Synaptic Transistors Based on PVA: Chitosan Biopolymer Blended Electric-Double-Layer with High Ionic Conductivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
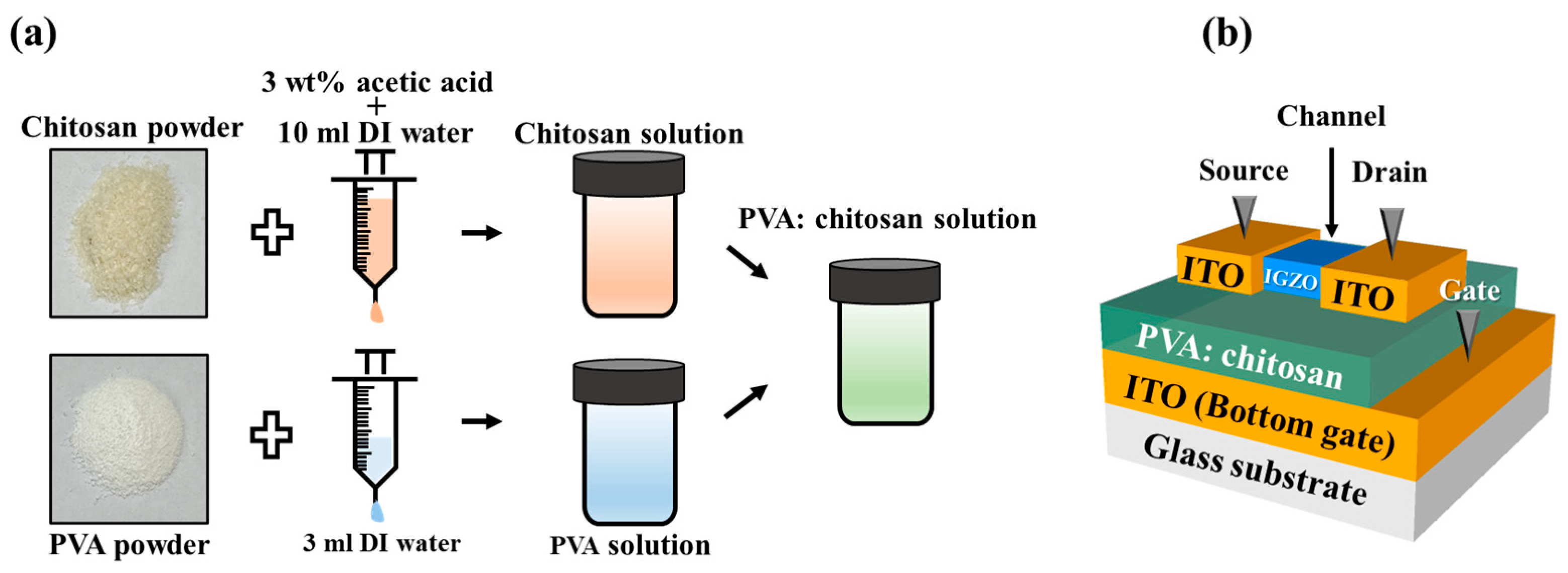
2.2. Preparation of PVA:CS Blended Solution
2.3. Fabrication of EDL Synaptic Transistors Based on PVA:CS Biocompatible Blended Electrolyte
2.4. Characterization
3. Results and Discussion
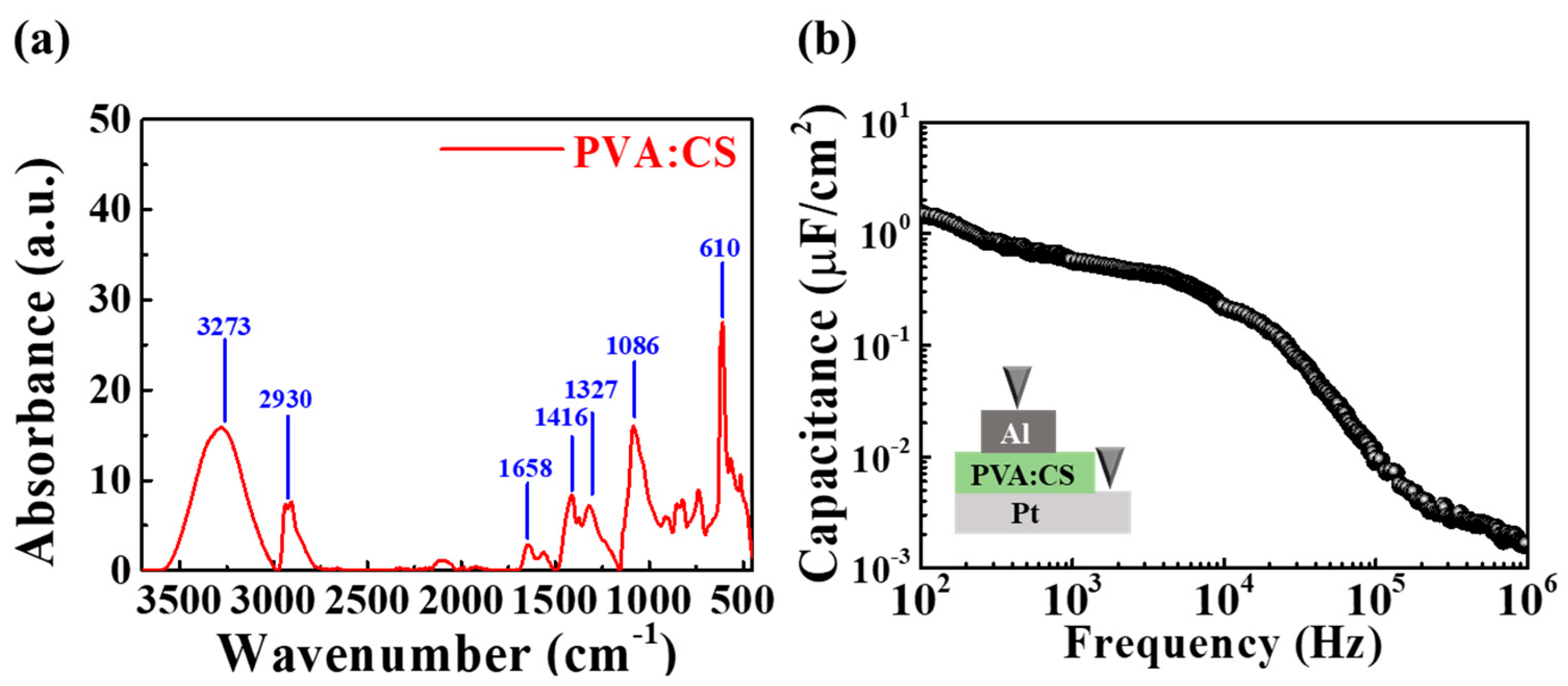
3.1. Characteristics of PVA:CS Biopolymer Blended EDL Membrane
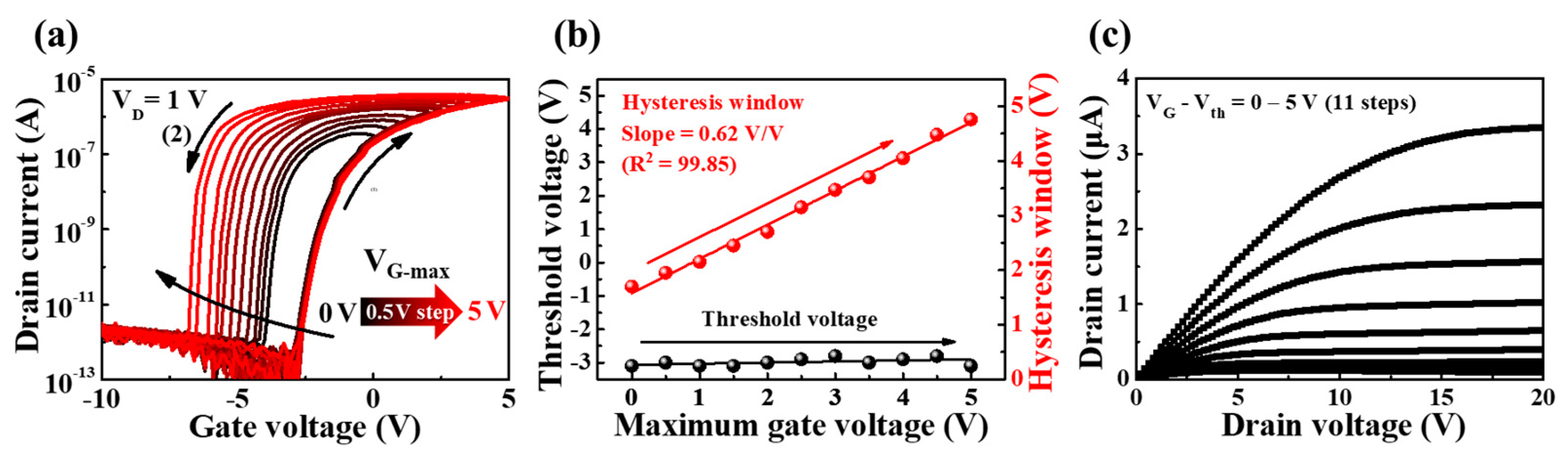
3.2. Electrical Characteristics of Synaptic Transistors Based on PVA:CS Biopolymer Blended EDL with High Ionic Conductivity
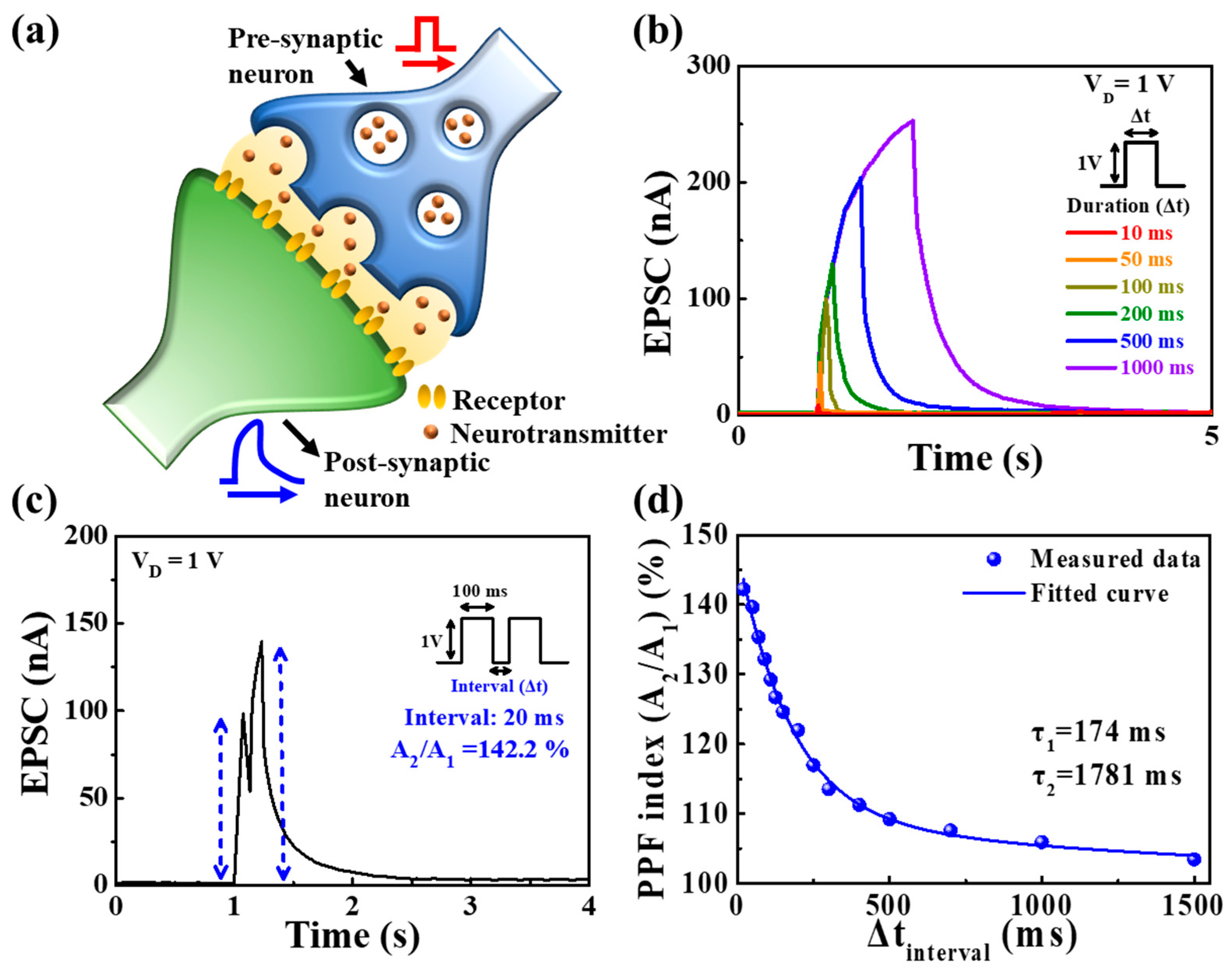
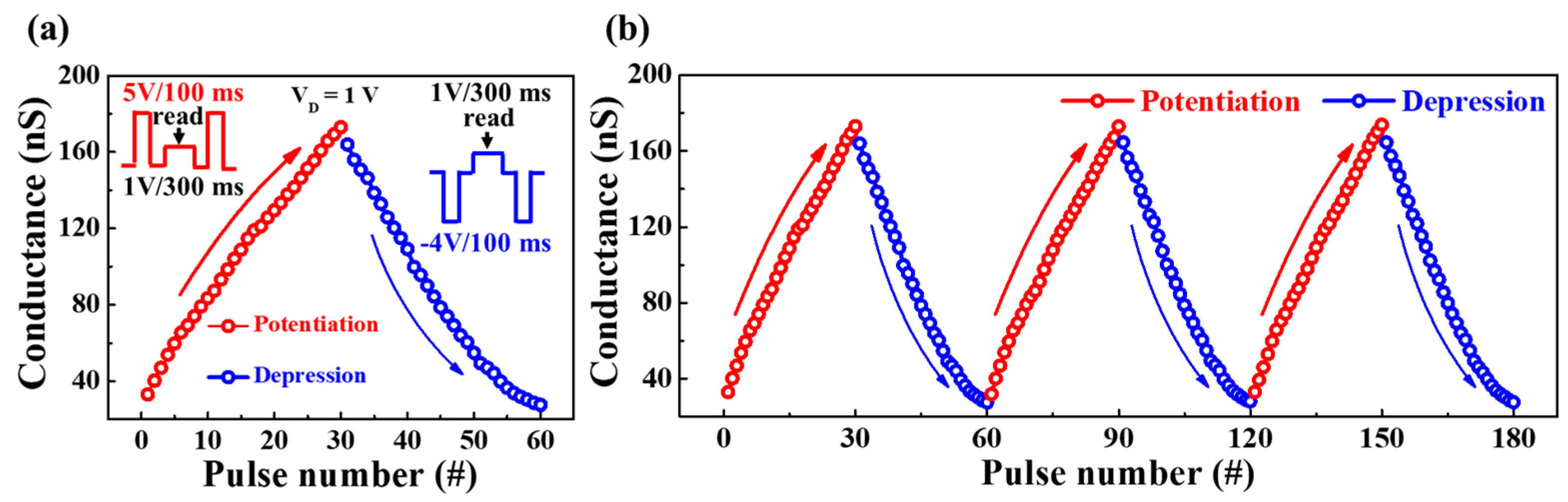
3.3. Synaptic Characteristics of PVA:CS Blended Electrolyte-Based EDL Synaptic Transistors
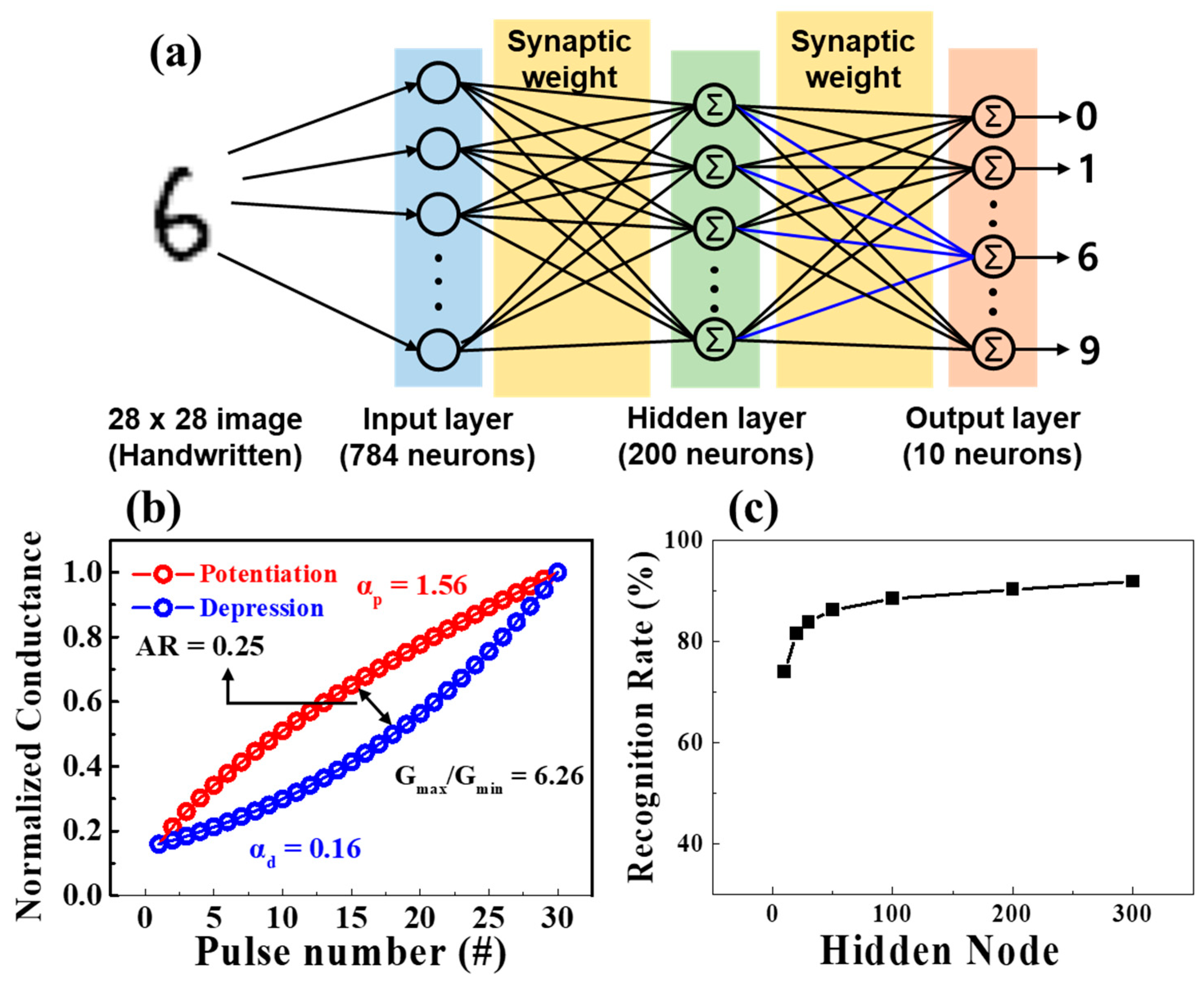
3.4. MNIST ANN Simulation of Proposed Synaptic Transistors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Slavakis, K.; Giannakis, G.B.; Mateos, G. Modeling and optimization for big data analytics:(statistical) learning tools for our era of data deluge. IEEE Signal Process. Mag. 2014, 31, 18–31. [Google Scholar] [CrossRef]
- Tavakoli, M.; Carriere, J.; Torabi, A. Robotics, Smart Wearable Technologies, and Autonomous Intelligent Systems for Healthcare During the COVID-19 Pandemic: An Analysis of the State of the Art and Future Vision. Adv. Intell. Syst. 2020, 2, 2000071. [Google Scholar] [CrossRef]
