Interfacial Bond Properties of Underwater Concrete Coated with Bisphenol A Epoxy Resins
Abstract
1. Introduction
2. Research Significance
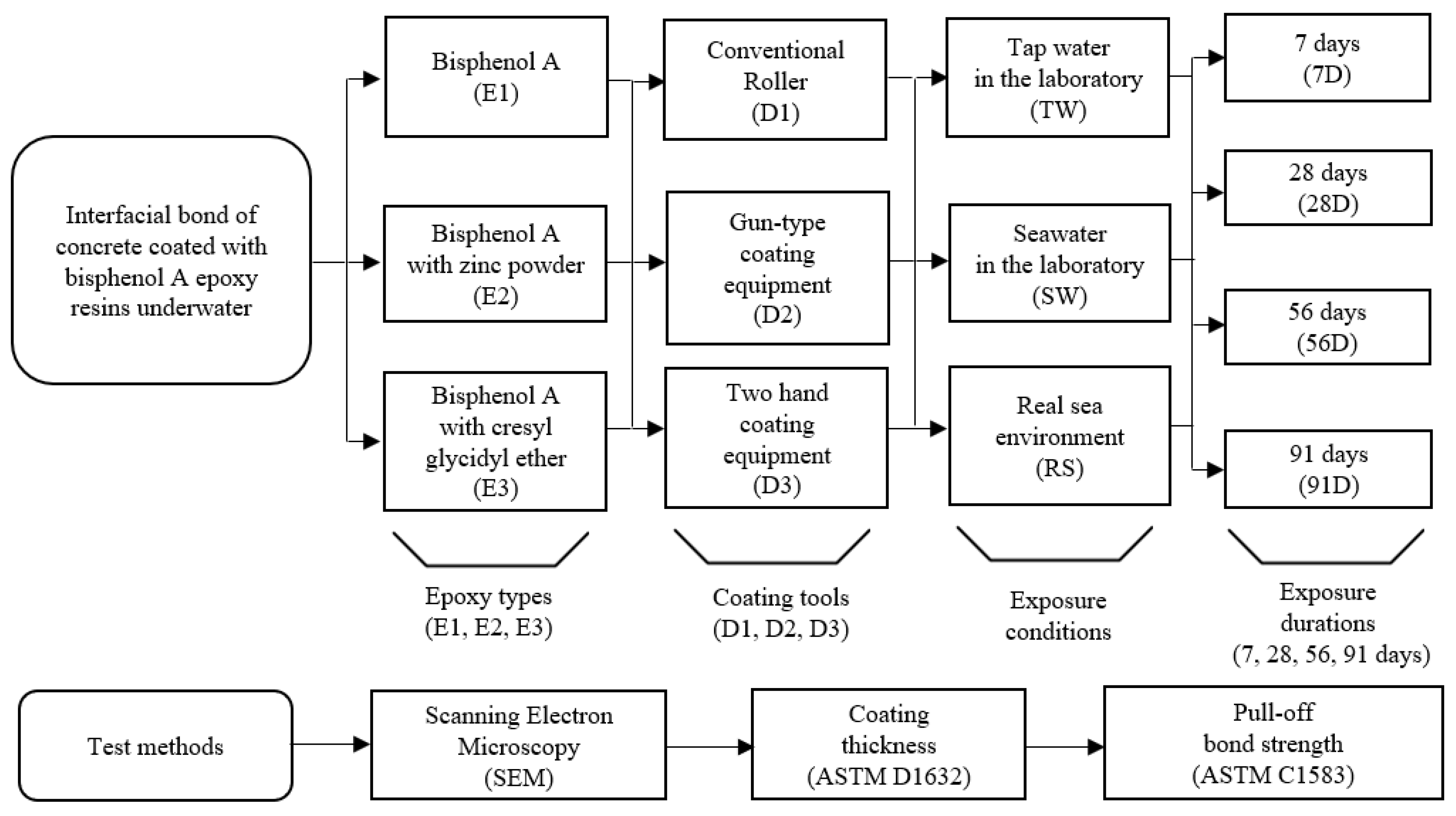
3. Experimental
