Depolymerization of Pine Wood Organosolv Lignin in Ethanol Medium over NiCu/SiO2 and NiCuMo/SiO2 Catalysts: Impact of Temperature and Catalyst Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction of Ethanol Lignin from Pine Wood
2.2. Catalyst Preparation
2.3. Thermal and Thermocatalytic Studies in Supercritical Ethanol
2.4. Composition and Structure of Ethanol Lignin and of Liquid Products of Its Transformation
3. Results
3.1. Catalyst Characterization
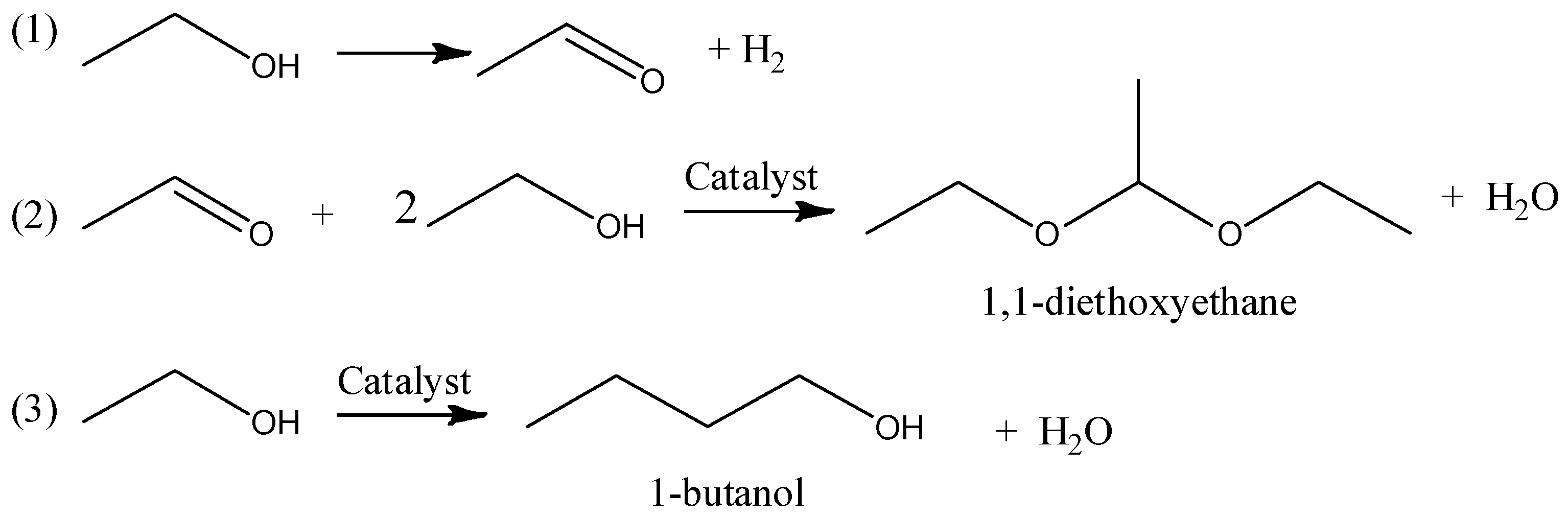
3.2. Thermal and Thermocatalytic Transformations of Ethanol
3.3. Thermocatalytic Conversion of Ethanol Lignin in Supercritical Ethanol
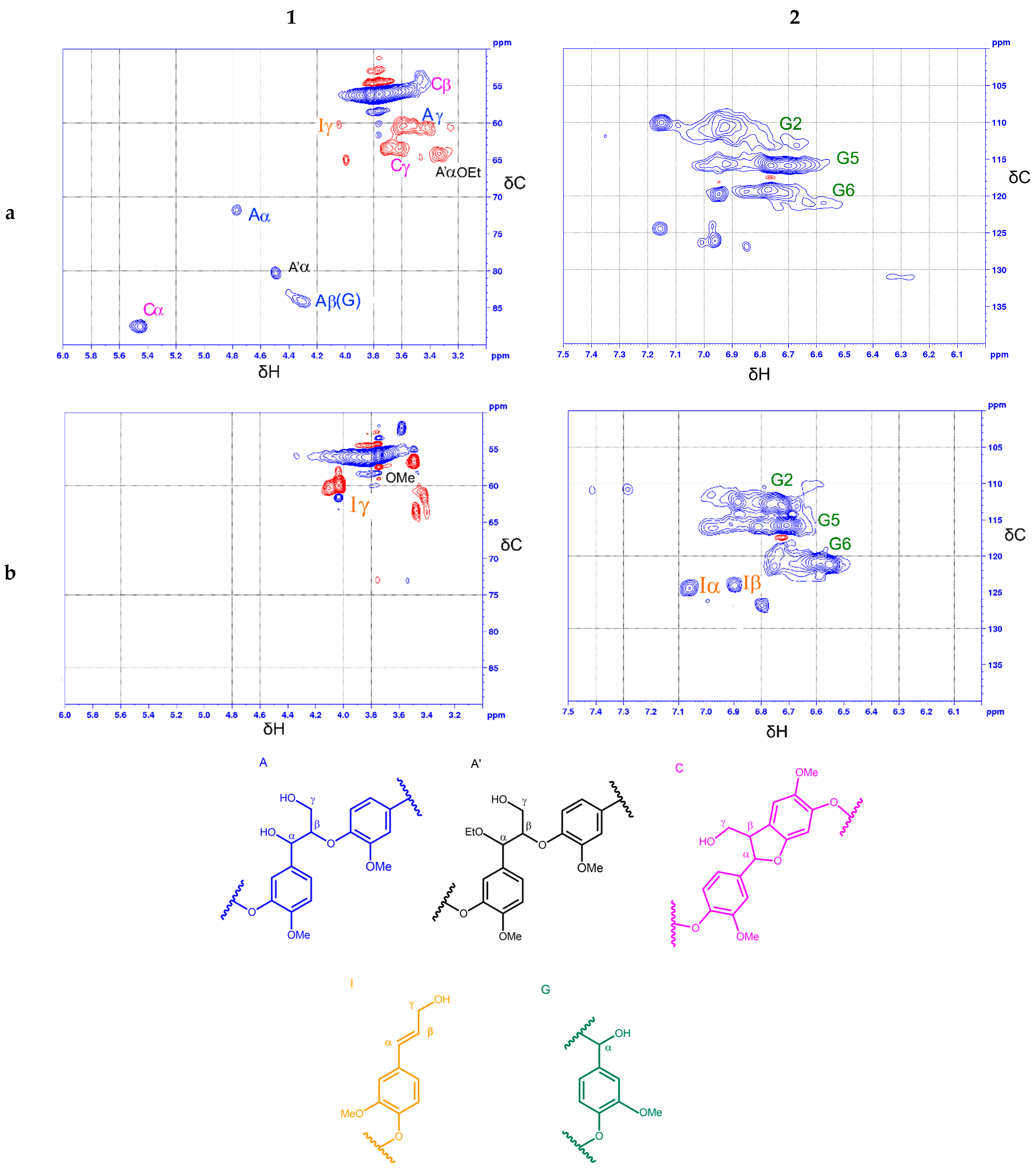
3.4. Structural Characterization of Ethanol Lignin and Liquid Products of Lignin Depolimerization via 2D HSQC NMR
3.5. Influence of Temperature and the Catalyst Composition on the Composition of Monomer Products of Ethanol Lignin Conversion
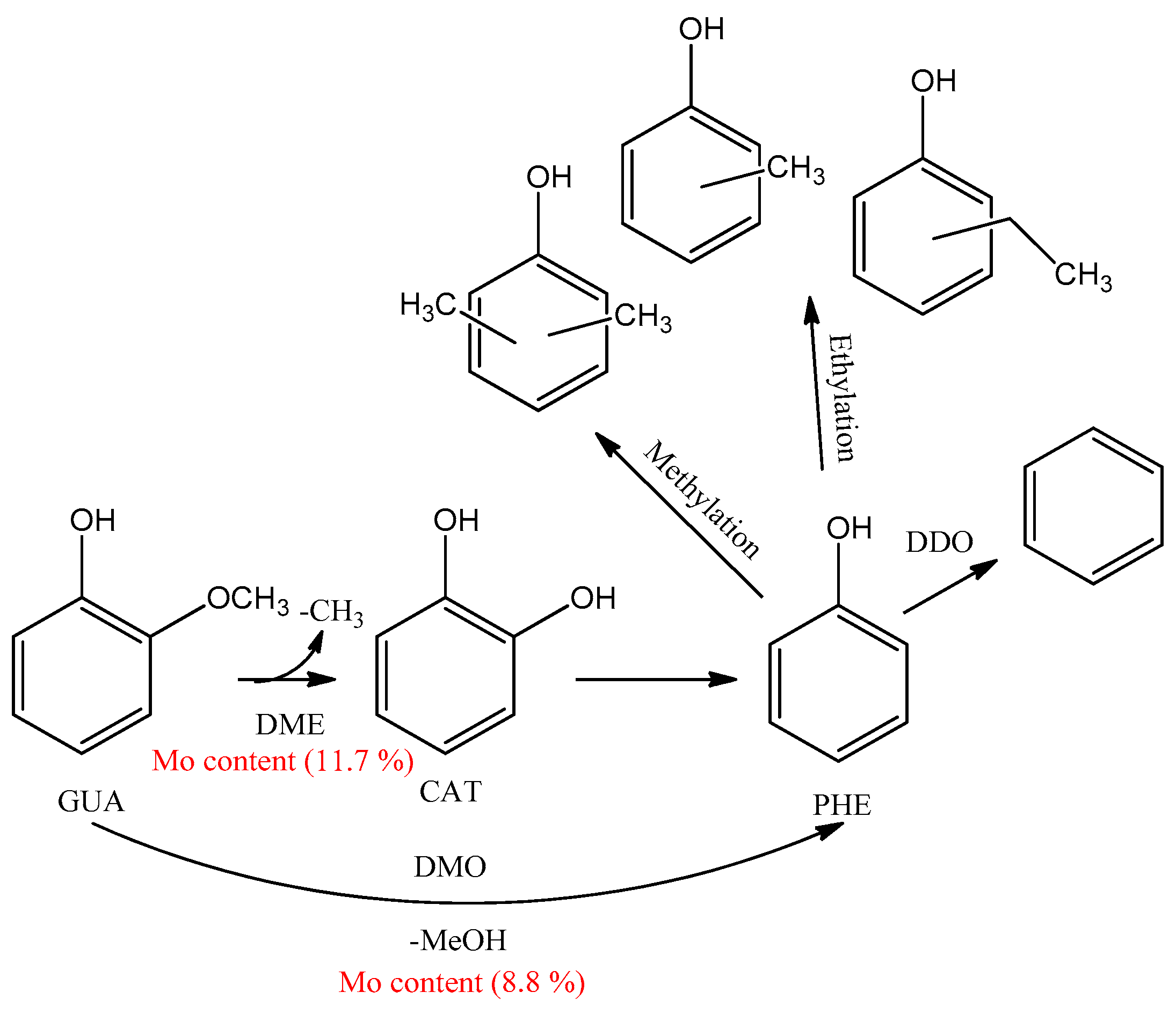
3.6. Reaction Pathways
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, C.-H.; Xia, X.; Lin, C.-X.; Tong, D.-S.; Beltramini, J. Catalytic conversion of lignocellulosic biomass to fine chemicals and fuels. Chem. Soc. Rev. 2011, 40, 5588–5617. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Arancon, R.A.D.; Labidi, J.; Luque, R. Lignin depolymerisation strategies: Towards valuable chemicals and fuels. Chem. Soc. Rev. 2014, 43, 7485–7500. [Google Scholar] [CrossRef] [PubMed]
- Nelson, V. Introduction to Renewable Energy; CRC Press: Boca Raton, FL, USA, 2011; 384p. [Google Scholar]
- Kim, J.-Y.; Oh, S.; Hwang, H.; Cho, T.-S.; Choi, I.-G.; Weon Choi, J. Effects of various reaction parameters on solvolytical depolymerization of lignin in sub- and supercritical ethanol. Chemosphere 2013, 93, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.P.; Kim, C.S. Lignin Depolymerization and Conversion: A Review of Thermochemical Methods. Chem. Eng. Technol. 2011, 34, 29–41. [Google Scholar] [CrossRef]
