The Effect of Polyamide 11 on the Thermal Stability and Light Transmittance of Silicone-Based Thermoplastic Vulcanizates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Characterization Methods
3. Results
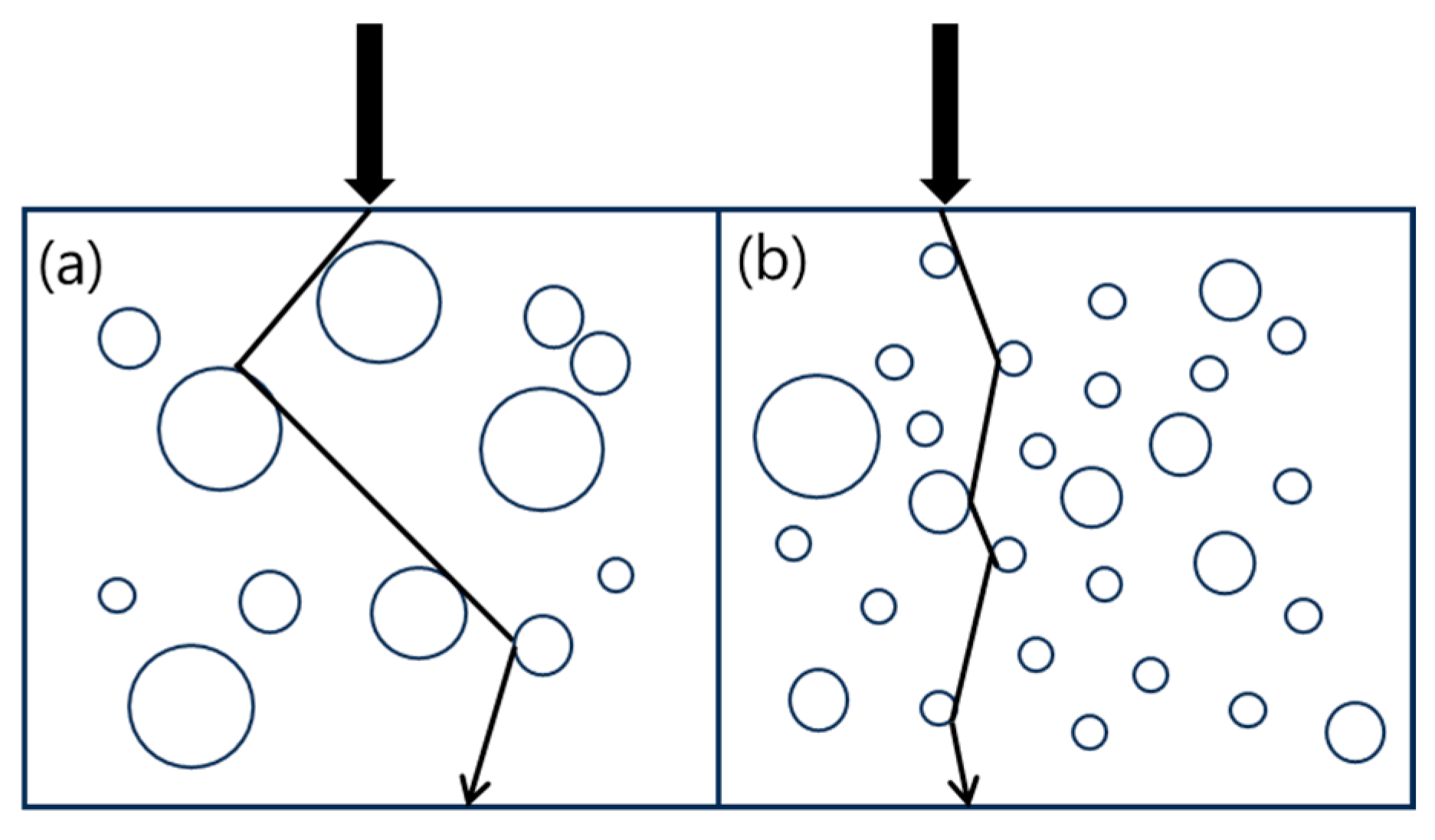
3.1. Morphology of TPVs
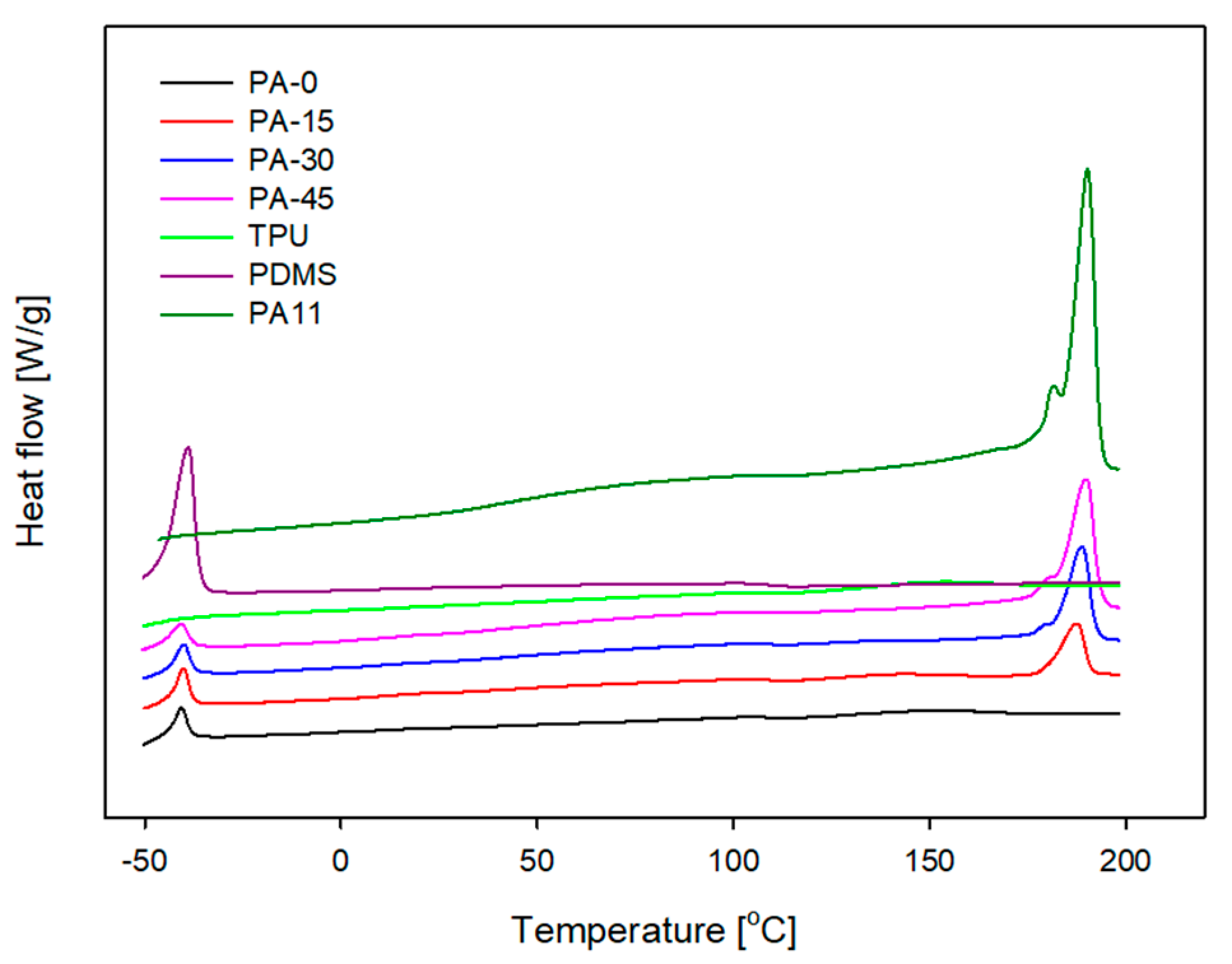
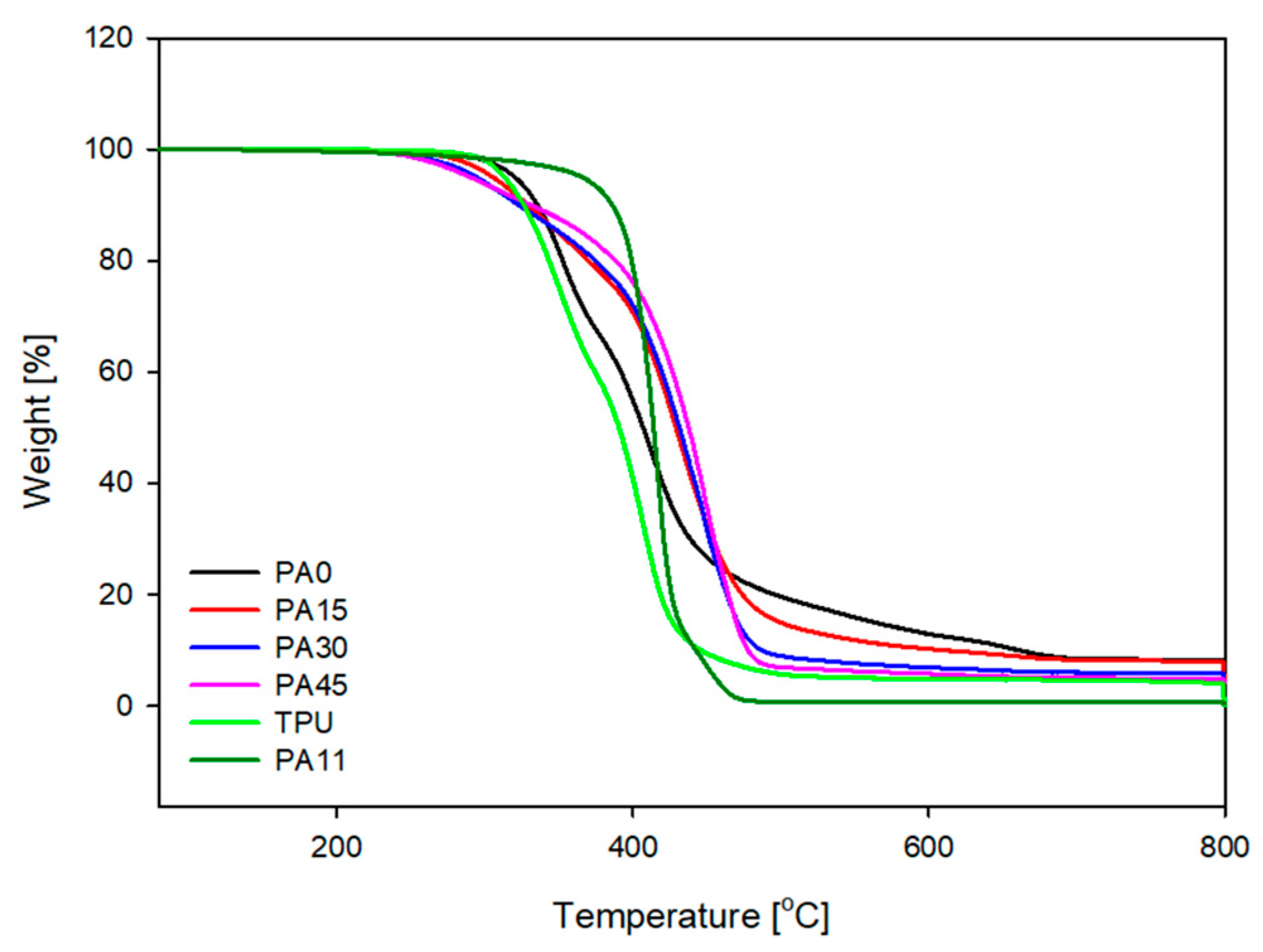
3.2. Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA)
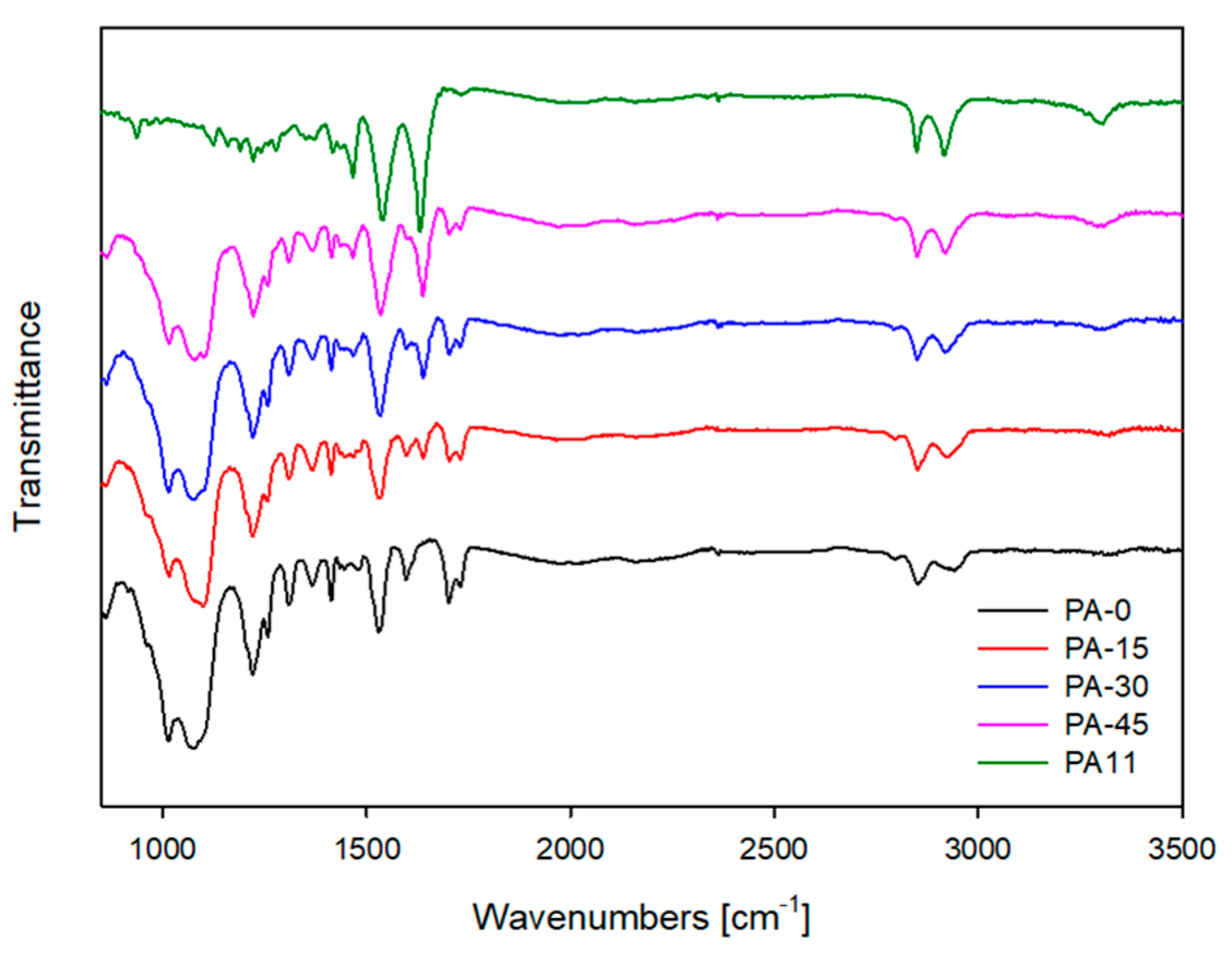
3.3. Attenuated Total Reflection Fourier-Transform Infrared Spectroscopy (ATR-FTIR)
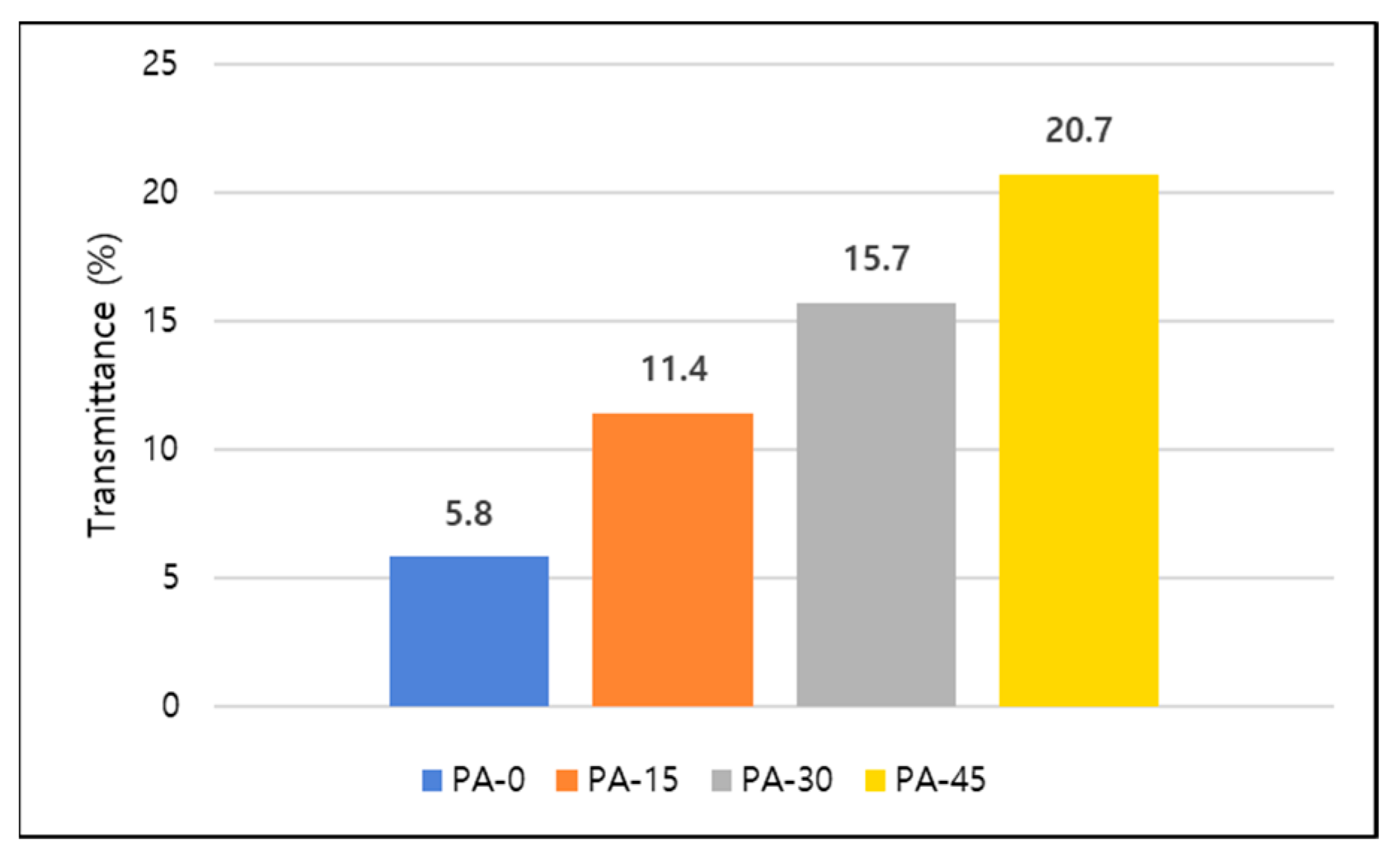
3.4. Light Transmittance
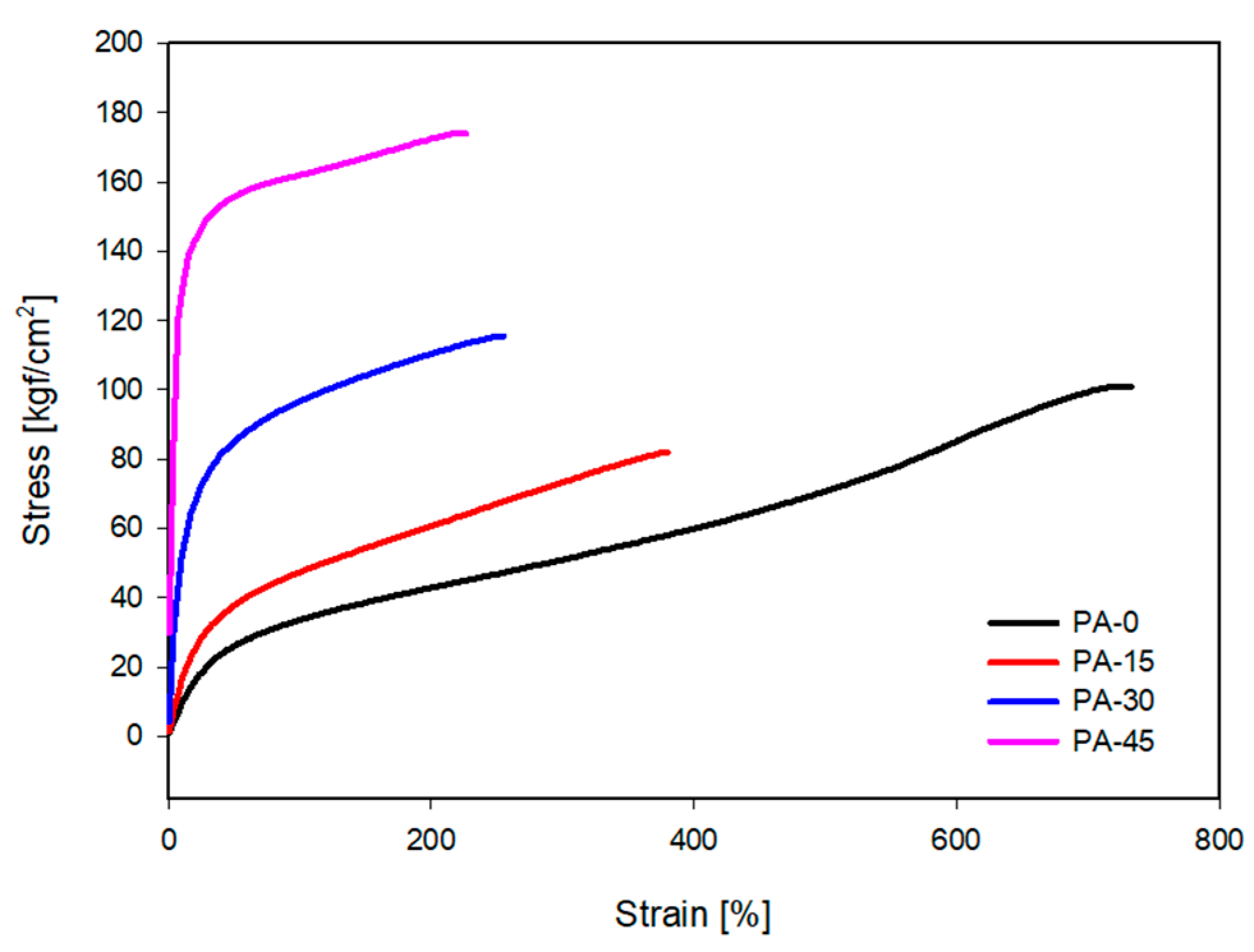
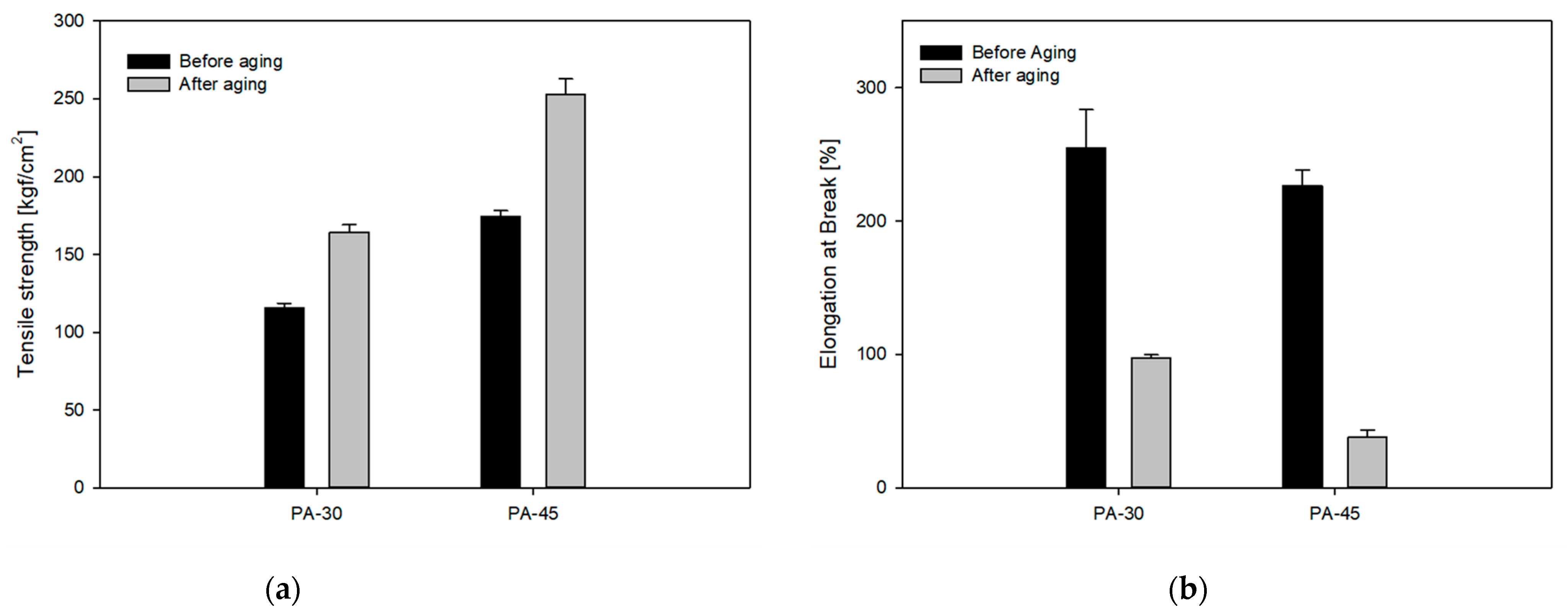
3.5. Mechanical and Thermo-Oxidative Aging Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Drobny, J.G. Handbook of Thermoplastic Elastomers; William Andrew Publishing: Norwich, NY, USA, 2007; p. 179. [Google Scholar]
- Oderkerk, J.; Groeninckx, G. Morphology Development by Reactive Compatibilization and Dynamic Vulcanization of Nylon6/EPDM Blends with A High Rubber Fraction. Polymer 2002, 43, 2219–2228. [Google Scholar] [CrossRef]
- Holden, G.; Kricheldrof, H.R.; Quirk, R.P. Thermoplastic Elastomers; Hanser: Blue Ash, OH, USA, 2004; p. 217. [Google Scholar]
- White, J.; De, S.K.; Naskar, K. Rubber Technologist’s Handbook Volume 2; Smithers Rapra: Shawbury, UK, 2009; p. 219. [Google Scholar]
- Bousmina, M.; Muller, R. Rheology/Morphology/Flow Conditions Relationships for Polymethylmethacrylate/Rubber Blend. Rheol. Acta 1996, 35, 369–381. [Google Scholar] [CrossRef]
- Abdou-Sabet, S.; Puydak, R.C.; Rader, C.P. Dynamically Vulcanized Thermoplastic Elastomers. Rubber Chem. Technol. 1996, 69, 476–494. [Google Scholar] [CrossRef]
