Abstract
Polyhydroxyalkanoates (PHA) are biodegradable plastic. Numerous bacteria produce PHAs under environmental stress conditions, such as excess carbon-rich organic matter and limitations of other nutritional elements such as potassium, magnesium, oxygen, phosphorus, and nitrogen. In addition to having physicochemical properties similar to fossil-fuel-based plastics, PHAs have unique features that make them ideal for medical devices, such as easy sterilization without damaging the material itself and easy dissolution following use. PHAs can replace traditional plastic materials used in the biomedical sector. PHAs can be used in a variety of biomedical applications, including medical devices, implants, drug delivery devices, wound dressings, artificial ligaments and tendons, and bone grafts. Unlike plastics, PHAs are not manufactured from petroleum products or fossil fuels and are, therefore, environment-friendly. In this review, a recent overview of applications of PHAs with special emphasis on biomedical sectors, including drug delivery, wound healing, tissue engineering, and biocontrols, are discussed.
1. Introduction
Polyhydroxyalkanoates (PHA) are a class of biodegradable polymer-based materials that have garnered substantial attention for their potential medical applications [1,2,3]. These polymers contain carbon and hydrogen, with ester linkages between the hydroxyl groups. Microorganisms produce esters via fermentation; the microbes consume sugar and convert it to fat for storage and energy production. These polymers are produced via fatty acid synthesis, and the enzymes involved are synthases. Unlike plastics, PHAs are environmentally friendly because they are not made from petroleum products or fossil fuels. Bioplastics have several advantages over conventional plastics, such as a low carbon footprint, energy efficiency, versatility, unique mechanical and thermal characteristics, and societal acceptance. Bioplastics are unique due to their biodegradability; moreover, they can be sterilized without causing damage to the material itself. These properties render PHA ideal for medical devices and implants [4]. In addition to being biocompatible and biodegradable, PHAs are inexpensive to produce compared with other bioplastics; therefore, its large-scale production is cost-effective [5]. The PHA extraction process depends upon the bacterial culture and physico-chemical properties of the polymer (molecular weight, polydispersity index). The main recovery approaches involve solvents (alcohols, alkanes, halogenated solvents, carbonates, esters, and ketones) and cell lysis (enzymes, oxidants, surfactants, acid, and alkaline compounds) [6]. Globally, the PHA market was valued at approximately USD 73.6 million in 2021 and is projected to grow to USD 167 million by 2027 [7,8]. Bioplastics with potential biomedical applications include PHA, polylactic acid, poly-3-hydroxybutyrate (PHB), biopolymers (based on cellulose, lipids, proteins, and starch), polyamide 11, and polyhydroxyurethanes [9]. Among the various applications of PHAs, tissue engineering, which includes restoring or replacing damaged organs or tissues, was expected to grow from USD 9.9 billion at a rate of 14.2% between 2019 and 2027 [10,11]. The status of bioplastic production capacities (~103 tons) varies by sector: (i) coatings and adhesives (35.2), (ii) electrics and electronics (~10), (iii) agriculture and horticulture (~90), (iv) consumer goods (~200), (v) fibers (~30), (vi) rigid packaging (~200), and (vii) flexible packaging (~400), and to a minor extent, for articles such as food, coffee pods, compostable cutlery, edible films, bags, biomedical tools, and bottles [12,13,14].
Several types of PHAs with different structures and properties can be used for various purposes. For example, some types are bioerodible, which means that they completely break down in the body over time, while others persist for longer. The different properties of each type of plastic make them more suitable for certain applications [15]. For example, copolymers of PHAs are mechanically stronger than homopolymers. Consequently, they are useful for devices, such as surgical sutures, that need to withstand considerable stress without tearing. PHB is a highly crystalline microbial polyester that belongs to the PHA family. It is currently being tested as a controlled-release implant for drug delivery to treat cancer and other chronic diseases. PHAs have many potential applications in biomedical engineering [16]. Recently, these plastics have been investigated more thoroughly, and new applications are constantly being developed. Because they do not contain toxic substances, they can be safely used in the human body without fear of adverse side effects. In addition, most types of PHAs can be synthesized from pure sugars such as xylose and hexanoates [17] and renewable resources such as corn, and many biowastes of plant and animal origin can be used as feed for the microbial production of PHAs, which makes it environmentally friendly [18,19,20,21,22,23]. However, PHAs have several disadvantages. Some are not suitable for use with biological tissues because they are water-soluble and not very stiff. In addition, the manufacturing process is expensive and requires specialized equipment. Therefore, further investigation is required before they become more cost-effective and applicable than plastics for medical applications. In fact, PHAs must be modified to produce scaffolds for biomedical applications [24].
Biodegradable and porous scaffolds have been manufactured via various techniques: (i) electrospinning—from (a) poly3-hydroxybutyrate-co-4-hydroxybutyrate (P34HB)—fiber scaffold, nanofiber membrane, (b) PHB—microfibers and nanofibers, nanotubes scaffolds, nanofiber scaffold, conduit, (c) PHB and poly(3-hydroxybutyrate-co-3-valerate (PHBV)—fiber, (d) PHBV—membrane, nanofibers, tissue-engineered vascular graft, patches, fibrous scaffold, nanofiber film, fibrous scaffolds, nanofibrous scaffold, composite nanofiber, hydrogel patches, nanofibrous mat, (ii) co-precipitation from PHB—implant, (iii) Salt leaching technique and 3D- printing from PHB—Bioactive biopolymer/mineral/hydrogel scaffold, bone grafts, (iv) solution casting from poly(3-hydroxybutyrate-co-3-hydroxyhexanoate (PHBHHx)—film, (v) solvent evaporation from P34HB—film, (vi) solvent casting- particulate leaching from PHBHHx—porous structure scaffold, (vii) solution casting from PHBV—film [25,26,27,28,29,30,31,32,33,34,35]. This article aims to provide a comprehensive overview of PHA as an alternative to synthetic plastics in advanced biotechnological applications, including drug delivery, wound healing, tissue engineering, and as biocontrol agents.
2. Biopolymer-Synthetic, Biopolymer-Inorganic Composites
The characteristics of various PHA devices can be engineered by producing their blends and composites. Desired mechanical strength, degradation rate, and biocompatibility can be achieved for successful tissue engineering and drug delivery [26,36]. A few examples of PHAs and their composite-based scaffolds for biomedical applications are as follows: (i) PHB/ZrO2/Herafill® prepared through Injection-moulded cylindrical pins resulted in enhancing bone growth on 30% HerafillR composite [37], (ii) PHBV microspheres coated 45S5 bioactive glass-based construct via foam replication and dip coating 3D scaffold had the following characteristics—well-regulated drug delivery and a significantly improved compressive mechanical stability of scaffold [38], (iii) PHBHHx)/Mesoporous 45S5 Bioglass® fabricated using 3D printing technology had a few unique features such as hierarchical pore architecture, improved strength, higher bioactivity and significantly higher bone formation [39], (iv) nano-HA were incorporated into a P(3HB) matrix, which showed significantly higher cell proliferation and differentiation [40], (v) PHBV)/β-Ca2SiO4 fabricated using solvent casting, and two size salt particles, particulates leaching resulted in a composite with highly interconnected pores, enhanced hydrophilicity, higher cell proliferation/differentiation [41], and (vi) cardiac patches based on P(3HO) had mechanical strength equivalent to that observed for cardiac muscle. High biocompatibility with neonatal ventricular rat myocytes also had cell viability, proliferation and adhesion on (P(3HO) films [28].
3. Biomedical Applications
Many natural polymers, such as collagen and fibrin, are biocompatible but lack the necessary biodegradability required for successful medical applications. Hence, developing novel synthetic materials suitable for both medical and industrial applications has been of interest. Applications of PHAs for biotechnological purposes have been exploited in diverse fields, ranging from aquaculture to human health. Their economic value is relatively high because of their biodegradability, biocompatibility, and non-toxic nature. Recently, the use of PHAs has become an essential area of research because of their potential use in producing functional and biodegradable materials for a wide variety of biomedical applications, including tissue engineering (cardiac- and coronary-related bone reconstruction), drug carriers and delivery, medical implants, and biocontrol agents (Figure 1) [15,16,42,43,44].
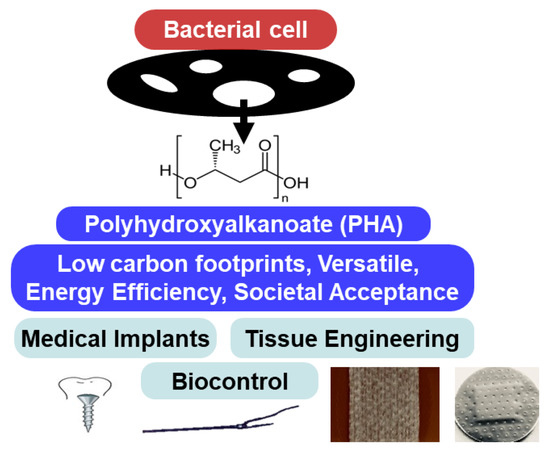
Figure 1.
Biomedical applications of polyhydroxyalkanoates.
3.1. Tissue Engineering
PHAs with varying structures and properties can be used to produce scaffolds for regenerative medicine [35]. Ultra-high-purity PHAs are required for tissue engineering applications. Biodegradable materials are essential because they minimize the risk of infection after implantation and enable the body to naturally heal the injured site sans surgical intervention [45]. However, the use of synthetic or non-biodegradable materials may result in complications such as scar tissue formation and subsequent lack of healing. Hence, PHA-based materials must be evaluated under in vivo conditions and, if necessary, modified to show the requisite properties for fabricating scaffolds and ensure time-bound biodegradation. Such well-engineered PHAs can be used to develop tissue-based products, achieve efficient tissue engineering, and meet the therapeutic needs of nerve tissues, heart valves, stents, and vascular grafts [25,26,27]. PHA modifications can help improve the mechanical strength and produce scaffolds that promote cell growth [46]. These properties can extend the range of biotechnological applications of PHAs to produce sutures, films, pins, and screws [47]. PHA copolymers based on multiple monomeric types of poly(3-hydroxybutyrate-co-4-hydroxyvalerate-co-3-hydroxyhexanoate) [P(3HB-4HB-3HV)] have been used for fabricating fibrous meshes, which can support the growth of stem cells [48]. Copolymeric PHA scaffolds have specific applications depending on their monomeric components: (i) poly(3-hydroxybutyrate-co-3-hydroxyvalerate-co-3-hydroxyhexanoate) [P(3HB-3HV-3HHx)] for liver tissues [49], (ii) 3-D scaffolds for nanofibers [50], and (iii) poly(3-hydroxybutyrate-co-polyhydroxyoctanoate) [P(3HB-3HO)] for cartilage repair [51]. PHAs combined with inorganic bioceramics have high flexibility and mechanical strength, and they can be easily blended, making them suitable for producing novel composites such as hydroxyapatite for engineering tissues [52,53]. To improve the efficiency of sutures, autodissolution after a certain period is highly desirable. Using a copolymer poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) P(3HB-3HHx)-based suture resulted in 58.5% weight loss over seven weeks [54].
