Dewetting-Induced Hierarchical Self-Assembly of Block Copolymers Templated by Colloidal Crystals
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Colloidal Particles and Multilayered Colloidal Crystals
2.2. Preparation of the Guiding Template
2.3. Dewetting of the BCP Film on the Guiding Template
2.4. Imaging, Characterization, and Pattern Analyses
3. Results
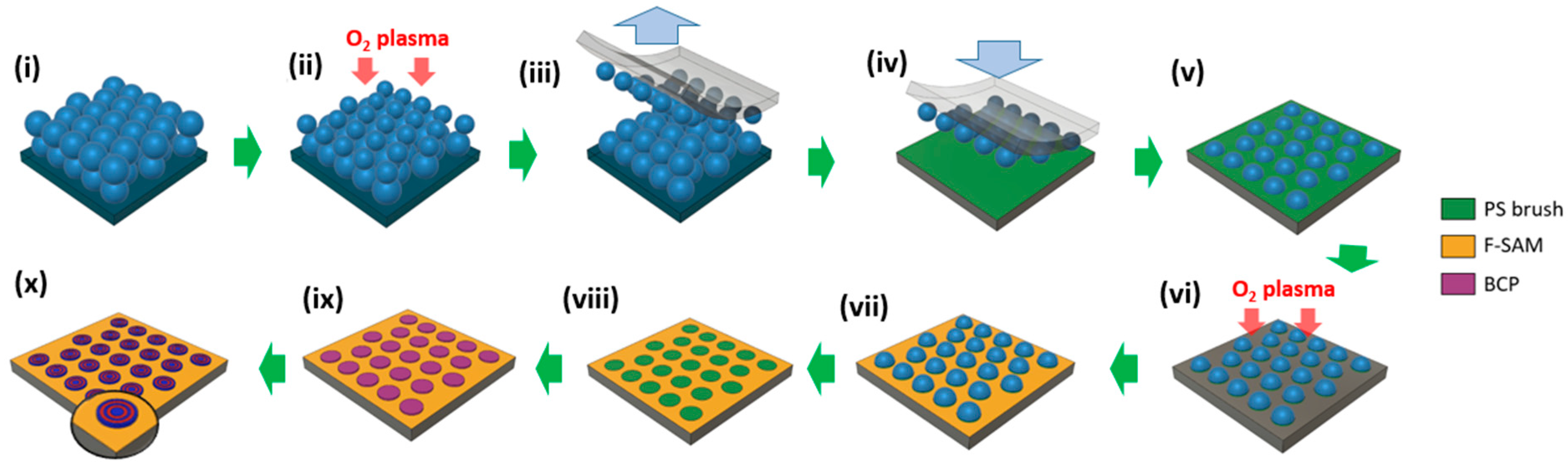
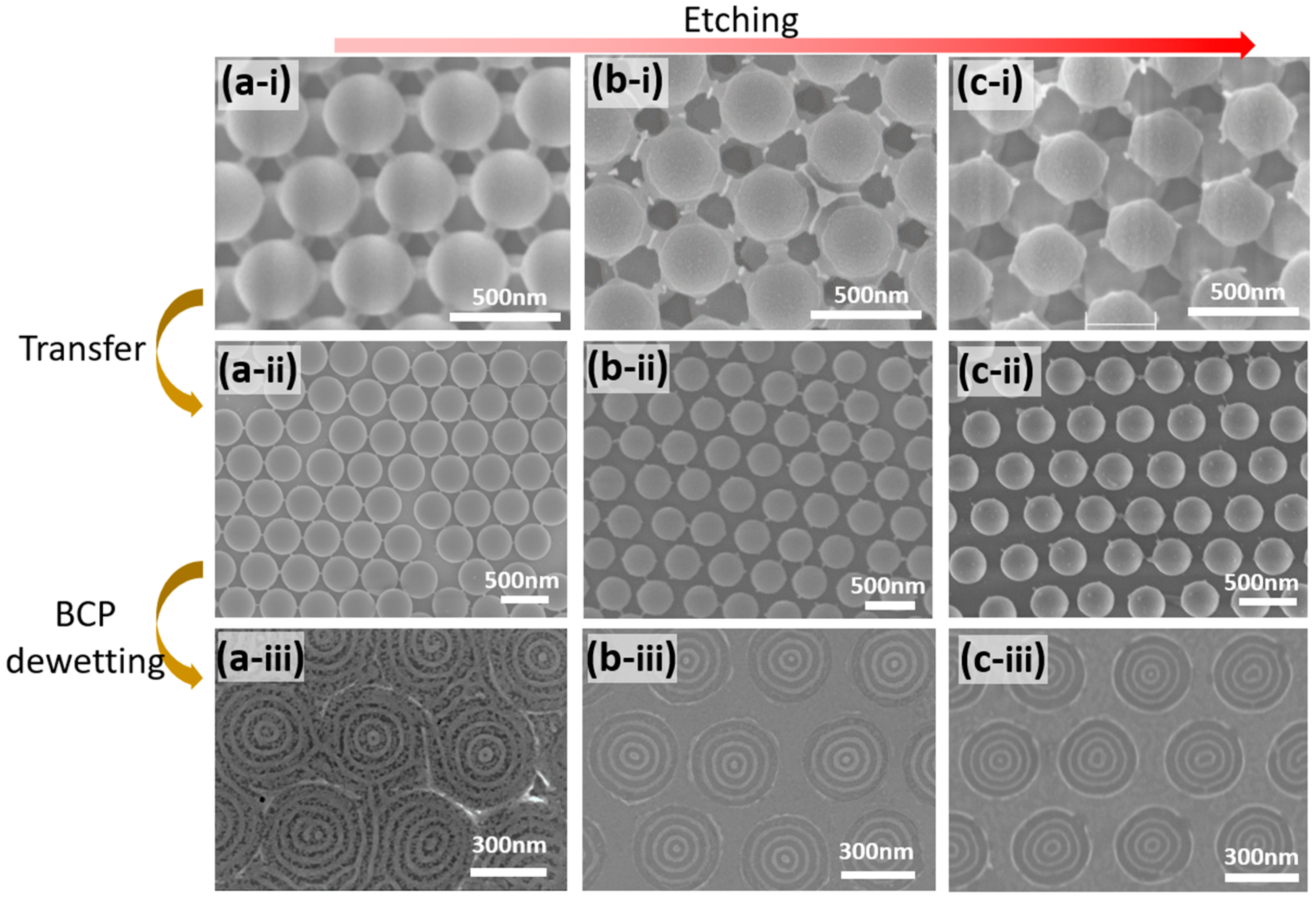
3.1. Fabrication of the Guiding Template
3.2. Dewetting of the BCP Film on the Guiding Template
3.3. Analysis of the BCP Concentric Ring Patterns
3.4. Analysis of Common Defective Features in the BCP Patterns
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Herr, D.J.C. Directed Block Copolymer Self-Assembly for Nanoelectronics Fabrication. J. Mater. Res. 2011, 26, 122–139. [Google Scholar] [CrossRef]
- Kim, H.-C.; Park, S.-M.; Hinsberg, W.D. Block Copolymer Based Nanostructures: Materials, Processes, and Applications to Electronics. Chem. Rev. 2010, 110, 146–177. [Google Scholar] [CrossRef]
- Ditte, K.; Perez, J.; Chae, S.; Hambsch, M.; Al-Hussein, M.; Komber, H.; Formanek, P.; Mannsfeld, S.C.B.; Fery, A.; Kiriy, A.; et al. Ultrasoft and High-Mobility Block Copolymers for Skin-Compatible Electronics. Adv. Mater. 2020, 33, 2005416. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, H.; Xu, J.; Hwang, M.-S.; Tian, D.; Wang, K.; Zhang, L.; Liao, Y.; Park, H.-G.; Yi, G.-R.; et al. Responsive Block Copolymer Photonic Microspheres. Adv. Mater. 2018, 30, 1707344. [Google Scholar] [CrossRef] [PubMed]
- Poutanen, M.; Guidetti, G.; Gröschel, T.I.; Borisov, O.V.; Vignolini, S.; Ikkala, O.; Gröschel, A.H. Block Copolymer Micelles for Photonic Fluids and Crystals. ACS Nano 2018, 12, 3149–3158. [Google Scholar] [CrossRef] [PubMed]
- Song, D.-P.; Li, C.; Li, W.; Watkins, J.J. Block Copolymer Nanocomposites with High Refractive Index Contrast for One-Step Photonics. ACS Nano 2016, 10, 1216–1223. [Google Scholar] [CrossRef]
- Park, T.H.; Eoh, H.; Jung, Y.; Lee, G.-W.; Lee, C.E.; Kang, H.S.; Lee, J.; Kim, K.-B.; Ryu, D.Y.; Yu, S.; et al. Thermo-Adaptive Block Copolymer Structural Color Electronics. Adv. Funct. Mater. 2020, 31, 2008548. [Google Scholar] [CrossRef]
- Hägglund, C.; Zeltzer, G.; Ruiz, R.; Thomann, I.; Lee, H.-B.-R.; Brongersma, M.L.; Bent, S.F. Self-Assembly Based Plasmonic Arrays Tuned by Atomic Layer Deposition for Extreme Visible Light Absorption. Nano Lett. 2013, 13, 3352–3357. [Google Scholar] [CrossRef]