- Rajeshbabu, R.; Gohs, U.; Naskar, K.; Thakur, V.; Wagenknecht, U.; Heinrich, G. Preparation of Poly-propylene (PP)/Ethylene Octene Copolymer (EOC) Thermoplastic Vulcanizates (tpvs) by High Energy Electron Reactive Processing. Radiat. Phys. Chem. 2011, 80, 1398–1405. [Google Scholar] [CrossRef]
- Mani, S. Fundamentals Aspects of Crosslinking Control of PDMS Rubber at High Temperatures Using Tempo Nitroxide. Ph.D. Thesis, Claude Bernard University Lyon 1, Villeurbanne, France, 2011. [Google Scholar]
- Hepburn, C. Polyurethane Elastomers; Elsevier Science: New York, NY, USA, 1992. [Google Scholar]
- Oertel, G. (Ed.) Polyurethane Handbook, 2nd ed.; Carl Hanser Verlag: Munich, Germany, 1994. [Google Scholar]
- Lin, S.B.; Hwang, K.S.; Tsay, S.Y.; Cooper, S.L. Segmental Orientation Studies of Polyether Polyurethane Block Copolymers with Different Hard Segment Lengths and Distributions. Colloid Polym. Sci. 1985, 263, 128–140. [Google Scholar] [CrossRef]
- Chang, C.L.; Don, T.M.; Lee, H.S.J.; Sha, Y.O. Studies on The Aminolysis of RTV Silicone Rubber and Modifications of Degradation Products. Polym. Degrad. Stab. 2004, 85, 769–777. [Google Scholar] [CrossRef]
- Hardan, B.; Torkelson, A. Encyclopedia of Polymer Science and Engineering; Wiley: New York, NY, USA, 1989; p. 204. [Google Scholar]
- Ning, N.; Li, S.; Wu, H.; Tian, H.; Yao, P.; Hu, G.-H.; Tian, M.; Zhang, L. Preparation, Microstructure, and Microstructure-Properties Relationship of Thermoplastic Vulcanizates (TPVs): A Review. Prog. Polym. Sci. 2018, 79, 61–97. [Google Scholar] [CrossRef]
