copolymerized Polydopamine and Zwitterionic Polymer. Sens. Actuators B 2020, 319, 128253. [Google Scholar] [CrossRef]
- Zhu, X.; Guo, W.; Wu, X. Frequency-and Temperature-Dependent Dielectric Properties of Fruit Juices Associated with Pasteurization by Dielectric Heating. J. Food Eng. 2012, 109, 258–266. [Google Scholar] [CrossRef]
- Tagmouti, S.; Bouzit, S.E.; Costa, L.C.; Graça, M.P.F.; Outzourhit, A. Impedance Spectroscopy of Nanofluids Based on Multiwall Carbon Nanotubes. Spectrosc. Lett. 2015, 48, 761–766. [Google Scholar] [CrossRef]
- Hammami, I.; Sales, A.M.J.; Benhamou, K.; Arous, M.; Costa, L.C.; da Cruz, J.A.; Kaddami, H. Dielectric Response and Molecular Dynamics of Nanocomposites Based on TEMPO-Oxidized Cellulose Nanofibrils and Polyvinyl Acetate. Therm. Sci. Eng. Prog. 2022, 34, 101428. [Google Scholar] [CrossRef]
- Graça, M.P.F.; da Silva, M.F.; Valente, M.A. Influence of thermal and thermoelectric treatments on structure and electric properties of B2O3–Li2O–Nb2O5 glasses. J. Non-Cryst. Solids 2008, 354, 901–908. [Google Scholar] [CrossRef]
- Graça, M.P.F.; Prezas, P.R.; Costa, M.M.; Valente, M.A. Structural and dielectric characterization of LiNbO3 nano-size powders obtained by Pechini method. J. Sol-Gel Sci. Technol. 2012, 64, 78–85. [Google Scholar] [CrossRef]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Hammami, I.; Gavinho, S.R.; Pádua, A.S.; Lança, M.d.C.; Borges, J.P.; Silva, J.C.; Sá-Nogueira, I.; Jakka, S.K.; Graça, M.P.F. Extensive Investigation on the Effect of Niobium Insertion on the Physical and Biological Properties of 45S5 Bioactive Glass for Dental Implant. Int. J. Mol. Sci. 2023, 24, 5244. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.C.; Valente, M.A.; Graça, M.P.F.; Sombra, A.S.B. The modulus formalism used in the dielectric analysis of hydroxyapatite and calcium phosphate with titanium formed by dry ball milling. J. Non-Cryst. Solids 2005, 351, 2945–2950. [Google Scholar] [CrossRef]
