Advances in Polymer Binder Materials for Lithium-Ion Battery Electrodes and Separators
Abstract
:1. Introduction
2. Anodes
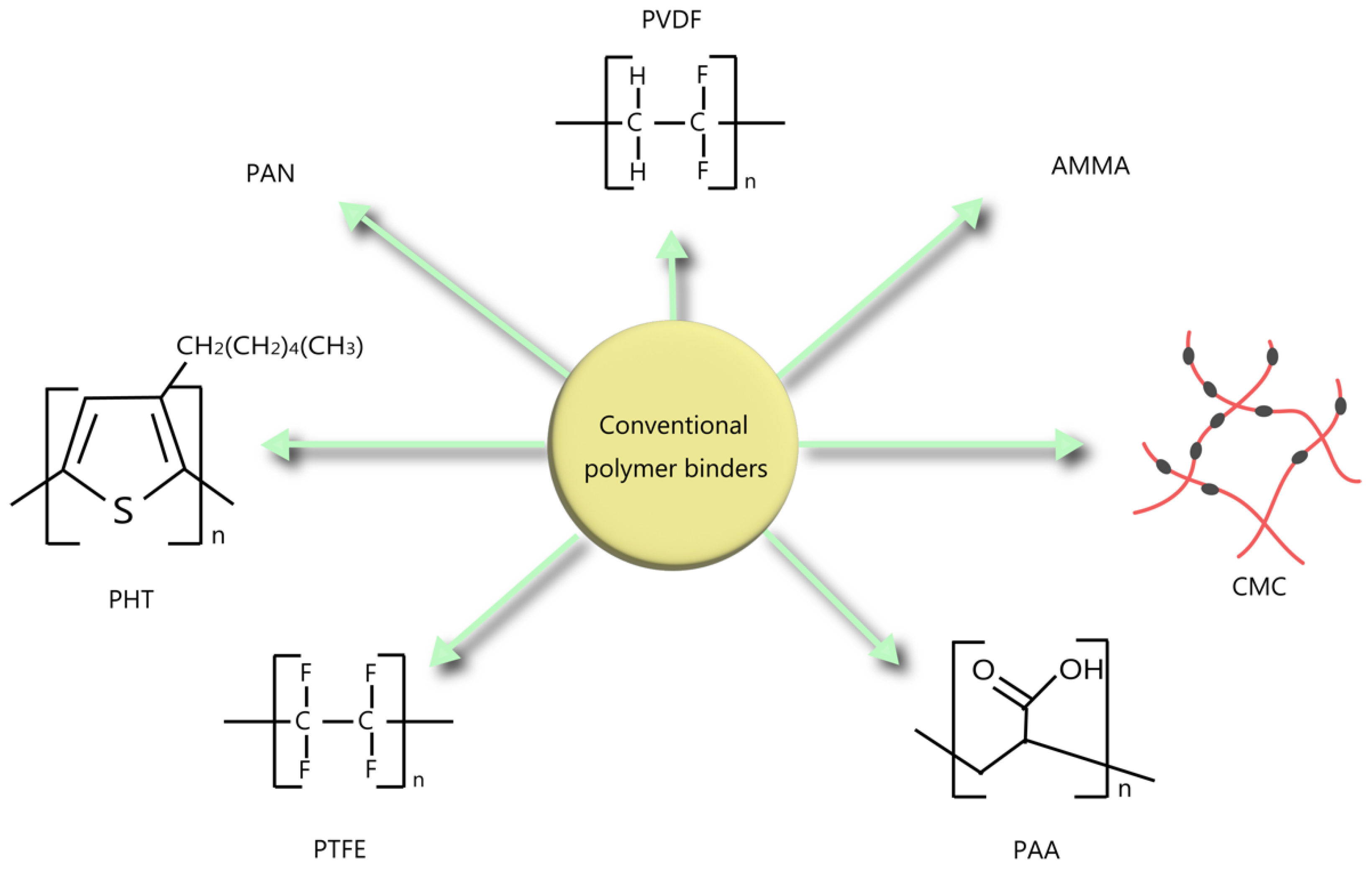
2.1. Conventional Binders for LIB Anodes
2.1.1. Graphite Anode Binders
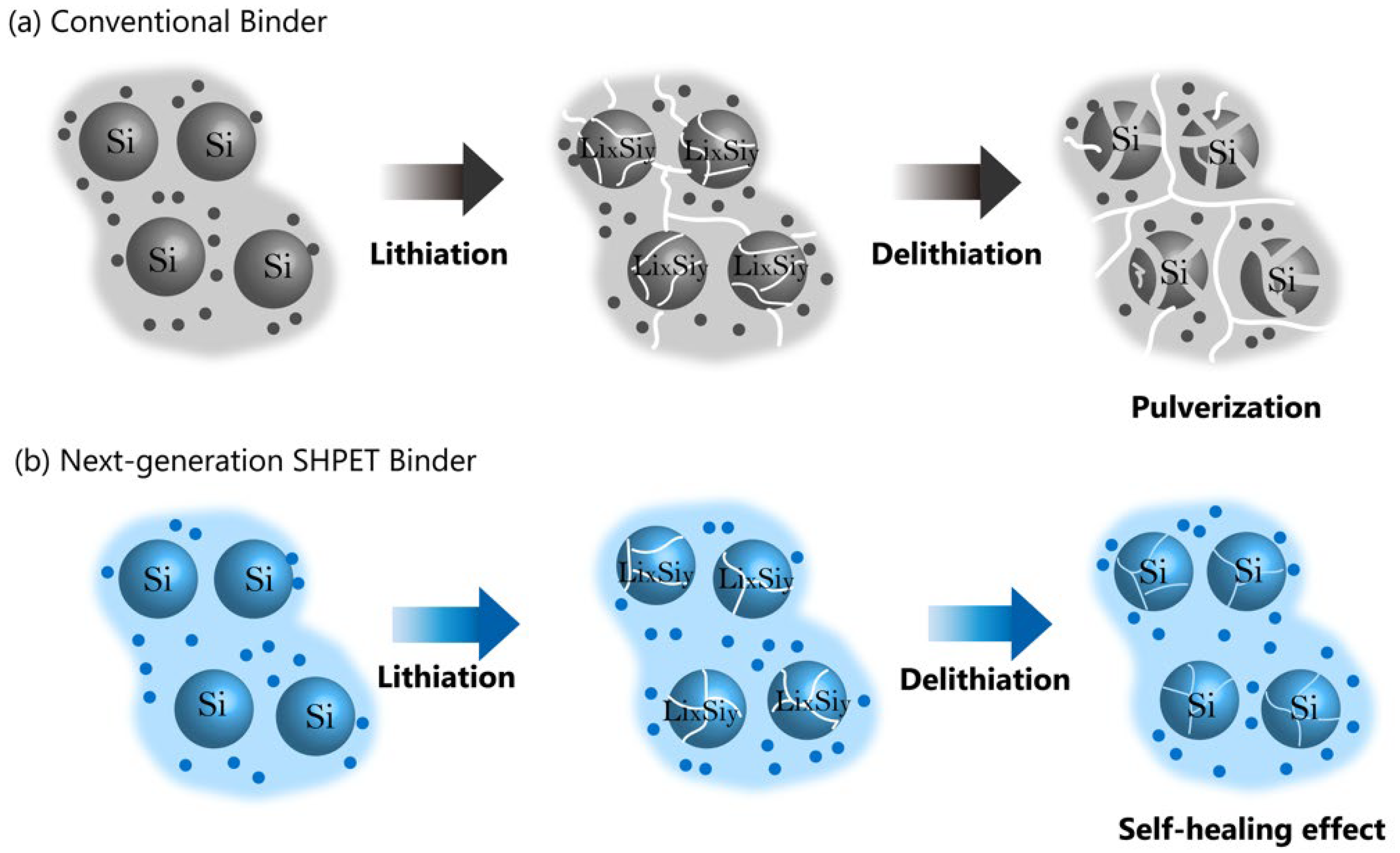
2.1.2. Si-Based Anode Binders
2.1.3. Other Types of Anode Binders
2.2. Next-Generation Anode Binders
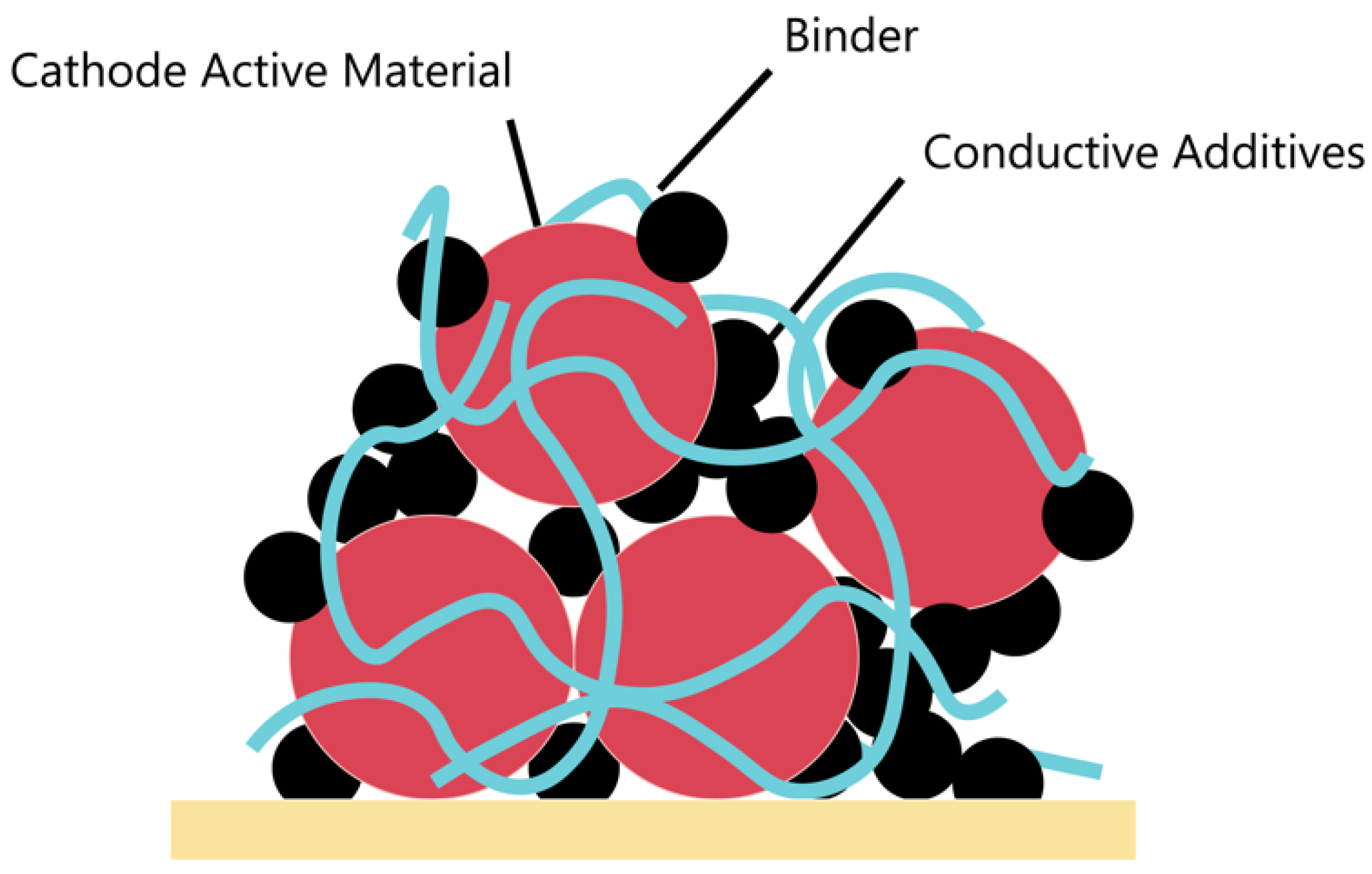
3. Cathodes
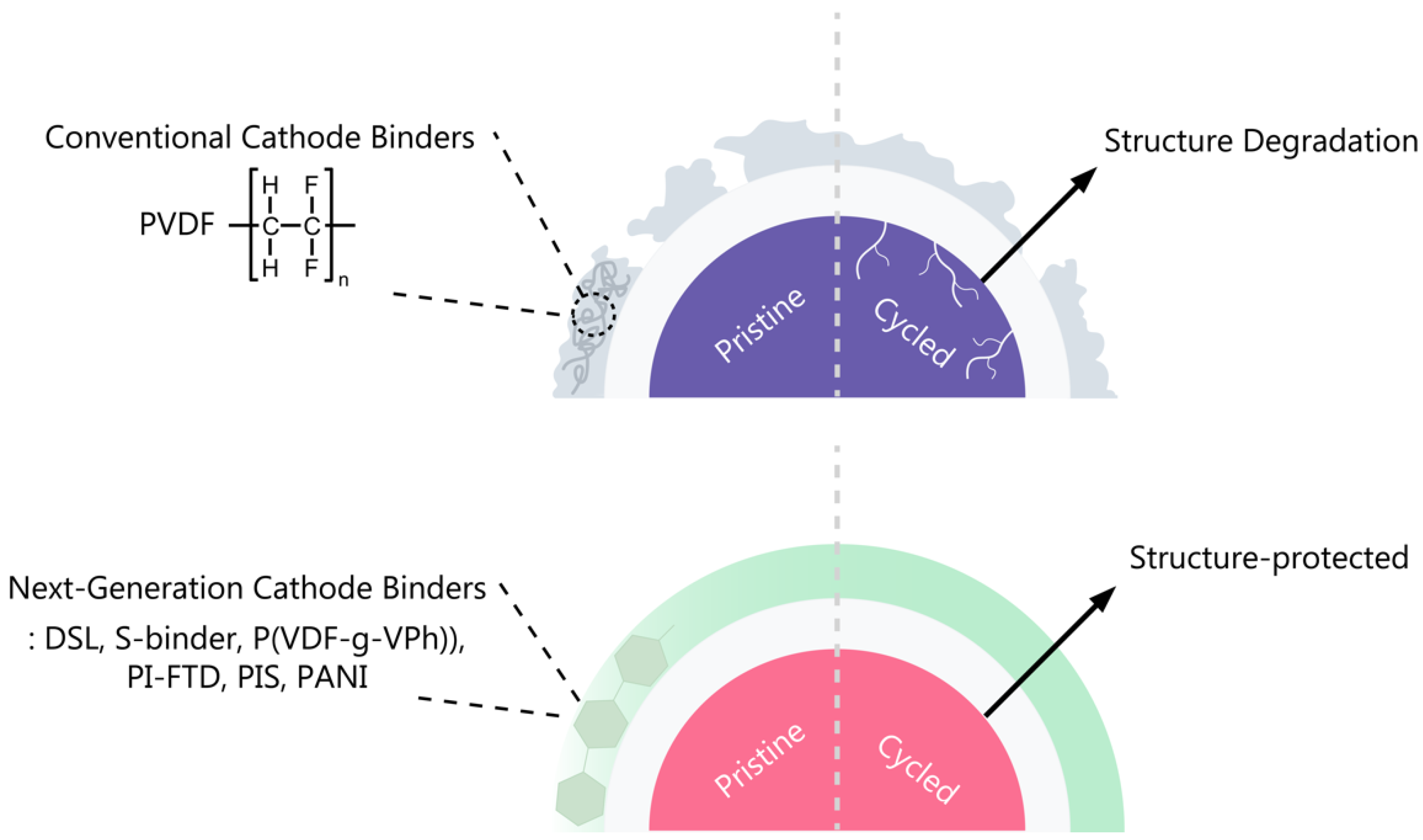
3.1. Conventional Binders for LIB Cathodes
3.2. Next-Generation Cathode Binders
3.2.1. LiCoO2
3.2.2. LiNi1−xMxO2
3.2.3. LiFePO4
4. Separators
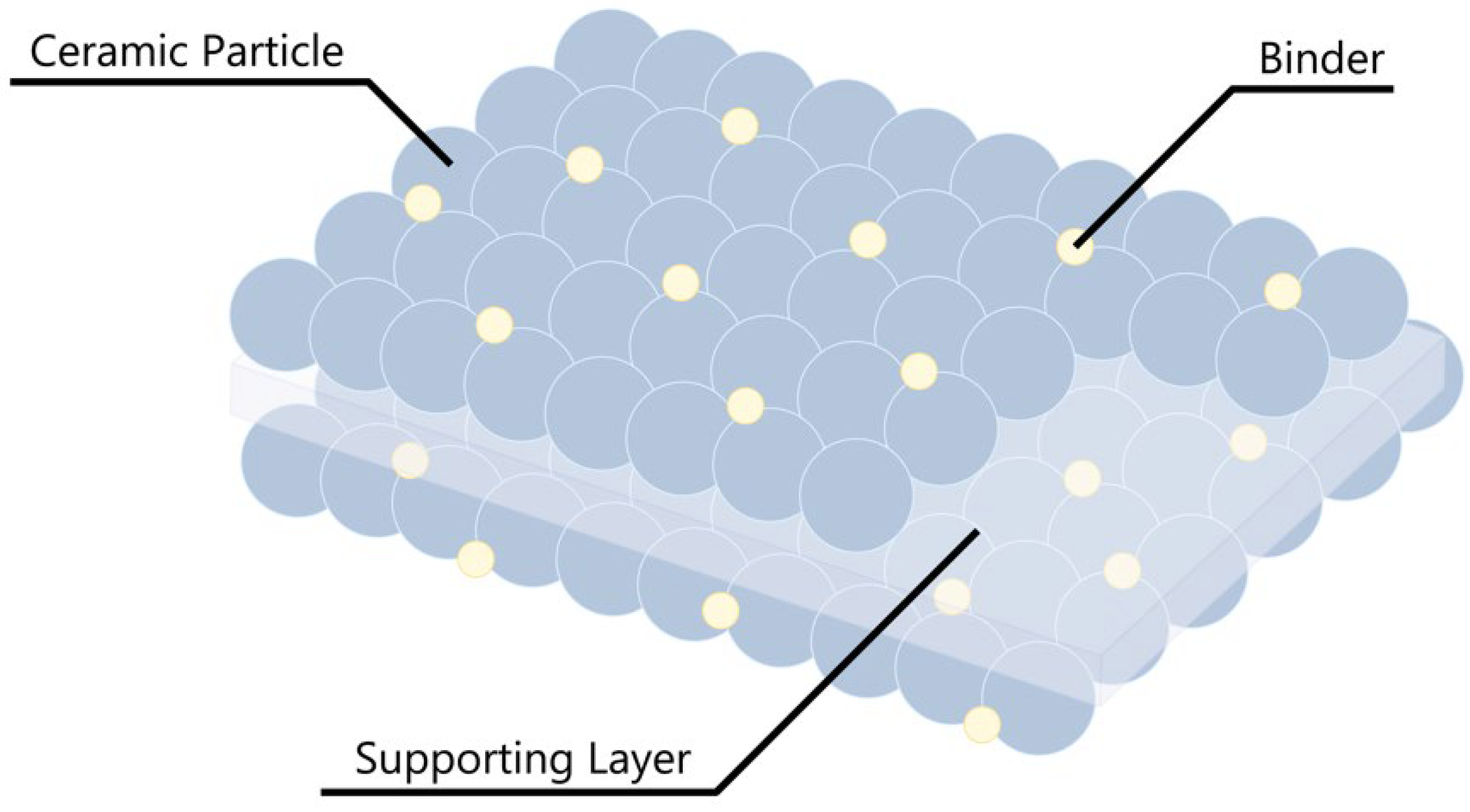
4.1. Conventional Binders for LIB Separators
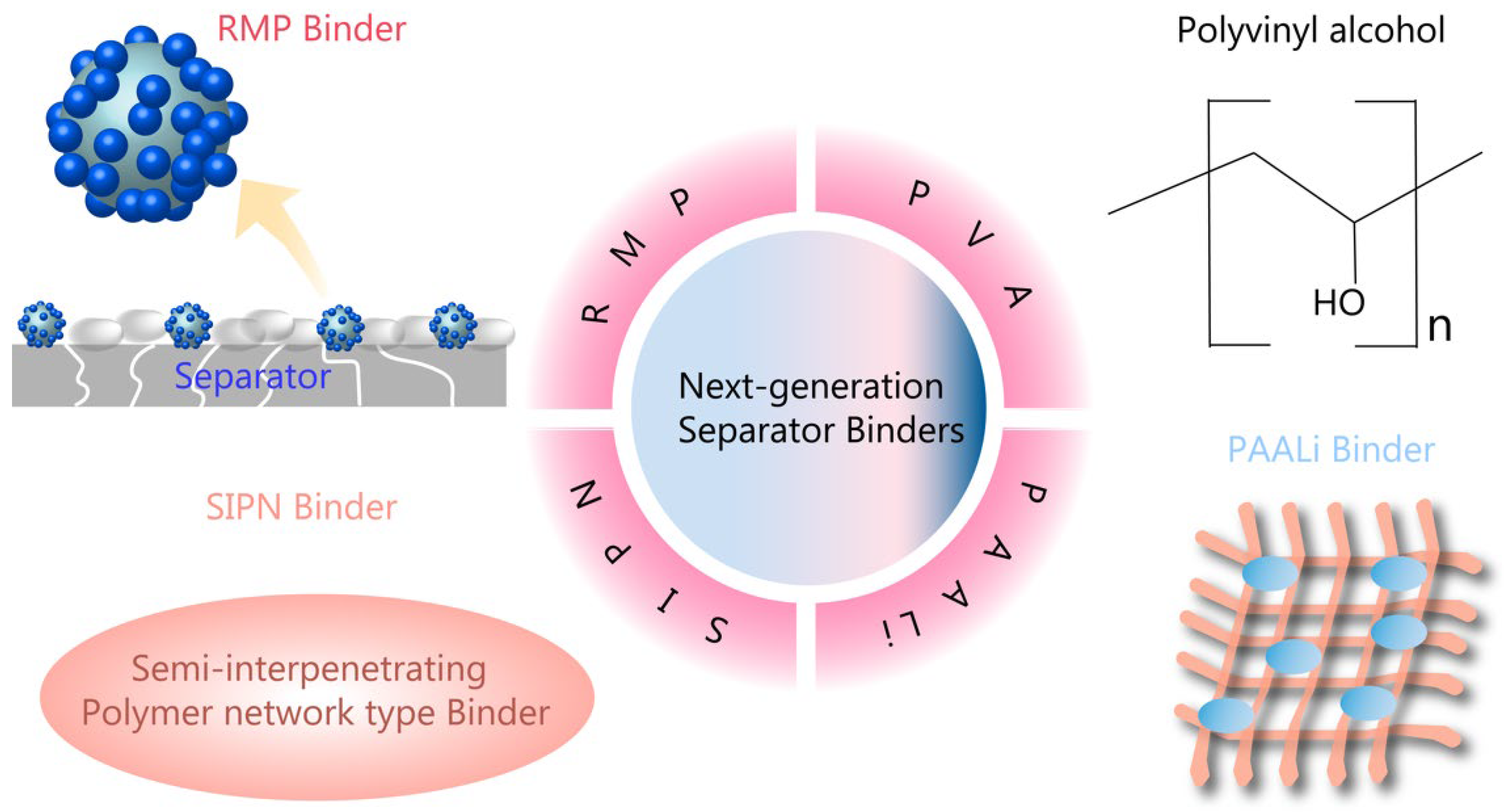
4.2. Next-Generation Separator Binders
5. Conclusions and Prospect
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LIB | Lithium-ion battery |
| NMP | N-methyl-2-pyrrolidone |
| EV | Electric vehicle |
| HEV | Hybrid electric vehicle |
| PVDF | Polyvinylidene fluoride |
| SHPET | Self-healing poly(ether-thioureas) |
| Si | Silicon |
| LCO | Lithium cobalt oxide |
| DSL | Dextran sulfate lithium |
| PI-FTD | Fluorinated polyimide |
| PIS | Poly(imide-siloxane) |
| Cyrene | Dihydrolevoglucosenone |
| NCM | Nickel cobalt manganese oxide |
| PTFE | Polytetrafluoroethylene |
| PAN | Polyacrylonitrile |
| GPE | Gel polymer electrolytes |
| AMMA | Poly(acrylonitrile-methyl methacrylate) |
| AN | Acrylonitrile |
| MMA | Methyl methacrylate |
| SEI | Solid electrolyte interface |
| PHT | Poly(3-n-hexylthiophene) |
| CMC | Carboxymethyl cellulose |
| PAA | Poly(acrylic acid) |
| LTO | Lithium titanium oxide |
| DMF | Dimethylformamide |
| FTIR | Fourier transform infrared |
| TGA | Thermogravimetric analysis |
| CV | Cyclic voltammetry |
| HPR | Hydroxypropyl polyrotaxane |
| AB | Acetylene black |
| HRTEM | High-resolution transmission electron microscopy |
| S-binder | Si-based binder |
| Sb-LCO | Lithium cobalt oxide electrode using S-binder |
| P(VDF-g-VPh) | Vinylphenol-grafted PVDF |
| CEI | Cathode–electrolyte interface |
| PANI | Polyaniline |
| PI | Polyimide |
| PDMS | Polydimethylsiloxanes |
| LFP | Lithium iron phosphate |
| PEDOT:PSS | Poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) |
| OIPC | Organic plastic crystal |
| C2mpyrFSI | N-ethyl-N-methylpyrrolidinium bis(fluorosulfonylimide) |