- Cha, S.K.; Mun, J.H.; Chang, T.; Kim, S.Y.; Kim, J.Y.; Jin, H.M.; Lee, J.Y.; Shin, J.; Kim, K.H.; Kim, S.O. Au-Ag Core-Shell Nanoparticle Array by Block Copolymer Lithography for Synergistic Broadband Plasmonic Properties. ACS Nano 2015, 9, 5536–5543. [Google Scholar] [CrossRef]
- Nunes, S.P.; Car, A. From Charge-Mosaic to Micelle Self-Assembly: Block Copolymer Membranes in the Last 40 Years. Ind. Eng. Chem. Res. 2013, 52, 993–1003. [Google Scholar] [CrossRef]
- Uehara, H.; Kakiage, M.; Sekiya, M.; Sakuma, D.; Yamonobe, T.; Takano, N.; Barraud, A.; Meurville, E.; Ryser, P. Size-Selective Diffusion in Nanoporous but Flexible Membranes for Glucose Sensors. ACS Nano 2009, 3, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, X.; Zhang, R.; Singh, M.; Ammar, A.; Cousins, D.; Hassan, M.K.; Ponnamma, D.; Adham, S.; Al-Maadeed, M.A.A.; et al. Vertically Oriented Nanoporous Block Copolymer Membranes for Oil/Water Separation and Filtration. Soft Matter 2020, 16, 9648–9654. [Google Scholar] [CrossRef] [PubMed]
- Young, W.-S.; Kuan, W.-F.; Epps, T.H. Block Copolymer Electrolytes for Rechargeable Lithium Batteries. J. Polym. Sci. B Polym. Phys. 2013, 52, 1–16. [Google Scholar] [CrossRef]
- Jeong, C.K.; Baek, K.M.; Niu, S.; Nam, T.W.; Hur, Y.H.; Park, D.Y.; Hwang, G.-T.; Byun, M.; Wang, Z.L.; Jung, Y.S.; et al. Topographically-Designed Triboelectric Nanogenerator via Block Copolymer Self-Assembly. Nano Lett. 2014, 14, 7031–7038. [Google Scholar] [CrossRef]
- Yang, H.; Shi, X.; Chu, S.; Shao, Z.; Wang, Y. Design of Block-Copolymer Nanoporous Membranes for Robust and Safer Lithium-Ion Battery Separators. Adv. Sci. 2021, 8, 2003096. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Guo, X.; Yang, Y.; Tang, M.; Wei, Q.; Yu, H. Block Copolymer Electrolyte with Adjustable Functional Units for Solid Polymer Lithium Metal Battery. J. Energy Chem. 2021, 52, 67–74. [Google Scholar] [CrossRef]
- Kim, S.O.; Solak, H.H.; Stoykovich, M.P.; Ferrier, N.J.; De Pablo, J.J.; Nealey, P.F. Epitaxial Self-Assembly of Block Copolymers on Lithographically Defined Nanopatterned Substrates. Nature 2003, 424, 411–414. [Google Scholar] [CrossRef]
- Stoykovich, M.P.; Müller, M.; Kim, S.O.; Solak, H.H.; Edwards, E.W.; De Pablo, J.J.; Nealey, P.F. Directed Assembly of Block Copolymer Blends into Nonregular Device-Oriented Structures. Science 2005, 308, 1442–1446. [Google Scholar] [CrossRef]
- Bita, I.; Yang, J.K.W.; Jung, Y.S.; Ross, C.A.; Thomas, E.L.; Berggren, K.K. Graphoepitaxy of Self-Assembled Block Copolymers on Two-Dimensional Periodic Patterned Templates. Science 2008, 321, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.K.W.; Jung, Y.S.; Chang, J.-B.; Mickiewicz, R.A.; Alexander-Katz, A.; Ross, C.A.; Berggren, K.K. Complex Self-Assembled Patterns Using Sparse Commensurate Templates with Locally Varying Motifs. Nat. Nanotechnol. 2010, 5, 256–260. [Google Scholar] [CrossRef]