3.1. Materials and Test Variables
3.2. Concrete Specimen Preparation
3.3. Test and Data Analysis
4. Test Results
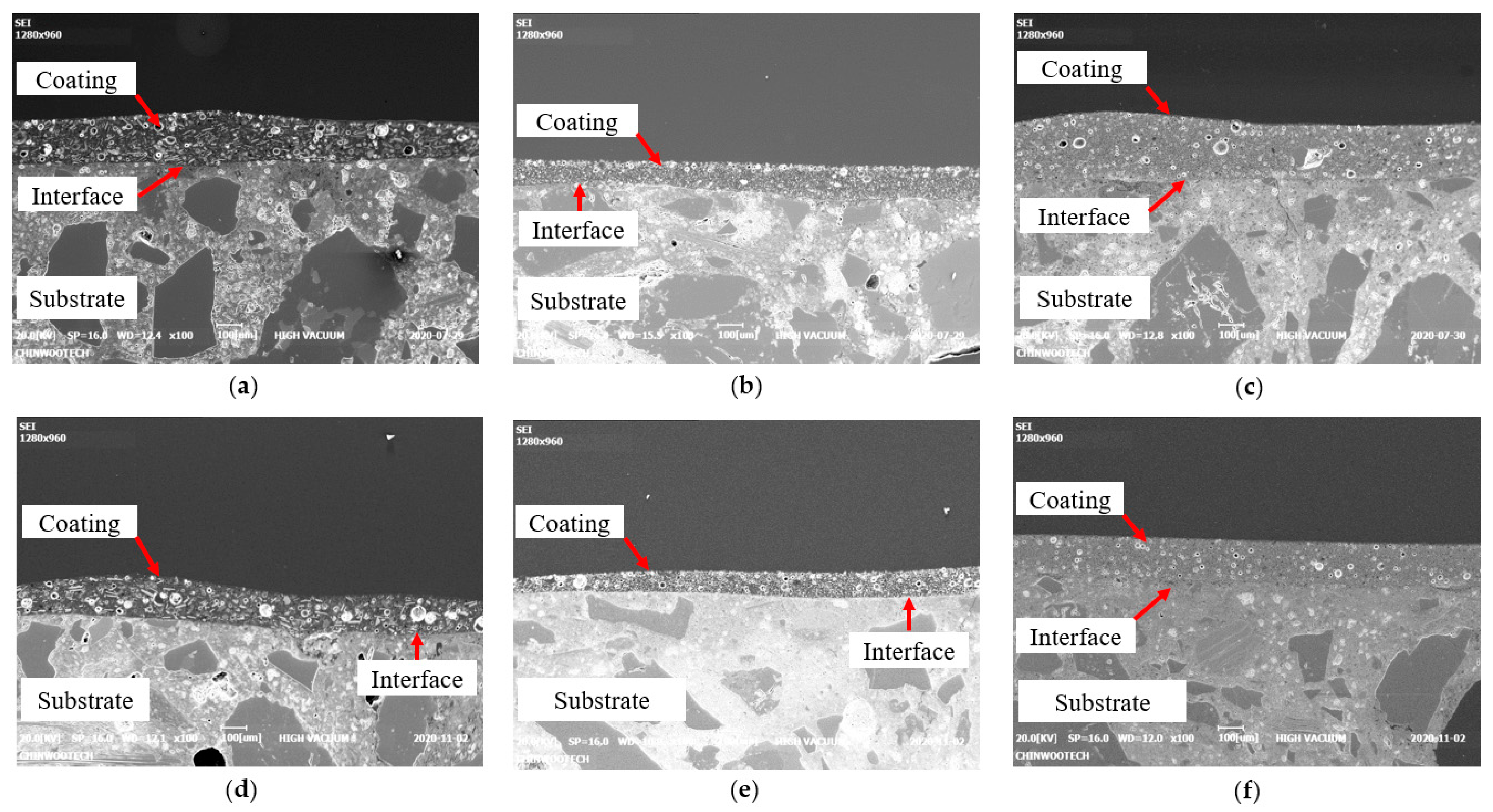
4.1. Measured Thickness
4.2. Measured Pull-Off Bond Strength
5. Analysis
