Form-Stable Composite Phase Change Materials Based on Porous Copper–Graphene Heterostructures for Solar Thermal Energy Conversion and Storage
Abstract
:1. Introduction
| Composites | Porosity | Thermal Conductivity (W/(m·K)) |
|---|---|---|
| Expanded graphite–paraffin composite [15] | 38.01% | 2.45 |
| Expanded graphite–hexadecane composite [17] | 80%, | 1.2402 |
| Melamine–paraffin composite [21] | 85.8%, | 0.096 |
| Copper–paraffin composite [37] | 95% | 1.04 |
| Copper–paraffin composite [40] | 97.3% | 2.879 |
| Nickel–paraffin composite [41] | 95.2% | 1.44 |
| Nickel–paraffin composite [42] | 90.61% | 2.33 |
| Copper–paraffin composite [43] | 95.92% | 1.439 |
| Copper–paraffin composite [43] | 97.59% | 1.238 |
| Nickel–myrtle alcohol composite [44] | 97% | 0.48 |
2. Materials and Methods
2.1. Materials
2.2. Preparation of Copper–Graphene Heterostructures
2.3. Preparation of the Graphene–Copper–Paraffin Composites
2.4. Measurement and Characterization
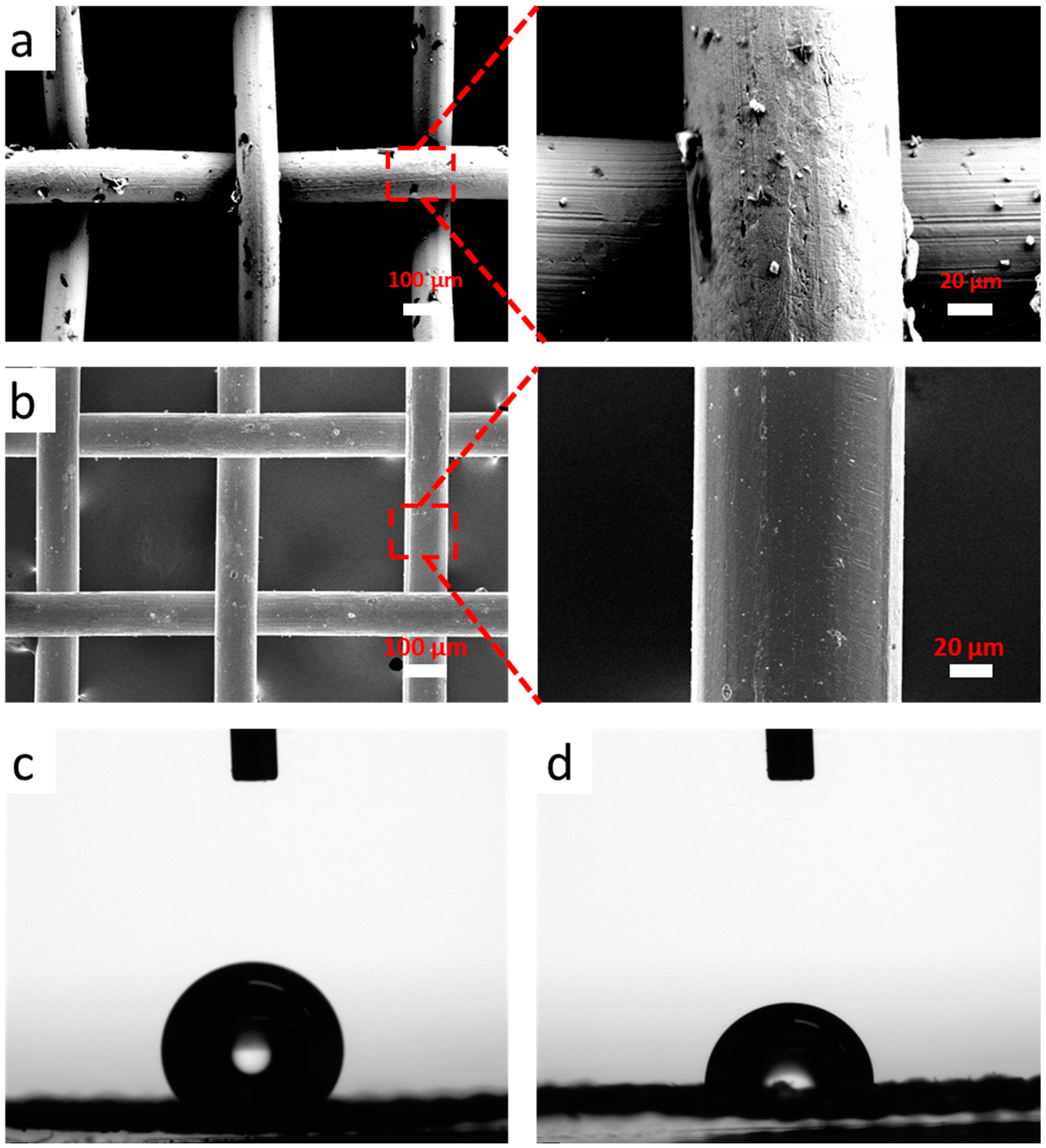
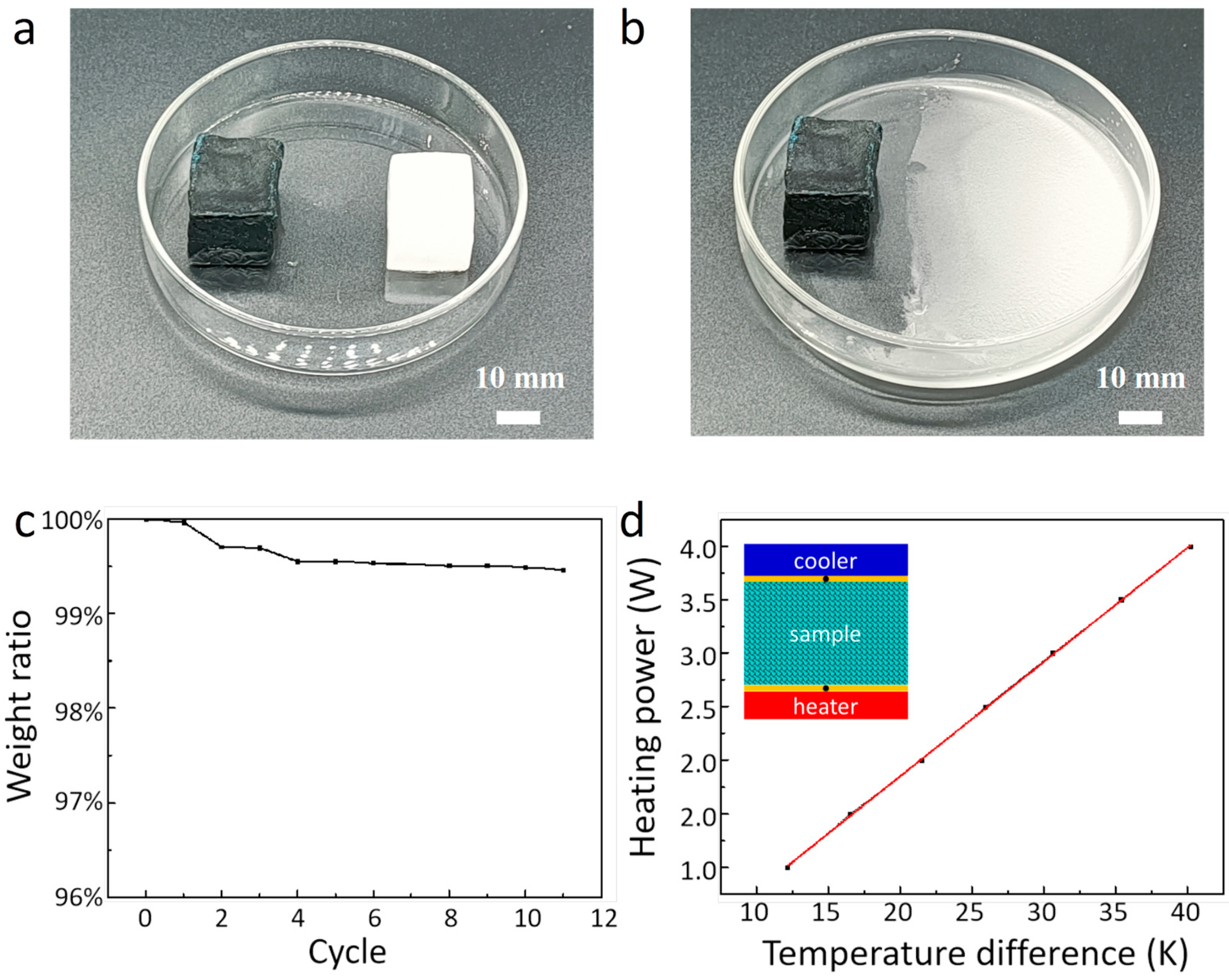
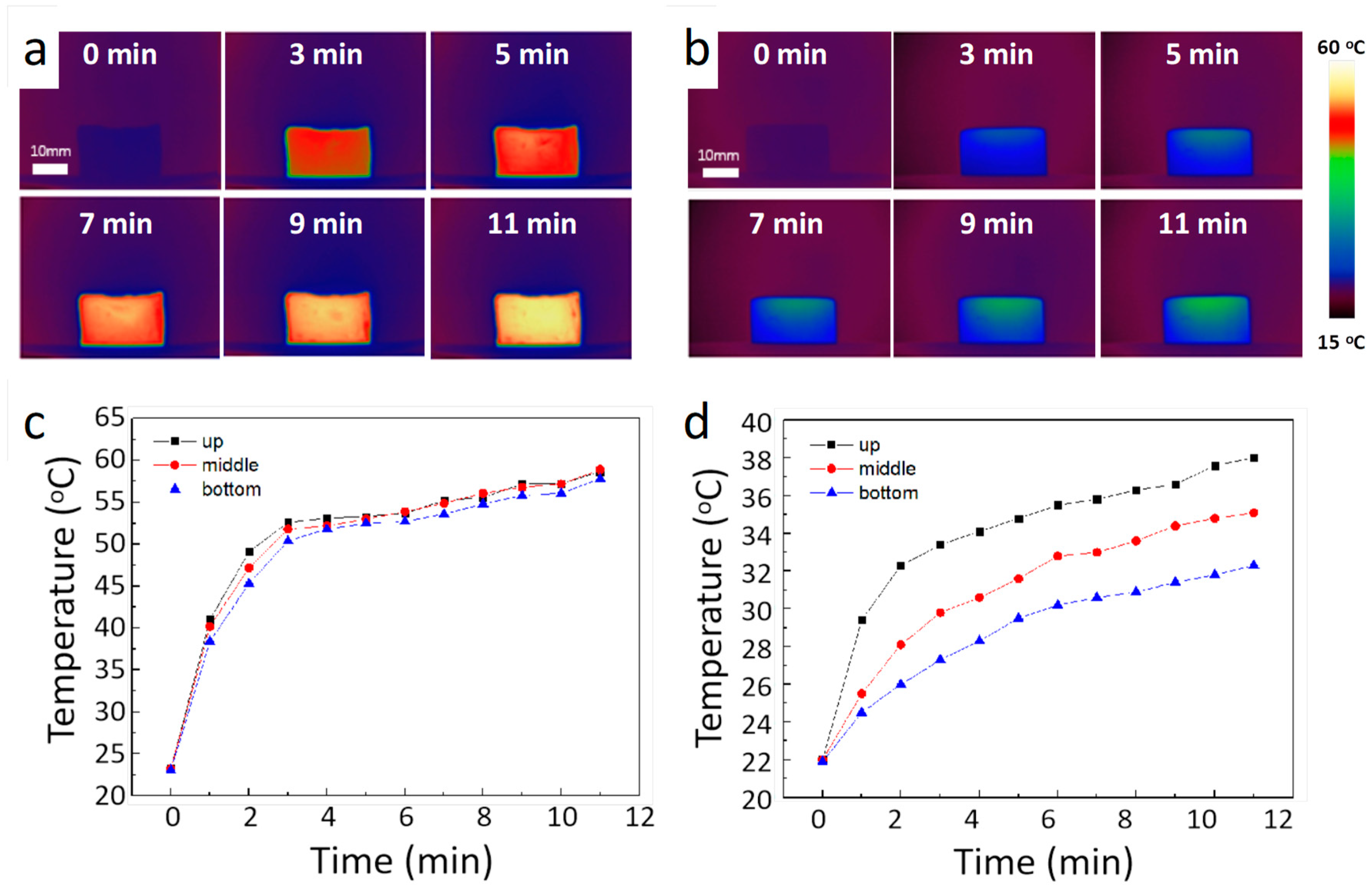
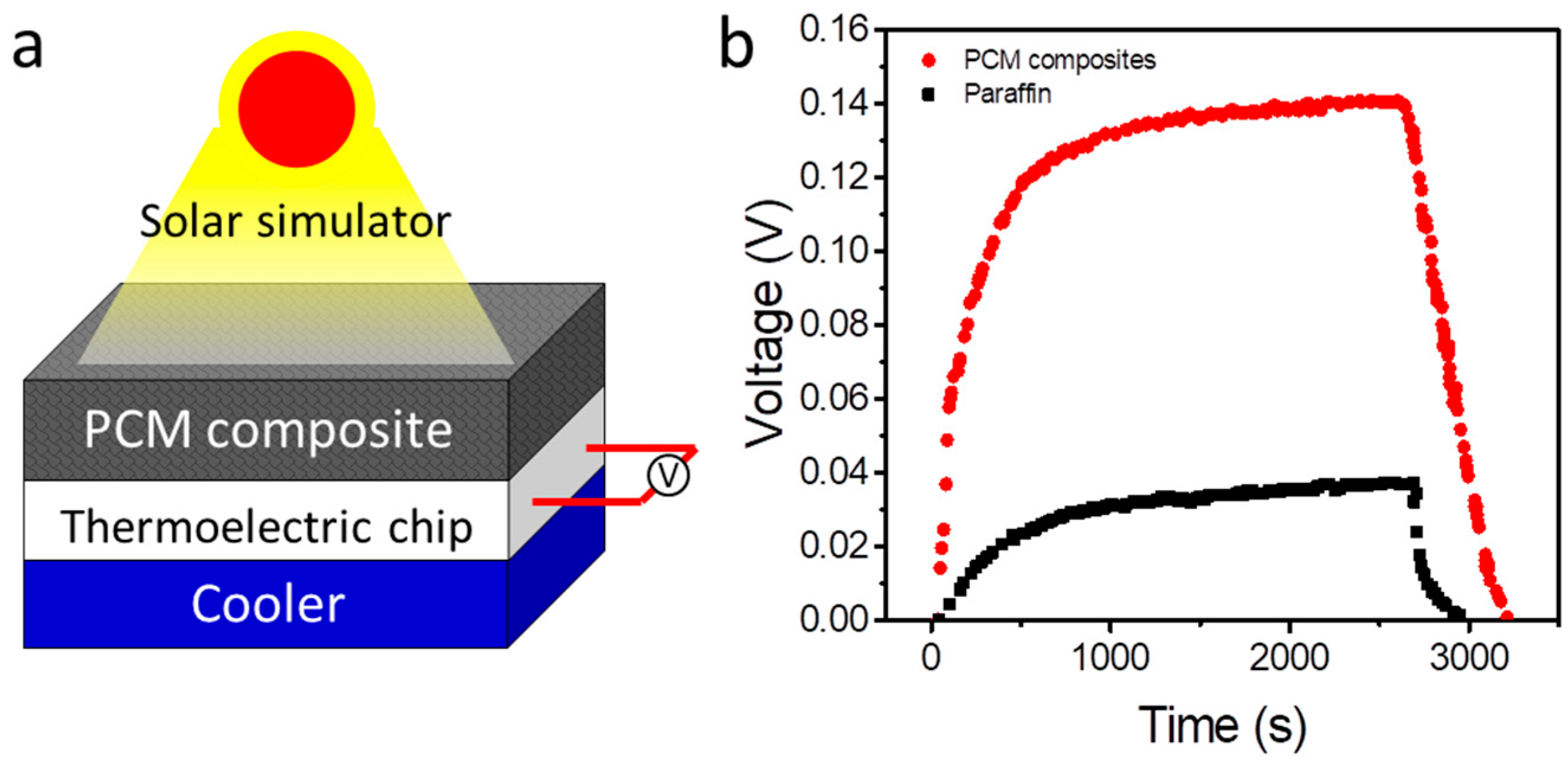
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carley, S.; Konisky, D.M. The justice and equity implications of the clean energy transition. Nat. Energy. 2020, 5, 569–577. [Google Scholar] [CrossRef]
- Kannan, N.; Vakeesan, D. Solar energy for future world: A review. Renew. Sust. Energy Rev. 2016, 62, 1092–1105. [Google Scholar] [CrossRef]
- Mekhilef, S.; Saidur, R.; Safari, A. A review on solar energy use in industries. Renew. Sust. Energy Rev. 2011, 15, 1777–1790. [Google Scholar] [CrossRef]
- Kumar, K.R.; Chaitanya, N.K.; Kumar, N.S. Solar thermal energy technologies and its applications for process heating and power generation–A review. J. Clean. Prod. 2021, 282, 125296. [Google Scholar] [CrossRef]