A few PHB-based items, such as non-woven patches and porous scaffolds (mesh-like structure), have been observed to assist in regenerating organs ranging from osseous, cardiac, intestinal, neural, and vascular tissues. The materials were found to induce vascularization in defective regions [55,56]. In contrast to homopolymers such as PHB, PHA copolymers including PHBV, poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHx), and poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHB-VHx) have been proven to be instrumental in stimulating the proliferation of HaCaT human keratinocytes [57]. Nanoparticles (NPs) of PHB-VHx (0.02–0.1 g/L) enhanced cell proliferation by activating cell division. This enhancement was due to an increase in the concentration of cytoplasmic calcium ions. Interestingly, the PHA metabolic degradation product 3-hydroxybutyric acid (3HB) (0.1–1.0 mM) also effectively activated cell division in L929 murine fibroblasts and HaCaT human keratinocytes. Moreover, fibroblast apoptosis and necrosis were suppressed [58,59]. The proliferation of pancreatic beta cells in mice and suppression of apoptosis, without any cytotoxicity, was observed in a wide range of homopolymers (3HB), PHA oligomers, and copolymers consisting of 4-hydroxybutyric acid and 3HB (20 µg/mL) [60]. A copolymer consisting of poly(3-hydroxybutyrate-co-3-hydroxyvalerate-co-2,3-dihydroxybutyrate), produced by Cupriavidus eutrophus was found to be effective in fabricating films suitable for regenerating soft tissues [61,62]. Scaffolds with low cytotoxicity have been used to enhance the osteogenic differentiation of osteoblasts and mesenchymal stem cells (MSCs) in humans and animals such as rats and rabbits [63,64,65,66,67]. This growth and differentiation are regulated by the topography and microstructure of the devices [68]. The PHA degradation product 3HB (>0.05 mM) promoted the osteogenic differentiation of osteoblasts [69]. Copolymers-PHBHx stimulates chondrogenic/neurogenic differentiation of MSCs by influencing the expression of the following genes: (i) sox9, pthrp, col2, and col10 [70], and (ii) encoding nestin, acidic protein, glial fibrillary, and βIII-tubulin [71,72]. Monomers of PHAs, 3HB, act as neuroprotective agents by providing energy to neurons and stimulating signal transduction. These metabolic processes are mandatory for enhancing memory and learning [73]. In certain cases, implantation is ineffective in regenerating defective bone tissues. In rat femur and skull models, the expression of type I collagen was determined as an osteogenic marker that regulated the bio-resorption of polymeric material and associated processes, such as vascularization and intergrowth into PHA scaffolds [28,56]. Tissue engineering scaffolds with myoblast cell lines showed very high biocompatibility when PHA copolymers, poly(3-hydroxyhexanoate-co-3-hydroxyoctanoate-co-3-hydroxydecanoate-co-3-hydroxydodecanoate) were produced from biodiesel industry waste as feed. The net gain was 72% higher than that of the C2C12 cell line [74]. Scaffolds with MSCs caused a 3.5-fold higher regeneration of bone defects in rats within a short span of 3–4 weeks [31]. The adhesion and lifespan of fibroblasts and neuronal cells were substantially improved when PHA-copolymer-based composites, films, and microfibers were used. Microbes, such as E. coli, Parabrkholderia, and Pseudomonas, are among the most effective PHA producers [75,76,77]. The main disadvantage of using PHAs for tissue engineering applications is their poor mechanical strength, which limits their use in load-bearing applications. Moreover, leaching bioactive molecules from scaffolds can lead to cytotoxic effects at the implantation sites. However, this limitation has been overcome by cross-linking poly[(R)-3-hydroxybutyrate-co-(R)-3-hydroxy-10-undecenoate] via thiol-one click chemistry. The cross-linked polyester had an increased tensile strength with physical characteristics relevant to soft-tissue replacement and did not exhibit any significant cytotoxicity [31]. Recent studies in tissue engineering further support the low or non-cytotoxicity of scaffolds [65,66,67]. Commercially produced PHB and PHA copolymers have enabled improvements in skin generation during wound healing, resulting in better cellular responsiveness in diabetic models and reducing unnecessary scar formation [29,30,32,33,34]. The diversity of biomedical applications of PHAs in tissue engineering has been presented in Table 1.

Table 1.
Diversity of biomedical applications of polyhydroxyalkanoates and their derivatives in tissue engineering.
The synthetic polymer poly(glycerol sebacate) was modified by 3D-printing technology using PHB and nano-HA to produce scaffolds useful for reconstructing the craniofacial bone [78,79]. PHA copolymer [PHBHHx (2 mol%)] produced on a large scale using a genetically modified Cupriavidus necator strain had unique characteristics (low melting point and glass transition temperatures) suitable for skin tissue engineering [80].
Tissue engineering scaffolds provide 3-dimensional support during tissue repair especially cell attachment and maturation [81,82]. In addition, functional scaffolding biomaterials such as bioactive glasses, nanocomposites of hydroxyapatite, and electrically conducting hydrogels play a vital role in regulating cell behavior. A few other features of importance in regenerative medicine are the antimicrobial surface coatings for biomedical implants and scaffolds and bioactive molecule-releasing scaffolds [83]. The biomolecule-releasing ability of the biocomposite scaffolds is desirable for improving bone regeneration efficiency. 3D nanofibrous scaffolds with the well-regulated release of growth factor (BMP-7) were fabricated by encapsulating them into the polymer microsphere. These were then immobilized on nanofibrous scaffolds. The controlled release of growth factors resulted in high ectopic bone formation [84]. Antimicrobials releasing scaffolds are designed to inhibit microbial colonization on implant sites. Poly(d, l-lactic acid) nanofibrous scaffolds treated with Silvadur ET released silver ions, which restricted bacterial growth [85]. Another feature important for tissue engineering and drug delivery is the stimuli-responsive materials [86]. These polymers can self-assemble or undergo morphology transformation or phase transitions. Piezoelectric scaffolds, which can cause electrical stimulation on cell growth, differentiation, and tissue growth, have potential applications in tissue repair and bone regeneration [87,88,89,90].
The structural features of scaffolds are critical in tissue engineering employed for the restoration and maintenance of injured tissues and organs [91,92]. Various fabrication techniques such as 3-D printing, etching, electrospinning, magnetic, and freeze-casting enable achieving scaffolds with varied topographic orientations. These features influence the efficiency of the regeneration of tissues and organs. The underlying mechanism in aligned and random orientation influences the biological responses in cells. Recent efforts are targeted to develop biomimetic scaffolds which stimulate the structure and composition of the extracellular matrix of the native tissues [93,94].
3.2. Drug Carriers and Delivery
Among the various approaches attempted to improve drug efficacy, well-regulated delivery has been found to be critical. Owing to their flexibility, durability, biodegradability, and biocompatibility, PHAs have been targeted as feed for producing NPs, and as scaffolds for eluting drugs [95,96]. Their use for controlled and sustained drug delivery to wounds improves the efficiency of therapeutic molecules with minimal side effects. For example, silicone, which has been frequently used to encapsulate hydrophobic drugs, must be replaced as it is carcinogenic [97,98]. Dendrimers are produced from 3HB monomers. These biopolymers have unique features, such as monodispersity and functional moieties on the surface, which promote their use as drug carriers [99,100]. Novel b- and c-peptides resistant to peptidases can be prepared from 3HB and 4-hydoxybutyric acid, which can be sustained for a long period within the mammalian serum by ensuring well-regulated drug delivery. The PHA monomer 3HB can be utilized for producing fragrance (S-citronellol) and sex hormones [101]. PHA copolymers—PHBHV)-and 3-hydroxybutyrate-co-4-hydroxybutyrate [P(3HB-4HB)]- based rods were used as implants that could release antibiotics. Microspheres fabricated from PHB were used to effectively transport and release hemoembolizing agents (rifamycin) on-site in a sustained manner [102]. The physicochemical properties of mcl-PHAs, such as their low crystallinity and melting point, proved effective for transdermal drug delivery. PHA-copolymers composed of monomers 3-hydroxy octanoic acid and 3HHx could adhere efficiently to python reticulatus skin. This enabled the easy dispersal of drugs, including clonidine, ketoprofen, and tamsulosin, owing to their enhanced permeability through the PHA matrix [103]. mcl-PHAs obtained from Pseudomonas fluorescens have multiple applications in diagnostic tools, such as drug delivery, immobilizing agents, and protein purification [104]. Modified PHAs as biologically active beads have been demonstrated to help in developing skin test reagents, diagnosis, production of recombinant proteins, delivery of vaccines, and removal of endotoxins [105,106,107,108,109,110,111,112,113,114,115,116].
Using PHA copolymer, P(3HB-HV)-based microspheres mixed with a surface stabilizer (polyvinyl alcohol) substantially improved tetracycline loading and a well-regulated release of the antibiotic used for treating periodontal infection caused by Porphyromonas gingivalis and Actinobacillus actinomycetemcomitans [117]. Loading antimicrobial compounds such as epirubicin, doxorubicin, or curcumin on PHA copolymers could enhance cell viability, which was accompanied by retardation in the growth of pathogenic bacteria [59,118,119,120]. Immobilization of lysozyme on electrospun sheets made up of P(3HB-30 mol% HHx) at the rate of 16.1 µg enzyme/9.5 mm3 discs could effectively inhibit 42% of the total biofilm formation by pathogenic bacteria. Biofilm inhibition was 12% higher than that achieved using solvent-cast sheets. Thus, wound dressings based on such sheets can more effectively eradicate bacterial infections [121].
Restricting cell proliferation is a strategy for cancer management. Pseudomonas putida CA-3 is known to produce medium-chain-length (mcl)-PHA, which has improved physicochemical properties compared to PHB. Depolymerized mcl-PHA generates monomers, largely (R)-3-hydroxy decanoic acid (R10), which, when conjugated with D-peptide DP18, showed a substantially enhanced anticancer proliferation activity. A 3.3–6.3 higher IC50 value was observed when conjugating peptides to hydroxylated decanoic acid (ω-hydroxy decanoic acid). The uptake of peptides into MiaPaCA and HeLa cells induced apoptosis [122]. PHA copolymers with a high hydroxy valerate content (P(3HB-3HV, 5–15 mol%)) obtained from B. cereus were used to produce drug nanocarriers. These nanocarriers were used to carry ellipticine (a plant alkaloid). The biomolecule content varied between 39–45%, which was influenced by the contribution of HV to the copolymer as PHA-polyvinyl alcohol (PHA-PVA). These anticancer agents are efficient because of the high biocompatibility of nanocarriers [123]. The delivery of anticancer drugs through these NPs based on PHA-polyethylene glycol (PHA-PEG) was efficient for a sustained response. The use of nanocarriers in cancer therapy has been demonstrated using cisplatin, which led to higher apoptotic activity in HT22 cells [124]. P(3HB)-based spherical polymeric nanocomposites with a higher anticancer drug (docetaxel) loading capacity (>17%) showed enhanced (>43%) encapsulation efficiency [125]. The information regarding the applications of PHAs in drug carriers and delivery has been presented in Table 2.

Table 2.
Diversity of biomedical applications of polyhydroxyalkanoates and their derivatives in drug carriers and delivery.
