Phase Morphology and Mechanical Properties of Super-Tough PLLA/TPE/EMA-GMA Ternary Blends
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Blends
2.3. Differential Scanning Calorimetry (DSC)
2.4. Mechanical Tests
2.5. Scanning Electron Microscopy (SEM)
3. Results and Discussion
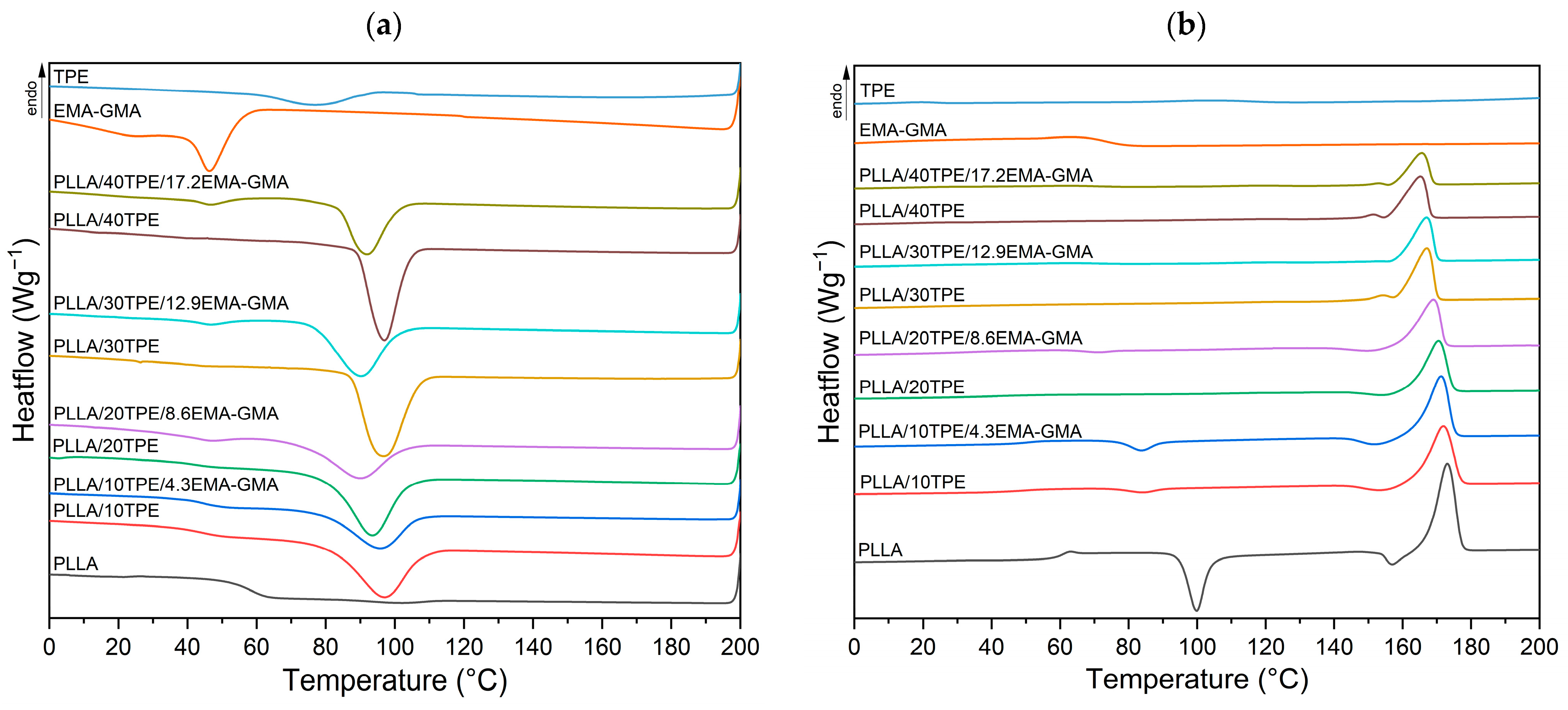
3.1. Thermal and Crystallization Behavior
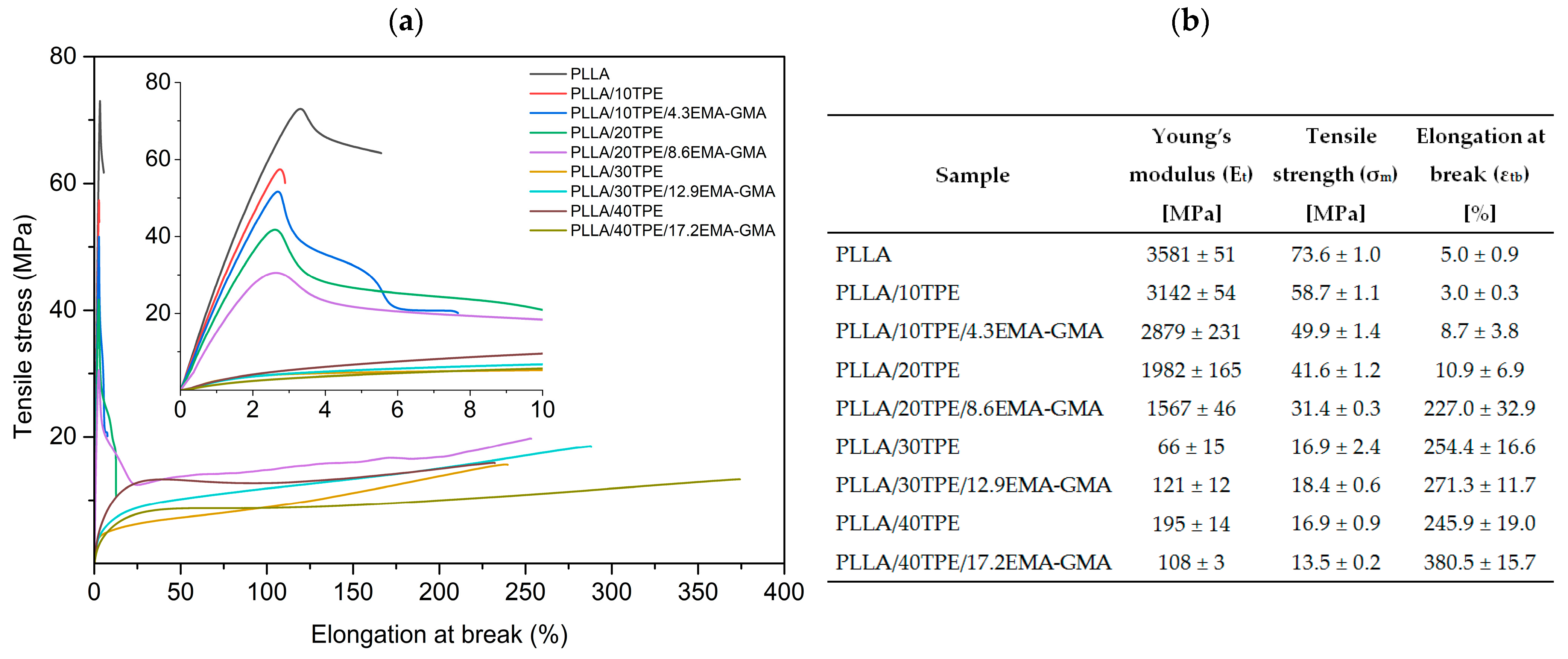
3.2. Tensile Properties
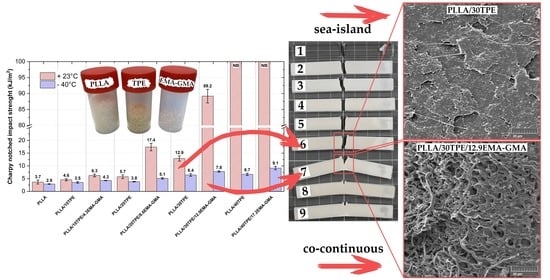
3.3. Impact Properties at Ambient and Sub-Zero Conditions
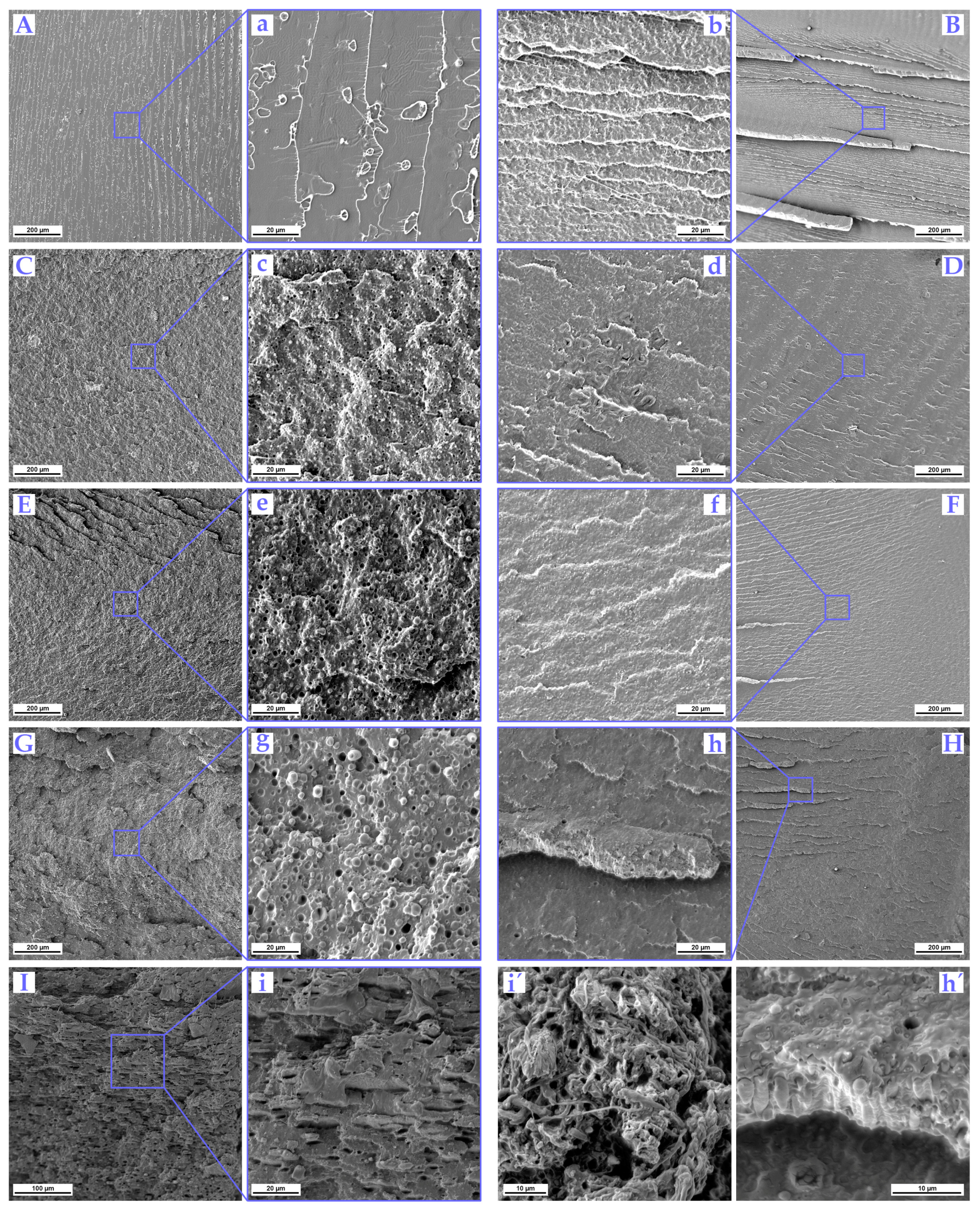
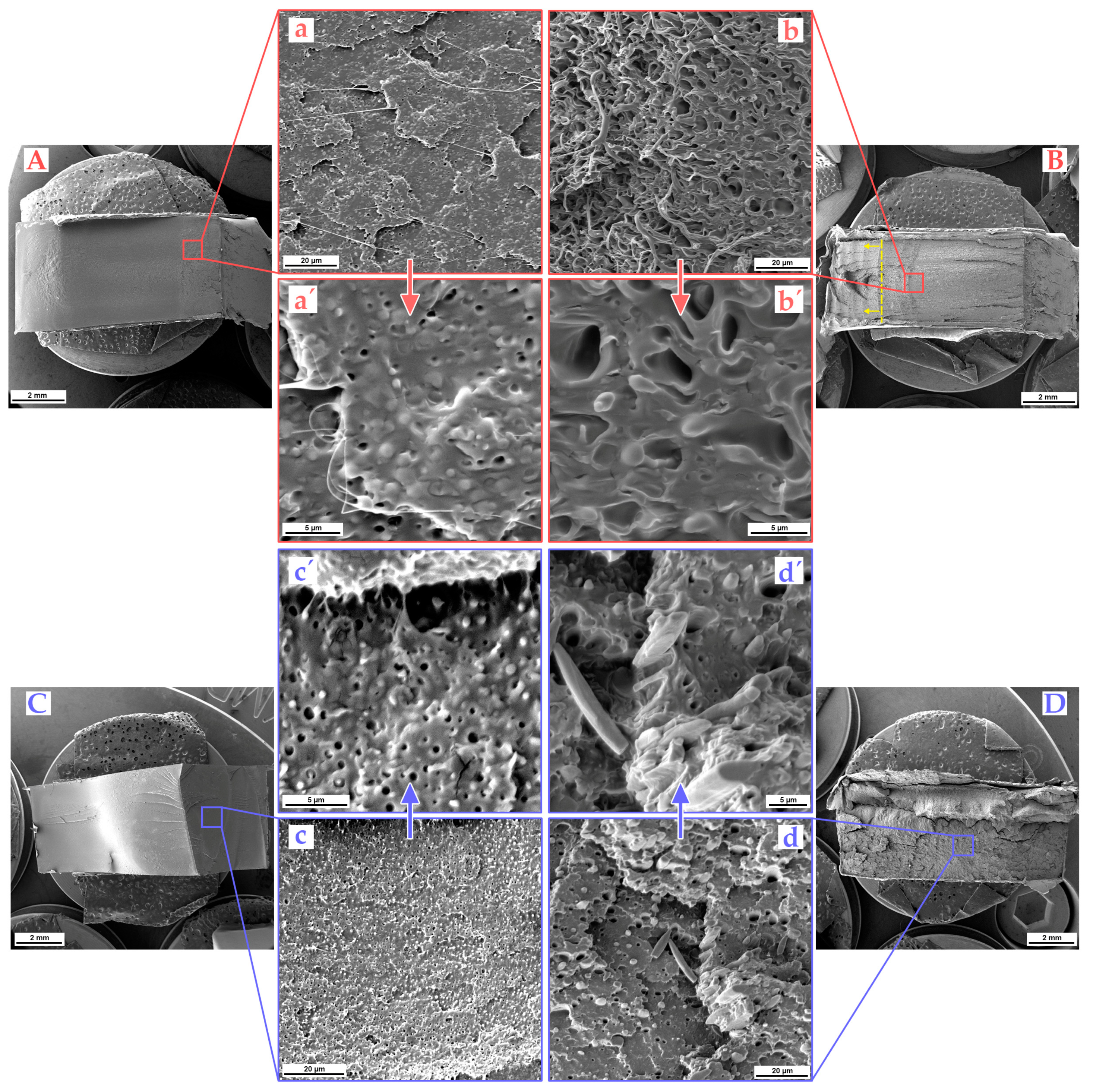
3.4. Impact Fractured Surface Morphology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Mülhaupt, R. Green Polymer Chemistry and Bio-based Plastics: Dreams and Reality. Macromol. Chem. Phys. 2013, 214, 159–174. [Google Scholar] [CrossRef]
- Plastics Europe Plastics—The Facts 2022. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022 (accessed on 11 December 2023).
- The Global Commitment 2022 Progress Report. Available online: https://www.ellenmacarthurfoundation.org/global-commitment-2022 (accessed on 11 December 2023).
- Mah, A. Future-Proofing Capitalism: The Paradox of the Circular Economy for Plastics. Glob. Environ. Politics 2021, 21, 121–142. [Google Scholar] [CrossRef]
- Ponte, S. Green Capital Accumulation: Business and Sustainability Management in a World of Global Value Chains. New Political Econ. 2020, 25, 72–84. [Google Scholar] [CrossRef]
- Dauvergne, P. Will Big Business Destroy Our Planet? John Wiley & Sons: Hoboken, NJ, USA, 2018; ISBN 1-5095-2404-5. [Google Scholar]
- Lisiecki, M.; Damgaard, A.; Ragaert, K.; Astrup, T. Circular Economy Initiatives Are No Guarantee for Increased Plastic Circularity: A Framework for the Systematic Comparison of Initiatives. Resour. Conserv. Recycl. 2023, 197, 107072. [Google Scholar] [CrossRef]
- Klotz, M.; Haupt, M.; Hellweg, S. Limited Utilization Options for Secondary Plastics May Restrict Their Circularity. Waste Manag. 2022, 141, 251–270. [Google Scholar] [CrossRef]
- Velenturf, A.P.; Purnell, P. Principles for a Sustainable Circular Economy. Sustain. Prod. Consum. 2021, 27, 1437–1457. [Google Scholar] [CrossRef]
- Hottle, T.A.; Bilec, M.M.; Landis, A.E. Sustainability Assessments of Bio-Based Polymers. Polym. Degrad. Stab. 2013, 98, 1898–1907. [Google Scholar] [CrossRef]
- Walker, S.; Rothman, R. Life Cycle Assessment of Bio-Based and Fossil-Based Plastic: A Review. J. Clean. Prod. 2020, 261, 121158. [Google Scholar] [CrossRef]
- Nessi, S.; Sinkko, T.; Bulgheroni, C.; Garcia-Gutierrez, P.; Giuntoli, J.; Konti, A.; Sanye Mengual, E.; Tonini, D.; Pant, R.; Marelli, L. Life Cycle Assessment (LCA) of Alternative Feedstocks for Plastics Production; Publications Office of the European Union: Luxembourg, 2021. [Google Scholar]
- Carus, M.; Puente, Á.; vom Berg, C.; Scharf, A. How Can the Environmental Effects of Bio-Based Polymers Be Compared with Those of Petrochemical Polymers on Equal Footing? Nova-Institut: Hürth, Germany, 2019. [Google Scholar]
- Rosenboom, J.-G.; Langer, R.; Traverso, G. Bioplastics for a Circular Economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef]