- Jeong, S.-J.; Kim, J.Y.; Kim, B.H.; Moon, H.-S.; Kim, S.O. Directed Self-Assembly of Block Copolymers for Next Generation Nanolithography. Mater. Today 2013, 16, 468–476. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Zhang, X.; Yang, B. Colloidal Self-Assembly Meets Nanofabrication: From Two-Dimensional Colloidal Crystals to Nanostructure Arrays. Adv. Mater. 2010, 22, 4249–4269. [Google Scholar] [CrossRef]
- Jeong, U.; Wang, Y.; Ibisate, M.; Xia, Y. Some New Developments in the Synthesis, Functionalization, and Utilization of Monodisperse Colloidal Spheres. Adv. Funct. Mater. 2005, 15, 1907–1921. [Google Scholar] [CrossRef]
- Ke, Y.; Ye, S.; Hu, P.; Jiang, H.; Wang, S.; Yang, B.; Zhang, J.; Long, Y. Unpacking the Toolbox of Two-Dimensional Nanostructures Derived from Nanosphere Templates. Mater. Horiz. 2019, 6, 1380–1408. [Google Scholar] [CrossRef]
- Oliveira, R.D.; Mouquinho, A.; Centeno, P.; Alexandre, M.; Haque, S.; Martins, R.; Fortunato, E.; Águas, H.; Mendes, M.J. Colloidal Lithography for Photovoltaics: An Attractive Route for Light Management. Nanomaterials 2021, 11, 1665. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.; Akinoglu, E.M.; Luo, B.; Konarova, M.; Yun, J.-H.; Gentle, I.R.; Wang, L. Nanosphere Lithography: A Versatile Approach to Develop Transparent Conductive Films for Optoelectronic Applications. Adv. Mater. 2022, 34, 2103842. [Google Scholar] [CrossRef]
- Li, J.; Hu, Y.; Yu, L.; Li, L.; Ji, D.; Li, L.; Hu, W.; Fuchs, H. Recent Advances of Nanospheres Lithography in Organic Electronics. Small 2021, 17, 2100724. [Google Scholar] [CrossRef]
- Brassat, K.; Kool, D.; Bürger, J.; Lindner, J.K.N. Hierarchical Nanopores Formed by Block Copolymer Lithography on the Surfaces of Different Materials Pre-Patterned by Nanosphere Lithography. Nanoscale 2018, 10, 10005–10017. [Google Scholar] [CrossRef]
- Baek, K.M.; Kim, J.M.; Jeong, J.W.; Lee, S.Y.; Jung, Y.S. Sequentially Self-Assembled Rings-in-Mesh Nanoplasmonic Arrays for Surface-Enhanced Raman Spectroscopy. Chem. Mater. 2015, 27, 5007–5013. [Google Scholar] [CrossRef]
- Brassat, K.; Kool, D.; Nallet, C.G.A.; Lindner, J.K.N. Understanding Film Thickness-Dependent Block Copolymer Self-Assembly by Controlled Polymer Dewetting on Prepatterned Surfaces. Adv. Mater. Interfaces 2019, 7, 1901605. [Google Scholar] [CrossRef]