To develop the wound dressing necessary for healing defects in the skin of Wistar rats, the copolymer P(3HB-4HB) was used. PHA-based membranes loaded with fibroblasts from mesenchymal stem cells facilitated the release of fibroblast-secreted matrix proteins. These could facilitate the migration of epidermal cells to the wound site, which proved to be effective in enhancing the wound healing process by 1.4-fold compared to a cell-free membrane and 3.5-fold more rapid than control-eschar. This improved wound-healing process also showed reduced skin inflammation [126]. To repair damaged tissue rapidly, new cells should be able to proliferate vigorously. PHB scaffolds and surface-modified electrospun (laminin) fibrous material together enabled the cellular viability of the murine neuroblastoma Neuro2a cell line to increase from 116% at 4 h to 187% after 72 h of seeding [127].
3.3. Medical Implants
The human body has a well-developed immune system that reacts strongly to foreign bodies by secreting pro-inflammatory cytokines. However, PHAs are biological in origin and, therefore, biocompatible. This property enables their use in medical devices such as surgical sutures and dental implants. Because PHAs degrade in the body, their impact on long-term side effects is quite low. PHAs are suitable for applications in which a permanent device is not desirable. PHAs are advantageous for medical implants because they are biocompatible and biodegradable. Owing to their physicochemical properties, thermoplastic polyesters (PHAs) can also be used to produce customized shapes and sizes for specific applications [128,129]. Medical implants based on PHB and its copolymers have several advantages. Because of their high biocompatibility, strength, and slow degradation, biopolymers have been proven to be suitable for fabricating a wide range of resorbable medical devices. These devices range from surgical sutures, porous matrices, scaffolds, microspheres, woven mesh endoprostheses, orthopedic pins, meniscus repair devices, screws, staples, stents, stacks, rivets, surgical meshes, sutured fasteners, and plug endoprostheses to scaffolds for application in mammalian and human cells, including potent drugs (psychoactive neurotransmitters), regeneration of soft tissue during repair of hernia, and cancer cells [21,130,131].
Matrices based on P3HB and P3HO lead to the extensive production of drug-eluting coronary stents to prevent arterial blockage [132]. Biodegradable subcutaneous implants in rats were supplemented with 10-undecanoic acid and octanoic acid produced by Pseudomonas oleovorans [133,134]. Nanocomposites based on poly(3HB-co-70%4HB) from Cupriavidus strain and Claytone APA (5% w/w) exhibited highly improved physicochemical properties such as high strength (17 MPa Young’s modulus), low melting temperature (Tm), and high transparency. These composites are highly suitable for producing green materials and regenerative medicine. The di-alkyl chain component of clay particles inhibited Staphylococcus aureus infections [135]. The anti-inflammatory effect of these biopolymer-based medical devices was observed to be due to the inhibition of cytokine expression, including monocyte chemoattractant protein, interleukins, C-reactive protein, tumor necrosis factor, and inducible nitric oxide synthase. The unique features of such medical devices are also linked to higher gene expression, especially those encoding proteins responsible for actively regenerating various tissues, such as intestinal, cardiac, neural, osseous, and vascular tissues. The genes involved in these tissues were cytokeratin, type I collagen, heparan sulfate proteoglycan, prostacyclin, caveolin-1, and thrombomodulin [55,136]. The excellent hemocompatibility of the PHB polymer at the insertion site was reflected by the presence of undetectable lymphocyte levels, indicating poor immune reactions. Thus, PHBV copolymers could be used to develop devices such as coronary stents and patches for the pericardial wall and pulmonary artery [56]. Implants (wound dressings) coated with lysozyme that prevent biofilm formation (via anti-adhesion) have been fabricated from these biopolymers [121,137]. A PHB copolymer fastener coating on implants allows for well-regulated drug release [138,139].
3.4. Biocontrol Agents
Recently, PHAs have increasingly been used as biomaterials and anti-infective agents. PHA-based nanocomposites have been developed as bioactive materials that can be used to combat bacterial infection. Some studies have shown that PHAs can be used to control bacterial growth in vivo. However, comprehensive reviews that highlight the role of PHAs in disease prevention and treatment are lacking. Of the few PHA antibiotics currently available, most are against gram-positive bacteria, and only a few can be used against gram-negative bacteria and fungi. Studies have shown that PHA binds and inhibits the function of some microbial cell wall structures, such as the lipid A component of lipopolysaccharides. PHA has also been shown to disrupt bacterial cell membranes and target specific cell wall structures. PHA binding to bacterial membranes caused loss of membrane integrity and bacterial cell death. Hence, PHA can potentially be used as an alternative to current antibiotics. As an alternative to conventional antibiotics, PHAs are relatively safe and can be harmlessly degraded in the environment.
The emergence of drug-resistant bacteria has negatively affected human health, agriculture, and aquaculture. Animal feed supplementation with antibiotics has been banned, and ecologically and economically sustainable biocontrol agents are being investigated [140]. The primary concern is the emergence of resistance among gastrointestinal microflora [141,142]. Modifying PHA functional groups, especially hydroxyl (–OH) and carboxylic acid (–COOH), can result in the production of [(R)-3-hydroxycarboxylic acids, (R)-3HA]. Depolymerization of PHAs to monomers can be performed using the P. fluorescens GK13 depolymerase for reducing S. aureus infections [143,144,145]. Conjugating these monomers with D-peptides acts as an anticancer agent [122], whereas P3HB/P4HB enhances the angiogenic characteristics of wound and skin healing [126,146]. A variety of hydroxycarboxylic acids, including β-lactones and 2-alkylated 3HB, can be produced by transforming 3HAs for use as oral drugs, such as carbapenem or macrolide antibiotics [99,100,147]. These synthetic molecules possess antimicrobial, antifungal, and antiviral properties. PHB has been used as a growth inhibitor against Vibrio spp., Salmonella spp., and Escherichia coli [148]. Initial studies involved testing these properties against the pathogenic Vibrio campbellii. This treatment resulted in a 2-to 3-fold increase in the survival rate of brine shrimp larvae. Indirectly, it proved the potential of PHAs to extend the prospects of providing health benefits to humans [149]. PHAs depolymerization can generate short-chain fatty acids, which are effective antimicrobials against pathogens that cause diseases in giant tiger prawns (Penaeus monodon) [150,151].
PHAs as biocontrol agents vary in efficacy depending on their monomeric composition. (R)-3HAs with 8–18C atoms and strong bactericidal activity were obtained from Streptomyces sp. JM3 (JN166713). At a minimal inhibitory concentration (MIC) of 1.2–25 mg/mL, it could act against E. coli O157:H7, Salmonella typhimurium (ATCC 14028), and Listeria monocytogenes (ATCC 7644). Chemically modified PHA(3-hydroxy octanoate) was effective as (i) antimicrobial against (a) bacterial pathogens (MIC 2.8–7.0 mM) and (b) the fungi Microsporum gypseum and Candida albicans (MIC:0.1–6.3 mM) and (ii) (E)-oct-2-enoic and 3-oxo octanoic acid (IC50 1.6–1.7 mM) against lung fibroblasts without affecting mammalian cell proliferation [152]. Biopolymeric films of a nanocomposite nature were produced by combining nanomelanin particles with PHB. This enabled the expression of antibacterial activity against MDR strains of S. aureus. It has been proposed for application in medical devices and food materials against microbial infection and oxidation [153].
Pretreating rat synovial fibroblasts (2.5 × 107) infected with S. typhimurium (ATCC 14028) (60 µL of 1 × 106 CFU/mL) with 0.8 mg/mL (R)-3HA resulted in higher viability [154]. PHACOS, a modified bacterial polyester, substantially inhibited the biofilm-producing potential of MRSA. This functional modification had a very low inflammatory effect and extremely low levels of cytotoxicity. This helped the adhesion of fibroblasts and considerably reduced the presence of neutrophils and macrophages around PHACOS implants [146]. A unique approach to protect the larvae of giant tiger prawns P. monodon against V. campbellii was achieved by fortifying the feed with PHB [151]. PHB metabolism led to monomers (3-HB), which, along with its modified form, 3HB methyl ester, has been shown to supply energy in the absence of glucose. This mechanism is expected to be effective during (i) severe brain injuries causing hypoglycemia, (ii) inhibition of reactive oxygen species generation in mice with Alzheimer’s disease (AD), and (iii) inhibition of apoptosis [73]. Based on this information, (D)-BHB, a ketone body, could act as an alternative source of glucose to supply energy. This isomeric form could prevent neuronal death by lowering the concentration of light chain 3 and its associated protein p62. It inhibited the accumulation of autophagosomes, thereby stimulating autophagic flux [155]. The efficiency and production cost of these PHA-based materials can be improved using jatropha oil, vegetable oils, and sugarcane molasses as feed for Bacillus, Cuprividus, and Pseudomonas spp. These modified biopolymeric materials have been used in wound dressing bandages, preventing shrimp mortality and improving food storage [59,121,156,157,158]. A brief presentation of the applications of PHAs as biocontrol agents has been presented in Table 3.

Table 3.
Diversity of biomedical applications of polyhydroxyalkanoates and their derivatives as biocontrol agents.
4. Perspectives
Biopolymers, such as PHAs, have a high potential for commercialization owing to their unique properties compared to many other biopolymeric substances. In the future, all medical devices could be replaced with PHA and their derivatives. Degradation of biopolymers in biological environments occurs through enzymatic and non-enzymatic hydrolysis. These processes don’t involve thermal oxidation, photolysis or radiolysis [159]. PHA degradation takes place with the help of depolymerizing enzymes: depolymerases, 3HB-oilgomer hydrolases, acetyl-CoA hydrolases, and dehydrogenases [143,145,147,160,161,162]. PHA depolymerase consists of a catalytic domain and a substrate-binding domain. Crystalline PHA binds to the substrate binding domain of the enzyme, whereas the catalytic domain initiates the cleaving of the polymer chain [163]. The degradation process is dependent upon the composition, stereoregularity, additives, crystallinity, and accessibility of the polymer. The end products of the degradation process are either (i) CO2 and water under aerobic conditions or (ii) methane, CO2, and water under anaerobic conditions [164]. The rate of degradation is affected by various factors: microbial population and diversity, temperature, pH, nutrient supply, moisture level, and properties of the polymer (composition, and crystallinity). The rate of polymer synthesis in Cupriavidus necator under N-free medium was 10-fold faster than its degradation [162]. Extracellular degradation is influenced by the length of the side chain of PHA. Higher degradability was observed in PHA with longer side chains [165]. PHA production in Bacillus species was observed to peak from 72–120 h, whereas its depolymerization was reported to be from 96–192 h of incubation. The duration of these processes varied with bacterial strain and feed [166]. These devices dissolve over time and are much easier to sterilize than traditional metal or plastic devices. This would be beneficial in cases where repeated surgery is required or where a device must be replaced due to wear and tear. One potential application of this technology is in developing artificial organs that can be implanted into the human body. These devices can also be used in prosthetics to help restore functionality in damaged limbs. PHAs can serve as templates to produce recombinant proteins, which can be used as therapeutic agents for treating various diseases. PHAs are also used to produce bone cement owing to their strong mechanical properties. These are only a few of the many potential biomedical applications of PHA. Novel technologies are being developed that will allow the use of these materials in even more applications and medical research efforts. Despite such promising benefits, few challenges must be addressed before full-scale commercialization [167]. Research on modifying the properties of PHAs must focus on lowering the melting point and glass transition temperature and improving the tensile strength, elastic modulus, and elongation. These features govern the molecular weight, which in turn is influenced by the monomers present in the copolymers [168,169]. To counter the feed cost, biowastes and culture conditions must be evaluated and optimized, genetically modifying the expression of PHA operons and synchronizing the expression of depolymerase genes and PHA biosynthesis with auto-cell lysis [170,171,172,173]. These manipulations are expected to lead to the cost-effective commercial production of PHAs.