- Wellenreuther, C.; Wolf, A.; Zander, N. Cost Competitiveness of Sustainable Bioplastic Feedstocks–A Monte Carlo Analysis for Polylactic Acid. Clean. Eng. Technol. 2022, 6, 100411. [Google Scholar] [CrossRef]
- de Oliveira, P.Z.; de Souza Vandenberghe, L.P.; de Mello, A.F.M.; Soccol, C.R. A Concise Update on Major Poly-Lactic Acid Bioprocessing Barriers. Bioresour. Technol. Rep. 2022, 18, 101094. [Google Scholar]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(Lactic Acid) Crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Yang, H.; Deri, F.; Ko, Y. Properties and Medical Applications of Polylactic Acid: A Review. Express Polym. Lett. 2015, 9, 435–455. [Google Scholar] [CrossRef]
- Cheng, Y.; Deng, S.; Chen, P.; Ruan, R. Polylactic Acid (PLA) Synthesis and Modifications: A Review. Front. Chem. China 2009, 4, 259–264. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(Lactic Acid) Modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J. Research Progress in Toughening Modification of Poly(Lactic Acid). J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1051–1083. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, L.; Xiong, Z.; Tang, Z.; Zhang, R.; Zhu, J. Research Progress in the Heat Resistance, Toughening and Filling Modification of PLA. Sci. China Chem. 2016, 59, 1355–1368. [Google Scholar] [CrossRef]
- Anderson, K.S.; Lim, S.H.; Hillmyer, M.A. Toughening of Polylactide by Melt Blending with Linear Low-density Polyethylene. J. Appl. Polym. Sci. 2003, 89, 3757–3768. [Google Scholar] [CrossRef]
- Kim, Y.F.; Choi, C.N.; Kim, Y.D.; Lee, K.Y.; Lee, M.S. Compatibilization of Immiscible Poly(l-Lactide) and Low Density Polyethylene Blends. Fibers Polym. 2004, 5, 270–274. [Google Scholar] [CrossRef]
- Anderson, K.S.; Hillmyer, M.A. The Influence of Block Copolymer Microstructure on the Toughness of Compatibilized Polylactide/Polyethylene Blends. Polymer 2004, 45, 8809–8823. [Google Scholar] [CrossRef]
- Machado, A.; Moura, I.; Duarte, F.; Botelho, G.; Nogueira, R.; Brito, A. Evaluation of Properties and Biodeterioration Potential of Polyethylene and Aliphatic Polyester Blends. Int. Polym. Process. 2007, 22, 512–518. [Google Scholar] [CrossRef]
- Pivsa-Art, S.; Kord-Sa-Ard, J.; Pivsa-Art, W.; Wongpajan, R.; Narongchai, O.; Pavasupree, S.; Hamada, H. Effect of Compatibilizer on PLA/PP Blend for Injection Molding. Energy Procedia 2016, 89, 353–360. [Google Scholar] [CrossRef]
- Sui, G.; Jing, M.; Zhao, J.; Wang, K.; Zhang, Q.; Fu, Q. A Comparison Study of High Shear Force and Compatibilizer on the Phase Morphologies and Properties of Polypropylene/Polylactide (PP/PLA) Blends. Polymer 2018, 154, 119–127. [Google Scholar] [CrossRef]
- Yoo, T.W.; Yoon, H.G.; Choi, S.J.; Kim, M.S.; Kim, Y.H.; Kim, W.N. Effects of Compatibilizers on the Mechanical Properties and Interfacial Tension of Polypropylene and Poly(Lactic Acid) Blends. Macromol. Res. 2010, 18, 583–588. [Google Scholar] [CrossRef]
- da Silva Barbosa Ferreira, E.; Luna, C.B.B.; Siqueira, D.D.; Araújo, E.M.; de França, D.C.; Wellen, R.M.R. Annealing Effect on Pla/Eva Blends Performance. J. Polym. Environ. 2022, 30, 541–554. [Google Scholar] [CrossRef]