5.1. Effect of Epoxy Type on the Measured Thickness and Bond Strength
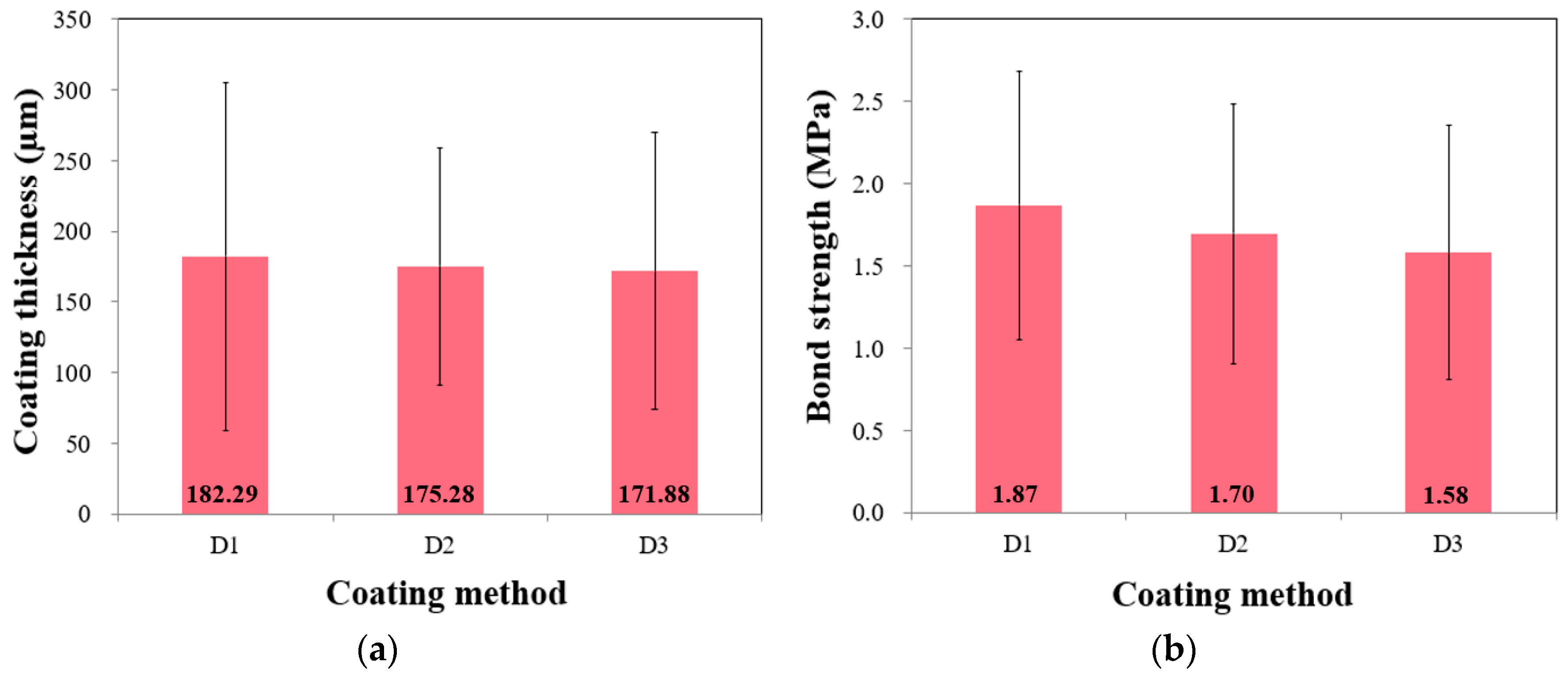
5.2. Effect of the Coating Method on the Measured Thickness and Bond Strength