- Galkin, M.V.; Smit, A.T.; Subbotina, E.; Artemenko, K.A.; Bergquist, J.; Huijgen, W.J.J.; Samec, J.S.M. Hydrogen-free catalytic fractionation of woody biomass. ChemSusChem 2016, 9, 3280–3287. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The Catalytic Valorization of Lignin for the Production of Renewable Chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Sharypov, V.; Kusnetsov, B.; Yakovlev, V.; Beregovtsova, N.; Baryshnikov, S. Studying the thermal conversion of acetone lignin in supercritical butanol in the presence of NiCuMo/SiO2 catalysts. Catal. Ind. 2017, 9, 170–179. [Google Scholar] [CrossRef]
- Zhang, K.; Pei, Z.J.; Wang, D. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: A review. Bioresour. Technol. 2015, 199, 21–33. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Sharypov, V.I.; Chesnokov, N.V.; Beregovtsova, N.G.; Baryshnikov, S.V.; Lavrenov, A.V.; Vosmerikov, A.V.; Agabekov, V.E. Lignin Conversion in Supercritical Ethanol in the Presence of Solid Acid Catalysts. Kinet. I Katal. 2015, 56, 8. [Google Scholar] [CrossRef]
- Macala, G.S.; Matson, T.D.; Johnson, C.L.; Lewis, R.S.; Iretskii, A.V.; Ford, P.C. Hydrogen Transfer from Supercritical Methanol over a Solid Base Catalyst: A Model for Lignin Depolymerization. ChemSusChem 2009, 2, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Løhre, C.; Barth, T.; Kleinert, M. The effect of solvent and input material pretreatment on product yield and composition of bio-oils from lignin solvolysis. J. Anal. Appl. Pyrolysis 2016, 119, 208–216. [Google Scholar] [CrossRef]
- Huang, X.; Korányi, T.I.; Boot, M.D.; Hensen, E.J.M. Catalytic Depolymerization of Lignin in Supercritical Ethanol. ChemSusChem 2014, 7, 2276–2288. [Google Scholar] [CrossRef] [PubMed]
- Chikunov, A.S.; Shashkov, M.V.; Pestunov, A.V.; Kazachenko, A.S.; Mishenko, T.I.; Taran, O.P. Hydrogenolysis of Birch Ethanol-Lignin in Supercritical Over Bifunctional Ru and Ni Catalysts Bifunctional Supported on Oxidized Carbon. J. Sib. Fed. Univ. Chem. 2018, 1, 131–150. [Google Scholar] [CrossRef]
- Lenihan, P.; Orozco, A.; O’Neill, E.; Ahmad, M.N.M.; Rooney, D.W.; Walker, G.M. Dilute acid hydrolysis of lignocellulosic biomass. Chem. Eng. J. 2010, 156, 395–403. [Google Scholar] [CrossRef]
- Asawaworarit, P.; Daorattanachai, P.; Laosiripojana, W.; Sakdaronnarong, C.; Shotipruk, A.; Laosiripojana, N. Catalytic depolymerization of organosolv lignin from bagasse by carbonaceous solid acids derived from hydrothermal of lignocellulosic compounds. Chem. Eng. J. 2019, 356, 461–471. [Google Scholar] [CrossRef]