5. Conclusions
The use of plastic-based materials has reached a stage where they have become indispensable. However, due to their non-biodegradable nature, their applications turn non-ecofriendly and contribute to the financial burden of controlling environmental pollution. Hence, bioplastics, especially co-polymers of PHAs, have provided an opportunity to use them in diverse fields, especially for medical applications. The major limitation of PHAs is their costly commercial-level production. The following two strategies can help overcome this limitation to a large extent. Firstly, using biowastes as feed can lead to a 45% reduction in production costs. Secondly, their usage for high-valued medical applications such as sutures, implants, drug delivery agents and carriers, wound healing meshes, tissue engineering, and biocontrol agents can contribute to their application.
Author Contributions
Conceptualization, V.C.K.; methodology, V.C.K. and S.K.S.P.; validation, V.C.K. and S.K.S.P.; formal analysis, V.C.K.; resources, J.-K.L.; data curation, V.C.K.; writing—original draft, V.C.K. and J.-K.L.; writing—review and editing, V.C.K.; supervision, V.C.K. and J.-K.L.; funding acquisition, J.-K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) and funded by the Ministry of Science, ICT & Future Planning (grant numbers NRF-2021R1A2B5B03002728, 2022M3A9I3082366, 2022M3A9I5015091).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Koller, M. Biodegradable and biocompatible polyhydroxyalkanoates (PHA): Auspicious microbial macromolecules for pharmaceutical and therapeutic applications. Molecules 2018, 23, 362. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.C.; Ray, S.; Patel, S.K.; Singh, M.; Singh, G.P. The dawn of novel biotechnological applications of polyhydroxyalkanoates. In Biotechnological Applications of Polyhydroxyalkanoates; Springer: Singapore, 2019; pp. 1–11. [Google Scholar]
- Sharma, V.; Sehgal, R.; Gupta, R. Polyhydroxyalkanoate (PHA): Properties and modifications. Polymer 2021, 212, 123161. [Google Scholar] [CrossRef]
- Gregory, D.A.; Taylor, C.S.; Fricker, A.T.R.; Asare, E.; Tetali, S.S.V.; Haycock, J.W.; Roy, I. Polyhydroxyalkanoates and their advances for biomedical applications. Trends Mol. Med. 2022, 28, 331–342. [Google Scholar] [CrossRef]
- Behera, S.; Vandana, M.P.; Das, S. Polyhydroxyalkanoates, the bioplastics of microbial origin: Properties, biochemical synthesis, and their applications. Chemosphere 2022, 294, 133723. [Google Scholar] [CrossRef]
- Pagliano, G.; Galletti, P.; Samori, C.; Zaghini, A.; Torri, C. Recovery of polyhydroxyalkanaotes from single and mixed microbial cultures: A review. Front. Bioeng. Biotechnol. 2021, 9, 624021. [Google Scholar] [CrossRef] [PubMed]
- Khatami, K.; Perez-Zabaleta, M.; Owusu-Agyeman, I.; Cetecioglu, Z. Waste to bioplastics: How close are we to sustainable polyhydroxyalkanoates production? Waste Manag. 2021, 119, 374–388. [Google Scholar] [CrossRef] [PubMed]
- Polyhydroxyalkanoate (PHA) Market. Available online: https://www.marketsandmarkets.com/Market-Reports/pha-market-395.html? (accessed on 23 February 2023).
- Kabir, E.; Kaur, R.; Lee, J.; Kim, K.H.; Kwon, E.E. Prospects of biopolymer technology as an alternative option for non-degradable plastics and sustainable management of plastic wastes. J. Clean. Prod. 2020, 258, 120536. [Google Scholar] [CrossRef]
- Tissue Engineering Market Size; Industry Analysis Report, 2000–2027. Available online: https://www.grandviewresearch.com/industry-analysis/tissue-engineering-and-regeneration-industry (accessed on 26 February 2023).
- Darie-Nita, R.N.; Rapa, M.; Frackowick, S. Special features of polyester-based materials for medical applications. Polymers 2022, 14, 951. [Google Scholar] [CrossRef]
- Nanda, S.; Patra, B.R.; Patel, R.; Bakos, J.; Dalai, A.K. Innovations in applications and prospects of bioplastics and biopolymers: A review. Environ. Chem. Lett. 2022, 20, 379–395. [Google Scholar] [CrossRef]
- Gregory, D.A.; Fricker, A.T.R.; Mitrev, P.; Ray, M.; Asare, E.; Sim, D.; Larpnimitchai, S.; Zhang, Z.; Ma, J.; Tetali, S.S.V.; et al. Additive manufacturing of polyhydroxyalkanoate-based blends using fused deposition modelling for the development of biomedical devices. J. Funct. Biomater. 2023, 14, 40. [Google Scholar] [CrossRef]
- European Bioplastics. 2021. Available online: https://www.european-bioplastics.org/market/ (accessed on 23 February 2023).
- Ray, S.; Kalia, V.C. Biomedical applications of polyhydroxyalkanoates. Indian J. Microbiol. 2017, 57, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Yang, K.; Qin, X.; Luo, R.; Wang, H.; Huang, R. Polyhydroxyalkanoates in tissue repair and regeneration. Eng. Regen. 2022, 3, 24–40. [Google Scholar] [CrossRef]
- Oliveira-Filho, E.R.; de Macedo, M.A.; Lemos, A.C.C.; Adams, F.; Merkel, O.M.; Taciro, M.K.; Gomez, J.G.V.; Silva, L.F. Engineering Burkholderia sacchari to enhance poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) [P(3HB-co-3HHx)] production from xylose and hexanoate. Int. J. Biol. Macromol. 2022, 213, 902–914. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.S.; Kumar, P.; Singh, S.; Lee, J.K.; Kalia, V.C. Integrative approach to produce hydrogen and polyhydroxybutyrate from biowaste using defined bacterial cultures. Bioresour. Technol. 2015, 176, 136–141. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Lee, J.K.; Kalia, V.C. Integrative approach for producing hydrogen and polyhydroxyalkanoate from mixed wastes of biological origin. Indian J. Microbiol. 2016, 56, 293–300. [Google Scholar] [CrossRef]
- Kalia, V.C.; Prakash, J.; Koul, S. Biorefinery for glycerol rich biodiesel industry waste. Indian J. Microbiol. 2016, 56, 113–125. [Google Scholar] [CrossRef]
- Ray, S.; Kalia, V.C. Co-metabolism of substrates by Bacillus thuringiensis regulates polyhydroxyalkanoate co-polymer composition. Bioresour. Technol. 2017, 224, 743–747. [Google Scholar] [CrossRef]
- Ray, S.; Kalia, V.C. Microbial cometabolism and polyhydroxyalkanoate co-polymers. Indian J. Microbiol. 2017, 57, 39–47. [Google Scholar] [CrossRef]
- Kavitha, G.; Rengasamy, R.; Inbakandan, D. Polyhydroxybutyrate production from marine source and its application. Int. J. Biol. Macromol. 2018, 111, 102–108. [Google Scholar] [CrossRef]
- Grigore, M.E.; Grigorescu, R.M.; Iancu, L.; Ion, R.-M.; Zaharia, C.; Andrei, E.R. Methods of synthesis, properties and biomedical applications of polyhydroxyalkanoates: A review. J. Biomater. Sci. Polym. Ed. 2019, 30, 695–712. [Google Scholar] [CrossRef]
- Levine, A.C.; Sparano, A.; Twigg, F.F.; Numata, K.; Nomura, C.T. Influence of cross-linking on the physical properties and cytotoxicity of polyhydroxyalkanoate (PHA) scaffolds for tissue engineering. ACS Biomater. Sci. Eng. 2015, 1, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Goonoo, N.; Bhaw-Luximon, A.; Passanha, P.; Esteves, S.R.; Jhurry, D. Third generation poly(hydroxyacid) composite scaffolds for tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1667–1684. [Google Scholar] [CrossRef]
- Ke, Y.; Zhang, X.Y.; Ramakrishna, S.; He, L.M.; Wu, G. Reactive blends based on polyhydroxyalkanoates: Preparation and biomedical application. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Bagdadi, A.V.; Safari, M.; Dubey, P.; Basnett, P.; Sofokleous, P.; Humphrey, E.; Locke, I.; Edirisinghe, M.; Terracciano, C.; Boccaccini, A.R.; et al. Poly(3-hydroxyoctanoate), a promising new material for cardiac tissue engineering. J. Tissue Eng. Regen. Med. 2018, 12, e495–e512. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A.; Patan, N.K.; Dalvi, Y.B.; Varghese, R.; Antony, A.; Unni, R.N.; Sandhyarani, N.; Moustafa, A.E.A. Cerium oxide nanoparticle incorporated electrospun poly(3-hydroxybutyrate-co-3-hydroxyvalerate) membranes for diabetic wound healing applications. ACS Biomater. Sci. Eng. 2020, 6, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Chen, J.; Wu, L.P.; Wu, J.; Xiang, H.; Leong, K.W.; Han, J. Prevention of excessive scar formation using nanofibrous meshes made of biodegradable elastomer poly(3-hydroxybutyrate-co-3-hydroxyvalerate). J. Tissue Eng. 2020, 11, 2041731420949332. [Google Scholar] [CrossRef]
- Volkov, A.V.; Muraev, A.A.; Zharkova, I.I.; Voinova, V.V.; Akoulina, E.A.; Zhuikov, V.A.; Khaydapova, D.D.; Chesnokova, D.V.; Menshikh, K.A.; Dudun, A.A.; et al. Poly(3-hydroxybutyrate)/hydroxyapatite/alginate scaffolds seeded with mesenchymal stem cells enhance the regeneration of critical-sized bone defect. Mater. Sci. Eng. C 2020, 114, 110991. [Google Scholar] [CrossRef]
- Ye, J.P.; Gong, J.S.; Su, C.; Liu, Y.G.; Jiang, M.; Pan, H.; Li, R.Y.; Geng, Y.; Xu, Z.H.; Shi, J.S. Fabrication and characterization of high molecular keratin based nanofibrous membranes for wound healing. Colloids Surf. B Biointerfaces 2020, 194, 111158. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A.; Dalvi, Y.B.; Rehman, S.R.U.; Varghese, R.; Unni, R.N.; Yalcin, H.C.; Alfkey, R.; Thomas, S.; Al Moustafa, A.E. Growth factor loaded in situ photocrosslinkable poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/gelatin methacryloyl hybrid patch for diabetic wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111519. [Google Scholar] [CrossRef]