- Lei, C.H.; Li, S.L.; Xu, R.J.; Xu, Y.Q. Thermoplastic Vulcanizates Based on Compatibilized Polyurethane and Silicone Rubber Blend. J. Elastomers Plast. 2012, 44, 563–574. [Google Scholar] [CrossRef]
- Xing, P.; Bousmina, M.; Rodrigue, D.; Kamal, M.R. Critical Experimental Comparison Between Five Techniques for the Determination of Interfacial Tension in Polymer Blends: Model System of Polystyrene/Polyamide-6. Macromolecules 2000, 33, 8020–8034. [Google Scholar] [CrossRef]
- Sundararaj, U.; Macosko, C.W. Drop Breakup and Coalescence in Polymer Blends: The Effects of Concentration and Compatibilization. Macromolecules 1995, 28, 2647–2657. [Google Scholar] [CrossRef]
- Robeson, L.M. Polymer Blends: A Comprehensive Review; Hanser: Blue Ash, OH, USA, 2007; Chapter 3; p. 6. [Google Scholar]
- Utracki, L.A. Melt Flow of Polymer Blends. Polym. Eng. Sci. 1983, 23, 602–609. [Google Scholar] [CrossRef]
- Coran, A.Y.; Patel, R.P. Thermoplastic Elastomers Based on Dynamically Vulcanized Elastomer/Thermoplastic Blends. In Thermoplastic Elastomers, 2nd ed.; Holden, G., Legge, N.R., Quirk, R., Schroeder, H.E., Eds.; Hanser: Blue Ash, OH, USA, 1996; pp. 349–394. [Google Scholar]
- De, S.K. Thermoplastic Elastomers from Rubber-Plastic Blends; Bhowmick, A.K., Ed.; Ellis Horwood: Herts, UK, 1990. [Google Scholar]
- Van, D.M. Crosslinking Systems for EPDM/PP-Based Thermoplastic Vulcanizates. In Proceedings of the International Rubber Conference, Birmingham, UK, 12–14 June 2001. [Google Scholar]
- Martin, G.; Barrès, C.; Sonntag, P.; Garois, N.; Cassagnau, P. Morphology Development in Thermoplastic Vulcanizates (TPV): Dispersion Mechanisms of a Pre-crosslinked EPDM Phase. Eur. Polym. J. 2009, 45, 3257–3268. [Google Scholar] [CrossRef]