- Ennaert, T.; Van Aelst, J.; Dijkmans, J.; De Clercq, R.; Schutyser, W.; Dusselier, M.; Verboekend, D.; Sels, B.F. Potential and challenges of zeolite chemistry in the catalytic conversion of biomass. Chem. Soc. Rev. 2016, 45, 584–611. [Google Scholar] [CrossRef]
- Wang, H.; Tucker, M.; Ji, Y. Recent Development in Chemical Depolymerization of Lignin: A Review. J. Appl. Chem. 2013, 2013, 838645. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Park, J.; Hwang, H.; Kim, J.K.; Kyu Song, I.; Weon Choi, J. Catalytic depolymerization of lignin macromolecule to alkylated phenols over various metal catalysts in supercritical tert-butanol. J. Anal. Appl. Pyrolysis 2014, 113, 99–106. [Google Scholar] [CrossRef]
- He, J.; Tang, D.; Hu, C.; Luo, Y.; Kim, C.K.; Su, Z. Mechanistic study on the depolymerization of typical lignin-derived oligomers catalyzed by Pd/NbOPO4. Mol. Catal. 2022, 528, 112500. [Google Scholar] [CrossRef]
- Sturgeon, M.; O’Brien, M.; Ciesielski, P.N.; Katahira, R.; Kruger, J.S.; Chmely, S.C.; Hamlin, J.; Lawrence, K.; Hunsinger, G.B.; Foust, T.; et al. Lignin depolymerisation by nickel supported layered-double hydroxide catalysts. Green Chem. 2014, 16, 824–835. [Google Scholar] [CrossRef]
- Oregui, M.; Gandarias, I.; Miletic, N.; Simonsen, S.F.; Kronstad, A.; Arias, P.L.; Barth, T. Thermocatalytic conversion of lignin in an ethanol/formic acid medium with NiMo catalysts: Role of the metal and acid sites. Appl. Catal. B Environ. 2017, 217, 353–364. [Google Scholar] [CrossRef]
- Joffres, B.; Lorentz, C.; Vidalie, M.; Laurenti, D.; Quoineaud, A.A.; Charon, N.; Daudin, A.; Quignard, A.; Geantet, C. Catalytic hydroconversion of a wheat straw soda lignin: Characterization of the products and the lignin residue. Appl. Catal. B Environ. 2014, 145, 167–176. [Google Scholar] [CrossRef]
- Horáček, J.; Homola, F.; Kubičková, I.; Kubička, D. Lignin to liquids over sulfided catalysts. Catal. Today 2012, 179, 191–198. [Google Scholar] [CrossRef]
- Sharypov, V.I.; Kuznetsov, B.N.; Yakovlev, B.A.; Beregovtsova, N.G.; Baryshnikov, S.V.; Djakovitch, L.; Pinelc, C. Composition of Liquid Products of Acetonlignin Conversion Over NiCu/SiO2 Catalysts in Supercritical Butanol. J. Sib. Fed. Univ. Chem. 2015, 8, 465–475. [Google Scholar] [CrossRef]