- Wan, Q.; Sharbati, M.T.; Erickson, J.R.; Du, Y.; Xiong, F. Emerging artificial synaptic devices for neuromorphic computing. Adv. Mater. Technol. 2019, 4, 1900037. [Google Scholar] [CrossRef]
- Oh, K.I.; Kim, S.E.; Bae, Y.H.; Park, K.H.; Kwon, Y.S. Trend of AI Neuromorphic Semiconductor Technology. Electron. Telecommun. Trends 2020, 35, 76–84. [Google Scholar]
- Nurvitadhi, E.; Sheffield, D.; Sim, J.; Mishra, A.; Venkatesh, G.; Marr, D. Accelerating Binarized Neural Networks: Comparison of FPGA, CPU, GPU, and ASIC. In Proceedings of the International Conference on Field-Programmable Technology (FPT), Xi’an, China, 7–9 December 2016; IEEE Publications: Piscataway, NJ, USA, 2016; pp. 77–84. [Google Scholar]
- Arikpo, I.I.; Ogban, F.U.; Eteng, I.E. Von neumann architecture and modern computers. Glob. J. Math. Sci. 2007, 6, 97–103. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, G.; Xin, Z.; Fu, C.; Wan, Q.; Shan, F. Solution-processed, electrolyte-gated In2O3 flexible synaptic transistors for brain-inspired neuromorphic applications. ACS Appl. Mater. Interfaces 2019, 12, 1061–1068. [Google Scholar] [CrossRef]
- Kuzum, D.; Yu, S.; Wong, H.P. Synaptic electronics: Materials, devices and applications. Nanotechnology 2013, 24, 382001. [Google Scholar] [CrossRef]
- Dellaferrera, G.; Woźniak, S.; Indiveri, G.; Pantazi, A.; Eleftheriou, E. Introducing principles of synaptic integration in the optimization of deep neural networks. Nat. Commun. 2022, 13, 1–14. [Google Scholar] [CrossRef]
- Royer, S.; Paré, D. Conservation of total synaptic weight through balanced synaptic depression and potentiation. Nature 2003, 422, 518–522. [Google Scholar] [CrossRef]