- Shi, L.; Wang, X.; Hu, Y.; He, Y.; Yan, Y. Solar-thermal conversion and steam generation: A review. Appl. Therm. Eng. 2020, 179, 115691. [Google Scholar] [CrossRef]
- Tao, P.; Chang, C.; Tong, Z.; Bao, H.; Song, C.; Wu, J.; Shang, W.; Deng, T. Magnetically-accelerated large-capacity solar-thermal energy storage within high-temperature phase-change materials. Energy Environ. Sci. 2019, 12, 16131621. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Khalid, M.; Rashmi, W.; Chan, A.; Shahbaz, K. Recent progress in solar thermal energy storage using nanomaterials. Renew. Sust. Energy Rev. 2017, 67, 450–460. [Google Scholar] [CrossRef]
- Alva, G.; Liu, L.; Huang, X.; Fang, G. Thermal energy storage materials and systems for solar energy applications. Renew. Sust. Energy Rev. 2017, 68, 693–706. [Google Scholar] [CrossRef]
- Suresh, C.; Saini, R.P. Review on solar thermal energy storage technologies and their geometrical configurations. Int. J. Energy Res. 2020, 44, 4163–4195. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, D.; Shi, L.; Wang, L.; Jin, Y.; Tian, L.; Li, Z.; Wang, G.; Zhao, L.; Yan, Y. A review of phase change heat transfer in shape-stabilized phase change materials (ss-PCMs) based on porous supports for thermal energy storage. Renew. Sust. Energy Rev. 2021, 135, 110127. [Google Scholar] [CrossRef]
- Ali, H.M. Recent advancements in PV cooling and efficiency enhancement integrating phase change materials based systems—A comprehensive review. Sol. Energy 2020, 197, 163–198. [Google Scholar] [CrossRef]
- Wu, S.; Yan, T.; Kuai, Z.; Pan, W. Thermal conductivity enhancement on phase change materials for thermal energy storage: A review. Energy Stor. Mater. 2020, 25, 251–295. [Google Scholar] [CrossRef]