- Xue, J.; Wang, D.; Li, X.; Li, G.; Wang, Z.; Liu, Y.; Li, X. A tandem strategy of mild preoxidation-hydrogenolysis for efficient depolymerization of lignin. Mol. Catal. 2023, 549, 113529. [Google Scholar] [CrossRef]
- Ermakova, M.A.; Ermakov, D.Y. High-loaded nickel–silica catalysts for hydrogenation, prepared by sol–gel: Route: Structure and catalytic behavior. Appl. Catal. A Gen. 2003, 245, 277–288. [Google Scholar] [CrossRef]
- Bykova, M.V.; Ermakov, D.Y.; Khromova, S.A.; Smirnov, A.A.; Lebedev, M.Y.; Yakovlev, V.A. Stabilized Ni-based catalysts for bio-oil hydrotreatment: Reactivity studies using guaiacol. Catal. Today 2014, 220–222, 21–31. [Google Scholar] [CrossRef]
- Arato, C.; Kendall Pye, E.; Gjennestad, G. The Lignol Approach to Biorefining of Woody Biomass to Produce Ethanol and Chemicals. Appl. Biochem. Biotechnol. 2005, 123, 871–882. [Google Scholar] [CrossRef]
- Alekseeva , M.V.; Rekhtina, M.A.; Lebedev, M.Y.; Zavarukhin, S.G.; Kaichev, V.V.; Venderbosch, R.H.; Yakovlev, V.A. Hydrotreatment of 2-Methoxyphenol over High Ni-Loaded Sol-Gel Catalysts: The Influence of Mo on Catalyst Activity and Reaction Pathways. ChemistrySelect 2018, 3, 5153–5164. [Google Scholar] [CrossRef]
- Miroshnikova, A.V.; Baryshnikov, S.V.; Malyar, Y.N.; Yakovlev, V.A.; Taran, O.P.; Djakovitch, L.; Kuznetsov, B.N. Depolymerization of Pine Ethanol Lignin in the Medium of Supercritical Ethanol in the Presence of Catalysts NiCu/SiO₂ and NiCuMo/SiO₂. J. Sib. Fed. Univ. Chem. 2020, 13, 247–259. [Google Scholar] [CrossRef]
- Mao, J.Z.; Zhang, L.; Xu, F. Fractional and structural characterization of alkaline lignins from carexmeyeriana kunth. Cellul. Chem. Technol. 2012, 46, 193–205. [Google Scholar]
- Yuan, T.-Q.; Sun, S.-N.; Xu, F.; Sun, R.-C. Structural Characterization of Lignin from Triploid of Populus tomentosa Carr. J. Agric. Food Chem. 2011, 59, 6605–6615. [Google Scholar] [CrossRef] [PubMed]
- Ralph, J.; Hatfield, R.D. Pyrolysis-GC-MS characterization of forage materials. J. Agric. Food Chem. 1991, 39, 1426–1437. [Google Scholar] [CrossRef]