- Sanhueza, C.; Hermosilla, J.; Bugallo-Casal, A.; Da Silva-Candal, A.; Taboada, C.; Millán, R.; Concheiro, A.; Alvarez-Lorenzo, C.; Acevedo, F. One-step electrospun scaffold of dual-sized gelatin/poly-3-hydroxybutyrate nano/microfibers for skin regeneration in diabetic wound. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111602. [Google Scholar] [CrossRef]
- Pulingam, T.; Appaturi, J.N.; Parumasivam, T.; Ahmad, A.; Sudesh, K. Biomedical applications of polyhydroxyalkanoate in tissue engineering. Polymers 2022, 14, 2141. [Google Scholar] [CrossRef]
- Lizarraga-Valderrama, L.; Thomas, C.; Cadiz-Miranda, J.; Roy, I. Tissue engineering: Polyhydroxyalkanoate-based materials and composites. In Encyclopaedia of Polymer Applications; Taylor & Francis Group: Boca Raton, FL, USA, 2018; pp. 2652–2675. [Google Scholar]
- Meischel, M.; Eichler, J.; Martinelli, E.; Karr, U.; Weigel, J.; Schmöller, G.; Tschegg, E.K.; Fischerauer, S.; Weinberg, A.M.; Stanzl-Tschegg, S.E. Adhesive strength of bone-implant interfaces and in-vivo degradation of PHB composites for load-bearing applications. J. Mech. Behav. Biomed. Mater. 2016, 53, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Zhu, H.; Li, J.; Li, J.; Feng, X.; Chen, J. Preparation and characterization of polyhydroxybutyrate-co-hydroxyvalerate/ silk fibroin nanofibrous scaffolds for skin tissue engineering. Polym. Eng. Sci. 2015, 55, 907–916. [Google Scholar] [CrossRef]
- Zhao, Y.; Feric, N.T.; Thavandiran, N.; Nunes, S.S.; Radisic, M. The role of tissue engineering and biomaterials in cardiac regenerative medicine. Can. J. Cardiol. 2014, 30, 1307–1322. [Google Scholar] [CrossRef] [PubMed]
- Saadat, A.; Behnamghader, A.; Karbasi, S.; Abedi, D.; Soleimani, M.; Shafiee, A. Comparison of acellular and cellular bioactivity of poly 3-hydroxybutyrate/hydroxyapatite nanocomposite and poly 3-hydroxybutyrate scaffolds. Biotechnol. Bioprocess Eng. 2013, 18, 587–593. [Google Scholar] [CrossRef]
- Wang, B.; Pugh, S.; Nielsen, D.R.; Zhang, W.; Meldrum, D.R. Engineering cyanobacteria for photosynthetic production of 3-hydroxybutyrate directly from CO2. Metab. Eng. 2013, 16, 68–77. [Google Scholar] [CrossRef]
- Goswami, M.; Rekhi, P.; Debnath, M.; Ramakrishna, S. Microbial Polyhydroxyalkanoates Granules: An Approach Targeting Biopolymer for Medical Applications and Developing Bone Scaffolds. Molecules 2021, 26, 860. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Udduttula, A.; Fan, Z.S.; Chen, J.H.; Sun, A.R.; Zhang, P. Microbial-Derived Polyhydroxyalkanoate-Based Scaffolds for Bone Tissue Engineering: Biosynthesis, Properties, and Perspectives. Front. Bioeng. Biotechnol. 2021, 9, 763031. [Google Scholar] [CrossRef]
- Lizarraga-Valderrama, L.R.; Ronchi, G.; Nigmatullin, R.; Fregnan, F.; Basnett, P.; Paxinou, A.; Geuna, S.; Roy, I. Preclinical study of peripheral nerve regeneration using nerve guidance conduits based on polyhydroxyalkanoates. Bioeng. Transl. Med. 2021, 6, e10223. [Google Scholar] [CrossRef]
- Basnett, P.; Matharu, R.K.; Taylor, C.S.; Illangakoon, U.; Dawson, J.I.; Kanczler, J.M.; Behbehani, M.; Humphrey, E.; Majid, Q.; Lukasiewicz, B.; et al. Harnessing polyhydroxyalkanoates and pressurized gyration for hard and soft tissue engineering. ACS Appl. Mater. Interfaces 2021, 13, 32624–32639. [Google Scholar] [CrossRef]
- Chen, W.; Tong, Y.W. PHBV microspheres as neural tissue engineering scaffold support neuronal cell growth and axon–dendrite polarization. Acta Biomater. 2012, 8, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Grande, D.; Ramier, J.; Versace, D.L.; Renard, E.; Langlois, V. Design of functionalized biodegradable PHA-based electrospun scaffolds meant for tissue engineering applications. New Biotechnol. 2017, 37, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Canadas, R.F.; Cavalheiro, J.M.B.T.; Guerreiro, J.D.T.; de Almeida, M.C.M.D.; Pollet, E.; da Silva, C.L.; da Fonseca, M.M.R.; Ferreira, F.C. Polyhydroxyalkanoates: Waste glycerol upgrade into electrospun fibrous scaffolds for stem cells culture. Int. J. Biol. Macromol. 2014, 71, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Li, P.; Wu, B.; Ma, H.; Wang, Y.; Liu, G.; Wei, X. PHBVHHx scaffolds loaded with umbilical cord-derived mesenchymal stem cells or hepatocyte-like cells differentiated from these cells for liver tissue engineering. Mater. Sci. Eng. C 2014, 45, 374–382. [Google Scholar] [CrossRef]
- Xu, X.Y.; Li, X.T.; Peng, S.W.; Xiao, J.F.; Liu, C.; Fang, G.; Chen, G.Q. The behaviour of neural stem cells on polyhydroxyalkanoate nanofiber scaffolds. Biomaterials 2010, 31, 3967–3975. [Google Scholar] [CrossRef]
- Ching, K.Y.; Andriotis, O.G.; Li, S.; Basnett, P.; Su, B.; Roy, I.; Stolz, M. Nanofibrous poly (3-hydroxybutyrate)/poly (3-hydroxyoctanoate) scaffolds provide a functional microenvironment for cartilage repair. J. Biomater. Appl. 2016, 31, 77–91. [Google Scholar] [CrossRef]
- Xi, J.; Zhang, L.; Zheng, Z.A.; Chen, G.; Gong, Y.; Zhao, N.; Zhang, X. Preparation and evaluation of porous poly (3-hydroxybutyrate-co-3-hydroxyhexanoate)—Hydroxyapatite composite scaffolds. J. Biomater. Appl. 2008, 22, 293–307. [Google Scholar] [CrossRef]
- Yucel, D.; Kose, G.T.; Hasirci, V. Polyester based nerve guidance conduit design. Biomaterials 2010, 31, 1596–1603. [Google Scholar] [CrossRef]
- Kehail, A.; Boominathan, V.; Fodor, K.; Chalivendra, V.; Ferreira, T.; Brigham, C. In vivo and in vitro degradation studies for Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) Biopolymer. J. Polym. Environ. 2017, 25, 296–307. [Google Scholar] [CrossRef]
- Castellano, D.; Blanes, M.; Marco, B.; Cerrada, I.; Ruiz-Sauri, A.; Pelacho, B.; Arana, M.; Montero, J.A.; Cambra, V.; Prosper, F.; et al. A comparison of electrospun polymers reveals poly(3-hydroxybutyrate) fiber as a superior scaffold for cardiac repair. Stem. Cells Dev. 2014, 23, 1479–1490. [Google Scholar] [CrossRef]
- Shumilova, A.A.; Myltygashev, M.P.; Kirichenko, A.K.; Nikolaeva, E.D.; Volova, T.G.; Shishatskaya, E.I. Porous 3D implants of degradable poly-3-hydroxybutyrate used to enhance regeneration of rat cranial defect. J. Biomed. Mater. Res. A 2017, 105, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Roether, J.A.; Knowles, J.C.; Mordan, N.; Salih, V.; Locke, I.C.; Michael, P.G.; MnCormick, A.; Mohn, D.; Stark, W.J.; et al. Highly elastomeric poly (3-hydroxyoctanoate) based natural polymer composite for enhanced keratinocyte regeneration. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 326–335. [Google Scholar] [CrossRef]
- Ji, Y.; Li, X.T.; Chen, G.Q. Interactions between a poly(3-hydroxybutyrate-co-3-hydroxyvalerate-co-3-hydroxyhexanoate) terpolyester and human keratinocytes. Biomaterials 2008, 29, 3807–3814. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, N.; Dutta, K.; Basu, R.K.; Kundu, P.P. Aromatic π-conjugated curcumin on surface modified polyaniline/polyhydroxyalkanoate based 3d porous scaffolds for tissue engineering applications. ACS Biomater. Sci. Eng. 2016, 2, 2365–2377. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.D.; Zou, X.H.; Dai, Z.W.; Luo, R.C.; Wei, C.J.; Chen, G.Q. Effects of oligo(3-hydroxyalkanoates) on the viability and insulin secretion of murine beta cells. J. Biomater. Sci. Polym. Ed. 2009, 20, 1729–1746. [Google Scholar] [CrossRef] [PubMed]
- Insomphun, C.; Chuah, J.A.; Kobayashi, S.; Fujiki, T.; Numata, K. Influence of hydroxyl groups on the cell viability of polyhydroxyalkanoate (PHA) scaffolds for tissue engineering. ACS Biomater. Sci. Eng. 2017, 3, 3064–3075. [Google Scholar] [CrossRef] [PubMed]
- Surmenev, R.A.; Chernozem, R.V.; Syromotina, D.S.; Oehr, C.; Baumbach, T.; Krause, B.; Boyandin, A.N.; Dvoinina, L.M.; Volova, T.G.; Surmeneva, M.A. Low-temperature argon and ammonia plasma treatment of poly-3-hydroxybutyrate films: Surface topography and chemistry changes affect fibroblast cells in vitro. Eur. Polym. J. 2019, 112, 137–145. [Google Scholar] [CrossRef]
- Misra, S.K.; Ansari, T.; Mohn, D.; Valappil, S.P.; Brunner, T.J.; Stark, W.J.; Roy, I.; Knowles, J.C.; Sibbons, P.D.; Jones, E.V.; et al. Effect of nanoparticulate bioactive glass particles on bioactivity and cytocompatibility of poly(3-hydroxybutyrate) composites. J. R. Soc. Interface 2010, 7, 453–465. [Google Scholar] [CrossRef]
- De Paula, A.C.; Zonari, A.A.; Martins, T.M.; Novikoff, S.; da Silva, A.R.; Correlo, V.M.; Reis, R.L.; Gomes, D.A.; Goes, A.M. Human serum is a suitable supplement for the osteogenic differentiation of human adipose-derived stem cells seeded on poly-3-hydroxibutyrate-co-3-hydroxyvalerate scaffolds. Tissue Eng. Part A 2013, 19, 277–289. [Google Scholar] [CrossRef]
- Akaraonye, E.; Filip, J.; Safarikova, M.; Salih, V.; Keshavarz, T.; Knowles, J.C.; Roy, I. Composite scaffolds for cartilage tissue engineering based on natural polymers of bacterial origin, thermoplastic poly (3-hydroxybutyrate) and micro-fibrillated bacterial cellulose. Polym. Int. 2016, 65, 780–791. [Google Scholar] [CrossRef]