- Qureshi, Z.A.; Ali, H.M.; Khushnood, S. Recent advances on thermal conductivity enhancement of phase change materials for energy storage system: A review. Int. J. Heat Mass Transf. 2018, 127, 838–856. [Google Scholar] [CrossRef]
- Cheng, P.; Chen, X.; Gao, H.; Zhang, X.; Tang, Z.; Li, A.; Wang, G. Different dimensional nanoadditives for thermal conductivity enhancement of phase change materials: Fundamentals and applications. Nano Energy 2021, 85, 105948. [Google Scholar] [CrossRef]
- Zhao, Y.; Jin, L.; Zou, B.; Qiao, G.; Zhang, T.; Cong, L.; Jiang, F.; Li, C.; Huang, Y.; Ding, Y. Expanded graphite–Paraffin composite phase change materials: Effect of particle size on the composite structure and properties. Appl. Therm. Eng. 2020, 171, 115015. [Google Scholar] [CrossRef]
- Wang, T.; Wang, K.; Ye, F.; Ren, Y.; Xu, C. Characterization and thermal properties of a shape-stable Na2CO3-K2CO3/coal fly ash/expanded graphite composite phase change materials for high-temperature thermal energy storage. J. Energy Storage 2021, 33, 102123. [Google Scholar] [CrossRef]
- Chriaa, I.; Karkri, M.; Trigui, A.; Jedidi, I.; Abdelmouleh, M.; Boudaya, C. The performances of expanded graphite on the phase change materials composites for thermal energy storage. Polymer 2021, 212, 123128. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Z.; Yu, F. Enhanced thermal conductivity of microencapsulated phase change materials based on graphene oxide and carbon nanotube hybrid filler. Sol. Energy Mater. Sol. Cells. 2019, 192, 72–80. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, B.; Zhang, S. Single-walled carbon nanotube/phase change material composites: Sunlight-driven, reversible, form-stable phase transitions for solar thermal energy storage. Adv. Funct. Mater. 2013, 23, 4354–4360. [Google Scholar] [CrossRef]