- Guillen, M.; L Ibargoitia, M. GC/MS analysis of lignin monomers, dimers and trimers in liquid smoke flavourings. J. Sci. Food Agric. 1999, 79, 1889–1903. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Oh, S.; Hwang, H.; Kim, U.-J.; Weon Choi, J. Structural features and thermal degradation properties of various lignin macromolecules obtained from poplar wood (Populus albaglandulosa). Polym. Degrad. Stab. 2013, 98, 1671–1678. [Google Scholar] [CrossRef]
- Alekseeva, M.V.; Otyuskaya, D.S.; Rekhtina, M.A.; Bulavchenko, O.A.; Stonkus, O.A.; Kaichev, V.V.; Zavarukhin, S.G.; Thybaut, J.W.; Alexiadis, V.; Venderbosch, R.H.; et al. NiCuMo-SiO2 catalyst for pyrolysis oil upgrading: Model acidic treatment study. Appl. Catal. A Gen. 2019, 573, 1–12. [Google Scholar] [CrossRef]
- Riittonen, T.; Toukoniitty, E.; Madnani, D.K.; Leino, A.-R.; Kordas, K.; Szabo, M.; Sapi, A.; Arve, K.; Wärnå, J.; Mikkola, J.-P. One-Pot Liquid-Phase Catalytic Conversion of Ethanol to 1-Butanol over Aluminium Oxide—The Effect of the Active Metal on the Selectivity. Catalysts 2012, 2, 68–84. [Google Scholar] [CrossRef]
- Wen, J.-L.; Sun, S.-L.; Yuan, T.-Q.; Xu, F.; Sun, R.-C. Structural Elucidation of Lignin Polymers of Eucalyptus Chips during Organosolv Pretreatment and Extended Delignification. J. Agric. Food Chem. 2013, 61, 11067–11075. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef]
- Song, Q.; Wang, F.; Cai, J.; Wang, Y.; Zhang, J.; Yu, W.; Xu, J. Lignin depolymerization (LDP) in alcohol over nickel-based catalysts via a fragmentation–hydrogenolysis process. Energy Environ. Sci. 2013, 6, 994–1007. [Google Scholar] [CrossRef]
- Liu, X.; Li, H.; Xiao, L.-P.; Sun, R.-C.; Song, G. Chemodivergent hydrogenolysis of eucalyptus lignin with Ni@ZIF-8 catalyst. Green Chem. 2019, 21, 1498–1504. [Google Scholar] [CrossRef]
- Lan, W.; Amiri, M.T.; Hunston, C.M.; Luterbacher, J.S. Protection Group Effects During α,γ-Diol Lignin Stabilization Promote High-Selectivity Monomer Production. Angew. Chem. Int. Ed. 2018, 57, 1356–1360. [Google Scholar] [CrossRef]
- Galkin, M.V.; Samec, J.S.M. Selective Route to 2-Propenyl Aryls Directly from Wood by a Tandem Organosolv and Palladium-Catalysed Transfer Hydrogenolysis. ChemSusChem 2014, 7, 2154–2158. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, K.; Xiao, L.-P.; Sun, R.-C.; Song, G. Total utilization of lignin and carbohydrates in Eucalyptus grandis: An integrated biorefinery strategy towards phenolics, levulinic acid, and furfural. Biotechnol. Biofuels 2020, 13, 2. [Google Scholar] [CrossRef]