- Tábi, T. The Application of the Synergistic Effect between the Crystal Structure of Poly(Lactic Acid)(PLA) and the Presence of Ethylene Vinyl Acetate Copolymer (EVA) to Produce Highly Ductile PLA/EVA Blends. J. Therm. Anal. Calorim. 2019, 138, 1287–1297. [Google Scholar] [CrossRef]
- Ferreira, E.d.S.B.; Luna, C.B.B.; dos Santos Filho, E.A.; Wellen, R.M.R.; Araújo, E.M. Use of Crosslinking Agent to Produce High-performance PLA/EVA Blends via Reactive Processing. J. Vinyl Addit. Technol. 2023, 29, 161–175. [Google Scholar] [CrossRef]
- Lohrasbi, P.; Yeganeh, J.K. Synergistic Toughening of Poly(Lactic Acid)/Poly(Ethylene Vinyl Acetate)(PLA/EVA) by Dynamic Vulcanization and Presence of Hydrophobic Nanoparticles. Polym. Adv. Technol. 2021, 32, 4326–4339. [Google Scholar] [CrossRef]
- Lee, C.M.; Kim, E.S.; Yoon, J. Reactive Blending of Poly(l-lactic Acid) with Poly(Ethylene-co-vinyl Alcohol). J. Appl. Polym. Sci. 2005, 98, 886–890. [Google Scholar] [CrossRef]
- Gui, Z.; Zhang, W.; Lu, C.; Cheng, S. Improving the Barrier Properties of Poly(Lactic Acid) by Blending with Poly(Ethylene-Co-Vinyl Alcohol). J. Macromol. Sci. Part B 2013, 52, 685–700. [Google Scholar] [CrossRef]
- Hao, Y.; Yang, H.; Pan, H.; Ran, X.; Zhang, H. The Effect of MBS on the Heat Resistant, Mechanical Properties, Thermal Behavior and Rheological Properties of PLA/EVOH Blend. J. Polym. Res. 2018, 25, 171. [Google Scholar] [CrossRef]
- Li, Y.; Shimizu, H. Improvement in Toughness of Poly(l-Lactide) (PLLA) through Reactive Blending with Acrylonitrile–Butadiene–Styrene Copolymer (ABS): Morphology and Properties. Eur. Polym. J. 2009, 45, 738–746. [Google Scholar] [CrossRef]
- Chaikeaw, C.; Srikulkit, K. Preparation and Properties of Poly(Lactic Acid)/PLA-g-ABS Blends. Fibers Polym. 2018, 19, 2016–2022. [Google Scholar] [CrossRef]
- Rigoussen, A.; Raquez, J.-M.; Dubois, P.; Verge, P. A Dual Approach to Compatibilize PLA/ABS Immiscible Blends with Epoxidized Cardanol Derivatives. Eur. Polym. J. 2019, 114, 118–126. [Google Scholar] [CrossRef]
- Costa, A.R.D.M.; Luna, C.B.B.; do Nascimento, E.P.; Ferreira, E.D.S.B.; Costa, C.D.M.; de Almeida, Y.M.B.; Araújo, E.M. Tailoring PLA/ABS Blends Compatibilized with SEBS-g-MA through Annealing Heat Treatment. Polymers 2023, 15, 3434. [Google Scholar] [CrossRef]
- Imre, B.; Bedő, D.; Domján, A.; Schön, P.; Vancso, G.J.; Pukánszky, B. Structure, Properties and Interfacial Interactions in Poly(Lactic Acid)/Polyurethane Blends Prepared by Reactive Processing. Eur. Polym. J. 2013, 49, 3104–3113. [Google Scholar] [CrossRef][Green Version]
- Lin, W.; Qu, J.-P. Enhancing Impact Toughness of Renewable Poly(Lactic Acid)/Thermoplastic Polyurethane Blends via Constructing Cocontinuous-like Phase Morphology Assisted by Ethylene–Methyl Acrylate–Glycidyl Methacrylate Copolymer. Ind. Eng. Chem. Res. 2019, 58, 10894–10907. [Google Scholar] [CrossRef]
- Mo, X.-Z.; Wei, F.-X.; Tan, D.-F.; Pang, J.-Y.; Lan, C.-B. The Compatibilization of PLA-g-TPU Graft Copolymer on Polylactide/Thermoplastic Polyurethane Blends. J. Polym. Res. 2020, 27, 33. [Google Scholar] [CrossRef]
- Feng, F.; Ye, L. Morphologies and Mechanical Properties of Polylactide/Thermoplastic Polyurethane Elastomer Blends. J. Appl. Polym. Sci. 2011, 119, 2778–2783. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.; Peng, X.; Turng, L. The Morphology, Properties, and Shape Memory Behavior of Polylactic Acid/Thermoplastic Polyurethane Blends. Polym. Eng. Sci. 2015, 55, 70–80. [Google Scholar] [CrossRef]