- Wang, W.; Umair, M.; Qiu, J.; Fan, X.; Cui, Z.; Yao, Y.; Tang, B. Electromagnetic and solar energy conversion and storage based on Fe3O4-functionalised graphene/phase change material nanocomposites. Energy Convers. Manag. 2019, 196, 1299–1305. [Google Scholar] [CrossRef]
- Wu, H.; Li, S.; Shao, Y.; Jin, X.; Qi, X.; Yang, J.; Zhou, Z.; Wang, Y. Melamine foam/reduced graphene oxide supported form-stable phase change materials with simultaneous shape memory property and light-to-thermal energy storage capability. Chem. Eng. J. 2020, 379, 122373. [Google Scholar] [CrossRef]
- Zhu, Y.; Qin, Y.; Liang, S.; Chen, K.; Tian, C.; Wang, J.; Luo, X.; Zhang, L. Graphene/SiO2/n-octadecane nanoencapsulated phase change material with flower like morphology, high thermal conductivity, and suppressed supercooling. Appl. Energy 2019, 250, 98–108. [Google Scholar] [CrossRef]
- Ghalambaz, M.; Zhang, J. Conjugate solid-liquid phase change heat transfer in heatsink filled with phase change material-metal foam. Int. J. Heat Mass Transf. 2020, 146, 118832. [Google Scholar] [CrossRef]
- Caliano, M.; Bianco, N.; Graditi, G.; Mongibello, L. Analysis of a phase change material-based unit and of an aluminum foam/phase change material composite-based unit for cold thermal energy storage by numerical simulation. Appl. Energy. 2019, 256, 113921. [Google Scholar] [CrossRef]
- Chang, C.; Chen, G.; Wu, F.; Han, Z.; Pei, L. Fabrication and Thermal Performance of 3D Copper-Mesh-Sintered Foam/Paraffin Phase Change Materials for Solar Thermal Energy Storage. Processes 2022, 10, 897. [Google Scholar] [CrossRef]
- Ye, Q.; Tao, P.; Chang, C.; Zhou, L.; Zeng, X.; Song, C.; Shang, W.; Wu, J.; Deng, T. Form-stable solar thermal heat packs prepared by impregnating phase-changing materials within carbon-coated copper foams. ACS Appl. Mater. Interfaces 2018, 11, 3417–3427. [Google Scholar] [CrossRef]