- Getachew, A.; Berhanu, A.; Birhane, A. Production of sterilized medium chain length polyhydroxyalkanoates (Smcl-PHA) as a biofilm to tissue engineering application. J. Tissue Sci. Eng. 2016, 7, 2. [Google Scholar] [CrossRef]
- Krishnan, S.; Chinnadurai, G.S.; Ravishankar, K.; Raghavachari, D.; Perumal, P. Statistical augmentation of polyhydroxybutyrate production by Isoptericola variabilis: Characterization, moulding, in vitro cytocompatibility and biodegradability evaluation. Int. J. Biol. Macromol. 2021, 166, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Criscenti, G.; Vasilevich, A.; Longoni, A.; De Maria, C.; van Blitterswijk, C.A.; Truckenmuller, R.; Vozzi, G.; De Boer, J.; Moroni, L. 3D screening device for the evaluation of cell response to different electrospun microtopographies. Acta Biomater. 2017, 55, 310–322. [Google Scholar] [CrossRef]
- Zhao, Y.; Zou, B.; Shi, Z.; Wu, Q.; Chen, G.Q. The effect of 3-hydroxybutyrate on the in vitro differentiation of murine osteoblast MC3T3-E1 and in vivo bone formation in ovariectomized rats. Biomaterials 2007, 28, 3063–3073. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, X.L.; Yang, S.C.; Lin, X.; He, Y.; Yan, C.; Wu, L.; Chen, G.Q.; Wang, Z.Y.; Wu, Q. MicroRNAs in the regulation of interfacial behaviors of MSCs cultured on microgrooved surface pattern. Biomaterials 2011, 32, 9207–9217. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Z.H.; Shen, C.Y.; You, M.L.; Xiao, J.F.; Chen, G.Q. Differentiation of human bone marrow mesenchymal stem cells grown in terpolyesters of 3-hydroxyalkanoates scaffolds into nerve cells. Biomaterials 2010, 31, 1691–1698. [Google Scholar] [CrossRef] [PubMed]
- Lizarraga-Valderrama, L.R.; Nigmatullin, R.; Taylor, C.; Haycock, J.W.; Claeyssens, F.; Knowles, J.C.; Roy, I. Nerve tissue engineering using blends of poly(3-hydroxyalkanoates) for peripheral nerve regeneration. Eng. Life Sci. 2015, 15, 612–621. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, Q.; Li, S.; Lu, X.; Zhao, Y.; Guan, J.S.; Chen, J.C.; Wu, Q.; Chen, G.Q. 3-Hydroxybutyrate methyl ester as a potential drug against Alzheimer’s disease via mitochondria protection mechanism. Biomaterials 2013, 34, 7552–7562. [Google Scholar] [CrossRef]
- Basnett, P.; Luckasiewicz, B.; Marcello, E.; Gura, H.K.; Knowles, J.C.; Roy, I. Production of a novel medium chain length poly(3-hydroxyalkanoate) using unprocessed biodiesel waste and its evaluation as a tissue engineering scaffold. Microb. Biotechnol. 2017, 10, 1384–1399. [Google Scholar] [CrossRef]
- Cerrone, F.; Pozner, T.; Siddiqui, A.; Ceppi, P.; Winner, B.; Rajendiran, M.; Babu, R.; Ibrahim, H.S.; Rodriguez, B.J.; Winkler, J.; et al. Polyhydroxyphenylvalerate/polycaprolactone nanofibers improve the lifespan and mechanoresponse of human IPSC-derived cortical neuronal cells. Mater. Sci. Eng. C 2020, 111, 110832. [Google Scholar] [CrossRef]
- Pereira, J.R.; Araújo, D.; Marques, A.C.; Neves, L.A.; Grandfils, C.; Sevrin, C.; Alves, V.D.; Fortunato, E.; Reis, M.A.M.; Freitas, F. Demonstration of the adhesive properties of the medium-chainlength polyhydroxyalkanoate produced by Pseudomonas chlororaphis subsp. aurantiaca from glycerol. Int. J. Biol. Macromol. 2019, 122, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Sanhueza, C.; Diaz-Rodriguez, P.; Villegas, P.; González, Á.; Seeger, M.; Suárez-González, J.; Concheiro, A.; Alvarez-Lorenzo, C.; Acevedo, F. Influence of the carbon source on the properties of poly-(3)-hydroxybutyrate produced by Paraburkholderia xenovorans LB400 and its electrospun fibers. Int. J. Biol. Macromol. 2020, 152, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Abazari, M.F.; Karizi, S.Z.; Samadian, H.; Nasiri, N.; Askari, H.; Asghari, M.; Frootan, F.; Bakhtiari, H.; Mahboudi, H.; Mansouri, V. Poly(glycerol sebacate) and polyhydroxybutyrate electrospun nanocomposite facilitates osteogenic differentiation of mesenchymal stem cells. J. Drug Deliv. Sci. Technol. 2021, 66, 102796. [Google Scholar] [CrossRef]
- Piszko, P.; Wlodarczyk, M.; Zielinska, S.; Gazinska, M.; Plocinski, P.; Rudnicka, K.; Szwed, A.; Krupa, A.; Grzymajlo, M.; Sobczak-Kupiec, A.; et al. PGS/HAp microporous composite scaffold obtained in the TIPS-TCL-SL Method: An innovation for bone tissue engineering. Int. J. Mol. Sci. 2021, 22, 8587. [Google Scholar] [CrossRef]
- Trakunjae, C.; Sudesh, K.; Neoh, S.Z.; Boondaeng, A.; Apiwatanapiwat, W.; Jancahi, P.; Vaithanomsat, P. Biosynthesis of P(3HB-co-3HHx) copolymers by a newly engineered strain of Cupriavidus necator PHB−4/pBBR_CnPro-phaCRp for skin tissue engineering application. Polymer 2022, 14, 4074. [Google Scholar] [CrossRef] [PubMed]
- Tallawi, M.; Rosellini, E.; Barbani, N.; Cascone, M.G.; Rai, R.; Saint-Pierre, G.; Boccaccini, A.R. Strategies for the chemical and biological functionalization iof scaffolds for cardiac tissue engineering: A review. J. R. Soc. Interface 2015, 12, 20150254. [Google Scholar] [CrossRef]
- Niemczyk-Soczynska, B.; Gradys, A.; Sajkiewicz, P. Hydrophilic surface functionalization of electrospun nanofibrous scaffolds in tissue engineering. Polymers 2020, 12, 2636. [Google Scholar] [CrossRef]
- Guo, B.; Lei, B.; Li, P.; Ma, P.X. Functionalized scaffolds to enhance tissue regeneration. Regen. Biomater. 2015, 2, 47–57. [Google Scholar] [CrossRef]
- Wei, G.B.; Jin, Q.M.; Giannobile, W.V.; Ma, P.X. The enhancement of osteogenesis by nano-fibrous scaffolds incorporating rhBMP-7 nanospheres. Biomaterials 2007, 28, 2087–2096. [Google Scholar] [CrossRef]
- Mohiti-Asli, M.; Pourdeyhimi, B.; Loboa, E.G. Novel, silver-ion-releasing nanofibrous scaffolds exhibit excellent antibacterial efficacy without the use of silver nanoparticles. Acta Biomater. 2014, 10, 2096–2104. [Google Scholar] [CrossRef]
- Municoy, S.; Álvarez Echazú, M.I.; Antezana, P.E.; Galdopórpora, J.M.; Olivetti, C.; Mebert, A.M.; Foglia, M.L.; Tuttolomondo, M.V.; Alvarez, G.S.; Hardy, J.G.; et al. Stimuli-sesponsive materials for tissue engineering and drug delivery. Int. J. Mol. Sci. 2020, 21, 4724. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, A.H.; Jaffe, M.; Arinzeh, T.L. Piezoelectric materials for tissue regeneration: A review. Acta Biomater. 2015, 24, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.; Sencadas, V.; Correia, D.M.; Lanceros-Méndez, S. Piezoelectric polymers as biomaterials for tissue engineering applications. Colloids Surfaces B Biointerfaces 2015, 136, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Lei, T.; Qin, Y.; He, J.H.; Yang, R. Design and application of piezoelectric biomaterials. J. Phys. D Appl. Phys. 2019, 52, 194002–194012. [Google Scholar] [CrossRef]
- Kapat, K.; Shubhra, Q.T.H.; Zhou, M.; Leeuwenburgh, S. Piezoelectric nano-biomaterials for biomedicine and tissue regeneration. Adv. Funct. Mater. 2020, 30, 1909045. [Google Scholar] [CrossRef]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes-different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef]
- Chi, J.; Wang, M.; Chen, J.; Hu, L.; Chen, Z.; Backman, L.J.; Zhang, W. Topographic orientation of scaffolds for tissue regeneration: Recent advances in biomaterial design and applications. Biomimetics 2022, 7, 131. [Google Scholar] [CrossRef]
- Tang, Y.; Tian, J.; Li, L.; Huang, L.; Shen, Q.; Guo, S.; Jiang, Y. Biomimetic biphasic electrospun scaffold for anterior cruciate ligament tissue engineering. Tissue Eng. Regen. Med. 2021, 18, 819–830. [Google Scholar] [CrossRef]
- Tonti, O.R.; Larson, H.; Lipp, S.N.; Luetkemeyer, C.M.; Makam, M.; Vargas, D.; Wilcox, S.M.; Calve, S. Tissue-specific parameters for the design of ECM-mimetic biomaterials. Acta Biomater. 2021, 132, 83–102. [Google Scholar] [CrossRef]
- Xiong, Y.C.; Yao, Y.C.; Zhan, X.Y.; Chen, G.Q. Application of polyhydroxyalkanoates nanoparticles as intracellular sustained drug-release vectors. J. Biomater. Sci. 2010, 21, 127–140. [Google Scholar] [CrossRef]
- Nigmatullin, R.; Thomas, P.; Lukasiewicz, B.; Puthussery, H.; Roy, I. Polyhydroxyalkanoates, a family of natural polymers, and their applications in drug delivery. J. Chem. Technol. Biotechnol. 2015, 90, 1209–1221. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, L.; Dong, Y.; Zhang, X.; Lin, J.; Chen, Z. Folate-mediated poly (3-hydroxybutyrate-co-3-hydroxyoctanoate) nanoparticles for targeting drug delivery. Eur. J. Pharm. Biopharm. 2010, 76, 10–16. [Google Scholar] [CrossRef]
- Pramual, S.; Assavanig, A.; Bergkvist, M.; Batt, C.A.; Sunintaboon, P.; Lirdprapamongkol, K.; Svasti, J.; Niamsiri, N. Development and characterization of bio-derived polyhydroxyalkanoate nanoparticles as a delivery system for hydrophobic photodynamic therapy agents. J. Mater. Sci. Mater. Med. 2016, 27, 40. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Q.; Wu, Q. Microbial production and applications of chiral hydroxyalkanoates. Appl. Microbiol. Biotechnol. 2005, 67, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Q.; Wu, Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 2005, 26, 6565–6578. [Google Scholar] [CrossRef] [PubMed]