- Zaman, H.U.; Song, J.C.; Park, L.-S.; Kang, I.-K.; Park, S.-Y.; Kwak, G.; Park, B.; Yoon, K.-B. Poly(Lactic Acid) Blends with Desired End-Use Properties by Addition of Thermoplastic Polyester Elastomer and MDI. Polym. Bull. 2011, 67, 187–198. [Google Scholar] [CrossRef]
- Wang, S.; Pang, S.; Pan, L.; Xu, N.; Li, T. Isothermal Cold Crystallization, Heat Resistance, and Tensile Performance of Polylactide/Thermoplastic Polyester Elastomer (PLA/TPEE) Blends: Effects of Annealing and Reactive Compatibilizer. Polymers 2016, 8, 417. [Google Scholar] [CrossRef]
- Lu, X.; Lv, Q.; Huang, X.; Song, Z.; Xu, N.; Pang, S.; Pan, L.; Li, T. Isothermal Melt Crystallization and Performance Evaluation of Polylactide/Thermoplastic Polyester Blends with Multi-functional Epoxy. J. Appl. Polym. Sci. 2018, 135, 6343. [Google Scholar] [CrossRef]
- Jin, H.-J.; Chin, I.-J.; Kim, M.-N.; Kim, S.-H.; Yoon, J.-S. Blending of Poly(l-Lactic Acid) with Poly(Cis-1,4-Isoprene). Eur. Polym. J. 2000, 36, 165–169. [Google Scholar] [CrossRef]
- Juntuek, P.; Ruksakulpiwat, C.; Chumsamrong, P.; Ruksakulpiwat, Y. Glycidyl Methacrylate Grafted Natural Rubber: Synthesis, Characterization, and Mechanical Property. J. Appl. Polym. Sci. 2011, 122, 3152–3159. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, C.; Pan, Y.; Wang, W.; Jiang, L.; Dan, Y. Study on the Effect of Dicumyl Peroxide on Structure and Properties of Poly(Lactic Acid)/Natural Rubber Blend. J. Polym. Environ. 2013, 21, 375–387. [Google Scholar] [CrossRef]
- Pongtanayut, K.; Thongpin, C.; Santawitee, O. The Effect of Rubber on Morphology, Thermal Properties and Mechanical Properties of PLA/NR and PLA/ENR Blends. Energy Procedia 2013, 34, 888–897. [Google Scholar] [CrossRef]
- Klinkajorn, J.; Tanrattanakul, V. Compatibilization of Poly(Lactic Acid)/Epoxidized Natural Rubber Blend with Maleic Anhydride. J. Appl. Polym. Sci. 2020, 137, 48297. [Google Scholar] [CrossRef]
- Rasselet, D.; Caro-Bretelle, A.-S.; Taguet, A.; Lopez-Cuesta, J.-M. Reactive Compatibilization of PLA/PA11 Blends and Their Application in Additive Manufacturing. Materials 2019, 12, 485. [Google Scholar] [CrossRef]
- Yu, X.; Wang, X.; Zhang, Z.; Peng, S.; Chen, H.; Zhao, X. High-Performance Fully Bio-Based Poly(Lactic Acid)/Polyamide11 (PLA/PA11) Blends by Reactive Blending with Multi-Functionalized Epoxy. Polym. Test. 2019, 78, 105980. [Google Scholar] [CrossRef]
- Zolali, A.M.; Heshmati, V.; Favis, B.D. Ultratough Co-Continuous PLA/PA11 by Interfacially Percolated Poly(Ether-b-Amide). Macromolecules 2017, 50, 264–274. [Google Scholar] [CrossRef]
- Vachon, A.; Pépin, K.; Balampanis, E.; Veilleux, J.; Vuillaume, P.Y. Compatibilization of PLA/PEBA Blends via Reactive Extrusion: A Comparison of Different Coupling Agents. J. Polym. Environ. 2017, 25, 812–827. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, G.; Feng, Y.; Hu, Y.; Zhao, G.; Jiang, W. Highly Toughened Poly(Lactic Acid) Blends Prepared by Reactive Blending with a Renewable Poly(Ether-block-amide) Elastomer. J. Appl. Polym. Sci. 2021, 138, 50097. [Google Scholar] [CrossRef]