- Kumar, P.; Anandkumar, R.; Mylsamy, K.; Prakash, K. Experimental investigation on thermal conductivity of nanoparticle dispersed paraffin (NDP). Mater. Today Proc. 2021, 45, 735–739. [Google Scholar] [CrossRef]
- Shama, A.; Kabeel, A.; Moharram, B.; Abosheiasha, H. Improvement of the thermal properties of paraffin wax using high conductive nanomaterial to appropriate the solar thermal applications. Appl. Nanosci. 2021, 11, 2033–2042. [Google Scholar] [CrossRef]
- Lei, C.; Wu, K.; Wu, L.; Liu, W.; Du, R.; Chen, F.; Fu, Q. Phase change material with anisotropically high thermal conductivity and excellent shape stability due to its robust cellulose/BNNSs skeleton. J. Mater. Chem. A 2019, 7, 19364–19373. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, L.; She, X.; Li, C.; Cang, D.; Liu, X.; Xuan, Y.; Ding, Y. Skeleton materials for shape-stabilization of high temperature salts based phase change materials: A critical review. Renew. Sust. Energy Rev. 2020, 119, 109539. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, Y.; Liu, Y.; Wang, S.; Guo, X.; Wang, H.; Cao, D. Paraffin/polyethylene/graphite composite phase change materials with enhanced thermal conductivity and leakage-proof. Adv. Compos. Hybrid Mater. 2021, 4, 543–551. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, K.; Wei, Q.; Ma, L.; Ye, W.; Li, H.; Zhou, B. Thermal conductivity enhancement of phase change materials with 3D porous diamond foam for thermal energy storage. Appl. Energy 2019, 233, 208–219. [Google Scholar]
- Wang, Z.; Zhang, H.; Dou, B.; Zhang, G.; Wu, W.; Zhou, X. Effect of copper metal foam proportion on heat transfer enhancement in the melting process of phase change materials. Appl. Therm. Eng. 2022, 201, 117778. [Google Scholar] [CrossRef]