- Kim, H.; Sah, M.P.; Yang, C.; Roska, T.; Chua, L.O. Neural synaptic weighting with a pulse-based memristor circuit. IEEE Trans. Circuits Syst. I Regul. Pap. 2011, 59, 148–158. [Google Scholar] [CrossRef]
- Dai, S.; Zhao, Y.; Wang, Y.; Zhang, J.; Fang, L.; Jin, S.; Shao, Y.; Huang, J. Recent advances in transistor-based artificial synapses. Adv. Funct. Mater. 2019, 29, 1903700. [Google Scholar] [CrossRef]
- Desbief, S.; Di Lauro, M.; Casalini, S.; Guerin, D.; Tortorella, S.; Barbalinardo, M.; Kyndiah, A.; Murgia, M.; Cramer, T.; Biscarini, F.; et al. Electrolyte-gated organic synapse transistor interfaced with neurons. Org. Electron. 2016, 38, 21–28. [Google Scholar] [CrossRef]
- Li, C.; Xiong, T.; Yu, P.; Fei, J.; Mao, L. Synaptic iontronic devices for brain-mimicking functions: Fundamentals and applications. ACS Appl. Bio Mater. 2020, 4, 71–84. [Google Scholar] [CrossRef]
- Tao, J.; Sarkar, D.; Kale, S.; Singh, P.K.; Kapadia, R. Engineering complex synaptic behaviors in a single device: Emulating consolidation of short-term memory to long-term memory in artificial synapses via dielectric band engineering. Nano Lett. 2020, 20, 7793–7801. [Google Scholar] [CrossRef]
- Tian, H.; Cao, X.; Xie, Y.; Yan, X.; Kostelec, A.; DiMarzio, D.; Chang, C.; Zhao, L.D.; Wu, W.; Tice, J.; et al. Emulating bilingual synaptic response using a junction-based artificial synaptic device. ACS Nano 2017, 11, 7156–7163. [Google Scholar] [CrossRef]