- Han, L.; Han, C.; Dong, L. Morphology and Properties of the Biosourced Poly(Lactic Acid)/Poly(Ethylene Oxide-b-amide-12) Blends. Polym. Compos. 2013, 34, 122–130. [Google Scholar] [CrossRef]
- Urquijo, J.; Guerrica-Echevarría, G.; Eguiazábal, J.I. Melt Processed PLA/PCL Blends: Effect of Processing Method on Phase Structure, Morphology, and Mechanical Properties. J. Appl. Polym. Sci. 2015, 132, 42641. [Google Scholar] [CrossRef]
- Fortelny, I.; Ujcic, A.; Fambri, L.; Slouf, M. Phase Structure, Compatibility, and Toughness of PLA/PCL Blends: A Review. Front. Mater. 2019, 6, 206. [Google Scholar] [CrossRef]
- Matta, A.; Rao, R.U.; Suman, K.; Rambabu, V. Preparation and Characterization of Biodegradable PLA/PCL Polymeric Blends. Procedia Mater. Sci. 2014, 6, 1266–1270. [Google Scholar] [CrossRef]
- Ostafinska, A.; Fortelny, I.; Nevoralova, M.; Hodan, J.; Kredatusova, J.; Slouf, M. Synergistic Effects in Mechanical Properties of PLA/PCL Blends with Optimized Composition, Processing, and Morphology. RSC Adv. 2015, 5, 98971–98982. [Google Scholar] [CrossRef]
- Luyt, A.; Gasmi, S. Influence of Blending and Blend Morphology on the Thermal Properties and Crystallization Behaviour of PLA and PCL in PLA/PCL Blends. J. Mater. Sci. 2016, 51, 4670–4681. [Google Scholar] [CrossRef]
- Noda, I.; Satkowski, M.M.; Dowrey, A.E.; Marcott, C. Polymer Alloys of Nodax Copolymers and Poly(Lactic Acid). Macromol. Biosci. 2004, 4, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Bartczak, Z.; Galeski, A.; Kowalczuk, M.; Sobota, M.; Malinowski, R. Tough Blends of Poly(Lactide) and Amorphous Poly([R,S]-3-Hydroxy Butyrate)–Morphology and Properties. Eur. Polym. J. 2013, 49, 3630–3641. [Google Scholar] [CrossRef]
- Ecker, J.V.; Burzic, I.; Haider, A.; Hild, S.; Rennhofer, H. Improving the Impact Strength of PLA and Its Blends with PHA in Fused Layer Modelling. Polym. Test. 2019, 78, 105929. [Google Scholar] [CrossRef]
- Yokohara, T.; Yamaguchi, M. Structure and Properties for Biomass-Based Polyester Blends of PLA and PBS. Eur. Polym. J. 2008, 44, 677–685. [Google Scholar] [CrossRef]
- Su, S.; Kopitzky, R.; Tolga, S.; Kabasci, S. Polylactide (PLA) and Its Blends with Poly(Butylene Succinate)(PBS): A Brief Review. Polymers 2019, 11, 1193. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.S.; Yildirim, R.; Kodal, M.; Ozkoc, G. Reactive Compatibilization of PLA/PBS Bio-blends via a New Generation of Hybrid Nanoparticles. J. Vinyl Addit. Technol. 2023, 29, 737–757. [Google Scholar] [CrossRef]
- Chang, F.-L.; Hu, B.; Huang, W.-T.; Chen, L.; Yin, X.-C.; Cao, X.-W.; He, G.-J. Improvement of Rheology and Mechanical Properties of PLA/PBS Blends by in-Situ UV-Induced Reactive Extrusion. Polymer 2022, 259, 125336. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, B.; Bian, X.; Feng, L.; Zhang, H.; Duan, R.; Sun, J.; Pang, X.; Chen, W.; Chen, X. Synergistic Effect of PLA–PBAT–PLA Tri-Block Copolymers with Two Molecular Weights as Compatibilizers on the Mechanical and Rheological Properties of PLA/PBAT Blends. RSC Adv. 2015, 5, 73842–73849. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Reactive Extrusion of PLA, PBAT with a Multi-Functional Epoxide: Physico-Chemical and Rheological Properties. Eur. Polym. J. 2014, 58, 90–102. [Google Scholar] [CrossRef]