| CPS | Ceramic powder-based separator |
| CCS | Ceramic-coated separator |
| SRS® | Safety Reinforced Separator® |
| FE-SEM | Field emission scanning electron microscope |
| SiO2/PMMA-BNP | SiO2/Poly(methyl methacrylate) binary nanoparticle |
| PLSS | Poly(lithium 4-styrenesulfonate) |
| cPET | Copolyester |
| SBR | Styrene-butadiene rubber |
| RMP | Raspberry-like micro-sized polymer |
| PVA | Polyvinyl alcohol |
| SIPN | Semi-interpenetrating polymer network |
| EPETA | Ethoxylated pentaerythritol tetraacrylate |
| PP | Polypropylene |
| PE | Polyethylene |
| PAALi | Lithium polyacrylate |
References
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A. 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Masias, A.; Marcicki, J.; Paxton, W.A. Opportunities and challenges of lithium ion batteries in automotive applications. ACS Energy Lett. 2021, 6, 621–630. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, Z.; Kang, Y.; Zhou, Y.; Li, Y.; He, X.; Wang, L.; Mai, W.; Wang, X.; Zhou, G.; et al. Rational design of functional binder systems for high-energy lithium-based rechargeable batteries. Energy Storage Mater. 2021, 35, 353–377. [Google Scholar] [CrossRef]
- Gendensuren, B.; Oh, E.-S. Dual-crosslinked network binder of alginate with polyacrylamide for silicon/graphite anodes of lithium ion battery. J. Power Sources 2018, 384, 379–386. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, X.; Zhao, P.; Fan, H.; Zhang, Z.; Deng, J.; Ungar, G.; Song, J. Gradient H-Bonding Binder Enables Stable High-Areal-Capacity Si-Based Anodes in Pouch Cells. Adv. Mater. 2021, 33, 2104416. [Google Scholar] [CrossRef]
- Chen, H.; Wu, Z.; Su, Z.; Chen, S.; Yan, C.; Al-Mamun, M.; Tang, Y.; Zhang, S. A mechanically robust self-healing binder for silicon anode in lithium ion batteries. Nano Energy 2021, 81, 105654. [Google Scholar] [CrossRef]
- Zhou, H.; Pei, B.; Fan, Q.; Xin, F.; Whittingham, M.S. Can greener cyrene replace NMP for electrode preparation of NMC 811 cathodes? J. Electrochem. 2021, 168, 040536. [Google Scholar] [CrossRef]
- Roy, P.; Srivastava, S.K. Nanostructured anode materials for lithium ion batteries. J. Mater. Chem. A 2015, 3, 2454–2484. [Google Scholar] [CrossRef]