- Van den Bosch, S.; Schutyser, W.; Vanholme, R.; Driessen, T.; Koelewijn, S.F.; Renders, T.; De Meester, B.; Huijgen, W.J.J.; Dehaen, W.; Courtin, C.M.; et al. Reductive lignocellulose fractionation into soluble lignin-derived phenolic monomers and dimers and processable carbohydrate pulps. Energy Environ. Sci. 2015, 8, 1748–1763. [Google Scholar] [CrossRef]
- Van den Bosch, S.; Renders, T.; Kennis, S.; Koelewijn, S.F.; Van den Bossche, G.; Vangeel, T.; Deneyer, A.; Depuydt, D.; Courtin, C.M.; Thevelein, J.M.; et al. Integrating lignin valorization and bio-ethanol production: On the role of Ni-Al2O3 catalyst pellets during lignin-first fractionation. Green Chem. 2017, 19, 3313–3326. [Google Scholar] [CrossRef]
- Chen, J.; Lu, F.; Si, X.; Nie, X.; Lu, R.; Xu, J. High Yield Production of Natural Phenolic Alcohols from Woody Biomass Using a Nickel-Based Catalyst. ChemSusChem 2016, 9, 3353–3360. [Google Scholar] [CrossRef]
- Hellinger, M.; Carvalho, H.W.P.; Baier, S.; Wang, D.; Kleist, W.; Grunwaldt, J.-D. Catalytic hydrodeoxygenation of guaiacol over platinum supported on metal oxides and zeolites. Appl. Catal. A Gen. 2015, 490, 181–192. [Google Scholar] [CrossRef]
- Bui, V.N.; Laurenti, D.; Afanasiev, P.; Geantet, C. Hydrodeoxygenation of guaiacol with CoMo catalysts. Part I: Promoting effect of cobalt on HDO selectivity and activity. Appl. Catal. B Environ. 2011, 101, 239–245. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Ling, L.-L.; Jiang, H. Selective hydrogenation of lignin to produce chemical commodities by using a biochar supported Ni–Mo2C catalyst obtained from biomass. Green Chem. 2016, 18, 4032–4041. [Google Scholar] [CrossRef]
- Shafaghat, H.; Sirous Rezaei, P.; Daud, W.M.A.W. Catalytic hydrogenation of phenol, cresol and guaiacol over physically mixed catalysts of Pd/C and zeolite solid acids. RSC Adv. 2015, 5, 33990–33998. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Wang, T.; Ma, L.; Yu, Y.; Chen, L. Hydrodeoxygenation of lignin-derived phenolic compounds to hydrocarbons over Ni/SiO2–ZrO2 catalysts. Bioresour. Technol. 