- Ding, Y.; Lu, B.; Wang, P.; Wang, G.; Ji, J. PLA-PBAT-PLA Tri-Block Copolymers: Effective Compatibilizers for Promotion of the Mechanical and Rheological Properties of PLA/PBAT Blends. Polym. Degrad. Stab. 2018, 147, 41–48. [Google Scholar] [CrossRef]
- Chen, X.; Zeng, Z.; Ju, Y.; Zhou, M.; Bai, H.; Fu, Q. Design of Biodegradable PLA/PBAT Blends with Balanced Toughness and Strength via Interfacial Compatibilization and Dynamic Vulcanization. Polymer 2023, 266, 125620. [Google Scholar] [CrossRef]
- Yang, H.-R.; Jia, G.; Wu, H.; Ye, C.; Yuan, K.; Liu, S.; Zhou, L.; Xu, H.; Gao, L.; Cui, J. Design of Fully Biodegradable Super-Toughened PLA/PBAT Blends with Asymmetric Composition via Reactive Compatibilization and Controlling Morphology. Mater. Lett. 2022, 329, 133067. [Google Scholar] [CrossRef]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Perspective on Polylactic Acid (PLA) Based Sustainable Materials for Durable Applications: Focus on Toughness and Heat Resistance. ACS Sustain. Chem. Eng. 2016, 4, 2899–2916. [Google Scholar] [CrossRef]
- Joziasse, C.; Topp, M.; Veenstra, H.; Grijpma, D.; Pennings, A. Supertough Poly(Lactide)s. Polym. Bull. 1994, 33, 599–605. [Google Scholar] [CrossRef]
- Wu, S. Chain Structure, Phase Morphology, and Toughness Relationships in Polymers and Blends. Polym. Eng. Sci. 1990, 30, 753–761. [Google Scholar] [CrossRef]
- Zhang, K.; Nagarajan, V.; Misra, M.; Mohanty, A.K. Supertoughened Renewable PLA Reactive Multiphase Blends System: Phase Morphology and Performance. ACS Appl. Mater. Interfaces 2014, 6, 12436–12448. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Luo, Y.; Bai, H.; Zhang, Q.; Fu, Q. Remarkably Enhanced Impact Toughness and Heat Resistance of Poly(L-Lactide)/Thermoplastic Polyurethane Blends by Constructing Stereocomplex Crystallites in the Matrix. ACS Sustain. Chem. Eng. 2016, 4, 111–120. [Google Scholar] [CrossRef]
- Dai, J.; Bai, H.; Liu, Z.; Chen, L.; Zhang, Q.; Fu, Q. Stereocomplex Crystallites Induce Simultaneous Enhancement in Impact Toughness and Heat Resistance of Injection-Molded Polylactide/Polyurethane Blends. RSC Adv. 2016, 6, 17008–17015. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, H. Mechanical Properties and Phase Morphology of Super-Tough PLA/PBAT/EMA-GMA Multicomponent Blends. Mater. Lett. 2017, 192, 17–20. [Google Scholar] [CrossRef]
- Wei, B.; Lin, Q.; Zheng, X.; Gu, X.; Zhao, L.; Li, J.; Li, Y. Reactive Splicing Compatibilization of Immiscible Polymer Blends: Compatibilizer Synthesis in the Melt State and Compatibilizer Architecture Effects. Polymer 2019, 185, 121952. [Google Scholar] [CrossRef]
- Henton, D.E.; Gruber, P.; Lunt, J.; Randall, J. Polylactic Acid Technology. Nat. Fibers Biopolym. Biocomposites 2005, 16, 527–577. [Google Scholar]
- Sarasua, J.-R.; Prud’Homme, R.E.; Wisniewski, M.; Le Borgne, A.; Spassky, N. Crystallization and Melting Behavior of Polylactides. Macromolecules 1998, 31, 3895–3905. [Google Scholar] [CrossRef]
- ISO 527-1:2019; Plastics—Determination of Tensile Properties—Part 1: General Principles. International Organization for Standarization—ISO Central Secretariat: Vernier, Switzerland, 2019.