- Fujimoto, T.; Awaga, K. Electric-double-layer field-effect transistors with ionic liquids. Phys. Chem. Chem. Phys. 2013, 15, 8983–9006. [Google Scholar] [CrossRef]
- Kim, S.H.; Hong, K.; Xie, W.; Lee, K.H.; Zhang, S.; Lodge, T.P.; Frisbie, C.D. Electrolyte-gated transistors for organic and printed electronics. Adv. Mater. 2013, 25, 1822–1846. [Google Scholar] [CrossRef]
- Tan, S.T.; Ng, E.Y.K. Numerical analysis of EDL effect on heat transfer characteristic of 3-D developing flow in a microchannel. Numer. Heat Transf. A Appl. 2006, 49, 991–1007. [Google Scholar] [CrossRef]
- Min, S.Y.; Cho, W.J. CMOS-compatible synaptic transistor gated by chitosan electrolyte-Ta2O5 hybrid electric double layer. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Y.; Shi, Y.; Wan, Q. Solution-processed chitosan-gated IZO-based transistors for mimicking synaptic plasticity. IEEE Electron. Dev. Lett. 2014, 35, 280–282. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hadi, M.J.; Dannoun, E.M.; Abdulwahid, R.T.; Saeed, R.S.; Marf, A.S.; Karim, W.O.; Kadir, M.F. The study of plasticized amorphous biopolymer blend electrolytes based on polyvinyl alcohol (PVA): Chitosan with high ion conductivity for energy storage electrical double-layer capacitors (EDLC) device application. Polymers 2020, 12, 1938. [Google Scholar] [CrossRef]
- Min, J.G.; Cho, W.J. Chitosan-Based Flexible Memristors with Embedded Carbon Nanotubes for Neuromorphic Electronics. Micromachines 2021, 12, 1259. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Rajabi-Abhari, A.; Oh, K.; Yang, G.; Youn, H.J.; Lee, H.L. Improving the barrier properties of packaging paper by polyvinyl alcohol based polymer coating—Effect of the base paper and nanoclay. Polymers 2021, 13, 1334. [Google Scholar] [CrossRef] [PubMed]