- Machado, A.V.; Van Duin, M. Dynamic Vulcanisation of EPDM/PE-Based Thermoplastic Vulcanisates Studied Along the Extruder Axis. Polymer 2005, 46, 6575–6586. [Google Scholar] [CrossRef]
- George, S.; Ramamurthy, K.; Anand, J.S.; Groeninckx, G.; Varughese, K.T.; Thomas, S. Rheological Behaviour of Thermoplastic Elastomers from Polypropylene/Acrylonitrile–Butadiene Rubber Blends: Effect of Blend Ratio, Reactive Compatibilization and Dynamic Vulcanization. Polymer 1999, 40, 4325–4344. [Google Scholar] [CrossRef]
- Martin, P.; Maquet, C.; Legras, R.; Bailly, C.; Leemans, L.; Van Gurp, M.; Van Duin, M. Conjugated Effects of the Compatibilization and the Dynamic Vulcanization on the Phase Inversion Behavior in Poly (Butylene Terephthalate)/Epoxide-Containing Rubber Reactive Polymer Blends. Polymer 2004, 45, 5111–5125. [Google Scholar] [CrossRef]
- Papke, N.; Karger-Kocsis, J. Thermoplastic Elastomers Based on Compatibilized Poly (Ethylene Terephthalate) Blends: Effect of Rubber Type and Dynamic Curing. Polymer 2001, 42, 1109–1120. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.L.; Gong, X.L.; Gong, X.Q.; Zhang, X.Z.; Jiang, W.Q.; Zhang, P.Q.; Chen, Z.Y. New Magnetorheological Elastomers Based on Polyurethane/Si-Rubber Hybrid. Polym. Test. 2005, 24, 324–329. [Google Scholar] [CrossRef]
- Maity, M.; Khatua, B.B.; Das, C.K. Polyblend Systems of Polyurethane Rubber and Silicone Rubber in the Presence of Silane Grafting Agent. J. Elastomers Plast. 2001, 33, 211–224. [Google Scholar] [CrossRef]
- Gerald, A.G.; Craig, S.G.; Mark, D.H. Thermoplastic Polyurethane–Silicone Elastomers. U.S. Patent 6,759,487, 6 July 2004. [Google Scholar]