- Das, A.; Mukherjee, R. Feature Size Modulation in Dewetting of Nanoparticle-Containing Ultrathin Polymer Films. Macromolecules 2021, 54, 2242–2255. [Google Scholar] [CrossRef]
- Huang, J.; Kim, F.; Tao, A.R.; Connor, S.; Yang, P. Spontaneous Formation of Nanoparticle Stripe Patterns through Dewetting. Nat. Mater. 2005, 4, 896–900. [Google Scholar] [CrossRef]
- Sehgal, A.; Ferreiro, V.; Douglas, J.F.; Amis, E.J.; Karim, A. Pattern-Directed Dewetting of Ultrathin Polymer Films. Langmuir 2002, 18, 7041–7048. [Google Scholar] [CrossRef]
- Giermann, A.L.; Thompson, C.V. Solid-State Dewetting for Ordered Arrays of Crystallographically Oriented Metal Particles. Appl. Phys. Lett. 2005, 86, 121903. [Google Scholar] [CrossRef]
- Bhandaru, N.; Mukherjee, R. Ordering in Dewetting of a Thin Polymer Bilayer with a Topographically Patterned Interface. Macromolecules 2021, 54, 4517–4530. [Google Scholar] [CrossRef]
- Choi, S.Y.; Lee, C.; Lee, J.W.; Park, C.; Kim, S.H. Dewetting-Induced Hierarchical Patterns in Block Copolymer Films. Macromolecules 2012, 45, 1492–1498. [Google Scholar] [CrossRef]
- Brassat, K.; Lindner, J.K.N. Nanoscale Block Copolymer Self-Assembly and Microscale Polymer Film Dewetting: Progress in Understanding the Role of Interfacial Energies in the Formation of Hierarchical Nanostructures. Adv. Mater. Interfaces 2019, 7, 1901565. [Google Scholar] [CrossRef]
- Farrell, R.A.; Kehagias, N.; Shaw, M.T.; Reboud, V.; Zelsmann, M.; Holmes, J.D.; Torres, C.M.S.; Morris, M.A. Surface-Directed Dewetting of a Block Copolymer for Fabricating Highly Uniform Nanostructured Microdroplets and Concentric Nanorings. ACS Nano 2011, 5, 1073–1085. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Sun, Y.-S.; Liou, J.-Y.; Chuang, W.-T. Hierarchically-Responded Assembly of Block Copolymer Thin Films with Stimuli of Varied Solvent Selectivity. Polymer 2012, 53, 5972–5981. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, H.-J.; Lee, C.; Song, S.W.; Lee, J.B.; Huh, D.; Nam, Y.S.; Jeon, D.Y.; Lee, H.; Jung, Y.S. Spontaneous Registration of Sub-10 nm Features Based on SubZero Celsius Spin-Casting of Self-Assembling Building Blocks Directed by Chemically Encoded Surfaces. ACS Nano 2018, 12, 8224–8233. [Google Scholar] [CrossRef]
- Kim, T.H.; Hwang, J.; Hwang, W.S.; Huh, J.; Kim, H.-C.; Kim, S.H.; Hong, J.M.; Thomas, E.L.; Park, C. Hierarchical Ordering of Block Copolymer Nanostructures by Solvent Annealing Combined with Controlled Dewetting. Adv. Mater. 2008, 20, 522–527. [Google Scholar] [CrossRef]