- Everard, C.D.; Fagan, C.C.; O’donnell, C.P.; O’callaghan, D.J.; Lyng, J.G. Dielectric Properties of Process Cheese from 0.3 to 3 GHz. J. Food Eng. 2006, 75, 415–422. [Google Scholar] [CrossRef]
- Yuen, S.-N.; Choi, S.-M.; Phillips, D.L.; Ma, C.-Y. Raman and FTIR Spectroscopic Study of Carboxymethylated Non-Starch Polysaccharides. Food Chem. 2009, 114, 1091–1098. [Google Scholar] [CrossRef]
- Kacurakova, M.; Capek, P.; Sasinkova, V.; Wellner, N.; Ebringerova, A. FT-IR Study of Plant Cell Wall Model Compounds: Pectic Polysaccharides and Hemicelluloses. Carbohydr. Pol. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Wang, J.; Somasundaran, P. Mechanisms of Ethyl (Hydroxyethyl) Cellulose–Solid Interaction: Influence of Hydrophobic Modification. J. Colloid. Interface Sci. 2006, 293, 322–332. [Google Scholar] [CrossRef]
- Figueiró, S.D.; Góes, J.C.; Moreira, R.A.; Sombra, A.S.B. On the Physico-Chemical and Dielectric Properties of Glutaraldehyde Crosslinked Galactomannan–Collagen Films. Carbohydr. Pol. 2004, 56, 313–320. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, B.P.; Choudhary, R.N.P.; Thakur, A.K. Characterization of Electrical Properties of Pb-Modified BaSnO3 Using Impedance Spectroscopy. Mater. Chem. Phys. 2006, 99, 150–159. [Google Scholar] [CrossRef]
- Woo, H.J.; Majid, S.R.; Arof, A.K. Dielectric Properties and Morphology of Polymer Electrolyte Based on Poly (ɛ-Caprolactone) and Ammonium Thiocyanate. Mater. Chem. Phys. 2012, 134, 755–761. [Google Scholar] [CrossRef]
- Cutler, P. Protein Purification Protocols; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008; Volume 244. [Google Scholar]
- Silva Neto, J.F.; Pereira, W.O.; Cavalcante, L.A.; Oliveira Neto, J.G.; Graça, M.P.F.; Gavinho, S.R.; Amaral, F.M.B.; Santos, A.O.; Sombra, A.S.B.; Mendes, F.; et al. Extraction, Purification and Electrical Characterization of Gross Galactomannan and Purified Galactomannan Obtained from Adenanthera pavonina L. Seeds. Chem. Biodivers. 2023, 20, e202200888. [Google Scholar] [CrossRef] [PubMed]