- Burdette-Trofimov, M.K.; Armstrong, B.L.; Korkosz, R.J.; Tyler, J.L.; McAuliffe, R.D.; Heroux, L.; Doucet, M.; Hoelzer, D.T.; Kanbargi, N.; Naskar, A.K. Understanding the solution dynamics and binding of a PVDF binder with silicon, graphite, and NMC materials and the influence on cycling performance. ACS Appl. Mater. Interfaces 2022, 14, 23322–23331. [Google Scholar] [CrossRef]
- Zhang, Z.; Fouchard, D.; Rea, J. Differential scanning calorimetry material studies: Implications for the safety of lithium-ion cells. J. Power Sources 1998, 70, 16–20. [Google Scholar] [CrossRef]
- Lingappan, N.; Kong, L.; Pecht, M. The significance of aqueous binders in lithium-ion batteries. Renew. Sustain. Energy Rev. 2021, 147, 111227. [Google Scholar] [CrossRef]
- Zhang, S.; Jow, T. Study of poly (acrylonitrile-methyl methacrylate) as binder for graphite anode and LiMn2O4 cathode of Li-ion batteries. J. Power Sources 2002, 109, 422–426. [Google Scholar] [CrossRef]
- Kuwabata, S.; Tsumura, N.; Goda, S.i.; Martin, C.R.; Yoneyama, H. Charge-Discharge Properties of Composite of Synthetic Graphite and Poly (3-n-hexylthiophene) as an Anode Active Material in Rechargeable Lithium-Ion Batteries. J. Electrochem. Soc. 1998, 145, 1415. [Google Scholar] [CrossRef]
- Parikh, P.; Sina, M.; Banerjee, A.; Wang, X.; D’Souza, M.S.; Doux, J.-M.; Wu, E.A.; Trieu, O.Y.; Gong, Y.; Zhou, Q. Role of polyacrylic acid (PAA) binder on the solid electrolyte interphase in silicon anodes. Chem. Mater. 2019, 31, 2535–2544. [Google Scholar] [CrossRef]
- Shen, L.; Shen, L.; Wang, Z.; Chen, L. In situ thermally cross-linked polyacrylonitrile as binder for high-performance silicon as lithium ion battery anode. ChemSusChem 2014, 7, 1951–1956. [Google Scholar] [CrossRef]
- Magasinski, A.; Zdyrko, B.; Kovalenko, I.; Hertzberg, B.; Burtovyy, R.; Huebner, C.F.; Fuller, T.F.; Luzinov, I.; Yushin, G. Toward efficient binders for Li-ion battery Si-based anodes: Polyacrylic acid. ACS Appl. Mater. Interfaces 2010, 2, 3004–3010. [Google Scholar] [CrossRef]
- Hu, B.; Shkrob, I.A.; Zhang, S.; Zhang, L.; Zhang, J.; Li, Y.; Liao, C.; Zhang, Z.; Lu, W.; Zhang, L. The existence of optimal molecular weight for poly (acrylic acid) binders in silicon/graphite composite anode for lithium-ion batteries. J. Power Sources 2018, 378, 671–676. [Google Scholar] [CrossRef]
- Gao, S.; Sun, F.; Brady, A.; Pan, Y.; Erwin, A.; Yang, D.; Tsukruk, V.; Stack, A.G.; Saito, T.; Yang, H. Ultra-efficient polymer binder for silicon anode in high-capacity lithium-ion batteries. Nano Energy 2020, 73, 104804. [Google Scholar] [CrossRef]