2013, 134, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Wang, H.; Lin, H.; Zheng, Y. Selective production of guaiacol from black liquor: Effect of solvents. Carbon Resour. Convers. 2019, 2, 1–12. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Miroshnikova, A.V.; Tarabanko, V.E.; Skripnikov, A.M.; Malyar, Y.N.; Borovkova, V.S.; Sychev, V.V.; Taran, O.P. Thermal Conversion of Flax Shives in Sub- and Supercritical Ethanol in the Presence of Ru/C Catalyst. Catalysts 2021, 11, 970. [Google Scholar] [CrossRef]
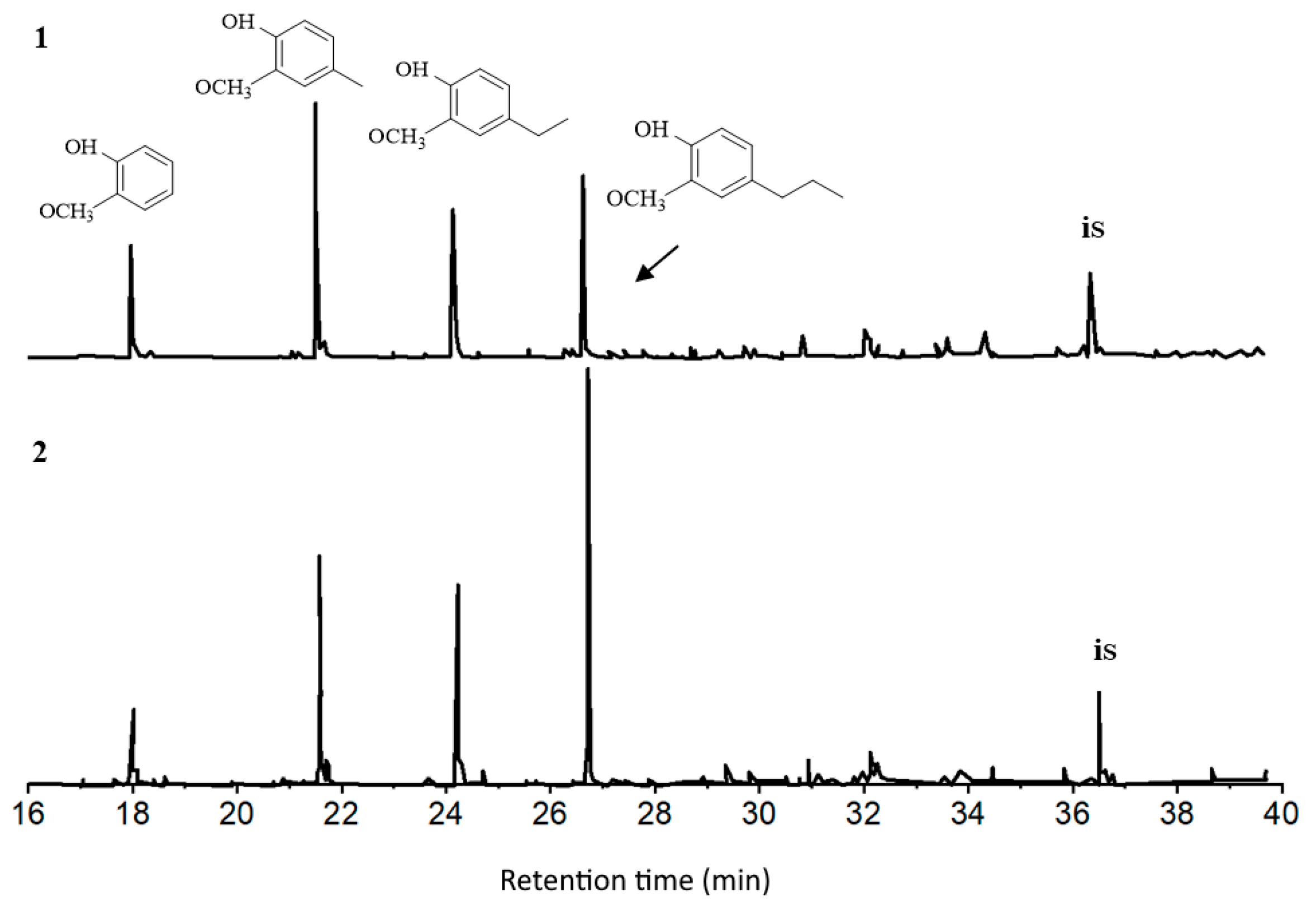

| RT | Substance | Structure | Yields, wt.% * | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No Catalyst | NiCu/SiO2 | NiCuMo/SiO2-1 | NiCuMo/SiO2-2 | |||||||
| 300 °C | 350 °C | 300 °C | 350 °C | 300 °C | 350 °C | 300 °C | 350 °C | |||
| 18.0 | Guaiacol | 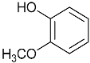 | 11.6 | 13.7 | 4.6 | 7.7 | 6.2 | 5.0 | 7.8 | 10.9 |
| 21.6 | Methyl guaiacol | 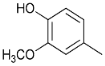 | 24.2 | 23.6 | 11.6 | 17.5 | 18.0 | 11.5 | 18.0 | 25.2 |
| 24.2 | Ethyl guaiacol |  | 14.9 | 16.2 | 14.0 | 15.8 | 15.9 | 14.8 | 15.9 | 15.8 |
| 26.7 | Propyl guaiacol | 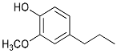 | 16.4 | 13.2 | 30.4 | 28.4 | 32.7 | 15.5 | 30.8 | 21.1 |
| 28.7 | Propenyl guaiacol | 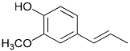 | 0.5 | 0.8 | 0.6 | 0.8 | 0.7 | n.d. | 0.8 | n.d. |
| 29.3 | Eugenol | 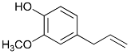 | 0.3 | n.d. | 1.5 | 0.4 | 1.6 | n.d. | 2.3 | 0.3 |
| 29.8 | Acetovanillone | 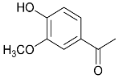 | 2.4 | 0.8 | 2.5 | 3.1 | 3.0 | 4.5 | 3.2 | 1.0 |
| 33.5 | Ethyl homovanillate |  | 2.1 | 0.7 | 2.2 | 3.9 | 2.0 | n.d. | 2.0 | 2.1 |
| 33.7 | Propanol guaiacol | 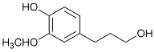 | 2.2 | n.d. | 0.7 | n.d. | 0.6 | 0.4 | 1.1 | 1.3 |
| 54.3 | Dimers | 23.3 | 8.3 | 29.9 | 11.1 | 17.4 | 3.2 | 15.3 | 1.8 | |
| Group composition | ||||||||||
| Phenol and its alkyl derivatives | n.d. | 10.6 | n.d. | 1.3 | n.d. | 36.0 | n.d. | 1.0 | ||
| Benzene derivatives | n.d. | 2.9 | n.d. | 2.9 | n.d. | 3.1 | n.d. | 0.6 | ||
| Esters of carboxylic acids, alcohols | 2.2 | 1.2 | 2.1 | 2.5 | 2.1 | 1.2 | 3.7 | 2.2 | ||
| Catechol alkyl derivatives | n.d. | 7.4 | n.d. | 3.5 | n.d. | 3.3 | n.d. | 16.6 | ||