- Philip, S.; Keshavarz, T.; Roy, I. Polyhydroxyalkanoates: Biodegradable polymers with a range of applications. J. Chem. Technol. Biotechnol. 2007, 82, 233–247. [Google Scholar] [CrossRef]
- Turesin, F.; Gursel, I.; Hasirci, V. Biodegradable polyhydroxyalkanoate implants for osteomyelitis therapy: In vitro antibiotic release. J. Biomater. Sci. Polym. Ed. 2001, 12, 195–207. [Google Scholar] [CrossRef]
- Mokhtarzadeh, A. Recent advances on biocompatible and biodegradable nanoparticles as gene carriers. Expert Opin. Biol. Ther. 2016, 16, 771–785. [Google Scholar] [CrossRef]
- Ihssen, J.; Magnani, D.; Thony-Meyer, L.; Ren, Q. Use of extracellular medium chain length polyhydroxyalkanoate depolymerase for targeted binding of proteins to artificial poly[(3-hydroxyoctanoate)-co-(3-hydroxyhexanoate)] granules. Biomacromolecules 2009, 10, 1854–1864. [Google Scholar] [CrossRef]
- Lee, S.J.; Park, J.P.; Park, T.J.; Lee, S.Y.; Lee, S.; Park, J.K. Selective immobilization of fusion proteins on poly(hydroxyalkanoate) microbeads. Anal. Chem. 2005, 77, 5755–5759. [Google Scholar] [CrossRef]
- Backstrom, B.T.; Brockelbank, J.A.; Rehm, B.H. Recombinant Escherichia coli produces tailor-made biopolyester granules for applications in fluorescence activated cell sorting: Functional display of the mouse interleukin-2 and myelin oligodendrocyte glycoprotein. BMC Biotechnol. 2007, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Jahns, A.C.; Haverkamp, R.G.; Rehm, B.H. Multifunctional inorganic-binding beads self-assembled inside engineered bacteria. Bioconjug. Chem. 2008, 19, 2072–2080. [Google Scholar] [CrossRef] [PubMed]
- Parlane, N.A.; Wedlock, D.N.; Buddle, B.M.; Rehm, B.H. Bacterial polyester inclusions engineered to display vaccine candidate antigens for use as a novel class of safe and efficient vaccine delivery agents. App. Environ. Microbiol. 2009, 75, 7739–7744. [Google Scholar] [CrossRef] [PubMed]
- Parlane, N.A.; Grage, K.; Lee, J.W.; Buddle, B.M.; Denis, M.; Rehm, B.H. Production of a particulate hepatitis C vaccine candidate by an engineered Lactococcus lactis strain. Appl. Environ. Microbiol. 2011, 77, 8516–8522. [Google Scholar] [CrossRef]
- Parlane, N.A.; Gupta, S.K.; Rubio-Reyes, P.; Chen, S.; Gonzalez-Miro, M.; Wedlock, D.N.; Rehm, B.H. Self-assembled protein coated polyhydroxyalkanoate beads: Properties and biomedical applications. ACS Biomater. Sci. Eng. 2017, 3, 3043–3057. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yu, H.; Xia, Y.; Kang, Z.; Qi, Q. Complete PHB mobilization in Escherichia coli enhances the stress tolerance: A potential biotechnological application. Microb. Cell Fact. 2009, 8, 1–9. [Google Scholar] [CrossRef]
- Geng, Y.; Wang, S.; Qi, Q. Expression of active recombinant human tissue-type plasminogen activator by using in vivo polyhydroxybutyrate granule display. App Environ. Microbiol. 2010, 76, 7226–7230. [Google Scholar] [CrossRef]
- Li, J.; Shang, G.; You, M.; Peng, S.; Wang, Z.; Wu, H.; Chen, G.Q. Endotoxin removing method based on lipopolysaccharide binding protein and polyhydroxyalkanoate binding protein PhaP. Biomacromolecules 2011, 12, 602–608. [Google Scholar] [CrossRef]
- Chen, S.; Parlane, N.A.; Lee, J.; Wedlock, D.N.; Buddle, B.M.; Rehm, B.H. New skin test for detection of bovine tuberculosis on the basis of antigen-displaying polyester inclusions produced by recombinant Escherichia coli. Appl. Environ. Microbiol. 2014, 80, 2526–2535. [Google Scholar] [CrossRef]
- Hay, I.D.; Du, J.; Reyes, P.R.; Rehm, B.H. In vivo polyester immobilized sortase for tagless protein purification. Microb. Cell Fact. 2015, 14, 190. [Google Scholar] [CrossRef]
- Martınez-Donato, G.; Piniella, B.; Aguilar, D.; Olivera, S.; Perez, A.; Castanedo, Y.; Alvarez-Lajonchere, L.; Duenas-Carrera, S.; Lee, J.W.; Burr, N.; et al. Protective T cell and antibody immune responses against Hepatitis C virus achieved using a biopolyester-bead-based vaccine delivery system. Clin. Vaccine Immunol. 2016, 23, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Panith, N.; Assavanig, A.; Lertsiri, S.; Bergkvist, M.; Surarit, R.; Niamsiri, N. Development of tunable biodegradable polyhydroxyalkanoates microspheres for controlled delivery of tetracycline for treating periodontal disease. J. Appl. Polym. Sci. 2016, 133, 44128–44141. [Google Scholar] [CrossRef]
- Murueva, A.V.; Shishatskaya, E.I.; Kuzmina, A.M.; Volova, T.G.; Sinskey, A.J. Microparticles prepared from biodegradable polyhydroxyalkanoates as matrix for encapsulation of cytostatic drug. J. Mater. Sci. Mater. Med. 2013, 24, 1905–1915. [Google Scholar] [CrossRef] [PubMed]
- Evangeline, S.; Sridharan, T.B. Biosynthesis and statistical optimization of polyhydroxyalkanoate (PHA) produced by Bacillus cereus VIT-SSR1 and fabrication of biopolymer films for sustained drug release. Int. J. Biol. Macromol. 2019, 135, 945–958. [Google Scholar] [CrossRef] [PubMed]
- Perveen, K.; Masood, F.; Hameed, A. Preparation, characterization and evaluation of antibacterial properties of epirubicin loaded PHB and PHBV nanoparticles. Int. J. Biol. Macromol. 2020, 144, 259–266. [Google Scholar] [CrossRef]
- Kehail, A.A.; Brigham, C.J. Anti-biofilm activity of solvent-cast and electrospun polyhydroxyalkanoate membranes treated with lysozyme. J. Polym. Environ. 2018, 26, 66–72. [Google Scholar] [CrossRef]
- O’Connor, S.; Szwej, E.; Nikodinovic-Runic, J.; O’Connor, A.; Byrne, A.T.; Devocelle, M.; O’Donovan, N.; Gallagher, W.M.; Babu, R.; Kenny, S.T.; et al. The anti-cancer activity of a cationic anti-microbial peptide derived from monomers of polyhydroxyalkanoate. Biomaterials 2013, 34, 2710–2718. [Google Scholar] [CrossRef]
- Masood, F.; Chen, P.; Yasin, T.; Fatima, N.; Hasan, F.; Hameed, A. Encapsulation of ellipticine in poly-(3-hydroxybutyrate-co-3-hydroxyvalerate) based nanoparticles and its in vitro application. Mater. Sci. Eng. 2013, 33, 1054–1060. [Google Scholar] [CrossRef]
- Shah, M.; Ullah, N.; Choi, M.H.; Yoon, S.C. Nanoscale poly (4-hydroxybutyrate)-mPEG carriers for anticancer drugs delivery. J. Nanosci. Nanotechnol. 2014, 14, 8416–8421. [Google Scholar] [CrossRef]
- Mascolo, D.D.; Basnett, P.; Palange, A.L.; Francardi, M.; Roy, I.; Decuzzi, P. Tuning core hydrophobicity of spherical polymeric nanoconstructs for docetaxel delivery. Polym. Int. 2016, 65, 741–746. [Google Scholar] [CrossRef]
- Shishatskaya, E.I.; Nikolaeva, E.D.; Vinogradova, O.N.; Volova, T.G. Experimental wound dressings of degradable PHA for skin defect repair. J. Mater. Sci. Mater. Med. 2016, 27, 165. [Google Scholar] [CrossRef] [PubMed]
- Sangsanoh, P.; Israsena, N.; Suwantong, O.; Supaphol, P. Effect of the surface topography and chemistry of poly(3-hydroxybutyrate) substrates on cellular behavior of the murine neuroblastoma Neuro2a cell line. Polym. Bull. 2017, 10, 4101–4118. [Google Scholar] [CrossRef]
- Williams, S.F.; Rizk, S.; Martin, D.P. Poly-4-hydroxybutyrate (P4HB): A new generation of resorbable medical devices for tissue repair and regeneration. Biomed. Technol. 2013, 58, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Romano, C.L.; Scarponi, S.; Gallazzi, E.; Romano, D.; Drago, L. Antibacterial coating of implants in orthopaedics and trauma: A classification proposal in an evolving panorama. J. Orthop. Surg. Res. 2015, 10, 157. [Google Scholar] [CrossRef]
- Chernozem, R.V.; Pariy, I.O.; Pryadko, A.; Bonartsev, A.P.; Voinova, V.V.; Zhuikov, V.A.; Makhina, T.K.; Bonartseva, G.A.; Shaitan, K.V.; Shvartsman, V.V.; et al. A comprehensive study of the structure and piezoelectric response of biodegradable polyhydroxybutyrate-based films for tissue engineering applications. Polym. J. 2022, 54, 1225–1236. [Google Scholar] [CrossRef]
- Dhania, S.; Bernela, M.; Rani, R.; Parsad, M.; Grewal, S.; Kumari, S.; Thakur, R. Scaffolds the backbone of tissue engineering: Advancements in use of polyhydroxyalkanoates (PHA). Int. Biol. Macromol. 2022, 208, 243–259. [Google Scholar] [CrossRef]
- Basnett, P.; Ching, K.Y.; Stolz, M.; Knowles, J.C.; Boccaccini, A.R.; Smith, C.; Locke, I.C.; Keshavarz, T.K.; Roy, I. Novel poly(3-hydroxyoctanoate)/poly(3-hydroxybutyrate) blends for medical applications. React. Funct. Polym. 2013, 73, 1340–1348. [Google Scholar] [CrossRef]
- Hazer, D.B.; Hazer, B.; Kaymaz, F. Synthesis of microbial elastomers based on soybean oily acids. Biocompatibility studies. Biomed. Mater. 2009, 4, 035011. [Google Scholar] [CrossRef]
- Hazer, D.B.; Hazer, B. The effect of gold clusters on the autoxidation of poly(3-hydroxy 10-undecenoate-co-3-hydroxyoctanoate) and tissue response evaluation. J. Polym. Res. 2011, 18, 251–262. [Google Scholar] [CrossRef]
- Hema, R.; Ng, P.N.; Amirul, A.A. Green nanobiocomposite: Reinforcement effect of montmorillonite clays on physical and biological advancement of various polyhydroxyalkanoates. Polym. Bull. 2013, 70, 755–771. [Google Scholar] [CrossRef]
- Pontailler, M.; Illangakoon, E.; Williams, G.R.; Marijon, C.; Bellamy, V.; Balvay, D.; Autret, G.; Vanneaux, V.; Larghero, J.; Planat-Benard, V.; et al. Polymer-based reconstruction of the inferior vena cava in rat: Stem cells or RGD peptide? Tissue Eng. Part A 2015, 21, 1552–1564. [Google Scholar] [CrossRef] [PubMed]