- Garsuch, R.R.; Le, D.-B.; Garsuch, A.; Li, J.; Wang, S.; Farooq, A.; Dahn, J. Studies of lithium-exchanged nafion as an electrode binder for alloy negatives in lithium-ion batteries. J. Electrochem. 2008, 155, A721. [Google Scholar] [CrossRef]
- Komaba, S.; Shimomura, K.; Yabuuchi, N.; Ozeki, T.; Yui, H.; Konno, K. Study on polymer binders for high-capacity SiO negative electrode of Li-ion batteries. J. Phys. Chem. C 2011, 115, 13487–13495. [Google Scholar] [CrossRef]
- Bridel, J.-S.; Azais, T.; Morcrette, M.; Tarascon, J.-M.; Larcher, D. Key parameters governing the reversibility of Si/carbon/CMC electrodes for Li-ion batteries. Chem. Mater. 2010, 22, 1229–1241. [Google Scholar] [CrossRef]
- Karuppiah, S.; Franger, S.; Nallathamby, K. Water-soluble green binder for Li4Ti5O12 anodes: Effect of binder choice on lithium storage. ChemElectroChem 2018, 5, 343–349. [Google Scholar] [CrossRef]
- Wang, R.; Feng, L.; Yang, W.; Zhang, Y.; Zhang, Y.; Bai, W.; Liu, B.; Zhang, W.; Chuan, Y.; Zheng, Z. Effect of different binders on the electrochemical performance of metal oxide anode for lithium-ion batteries. Nanoscale Res. Lett. 2017, 12, 1–11. [Google Scholar] [CrossRef]
- Xie, Z.H.; Rong, M.Z.; Zhang, M.Q. Dynamically cross-linked polymeric binder-made durable silicon anode of a wide operating temperature Li-ion battery. ACS Appl. Mater. Interfaces 2021, 13, 28737–28748. [Google Scholar] [CrossRef]
- Chen, H.; Ling, M.; Hencz, L.; Ling, H.Y.; Li, G.; Lin, Z.; Liu, G.; Zhang, S. Exploring chemical, mechanical, and electrical functionalities of binders for advanced energy-storage devices. Chem. Rev. 2018, 118, 8936–8982. [Google Scholar] [CrossRef]
- Oh, J.-M.; Geiculescu, O.; DesMarteau, D.; Creager, S. Ionomer binders can improve discharge rate capability in lithium-ion battery cathodes. J. Electrochem. Soc. 2010, 158, A207. [Google Scholar] [CrossRef]
- Singhal, A.; Skandan, G.; Amatucci, G.; Badway, F.; Ye, N.; Manthiram, A.; Ye, H.; Xu, J. Nanostructured electrodes for next generation rechargeable electrochemical devices. J. Power Sources 2004, 129, 38–44. [Google Scholar] [CrossRef]
- Wei, G.Z.; Lu, X.; Ke, F.S.; Huang, L.; Li, J.T.; Wang, Z.X.; Zhou, Z.Y.; Sun, S.G. Crystal habit-tuned nanoplate material of Li [Li1/3–2x/3NixMn2/3–x/3] O2 for high-rate performance lithium-ion batteries. Adv. Mater. 2010, 22, 4364–4367. [Google Scholar] [CrossRef]