| Total Yield of Phenolic Products and Yield of Main Substances | No Catalyst | NiCu/SiO2 | NiCuMo/SiO2-1 | NiCuMo /SiO2-2 |
|---|---|---|---|---|
| 300 °C | ||||
| Total yield of aromatic products, incl. | 6.36 | 8.69 | 12.11 | 12.67 |
| Methyl guaiacol | 1.54 | 0.99 | 2.20 | 2.28 |
| Ethyl guaiacol | 0.95 | 1.20 | 1.94 | 2.02 |
| Propyl guaiacol | 1.04 | 2.60 | 4.00 | 3.77 |
| Dimers | 1.48 | 2.65 | 2.12 | 1.94 |
| 350 °C | ||||
| Total yield of aromatic products, incl. | 9.32 | 10.08 | 11.8 | 6.59 |
| Methyl guaiacol | 2.49 | 1.9 | 1.47 | 2.06 |
| Ethyl guaiacol | 1.71 | 1.75 | 1.88 | 1.29 |
| Propyl guaiacol | 1.39 | 3.14 | 1.98 | 1.73 |
| Dimers | 0.87 | 1.23 | 0.48 | 0.15 |
| phenol and its alkyl derivatives | 1.21 | 0.15 | 4.58 | 0.08 |
| Alkyl Catechols | 0.78 | 0.39 | 0.41 | 1.36 |
| Benzene derivatives | 0.31 | 0.32 | 0.39 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miroshnikova, A.V.; Baryshnikov, S.V.; Malyar, Y.N.; Li, X.; Alekseeva, M.V.; Kuznetsov, B.N.; Taran, O.P. Depolymerization of Pine Wood Organosolv Lignin in Ethanol Medium over NiCu/SiO2 and NiCuMo/SiO2 Catalysts: Impact of Temperature and Catalyst Composition. Polymers 2023, 15, 4722. https://doi.org/10.3390/polym15244722
Miroshnikova AV, Baryshnikov SV, Malyar YN, Li X, Alekseeva MV, Kuznetsov BN, Taran OP. Depolymerization of Pine Wood Organosolv Lignin in Ethanol Medium over NiCu/SiO2 and NiCuMo/SiO2 Catalysts: Impact of Temperature and Catalyst Composition. Polymers. 2023; 15(24):4722. https://doi.org/10.3390/polym15244722
Chicago/Turabian StyleMiroshnikova, Angelina V., Sergey V. Baryshnikov, Yuriy N. Malyar, Xiaomin Li, Maria V. Alekseeva, Boris N. Kuznetsov, and Oxana P. Taran. 2023. "Depolymerization of Pine Wood Organosolv Lignin in Ethanol Medium over NiCu/SiO2 and NiCuMo/SiO2 Catalysts: Impact of Temperature and Catalyst Composition" Polymers 15, no. 24: 4722. https://doi.org/10.3390/polym15244722
APA StyleMiroshnikova, A. V., Baryshnikov, S. V., Malyar, Y. N., Li, X., Alekseeva, M. V., Kuznetsov, B. N., & Taran, O. P. (2023). Depolymerization of Pine Wood Organosolv Lignin in Ethanol Medium over NiCu/SiO2 and NiCuMo/SiO2 Catalysts: Impact of Temperature and Catalyst Composition. Polymers, 15(24), 4722. https://doi.org/10.3390/polym15244722