- El Mghari, H.; Huot, J.; Tong, L.; Xiao, J. Selection of phase change materials, metal foams and geometries for improving metal hydride performance. Int. J. Hydrog. Energy 2020, 45, 14922–14939. [Google Scholar] [CrossRef]
- Cui, W.; Si, T.; Li, X.; Li, X.; Lu, L.; Ma, T.; Wang, Q. Heat transfer analysis of phase change material composited with metal foam-fin hybrid structure in inclination container by numerical simulation and artificial neural network. Energy Rep. 2022, 8, 10203–10218. [Google Scholar] [CrossRef]
- Marri, G.; Balaji, C. Experimental and numerical investigations on the effect of porosity and PPI gradients of metal foams on the thermal performance of a composite phase change material heat sink. Int. J. Heat Mass Transf. 2021, 164, 120454. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Xi, S.; Xie, H.; Yu, W. 3D porous copper foam-based shape-stabilized composite phase change materials for high photothermal conversion, thermal conductivity and storage. Renew Energy 2021, 175, 307–317. [Google Scholar] [CrossRef]
- Zheng, X.; Gao, X.; Huang, Z.; Li, Z.; Fang, Y.; Zhang, Z. Form-stable paraffin/graphene aerogel/copper foam composite phase change material for solar energy conversion and storage. Sol. Energy Mater. Sol. Cells 2021, 226, 111083. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, C.; Liu, Q.; Tian, Z.; Fan, X. Thermal performance of copper foam/paraffin composite phase change material. Energy Convers. Manag. 2018, 157, 372–381. [Google Scholar] [CrossRef]
- Wang, C.; Lin, T.; Li, N.; Zheng, H. Heat Transfer Enhancement of Phase Change Composite Material: Copper Foam/Paraffin. Renew Energy 2016, 96, 960–965. [Google Scholar] [CrossRef]