- Reulier, M.; Avérous, L. Elaboration, Morphology and Properties of Renewable Thermoplastics Blends, Based on Polyamide and Polyurethane Synthesized from Dimer Fatty Acids. Eur. Polym. J. 2015, 67, 418–427. [Google Scholar] [CrossRef]
- Zhang, S.L.; Wang, G.B.; Jiang, Z.H.; Wang, D.; Ma, R.T.; Wu, Z.W. Impact Properties, Phase Structure, Compatibility, and Fracture Morphology of Polyamide-1010/Thermoplastic Poly (Ester Urethane) Elastomer Blends. J. Polym. Sci. B Polym. Phys. 2005, 43, 1177. [Google Scholar] [CrossRef]
- Kim, B.C.; Kim, W.Y. Mechanical and Thermal Properties of Thermoplastic Polyurethanes Modified with Polyamide-11. J. Ind. Eng. Chem. 2000, 6, 182–187. [Google Scholar]
- Huang, S.; Wang, M.; Liu, T.; Zhang, W.D.; Tjiu, W.C.; He, C.; Lu, X. Morphology, Thermal, and Rheological Behavior of Nylon 11/Multi-Walled Carbon Nanotube Nanocomposites Prepared by Melt Compounding. Polym. Eng. Sci. 2009, 49, 1063–1068. [Google Scholar] [CrossRef]
- ASTM D412-16(2021); Standard Test Methods for Vulcanized Rubber and Thermoplastic Elastomers—Tension. ASTM: West Conshohocken, PA, USA, 2021.
- ASTM D1003-21; Standard Test Method for Haze and Luminous Transmittance of Transparent Plastics. ASTM: West Conshohocken, PA, USA, 2021.
- Li, W.; Liu, J.; Hao, C.; Jiang, K.; Xu, D.; Wang, D. Interaction of Thermoplastic Polyurethane with Polyamide 1212 and Its Influence on The Thermal and Mechanical Properties of TPU/PA1212 Blends. Polym. Eng. Sci. 2008, 48, 249–256. [Google Scholar] [CrossRef]
- López-Barrón, C.R.; Macosko, C.W. Rheological and Morphological Study of Co-continuous Polymer Blends During Coarsening. J. Rheol. 2012, 56, 1315–1334. [Google Scholar] [CrossRef]