- Paula, C.; Luca, M.; Francesco, T.; Marco, A. PVDF Latex As a Binder for Positive Electrodes in Lithium-Ion Batteries. Ind. Eng. Chem. Res. 2014, 53, 9094–9100. [Google Scholar]
- Zheng, H.; Yang, R.; Liu, G.; Song, X.; Battaglia, V.S. Cooperation between active material, polymeric binder and conductive carbon additive in lithium ion battery cathode. J. Phys. Chem. C 2012, 116, 4875–4882. [Google Scholar] [CrossRef]
- Huang, H.; Li, Z.; Gu, S.; Bian, J.; Li, Y.; Chen, J.; Liao, K.; Gan, Q.; Wang, Y.; Wu, S. Dextran sulfate lithium as versatile binder to stabilize high-voltage LiCoO2 to 4.6 V. Adv. Energy Mater. 2021, 11, 2101864. [Google Scholar] [CrossRef]
- Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 2020, 11, 1550. [Google Scholar] [CrossRef]
- Zhang, S.D.; Qi, M.Y.; Guo, S.J.; Sun, Y.G.; Tan, X.X.; Ma, P.Z.; Li, J.Y.; Yuan, R.Z.; Cao, A.M.; Wan, L.J. Advancing to 4.6 V Review and Prospect in Developing High-Energy-Density LiCoO2 Cathode for Lithium-Ion Batteries. Small Methods 2022, 6, 2200148. [Google Scholar] [CrossRef]
- Ahn, J.; Im, H.-G.; Lee, Y.; Lee, D.; Jang, H.; Oh, Y.; Chung, K.; Park, T.; Um, M.-K.; Yi, J.W. A novel organosilicon-type binder for LiCoO2 cathode in Li-ion batteries. Energy Stor. Mater. 2022, 49, 58–66. [Google Scholar]
- Liu, S.; Xiong, L.; He, C. Long cycle life lithium ion battery with lithium nickel cobalt manganese oxide (NCM) cathode. J. Power Sources 2014, 261, 285–291. [Google Scholar] [CrossRef]
- Li, W.; Erickson, E.M.; Manthiram, A. High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat. Energy. 2020, 5, 26–34. [Google Scholar] [CrossRef]
- Xu, G.L.; Liu, X.; Daali, A.; Amine, R.; Chen, Z.; Amine, K. Challenges and strategies to advance high-energy nickel-rich layered lithium transition metal oxide cathodes for harsh operation. Adv. Funct. Mater. 2020, 30, 2004748. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, T.; Mu, P.; Zhang, H.; Liu, W.; Cui, G. Interfacial chemistry of vinylphenol-grafted PVDF binder ensuring compatible cathode interphase for lithium batteries. Chem. Eng. J. 2022, 446, 136798. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, L.; Xu, H.; Song, Y.; He, X. Polyimides as Promising Materials for Lithium-Ion Batteries: A Review. Nanomicro Lett. 2023, 15, 1–29. [Google Scholar] [CrossRef]