- El Idi, M.M.; Karkri, M.; Kraiem, M. Preparation and effective thermal conductivity of a Paraffin/Metal Foam composite. J. Energy Storage 2021, 33, 102077. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, P.; Li, M. Effective thermal conductivity of open-cell metal foams impregnated with pure paraffin for latent heat storage. Int. J. Therm. Sci. 2014, 81, 94–105. [Google Scholar] [CrossRef]
- Shang, B.; Hu, J.; Hu, R.; Cheng, J.; Luo, X. Modularized thermal storage unit of metal foam/paraffin composite. Int. J. Heat Mass Transf. 2018, 125, 596–603. [Google Scholar] [CrossRef]
- Huang, X.; Lin, Y.; Alva, G.; Fang, G. Thermal properties and thermal conductivity enhancement of composite phase change materials using myristyl alcohol/metal foam for solar thermal storage. Sol. Energy Mater. Sol. Cells 2017, 170, 68–76. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.; Li, B.; Fu, B.; Yang, X.; Ji, Y. Form-Stable Composite Phase Change Materials Based on Porous Copper–Graphene Heterostructures for Solar Thermal Energy Conversion and Storage. Polymers 2023, 15, 4723. https://doi.org/10.3390/polym15244723
Chang C, Li B, Fu B, Yang X, Ji Y. Form-Stable Composite Phase Change Materials Based on Porous Copper–Graphene Heterostructures for Solar Thermal Energy Conversion and Storage. Polymers. 2023; 15(24):4723. https://doi.org/10.3390/polym15244723
Chicago/Turabian StyleChang, Chao, Bo Li, Baocai Fu, Xu Yang, and Yulong Ji. 2023. "Form-Stable Composite Phase Change Materials Based on Porous Copper–Graphene Heterostructures for Solar Thermal Energy Conversion and Storage" Polymers 15, no. 24: 4723. https://doi.org/10.3390/polym15244723
APA StyleChang, C., Li, B., Fu, B., Yang, X., & Ji, Y. (2023). Form-Stable Composite Phase Change Materials Based on Porous Copper–Graphene Heterostructures for Solar Thermal Energy Conversion and Storage. Polymers, 15(24), 4723. https://doi.org/10.3390/polym15244723