- Schick, C. Differential Scanning Calorimetry (DSC) of Semicrystalline Polymers. Anal. Bioanal. Chem. 2009, 395, 1589–1611. [Google Scholar] [CrossRef] [PubMed]
- Frick, A.; Rochman, A. Characterization of TPU-Elastomers by Thermal Analysis (DSC). Polym. Test. 2004, 23, 413–417. [Google Scholar] [CrossRef]
- Kunchimon, S.Z.; Tausif, M.; Goswami, P.; Cheung, V. Polyamide 6 and Thermoplastic Polyurethane Recycled Hybrid Fibres via Twin-Screw Melt Extrusion. J. Polym. Res. 2019, 26, 162. [Google Scholar] [CrossRef]
- Todros, S.; Venturato, C.; Natali, A.N.; Pace, G.; Di Noto, V. Effect of Steam on Structure and Mechanical Properties of Biomedical Block Copolymers. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 1337–1346. [Google Scholar] [CrossRef]
- Rashmi, B.J.; Loux, C.; Prashantha, K. Bio-Based Thermoplastic Polyurethane and Polyamide 11 Bioalloys with Excellent Shape Memory Behavior. J. Appl. Polym. Sci. 2017, 134, 44794. [Google Scholar] [CrossRef]
- Zhang, J.; Tu, W.; Dai, Z. Transparent Polyester Polyol-Based Polyurethane Coatings: The Effect of Alcohols. J. Coat. Technol. Res. 2013, 10, 887–895. [Google Scholar] [CrossRef]
- Puszka, A.; Kultys, A. New Thermoplastic Polyurethane Elastomers Based on Aliphatic Diisocyanate. J. Therm. Anal. Calorim. 2017, 128, 407–416. [Google Scholar] [CrossRef]
- John, B.; Furukawa, M. Enhanced Mechanical Properties of Polyamide 6 Fibers Coated with a Polyurethane Thin Film. Polym. Eng. Sci. 2009, 49, 1970–1978. [Google Scholar] [CrossRef]
- Xiang, C.; Chen, H.; Wang, W.; Dai, Q.; Liu, Z.; Yang, B.; Zhou, Y.; Zhou, Y. Transparency-Tunable and Moderate-Temperature Healable Thermoplastic Polyurethane Elastomer Based on Bisphenol A Chain-Extender. J. Appl. Polym. Sci. 2021, 138, 49794. [Google Scholar] [CrossRef]