- Zanyar, M.; Rehman, S.; Rehman, I.U. Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar]
- Queiroz, M.F.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the use of chitosan contribute to oxalate kidney stone formation? Mar. Drugs 2014, 13, 141158. [Google Scholar]
- Jipa, I.M.; Stoica, A.; Stroescu, M.; Dobre, L.M.; Dobre, T.; Jinga, S.; Tardei, C. Potassium sorbate release from poly (vinyl alcohol)-bacterial cellulose films. Chem. Pap. 2012, 66, 138–143. [Google Scholar] [CrossRef]
- Escobar-Sierra, D.M.; Perea-Mesa, Y.P. Manufacturing and evaluation of Chitosan, PVA and Aloe Vera hydrogels for skin applications. Dyna 2017, 84, 134–142. [Google Scholar] [CrossRef]
- Gao, W.T.; Zhu, L.Q.; Tao, J.; Wan, D.Y.; Xiao, H.; Yu, F. Dendrite integration mimicked on starch-based electrolyte-gated oxide dendrite transistors. ACS Appl. Mater. Interfaces 2018, 10, 40008–40013. [Google Scholar] [CrossRef]
- Wu, G.; Feng, P.; Wan, X.; Zhu, L.; Shi, Y.; Wan, Q. Artificial synaptic devices based on natural chicken albumen coupled electric-double-layer transistors. Sci. Rep. 2016, 6, 23578. [Google Scholar] [CrossRef]
- Zhu, L.Q.; Wan, C.J.; Guo, L.Q.; Shi, Y.; Wan, Q. Artificial synapse network on inorganic proton conductor for neuromorphic systems. Nat. Commun. 2014, 5, 1–7. [Google Scholar] [CrossRef]