- Kim, T.-H.; Hwang, J.; Acharya, H.; Park, C. Ordered Nanostructure of PS-b-PEO Copolymer by Solvent Annealing with Mixture of Benzene/Water Vapor and Its Micropattern Fabrication. J. Nanosci. Nanotechnol. 2010, 10, 6883–6887. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Chainey, M.; El-Aasser, M.S.; Vanderhoff, J.W. Emulsifier-Free Emulsion Copolymerization of Styrene and Sodium Styrene Sulfonate. J. Polym. Sci. A Polym. Chem. 1992, 30, 171–183. [Google Scholar] [CrossRef]
- Ha, S.T.; Park, O.O.; Im, S.H. Size Control of Highly Monodisperse Polystyrene Particles by Modified Dispersion Polymerization. Macromol. Res. 2010, 18, 935–943. [Google Scholar] [CrossRef]
- Choi, H.K.; Kim, M.H.; Im, S.H.; Park, O.O. Fabrication of Ordered Nanostructured Arrays Using Poly(Dimethylsiloxane) Replica Molds Based on Three-Dimensional Colloidal Crystals. Adv. Funct. Mater. 2009, 19, 1594–1600. [Google Scholar] [CrossRef]
- Choi, H.K.; Yang, Y.J.; Park, O.O. Hemispherical Arrays of Colloidal Crystals Fabricated by Transfer Printing. Langmuir 2014, 30, 103–109. [Google Scholar] [CrossRef]
- Jung, Y.S.; Ross, C.A. Solvent-Vapor-Induced Tunability of Self-Assembled Block Copolymer Patterns. Adv. Mater. 2009, 21, 2540–2545. [Google Scholar] [CrossRef]
- Son, J.G.; Hannon, A.F.; Gotrik, K.W.; Alexander-Katz, A.; Ross, C.A. Hierarchical Nanostructures by Sequential Self-Assembly of Styrene-Dimethylsiloxane Block Copolymers of Different Periods. Adv. Mater. 2010, 23, 634–639. [Google Scholar] [CrossRef]
- Barriet, D.; Lee, T.R. Fluorinated Self-Assembled Monolayers: Composition, Structure and Interfacial Properties. Curr. Opin. Colloid Interface Sci. 2003, 8, 236–242. [Google Scholar] [CrossRef]
- Jasper, J.J. The Surface Tension of Pure Liquid Compounds. J. Phys. Chem. Ref. Data 1972, 1, 841. [Google Scholar] [CrossRef]
- Yang, P.-C.; Ting, Y.-X.; Gu, S.; Gandomi, Y.A.; Li, J.; Hsieh, C.-T. Effect of Solvent on Fluorescence Emission from Polyethylene Glycol-Coated Graphene Quantum Dots under Blue Light Illumination. Nanomaterials 2021, 11, 1383. [Google Scholar] [CrossRef]
- Jung, Y.S.; Ross, C.A. Well-Ordered Thin-Film Nanopore Arrays Formed Using a Block-Copolymer Template. Small 2009, 5, 1654–1659. [Google Scholar] [CrossRef]
- Ruiz, R.; Kang, H.; Detcheverry, F.; Dobisz, E.; Kercher, D.S.; Albrecht, T.R.; De Pablo, J.J.; Nealey, P.F. Density Multiplication and Improved Lithography by Directed Block Copolymer Assembly. Science 2008, 321, 936–939. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Jung, W.; Ross, C.A. Nanofabricated Concentric Ring Structures by Templated Self-Assembly of a Diblock Copolymer. Nano Lett. 2008, 8, 2975–2981. [Google Scholar] [CrossRef]