- Wu, H.; Tian, M.; Zhang, L.; Tian, H.; Wu, Y.; Ning, N.; Hu, G.H. Effect of Rubber Nanoparticle Agglomeration on Properties of Thermoplastic Vulcanizates During Dynamic Vulcanization. Polymers 2016, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.A.; van Duin, M.; Spoelstra, A.B.; Goossens, J.G. The Rubber Particle Size to Control the Properties-Processing Balance of Thermoplastic/Cross-Linked Elastomer Blends. Soft Matter 2010, 6, 1758–1768. [Google Scholar]
- Ma, P.; Xu, P.; Zhai, Y.; Dong, W.; Zhang, Y.; Chen, M. Biobased Poly (Lactide)/Ethylene-co-Vinyl Acetate Thermoplastic Vulcanizates: Morphology Evolution, Superior Properties, and Partial Degradability. ACS Sustain. Chem. Eng. 2015, 3, 2211–2219. [Google Scholar] [CrossRef]

| Sample | PA-0 | PA-15 | PA-30 | PA-45 |
|---|---|---|---|---|
| TPU | 60 | 45 | 30 | 15 |
| PA11 | - | 15 | 30 | 45 |
| TPU/PDMS M/B 1 | 40 | 40 | 40 | 40 |
| Chain extender | 0.5 | |||
| UV stabilizer | 0.3 | |||
| Anti-oxidant | 0.5 | |||
| Triganox 101-50D 2 | 0.5 | |||
| Sample | Tm (TPU) (°C) | Tm (PA11) (°C) | ∆Hm (PA11) (J/g) | XPA (%) |
|---|---|---|---|---|
| PA11 | - | 190.1 | 54.0 | 26.2 |
| PA-0 | 148.5 | - | - | - |
| PA-15 | 154.3 | 187.6 | 4.6 | 14.9 |
| PA-30 | - | 188.7 | 9.7 | 15.7 |
| PA-45 | - | 189.9 | 17.6 | 19.0 |
| Sample | Td (°C) | T20 (°C) | T50 (°C) | T75 (°C) |
|---|---|---|---|---|
| PA-0 | 320 | 352 | 406 | 458 |
| PA-15 | 305 | 370 | 429 | 462 |
| PA-30 | 294 | 374 | 431 | 464 |
| PA-45 | 290 | 388 | 439 | 465 |
| Mechanical Properties | PA-0 | PA-15 | PA-30 | PA-45 |
|---|---|---|---|---|