- Wee, G.; Larsson, O.; Srinivasan, M.; Berggren, M.; Crispin, X.; Mhaisalkar, S. Effect of the Ionic Conductivity on the Performance of Polyelectrolyte-Based Supercapacitors. Adv. Funct. Mater. 2010, 20, 4344–4350. [Google Scholar] [CrossRef]
- Veletić, M.; Balasingham, I. An information theory of neuro-transmission in multiple-access synaptic channels. IEEE Trans. Commun. 2019, 68, 841–853. [Google Scholar] [CrossRef]
- Veleti´c, M.; Mesiti, F.; Floor, P.A.; Balasingham, I. Communication Theory Aspects of Synaptic Transmission. In Proceedings of the IEEE International Conference on Communications (ICC), London, UK, 8–12 June 2015; IEEE Publications: Piscataway, NJ, USA, 2015; Volume 2015, pp. 1116–1121. [Google Scholar]
- Li, H.K.; Chen, T.P.; Liu, P.; Hu, S.G.; Liu, Y.; Zhang, Q.; Lee, P.S. A light-stimulated synaptic transistor with synaptic plasticity and memory functions based on InGaZnOx–Al2O3 thin film structure. J. Appl. Phys. 2016, 119, 244505. [Google Scholar] [CrossRef]
- Majumdar, S.; Tan, H.; Qin, Q.H.; van Dijken, S. Energy-efficient organic ferroelectric tunnel junction memristors for neuromorphic computing. Adv. Electron. Mater. 2019, 5, 1800795. [Google Scholar] [CrossRef]
- Zucker, R.S.; Regehr, W.G. Short-term synaptic plasticity. Annu. Rev. Physiol. 2022, 64, 355–405. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.Q.; Wan, C.J.; Gao, P.Q.; Liu, Y.H.; Xiao, H.; Ye, J.C.; Wan, Q. Flexible proton-gated oxide synaptic transistors on Si membrane. ACS Appl. Mater. Interfaces 2016, 8, 21770–21775. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, J.; Yu, R.; Zhou, Y.; Yang, Q.; Li, E.; Chen, Q.; Chen, H.; Guo, T. Tuning the synaptic behaviors of biocompatible synaptic transistor through ion-doping. Org. Electron. 2021, 89, 106019. [Google Scholar] [CrossRef]