- Choi, H.K.; Chang, J.-B.; Hannon, A.F.; Yang, J.K.W.; Berggren, K.K.; Alexander-Katz, A.; Ross, C.A. Nanoscale Spirals by Directed Self-Assembly. Nano Futures 2017, 1, 015001. [Google Scholar] [CrossRef]
- Turner, M.S. Equilibrium Properties of a Diblock Copolymer Lamellar Phase Confined between Flat Plates. Phys. Rev. Lett. 1992, 69, 1788. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.S.; Do, H.W.; Berggren, K.K.; Ross, C.A.; Choi, H.K. Commensurability-Driven Orientation Control during Block Copolymer Directed Self-Assembly. ACS Appl. Mater. Interfaces 2020, 12, 10852–10857. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Russell, T.P.; Checco, A. Lattice Deformation and Domain Distortion in the Self-Assembly of Block Copolymer Thin Films on Chemical Patterns. Small 2012, 9, 779–784. [Google Scholar] [CrossRef]
- Lee, G.H.; Kim, S.; Kim, Y.; Jang, M.S.; Jung, Y.S. Simulation and Fabrication of Nanoscale Spirals Based on Dual-Scale Self-Assemblies. ACS Appl. Mater. Interfaces 2020, 12, 46678–46685. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.W.; Park, W.I.; Kim, M.-J.; Ross, C.A.; Jung, Y.S. Highly Tunable Self-Assembled Nanostructures from a Poly(2-vinylpyridine-b-dimethylsiloxane) Block Copolymer. Nano Lett. 2011, 11, 4095–4101. [Google Scholar] [CrossRef] [PubMed]
- Black, C.T.; Bezencenet, O. Nanometer-Scale Pattern Registration and Alignment by Directed Diblock Copolymer Self-Assembly. IEEE Trans. Nanotechnol. 2004, 3, 412–415. [Google Scholar] [CrossRef]
- Barton, A.F. CRC Handbook of Solubility Parameters and Other Cohesion Parameters, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.H.; Kwon, H.G.; Choi, H.K. Dewetting-Induced Hierarchical Self-Assembly of Block Copolymers Templated by Colloidal Crystals. Polymers 2023, 15, 897. https://doi.org/10.3390/polym15040897
Kim DH, Kwon HG, Choi HK. Dewetting-Induced Hierarchical Self-Assembly of Block Copolymers Templated by Colloidal Crystals. Polymers. 2023; 15(4):897. https://doi.org/10.3390/polym15040897
Chicago/Turabian StyleKim, Dong Hwan, Hong Gu Kwon, and Hong Kyoon Choi. 2023. "Dewetting-Induced Hierarchical Self-Assembly of Block Copolymers Templated by Colloidal Crystals" Polymers 15, no. 4: 897. https://doi.org/10.3390/polym15040897
APA StyleKim, D. H., Kwon, H. G., & Choi, H. K. (2023). Dewetting-Induced Hierarchical Self-Assembly of Block Copolymers Templated by Colloidal Crystals. Polymers, 15(4), 897. https://doi.org/10.3390/polym15040897