| Hardness (Shore A) | 73.0 | 78.0 | 89.09 | 96.0 |
| Tensile strength (kgf/cm2) | 108.4 | 81.9 | 115.6 | 174.1 |
| Elongation at break (%) | 736.8 | 379.8 | 254.9 | 226.6 |
| Modulus at 100% (kgf/cm2) | 33.7 | 47.4 | 96.9 | 162.1 |
| After Aging (168 h 160 °C) | PA-0 | PA-15 | PA-30 | PA-45 |
|---|---|---|---|---|
| Hardness (Shore A) | - | - | 91.0 | 97.0 |
| Tensile strength (kgf/cm2) | - | - | 164.1 | 252.9 |
| Elongation at break (%) | - | - | 41.9 | 37.9 |
| Change in hardness (Shore A) (pts) | - | - | 2.0 | 1.0 |
| Change in tensile strength (%) | - | - | 41.9 | 45.3 |
| Change in elongation at break (%) | - | - | −61.9 | −83.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iz, M.; Lee, J.; Choi, M.; Yun, Y.; Bae, J. The Effect of Polyamide 11 on the Thermal Stability and Light Transmittance of Silicone-Based Thermoplastic Vulcanizates. Polymers 2024, 16, 324. https://doi.org/10.3390/polym16030324
Iz M, Lee J, Choi M, Yun Y, Bae J. The Effect of Polyamide 11 on the Thermal Stability and Light Transmittance of Silicone-Based Thermoplastic Vulcanizates. Polymers. 2024; 16(3):324. https://doi.org/10.3390/polym16030324
Chicago/Turabian StyleIz, Muhammet, Jinhyok Lee, Myungchan Choi, Yumi Yun, and Jongwoo Bae. 2024. "The Effect of Polyamide 11 on the Thermal Stability and Light Transmittance of Silicone-Based Thermoplastic Vulcanizates" Polymers 16, no. 3: 324. https://doi.org/10.3390/polym16030324
APA StyleIz, M., Lee, J., Choi, M., Yun, Y., & Bae, J. (2024). The Effect of Polyamide 11 on the Thermal Stability and Light Transmittance of Silicone-Based Thermoplastic Vulcanizates. Polymers, 16(3), 324. https://doi.org/10.3390/polym16030324