- Bliss, T.V.P.; Cooke, S.F. Long-term potentiation and long-term depression: A clinical perspective. Clinics 2011, 66, 3–17. [Google Scholar] [CrossRef]
- Bliss, T.V.; Collingridge, G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 1993, 361, 31–39. [Google Scholar] [CrossRef]
- Dan, Y.; Poo, M.M. Spike timing-dependent plasticity: From synapse to perception. Physiol. Rev. 2006, 86, 1033–1048. [Google Scholar] [CrossRef]
- Bi, G.Q.; Poo, M.M. Synaptic modifications in cultured hippocampal neurons: Dependence on spike timing, synaptic strength, and postsynaptic cell type. J. Neurosci. 1998, 18, 10464–10472. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Miller, K.D.; Abbott, L.F. Competitive Hebbian learning through spike-timing-dependent synaptic plasticity. Nat. Neurosci. 2000, 3, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.E. The spike-timing dependence of plasticity. Neuron 2012, 75, 556–571. [Google Scholar] [CrossRef]
- Xi, F.; Han, Y.; Liu, M.; Bae, J.H.; Tiedemann, A.; Grützmacher, D.; Zhao, Q.T. Artificial synapses based on ferroelectric Schottky barrier field-effect transistors for neuromorphic applications. ACS Appl. Mater. Interfaces 2021, 13, 32005–32012. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, Y.; Wang, Y.; Xu, X.; Fu, M.; Liu, Y.; Lin, Z.; Ling, H.; Gkoupidenis, P.; Yi, M.; et al. Thin-film transistors for emerging neuromorphic electronics: Fundamentals, materials, and pattern recognition. J. Mater. Chem. C 2021, 9, 11464–11483. [Google Scholar] [CrossRef]