- Gallo, J.; Holinka, M.; Moucha, C.S. Antibacterial surface treatment for orthopaedic implants. Int. J. Mol. Sci. 2014, 15, 13849–13880. [Google Scholar] [CrossRef] [PubMed]
- Raoga, O.; Sima, L.; Chirioiu, M.; Popescu-Pelin, G.; Fufa, O.; Grumezescu, O.; Socol, M.; Stanculescu, A.; Zgura, I.; Socol, G. Biocomposite coatings based on poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/calcium phosphates obtained by MAPLE for bone tissue engineering. Appl. Surf. Sci. 2017, 417, 204–212. [Google Scholar] [CrossRef]
- Rodrıguez-Contreras, A.; Garcıa, Y.; Manero, J.M.; Ruperez, E. Antibacterial PHAs coating for titanium implants. Eur. Polym. J. 2017, 90, 66–78. [Google Scholar] [CrossRef]
- Kalia, V.C.; Shim, W.Y.; Patel, S.K.S.; Gong, C.; Lee, J.K. Recent developments in antimicrobial growth promoters in chicken health: Opportunities and challenges. Sci. Total Environ. 2022, 834, 55300. [Google Scholar] [CrossRef]
- Bangera, R.; Correa, K.; Lhorente, J.P.; Figueroa, R.; Yanez, J.M. Genomic predictions can accelerate selection for resistance against Piscirickettsia salmonis in Atlantic salmon (Salmo salar). BMC Genom. 2017, 18, 121. [Google Scholar] [CrossRef]
- Martinez, J.L. Effect of antibiotics on bacterial populations: A multi-hierachical selection process. F1000 Res. 2017, 6, 51. [Google Scholar] [CrossRef]
- Cai, L.; Yuan, M.Q.; Liu, F.; Jian, J.; Chen, G.Q. Enhanced production of medium-chain-length polyhydroxyalkanoates (PHA) by PHA depolymerase knockout mutant of Pseudomonas putida KT2442. Bioresour. Technol. 2009, 100, 2265–2270. [Google Scholar] [CrossRef]
- De Eugenio, L.I.; Escapa, I.F.; Morales, V.; Dinjaski, N.; Galan, B.; Garcia, J.L.; Prieto, M.A. The turnover of medium-chain length polyhydroxyalkanoates in KT2442 and the fundamental role of PhaZ depolymerase for the metabolic balance. Environ. Microbiol. 2010, 12, 207–221. [Google Scholar] [CrossRef]
- Martinez, V.; Dinjaski, N.; De Eugenio, L.I.; De la Pena, F.; Prieto, M.A. Cell system engineering to produce extracellular polyhydroxyalkanoate depolymerase with targeted applications. Int. J. Biol. Macromol. 2014, 71, 28–33. [Google Scholar] [CrossRef]
- Dinjaski, N.; Fernandez-Gutierrez, M.; Selvam, S.; Parra-Ruiz, F.J.; Lehman, S.M.; San Roman, J.; Garcia, E.; Garcia, J.L.; Garcia, A.J.; Prieto, M.A. PHACOS, a functionalized bacterial polyester with bactericidal activity against methicillin-resistant Staphylococcus aureus. Biomaterials 2014, 35, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Shivakumar, S.; Jagadish, S.J.; Zatakia, H.; Dutta, J. Purification, characterization and kinetic studies of a novel poly(b)hydroxybutyrate (PHB) depolymerase PhaZ from Penicillium citrinum S2. Appl. Biochem. Biotechnol. 2011, 164, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Defoirdt, T.; Boon, N.; Sorgeloos, P.; Verstraete, W.; Bossier, P. Short-chain fatty acids and poly-b-hydroxyalkanoates: (new) biocontrol agents for a sustainable animal production. Biotechnol. Adv. 2009, 27, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; De Schryver, P.; Van Delsen, B.; Maignien, L.; Boon, N.; Sorgeloos, P.; Verstraete, W.; Bossier, P.; Defoirdt, T. PHB-degrading bacteria isolated from the gastrointestinal tract of aquatic animals as protective actors against luminescent vibriosis. FEMS Microbiol. Ecol. 2010, 74, 196–204. [Google Scholar] [CrossRef]
- Martınez, V.; de la Pena, F.; Garcıa-Hidalgo, J.; de la Mata, I.; Garcıa, J.L.; Prieto, M.A. Identification and biochemical evidence of a medium-chain-length polyhydroxyalkanoate depolymerase in the Bdellovibrio bacteriovorus predatory hydrolytic arsenal. Appl. Environ. Microbiol. 2012, 78, 6017–6026. [Google Scholar] [CrossRef]
- Ludevese-Pascual, G.; Laranja, J.L.Q.; Amar, E.C.; Sorgeloos, P.; Bossier, P.; De Schryver, P. Poly-beta-hydroxybutyrate enriched Artemia sp. for giant tiger prawn Penaeus monodon larviculture. Aquaculture 2016, 23, 422–429. [Google Scholar]
- Radivojevic, J.; Skaro, S.; Senerovic, L.; Vasiljevic, B.; Guzik, M.; Kenny, S.T.; Maslak, V.; Nikodinovic-Runic, J.; O’Connor, K.E. Polyhydroxyalkanoate-based 3-hydroxyoctanoic acid and its derivatives as a platform of bioactive compounds. Appl. Microbiol. Biotechnol. 2016, 100, 161–172. [Google Scholar] [CrossRef]
- Kiran, G.S.; Jackson, S.A.; Priyadharsini, S.; Dobson, A.D.W.; Selvin, J. Synthesis of Nm-PHB (nanomelanin-polyhydroxy butyrate) nanocomposite film and its protective effect against biofilmforming multi drug resistant Staphylococcus aureus. Sci. Rep. 2017, 7, 9167. [Google Scholar] [CrossRef]
- Allen, A.D.; Daley, P.; Ayorinde, F.O.; Gugssa, A.; Anderson, W.A.; Eribo, B.E. Characterization of medium chain length (R)-3-hydroxycarboxylic acids produced by Streptomyces sp. JM3 and the evaluation of their antimicrobial properties. World. J. Microbiol. Biotechnol. 2012, 28, 2791–2800. [Google Scholar] [CrossRef]
- Camberos-Luna, L.; Gerónimo-Olvera, C.; Montiel, T.; Rincon-Heredia, R.; Massieu, L. The ketone body, β-Hydroxybutyrate stimulates the autophagic flux and prevents neuronal death induced by glucose deprivation in cortical cultured neurons. Neurochem. Res. 2016, 41, 600–609. [Google Scholar] [CrossRef]
- Basnett, P.; Marcello, E.; Lukasiewicz, B.; Nigmatullin, R.; Paxinou, A.; Ahmad, M.H.; Gurumayum, B.; Roy, I. Antimicrobial materials with lime oil and a poly(3-hydroxyalkanoate) produced via valorisation of sugar cane molasses. J. Funct. Mater. 2020, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cicek, N.; Levin, D.B.; Logsetty, S.; Liu, S. Bacteria-triggered release of a potent biocide from core shell polyhydroxyalkanoate (PHA)-based nanofibers for wound dressing applications. J. Biomater. Sci. Polym. Ed. 2020, 31, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Xavier, J.R.; Babusha, S.T.; George, J.; Ramana, K.V. Material properties and antimicrobial activity of polyhydroxybutyrate (PHB) films incorporated with vanillin. Appl. Biochem. Biotechnol. 2015, 176, 1498–1510. [Google Scholar] [CrossRef]
- Ikada, Y.; Tsuji, H. Biodegradable polyesters for medical and ecological applications. Macromol. Rapid Commun. 2002, 21, 117–132. [Google Scholar] [CrossRef]
- Sugiyama, A.; Kobayashi, T.; Shiraki, M.; Saito, T. Roles of poly (3-hydroxybutyrate) depolymerase and 3HB-oligomer hydrolase in bacterial PHB metabolism. Curr. Microbiol. 2004, 48, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Uchino, K.; Saito, T.; Gebauer, B.; Jendrossek, D. Isolated poly (3-hydroxybutyrate)(PHB) granules are complex bacterial organelles catalyzing formation of PHB from acetyl coenzyme A (CoA) and degradation of PHB to acetyl-CoA. J. Bacteriol. 2007, 189, 8250–8256. [Google Scholar] [CrossRef]
- Yean, O.S.; Yee, C.J.; Kumar, S. Degradation of polyhydroxyalkanaote (PHA): A review. J. Sib. Fed. Univ. Biol. 2017, 10, 211–225. [Google Scholar]
- Numata, K.; Abe, H.; Iwata, T. Biodegradability of poly (hydroxyalkanoate) materials. Materials 2009, 2, 1104–1126. [Google Scholar] [CrossRef]
- Jendrossek, D.; Handrick, R. Microbial degradation of polyhydroxyalkanoates. Annu. Rev. Microbiol. 2002, 56, 403–432. [Google Scholar] [CrossRef]
- Li, Z.; Lin, H.; Ishii, N.; Chen, G.Q.; Inoue, Y. Study of enzymatic degradation of microbial copolyesters consisting of 3 -hydroxybutyrate and medium-chain-length 3 -hydroxyalkanoates. Polym. Degrad. Stab. 2007, 92, 1708–1714. [Google Scholar] [CrossRef]
- Ray, S.; Kalia, V.C. Polyhydroxyalkanoate production and degradation patterns in Bacillus species. Indian J. Microbiol. 2017, 57, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kumar, P.; Ray, S.; Kalia, V.C. Challenges and opportunities for the customizing polyhydroxyalkanoates. Indian J. Microbiol. 2015, 55, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kumar, P.; Patel, S.K.S.; Kalia, V.C. Production of polyhydroxyalkanoate co-polymer by Bacillus thuringiensis. Indian J. Microbiol. 2013, 53, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Singh, M.; Mehariya, S.; Patel, S.K.S.; Lee, J.K.; Kalia, V.C. Ecobiotechnological approach for exploiting the abilities of Bacillus to produce co-polymer of polyhydroxyalkanoate. Indian J. Microbiol. 2014, 54, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Patel, S.K.S.; Kalia, V.C. Bacillus subtilis as potential producer for polyhydroxyalkanoates. Microb. Cell Fact. 2009, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Mehariya, S.; Ray, S.; Mishra, A.; Kalia, V.C. Biodiesel industry waste: A potential source of bioenergy and biopolymers. Indian J. Microbiol. 2015, 55, 1–7. [Google Scholar] [CrossRef]
- Kumar, P.; Mehariya, S.; Ray, S.; Mishra, A.; Kalia, V.C. Biotechnology in aid of biodiesel industry effluent (glycerol): Biofuels and bioplastics. In Microbial Factories; Kalia, V.C., Ed.; Springer: New Delhi, India, 2015; pp. 105–119. [Google Scholar]
- Kumar, P.; Ray, S.; Patel, S.K.S.; Lee, J.K.; Kalia, V.C. Bioconversion of crude glycerol to polyhydroxyalkanoate by Bacillus thuringiensis under non-limiting nitrogen conditions. Int. J. Biol. Macromol. 2015, 78, 9–16. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).