- Pham, H.Q.; Lee, J.; Jung, H.M.; Song, S.-W. Non-flammable LiNi0. 8Co0. 1Mn0. 1O2 cathode via functional binder; stabilizing high-voltage interface and performance for safer and high-energy lithium rechargeable batteries. Electrochim. Acta 2019, 317, 711–721. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, N.; Liu, B.; Qi, K.; Tian, G.; Qi, S.; Wu, D. Enhanced electrochemical performance of the LiNi0.8Co0.1Mn0.1O2 cathode via in-situ nanoscale surface modification with poly (imide-siloxane) binder. Chem. Eng. J. 2022, 450, 137959. [Google Scholar] [CrossRef]
- Li, J.; Chang, C.-H.; Manthiram, A. Toward long-life, ultrahigh-nickel layered oxide cathodes for lithium-ion batteries: Optimizing the interphase chemistry with a dual-functional polymer. Chem. Mater. 2019, 32, 759–768. [Google Scholar] [CrossRef]
- Erabhoina, H.; Thelakkat, M. Tuning of composition and morphology of LiFePO4 cathode for applications in all solid-state lithium metal batteries. Sci. Rep. 2022, 12, 5454. [Google Scholar] [CrossRef]
- Yuan, L.-X.; Wang, Z.-H.; Zhang, W.-X.; Hu, X.-L.; Chen, J.-T.; Huang, Y.-H.; Goodenough, J.B. Development and challenges of LiFePO 4 cathode material for lithium-ion batteries. Energy Environ. Sci. 2011, 4, 269–284. [Google Scholar] [CrossRef]
- del Olmo, R.; Mendes, T.C.; Forsyth, M.; Casado, N. Mixed ionic and electronic conducting binders containing PEDOT: PSS and organic ionic plastic crystals toward carbon-free solid-state battery cathodes. J. Mater. Chem. A 2022, 10, 19777–19786. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, Z. Battery separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef]
- Takemura, D.; Aihara, S.; Hamano, K.; Kise, M.; Nishimura, T.; Urushibata, H.; Yoshiyasu, H. A powder particle size effect on ceramic powder based separator for lithium rechargeable battery. J. Power Sources 2005, 146, 779–783. [Google Scholar] [CrossRef]
- Jeong, H.-S.; Lee, S.-Y. Closely packed SiO2 nanoparticles/poly (vinylidene fluoride-hexafluoropropylene) layers-coated polyethylene separators for lithium-ion batteries. J. Power Sources 2011, 196, 6716–6722. [Google Scholar] [CrossRef]