- Padmasree, K.P.; Kanchan, D.K.; Kulkarni, A.R. Impedance and Modulus Studies of the Solid Electrolyte System 20CdI2–80 [xAg2O–y(0.7 V2O5–0.3 B2O3)], where 1 ≤ x/y ≤ 3. Solid. State Ionics 2006, 177, 475–482. [Google Scholar] [CrossRef]
- Içier, F.; Baysal, T. Dielectrical Properties of Food Materials—1: Factors Affecting and Industrial Uses. Crit. Rev. Food Sci. Nutr. 2004, 44, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.T.; Cerqueira, M.A.; Bourbon, A.I.; Pinheiro, A.C.; Souza, B.W.; Vicente, A.A. Synergistic effects between κ-carrageenan and locust bean gum on physicochemical properties of edible films made thereof. Food Hydrocoll. 2012, 29, 280–289. [Google Scholar] [CrossRef]
- Baldo, M.A.; Forrest, S.R. Interface-limited injection in amorphous organic semiconductors. Phys. Rev. B Condens. Matter 2001, 64, 085201. [Google Scholar] [CrossRef]







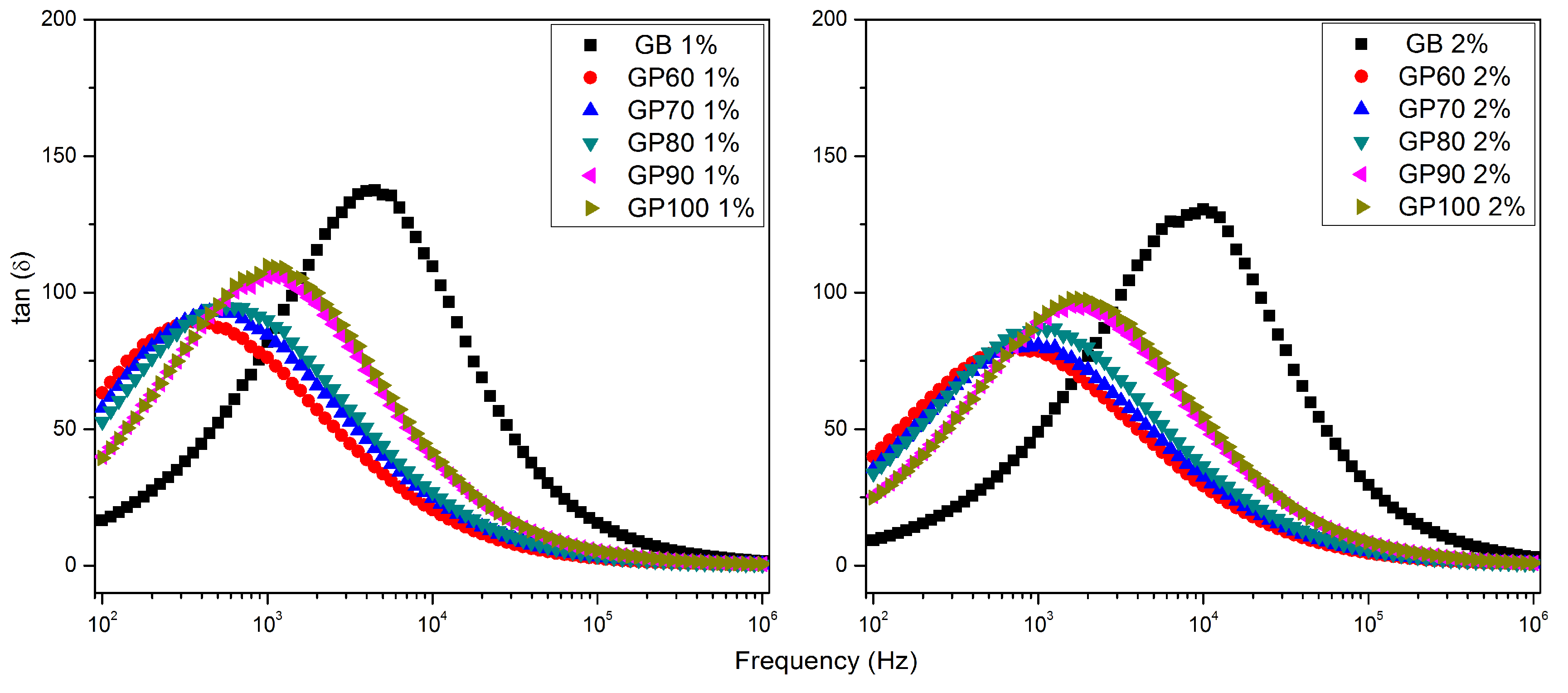
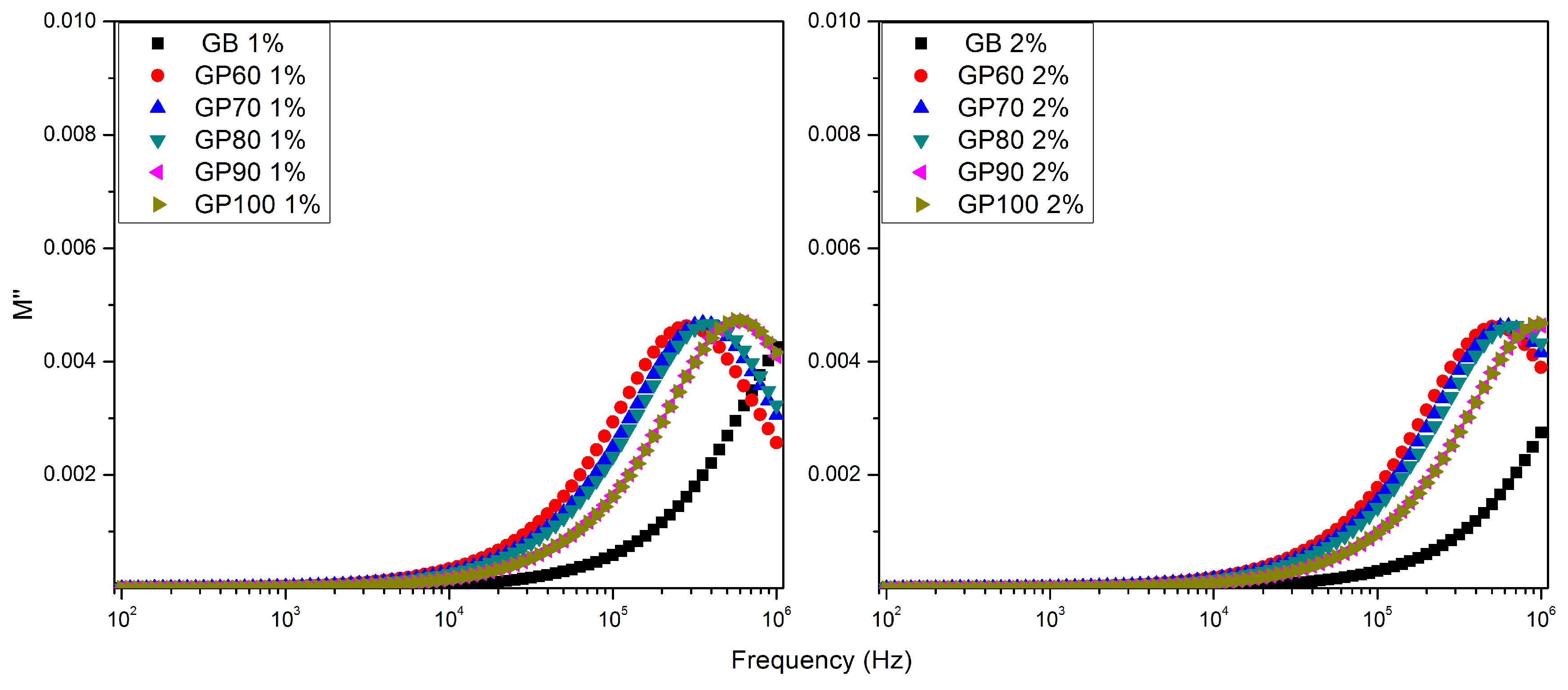


| ε′ | tan δ | σac (Ω−1m−1) | f (tan δ max) (Hz) | τ (tan δ) (μs) | f (M″ max) (Hz) | τ (M″) (μs) | |
|---|---|---|---|---|---|---|---|
| f = 1 kHz | |||||||
| GB 1% | 2051.34 | 81.90 | 0.0093 | 4467 | 35.63 | ----- | ----- |
| GB60 1% | 378.56 | 76.17 | 0.0016 | 355 | 448.56 | 281,838 | 0.56 |
| GB70 1% | 419.88 | 84.57 | 0.0020 | 447 | 356.30 | 354,813 | 0.45 |
| GB80 1% | 429.75 | 89.98 | 0.0022 | 562 | 283.02 | 398,107 | 0.40 |
| GB90 1% | 547.53 | 105.81 | 0.0032 | 1000 | 159.15 | 562,341 | 0.28 |
| GB100 1% | 538.09 | 109.82 | 0.0033 | 1000 | 159.15 | 562,341 | 0.28 |
| ε′ | tan δ | σac (Ω−1m−1) | f (tan δ max) (Hz) | τ (tan δ) (μs) | f (M″ max) (Hz) | τ (M″) (μs) | |
|---|---|---|---|---|---|---|---|
| f = 1 kHz | |||||||