- Jang, J.; Park, S.; Jeong, Y.; Hwang, H. ReRAM-based synaptic device for neuromorphic computing. In Proceedings of the 2014 IEEE International Symposium on Circuits and Systems (ISCAS), Melbourne, VIC, Australia, 1–5 June 2014; IEEE: Piscataway Township, NJ, USA, 2014. [Google Scholar]
- Jang, J.W.; Park, S.; Burr, G.W.; Hwang, H.; Jeong, Y.H. Optimization of conductance change in Pr1–xCaxMnO3-based synaptic devices for neuromorphic systems. IEEE Electron. Dev. Lett. 2015, 36, 457–459. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.-H.; Park, H.; Cho, W.-J. Synaptic Transistors Based on PVA: Chitosan Biopolymer Blended Electric-Double-Layer with High Ionic Conductivity. Polymers 2023, 15, 896. https://doi.org/10.3390/polym15040896
Lee D-H, Park H, Cho W-J. Synaptic Transistors Based on PVA: Chitosan Biopolymer Blended Electric-Double-Layer with High Ionic Conductivity. Polymers. 2023; 15(4):896. https://doi.org/10.3390/polym15040896
Chicago/Turabian StyleLee, Dong-Hee, Hamin Park, and Won-Ju Cho. 2023. "Synaptic Transistors Based on PVA: Chitosan Biopolymer Blended Electric-Double-Layer with High Ionic Conductivity" Polymers 15, no. 4: 896. https://doi.org/10.3390/polym15040896
APA StyleLee, D.-H., Park, H., & Cho, W.-J. (2023). Synaptic Transistors Based on PVA: Chitosan Biopolymer Blended Electric-Double-Layer with High Ionic Conductivity. Polymers, 15(4), 896. https://doi.org/10.3390/polym15040896