- Park, J.-H.; Cho, J.-H.; Park, W.; Ryoo, D.; Yoon, S.-J.; Kim, J.H.; Jeong, Y.U.; Lee, S.-Y. Close-packed SiO2/poly (methyl methacrylate) binary nanoparticles-coated polyethylene separators for lithium-ion batteries. J. Power Sources 2010, 195, 8306–8310. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, K.; Jow, T. An inorganic composite membrane as the separator of Li-ion batteries. J. Power Sources 2005, 140, 361–364. [Google Scholar] [CrossRef]
- Xing, J.; Bliznakov, S.; Bonville, L.; Oljaca, M.; Maric, R. A Review of Nonaqueous Electrolytes, Binders, and Separators for Lithium-Ion Batteries. Electrochem. Energ. Rev. 2022, 5, 14. [Google Scholar] [CrossRef]
- Kim, M.; Han, G.Y.; Yoon, K.J.; Park, J.H. Preparation of a trilayer separator and its application to lithium-ion batteries. J. Power Sources 2010, 195, 8302–8305. [Google Scholar] [CrossRef]
- Choi, J.-A.; Kim, S.H.; Kim, D.-W. Enhancement of thermal stability and cycling performance in lithium-ion cells through the use of ceramic-coated separators. J. Power Sources 2010, 195, 6192–6196. [Google Scholar] [CrossRef]
- Ko, Y.; Yoo, H.; Kim, J. Curable polymeric binder–ceramic composite-coated superior heat-resistant polyethylene separator for lithium ion batteries. RSC Adv. 2014, 4, 19229–19233. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, P.; Chen, L.; Yang, P.; Zhao, J. Effect of a thin ceramic-coating layer on thermal and electrochemical properties of polyethylene separator for lithium-ion batteries. J. Power Sources 2014, 270, 547–553. [Google Scholar] [CrossRef]
- Muddasar, M.; Beaucamp, A.; Culebras, M.; Collins, M.N. Cellulose: Characteristics and applications for rechargeable batteries. Int. J. Biol. Macromol. 2022, 219, 788–803. [Google Scholar] [CrossRef]
- Luo, H.; Ma, S.; Liu, J.; Luo, Y.; Gao, X. Raspberry-Like Micro-Size Polymer with High Spherical Shape-Retention Capability and Adhesion as Binder for Ceramic Separators. Eur. Polym. J. 2023, 194, 112184. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kang, S.H.; Park, Y.-S.; Choi, J.; Jin, H.M.; Shin, D.O.; Lee, M.J.; Lee, Y.-G. Multifunctional separators for lithium secondary batteries via in-situ surface modification of hydrophobic separator using aqueous binders. Surf. Interfaces 2023, 38, 102828. [Google Scholar] [CrossRef]
- Kim, P.S.; Le Mong, A.; Kim, D. Thermal, mechanical, and electrochemical stability enhancement of Al2O3 coated polypropylene/polyethylene/polypropylene separator via poly (vinylidene fluoride)-poly (ethoxylated pentaerythritol tetraacrylate) semi-interpenetrating network binder. J. Membr. Sci. 2020, 612, 118481. [Google Scholar] [CrossRef]