- ISO 179-1:2023; Plastics—Determination of Charpy Impact Properties—Part 1: Non-Instrumented Impact Test. International Organization for Standarization—ISO Central Secretariat: Vernier, Switzerland, 2023.
- Li, H.; Huneault, M.A. Effect of Nucleation and Plasticization on the Crystallization of Poly(Lactic Acid). Polymer 2007, 48, 6855–6866. [Google Scholar] [CrossRef]
- Li, Y.; Wu, H.; Wang, Y.; Liu, L.; Han, L.; Wu, J.; Xiang, F. Synergistic Effects of PEG and MWCNTs on Crystallization Behavior of PLLA. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 520–528. [Google Scholar] [CrossRef]
- Borůvka, M.; Běhálek, L.; Novák, J. Properties and Crystallization of PLLA Biopolymers with Cellulose Nanocrystals and Organic Plasticizer. MM Sci. J 2020, 2020, 4080–4085. [Google Scholar] [CrossRef]
- Samaniego, K.; Matos, A.; Sánchez-Safont, E.; Candal, M.V.; Lagaron, J.M.; Cabedo, L.; Gamez-Perez, J. Role of Plasticizers on PHB/Bio-TPE Blends Compatibilized by Reactive Extrusion. Materials 2022, 15, 1226. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.W. On the Equilibrium of Heterogeneous Substances. Am. J. Sci. 1878, 3, 441–458. [Google Scholar] [CrossRef]
- Rybnikář, F. Secondary Crystallization of Polymers. J. Polym. Sci. 1960, 44, 517–522. [Google Scholar] [CrossRef]
- Kawai, T.; Rahman, N.; Matsuba, G.; Nishida, K.; Kanaya, T.; Nakano, M.; Okamoto, H.; Kawada, J.; Usuki, A.; Honma, N. Crystallization and Melting Behavior of Poly(l-Lactic Acid). Macromolecules 2007, 40, 9463–9469. [Google Scholar] [CrossRef]
- Yasuniwa, M.; Sakamo, K.; Ono, Y.; Kawahara, W. Melting Behavior of Poly(l-Lactic Acid): X-ray and DSC Analyses of the Melting Process. Polymer 2008, 49, 1943–1951. [Google Scholar] [CrossRef]
- Zhang, J.; Tashiro, K.; Tsuji, H.; Domb, A.J. Disorder-to-Order Phase Transition and Multiple Melting Behavior of Poly(L-Lactide) Investigated by Simultaneous Measurements of WAXD and DSC. Macromolecules 2008, 41, 1352–1357. [Google Scholar] [CrossRef]
- Pan, P.; Kai, W.; Zhu, B.; Dong, T.; Inoue, Y. Polymorphous Crystallization and Multiple Melting Behavior of Poly(l-Lactide): Molecular Weight Dependence. Macromolecules 2007, 40, 6898–6905. [Google Scholar] [CrossRef]
- Androsch, R.; Schick, C.; Di Lorenzo, M.L. Melting of Conformationally Disordered Crystals (α′-Phase) of Poly(l-lactic Acid). Macromol. Chem. Phys. 2014, 215, 1134–1139. [Google Scholar] [CrossRef]
- Wasanasuk, K.; Tashiro, K. Structural Regularization in the Crystallization Process from the Glass or Melt of Poly(l-Lactic Acid) Viewed from the Temperature-Dependent and Time-Resolved Measurements of FTIR and Wide-Angle/Small-Angle X-ray Scatterings. Macromolecules 2011, 44, 9650–9660. [Google Scholar] [CrossRef]
- Willemse, R.; Speijer, A.; Langeraar, A.; De Boer, A.P. Tensile Moduli of Co-Continuous Polymer Blends. Polymer 1999, 40, 6645–6650. [Google Scholar] [CrossRef]
- Meekum, U.; Khiansanoi, A. PLA and Single Component Silicone Rubber Blends for Sub-Zero Temperature Blown Film Packaging Applications. Results Phys. 2018, 9, 1127–1135. [Google Scholar] [CrossRef]
- Mysiukiewicz, O.; Barczewski, M.; Kloziński, A. The Influence of Sub-Zero Conditions on the Mechanical Properties of Polylactide-Based Composites. Materials 2020, 13, 5789. [Google Scholar] [CrossRef]
- Lampman, S. Characterization and Failure Analysis of Plastics; Asm International: Almere, The Netherlands, 2003; ISBN 1-61503-073-5. [Google Scholar]
- Hull, D. Fractography: Observing, Measuring and Interpreting Fracture Surface Topography; Cambridge University Press: Cambridge, UK, 1999; ISBN 0-521-64684-7. [Google Scholar]
- Horiuchi, S.; Matchariyakul, N.; Yase, K.; Kitano, T. Morphology Development through an Interfacial Reaction in Ternary Immiscible Polymer Blends. Macromolecules 1997, 30, 3664–3670. [Google Scholar] [CrossRef]
- Reignier, J.; Favis, B.D. Control of the Subinclusion Microstructure in HDPE/PS/PMMA Ternary Blends. Macromolecules 2000, 33, 6998–7008. [Google Scholar] [CrossRef]