| GB 2% | 6624.99 | 49.09 | 0.0181 | 10,000 | 15.92 | ----- | ----- |
| GB60 2% | 656.22 | 78.58 | 0.0029 | 708 | 224.81 | 501,187 | 0.32 |
| GB70 2% | 734.77 | 80.90 | 0.0033 | 1000 | 159.15 | 630,957 | 0.25 |
| GB80 2% | 757.95 | 87.12 | 0.0037 | 1259 | 126.42 | 707,946 | 0.22 |
| GB90 2% | 1096.66 | 89.24 | 0.0054 | 1585 | 100.42 | ----- | ----- |
| GB100 2% | 1103.24 | 90.28 | 0.0055 | 1778 | 89.50 | ----- | ----- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devesa, S.; Graça, M.P.F.; Pereira, W.O.; Santos, G.L.; da Silva Neto, J.F.; Amaral, F.M.B.; Hammami, I.; Mendes, F.; Macêdo, A.A.M. Dielectric Characterization of Solutions of Galactomannan Extracted from Adenanthera pavonina L.: Effects of Purification and Ethanol Concentration. Polymers 2024, 16, 1476. https://doi.org/10.3390/polym16111476
Devesa S, Graça MPF, Pereira WO, Santos GL, da Silva Neto JF, Amaral FMB, Hammami I, Mendes F, Macêdo AAM. Dielectric Characterization of Solutions of Galactomannan Extracted from Adenanthera pavonina L.: Effects of Purification and Ethanol Concentration. Polymers. 2024; 16(11):1476. https://doi.org/10.3390/polym16111476
Chicago/Turabian StyleDevesa, Susana, Manuel P. F. Graça, Walajhone O. Pereira, Guilherme L. Santos, João F. da Silva Neto, Filipe M. B. Amaral, Imen Hammami, Fernando Mendes, and Ana A. M. Macêdo. 2024. "Dielectric Characterization of Solutions of Galactomannan Extracted from Adenanthera pavonina L.: Effects of Purification and Ethanol Concentration" Polymers 16, no. 11: 1476. https://doi.org/10.3390/polym16111476
APA StyleDevesa, S., Graça, M. P. F., Pereira, W. O., Santos, G. L., da Silva Neto, J. F., Amaral, F. M. B., Hammami, I., Mendes, F., & Macêdo, A. A. M. (2024). Dielectric Characterization of Solutions of Galactomannan Extracted from Adenanthera pavonina L.: Effects of Purification and Ethanol Concentration. Polymers, 16(11), 1476. https://doi.org/10.3390/polym16111476