- Li, M.; Sheng, L.; Xu, R.; Yang, Y.; Bai, Y.; Song, S.; Liu, G.; Wang, T.; Huang, X.; He, J. Enhanced the mechanical strength of polyimide (PI) nanofiber separator via PAALi binder for lithium ion battery. Compos. Commun. 2021, 24, 100607. [Google Scholar] [CrossRef]
| Binders | PVDF | PAA | CMC | Ref. |
|---|---|---|---|---|
| Density g/cm3 | 1.8480 ± 0.0036 | 1.5121 ± 0.0276 | 1.6511 ± 0.0148 | [18] |
| Binders | PVDF | PAA | CMC | Ref. |
|---|---|---|---|---|
| Specific Capacity (mAh g−1, 100th) | 118.3 | 1155.7 | 1125.5 | [18] |
| Capacity Retention (%, 100th) | 8.04 | 45.17 | 52.07 | [18] |
| Binders | Si Content (%) | Electrode Mass Loading (mg/cm2) | Electrochemical Performance | Specific Capacity Normalized to Total Electrode (mAh/g) | Ref. |
|---|---|---|---|---|---|
| PVDF | 80 | 2.6 | ~600 mAh/g @ 0.15 A/g, 50th | 480 | [19] |
| PAA | 80 | 0.89–1.7 | ~700 mAh/g @ 0.1 A/g, 50th | 560 | [20] |
| CMC | 33.3 | 2.26–2.83 | ~3000 mAh/g @ ~0.13 A/g, 100th | 1000 | [21] |
| Active Materials | Mixing Ratio | Electrochemical Performance | Capacity Retention (%) | Ref. |
|---|---|---|---|---|
| Li(Li0.17Ni0.25Mn0.58)O2 | 8:1:1 | ~238 mAh/g, 100th | 80 | [28] |
| Cu-LCO | 54:3:10.4 | ~100.9 mAh/g, 27th | 97 | [29] |
| LiNi0.8Co0.15Al0.05O2 | 89.4:8:4.8 | ~173 mAh/g, 600th | 90 | [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Koo, H.; Kang, H.S.; Oh, K.-H.; Nam, K.W. Advances in Polymer Binder Materials for Lithium-Ion Battery Electrodes and Separators. Polymers 2023, 15, 4477. https://doi.org/10.3390/polym15234477
Lee S, Koo H, Kang HS, Oh K-H, Nam KW. Advances in Polymer Binder Materials for Lithium-Ion Battery Electrodes and Separators. Polymers. 2023; 15(23):4477. https://doi.org/10.3390/polym15234477
Chicago/Turabian StyleLee, Siyeon, Heejin Koo, Hong Suk Kang, Keun-Hwan Oh, and Kwan Woo Nam. 2023. "Advances in Polymer Binder Materials for Lithium-Ion Battery Electrodes and Separators" Polymers 15, no. 23: 4477. https://doi.org/10.3390/polym15234477
APA StyleLee, S., Koo, H., Kang, H. S., Oh, K.-H., & Nam, K. W. (2023). Advances in Polymer Binder Materials for Lithium-Ion Battery Electrodes and Separators. Polymers, 15(23), 4477. https://doi.org/10.3390/polym15234477