- Park, S.D.; Todo, M.; Arakawa, K.; Koganemaru, M. Effect of Crystallinity and Loading-Rate on Mode I Fracture Behavior of Poly(Lactic Acid). Polymer 2006, 47, 1357–1363. [Google Scholar] [CrossRef]
- Wang, M.; Wu, Y.; Li, Y.-D.; Zeng, J.-B. Progress in Toughening Poly(Lactic Acid) with Renewable Polymers. Polym. Rev. 2017, 57, 557–593. [Google Scholar] [CrossRef]
- Liu, G.-C.; He, Y.-S.; Zeng, J.-B.; Xu, Y.; Wang, Y.-Z. In Situ Formed Crosslinked Polyurethane Toughened Polylactide. Polym. Chem. 2014, 5, 2530–2539. [Google Scholar] [CrossRef]
- Liu, H.; Song, W.; Chen, F.; Guo, L.; Zhang, J. Interaction of Microstructure and Interfacial Adhesion on Impact Performance of Polylactide (PLA) Ternary Blends. Macromolecules 2011, 44, 1513–1522. [Google Scholar] [CrossRef]
- He, Y.-S.; Zeng, J.-B.; Liu, G.-C.; Li, Q.-T.; Wang, Y.-Z. Super-Tough Poly(l-Lactide)/Crosslinked Polyurethane Blends with Tunable Impact Toughness. RSC Adv. 2014, 4, 12857–12866. [Google Scholar] [CrossRef]
- Bai, H.; Huang, C.; Xiu, H.; Gao, Y.; Zhang, Q.; Fu, Q. Toughening of Poly(l-Lactide) with Poly(ε-Caprolactone): Combined Effects of Matrix Crystallization and Impact Modifier Particle Size. Polymer 2013, 54, 5257–5266. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, X.; Wu, L.; Li, Y.; Qi, Z.; Choy, C.; Wang, F. Effects of Interfacial Adhesion on the Rubber Toughening of Poly(Vinyl Chloride) Part 1. Impact Tests. Polymer 2001, 42, 737–746. [Google Scholar] [CrossRef]


| Sample | PLLA (wt. %) | TPE (wt. %) | EMA-GMA (wt. %) |
|---|---|---|---|
| PLLA | 100 | 0 | 0 |
| PLLA/10TPE | 90 | 10 | 0 |
| PLLA/10TPE/4.3EMA-GMA | 85.7 | 10 | 4.3 |
| PLLA/20TPE | 80 | 20 | 0 |
| PLLA/20TPE/8.6EMA-GMA | 71.4 | 20 | 8.6 |
| PLLA/30TPE | 70 | 30 | 0 |
| PLLA/30TPE/12.9EMA-GMA | 57.1 | 30 | 12.9 |
| PLLA/40TPE | 60 | 40 | 0 |
| PLLA/40TPE/17.2EMA-GMA | 42.8 | 40 | 17.2 |
| Sample | Cooling | 2nd Heating | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tmc1 | ΔHmc1 | Tmc2 | ΔHmc2 | Tg | Tcc | ΔHcc | Trc (Thmα′) | ΔHrc | Thm | ΔHhm | χc | |
| [°C] | [J∙g−1] | [°C] | [J∙g−1] | [°C] | [°C] | [J∙g−1] | [°C] | [J∙g−1] | [°C] | [J∙g−1] | [%] | |
| PLLA | 101.1 | 1.2 | - | - | 59.4 | 99.8 | 30.9 | 156.9 | 6.2 | 173.0 | 49.5 | 11.7 |
| PLLA/10TPE | 97.0 | 23.3 | - | - | 48.4 | 83.9 | 3.7 | 153.4 | 4.1 | 172.1 | 42.2 | 36.0 |
| PLLA/10TPE/4.3EMA-GMA | 95.6 | 16.0 | - | - | 49.7 | 83.8 | 7.8 | 151.9 | 5.3 | 171.4 | 40.3 | 30.0 |
| PLLA/20TPE | 93.5 | 26.1 | - | - | 38.5 | - | - | 153.9 | 2.2 | 170.5 | 34.9 | 38.5 |
| PLLA/20TPE/8.6EMA-GMA | 89.8 | 17.9 | 46.6 | 1.8 | 36.5 | 71.1 | 1.6 | 149.9 | 3.0 | 168.9 | 33.2 | 37.8 |
| PLLA/30TPE | 96.7 | 28.2 | - | - | - | - | - | (154.0) | - | 166.9 | 34.6 | 46.6 |
| PLLA/30TPE/12.9EMA-GMA | 90.2 | 20.6 | 46.7 | 2.1 | - | 84.6 | 3.0 | (152.9) | - | 167.0 | 26.9 | 39.4 |
| PLLA/40TPE | 97.1 | 24.7 | - | - | - | - | - | (151.4) | - | 165.0 | 28.6 | 44.9 |
| PLLA/40TPE/17.2EMA-GMA | 91.8 | 16.9 | 46.6 | 2.8 | - | 81.9 | 3.2 | (152.9) | - | 165.5 | 19.6 | 36.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boruvka, M.; Base, R.; Novak, J.; Brdlik, P.; Behalek, L.; Ngaowthong, C. Phase Morphology and Mechanical Properties of Super-Tough PLLA/TPE/EMA-GMA Ternary Blends. Polymers 2024, 16, 192. https://doi.org/10.3390/polym16020192
Boruvka M, Base R, Novak J, Brdlik P, Behalek L, Ngaowthong C. Phase Morphology and Mechanical Properties of Super-Tough PLLA/TPE/EMA-GMA Ternary Blends. Polymers. 2024; 16(2):192. https://doi.org/10.3390/polym16020192
Chicago/Turabian StyleBoruvka, Martin, Roman Base, Jan Novak, Pavel Brdlik, Lubos Behalek, and Chakaphan Ngaowthong. 2024. "Phase Morphology and Mechanical Properties of Super-Tough PLLA/TPE/EMA-GMA Ternary Blends" Polymers 16, no. 2: 192. https://doi.org/10.3390/polym16020192
APA StyleBoruvka, M., Base, R., Novak, J., Brdlik, P., Behalek, L., & Ngaowthong, C. (2024). Phase Morphology and Mechanical Properties of Super-Tough PLLA/TPE/EMA-GMA Ternary Blends. Polymers, 16(2), 192. https://doi.org/10.3390/polym16020192