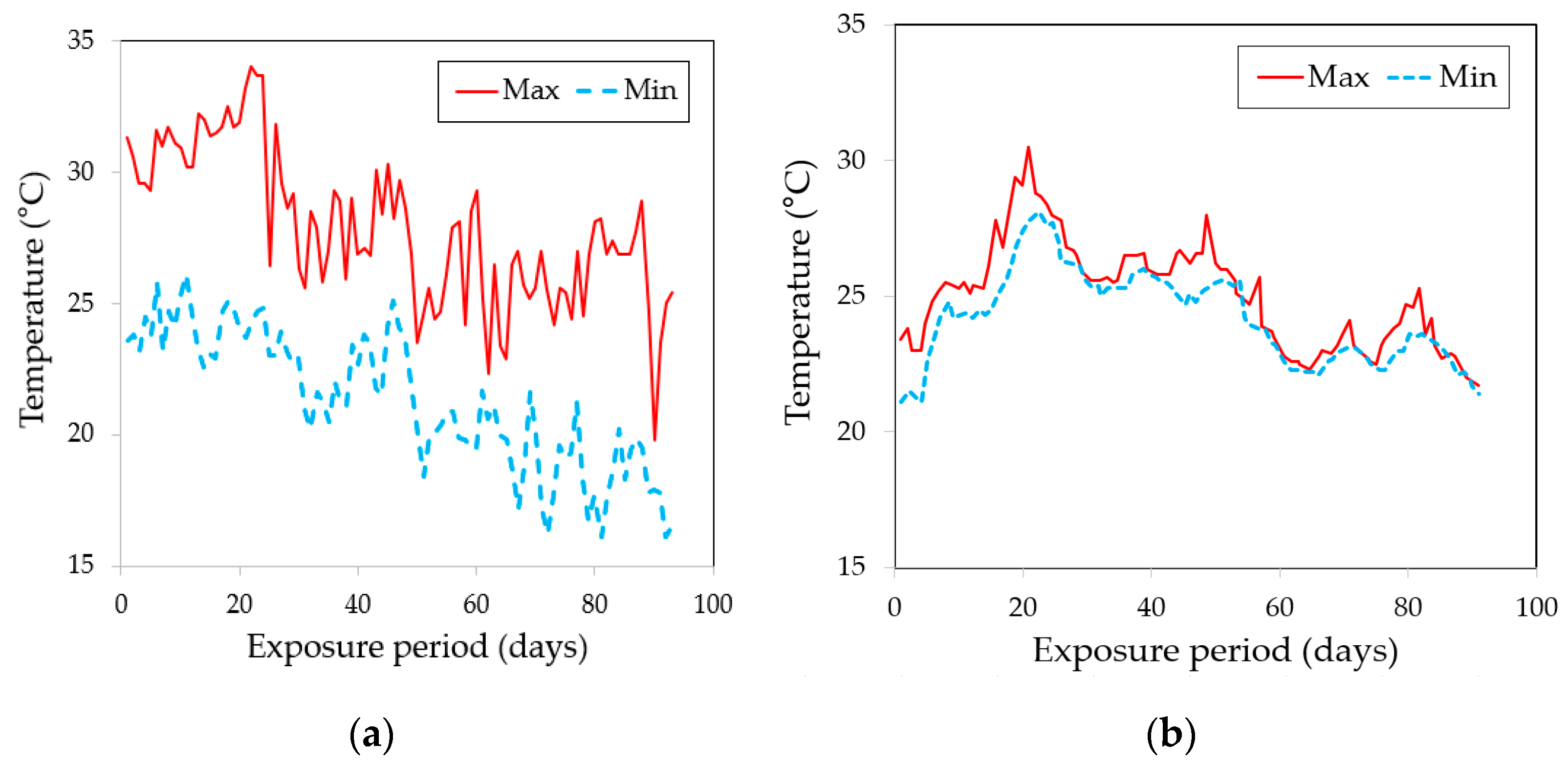
5.3. Effect of Exposure Condition on the Measured Thickness and Bond Strength
5.4. Effect of Exposure Periods on the Measured Thickness and Bond Strength
6. Discussion
7. Conclusions
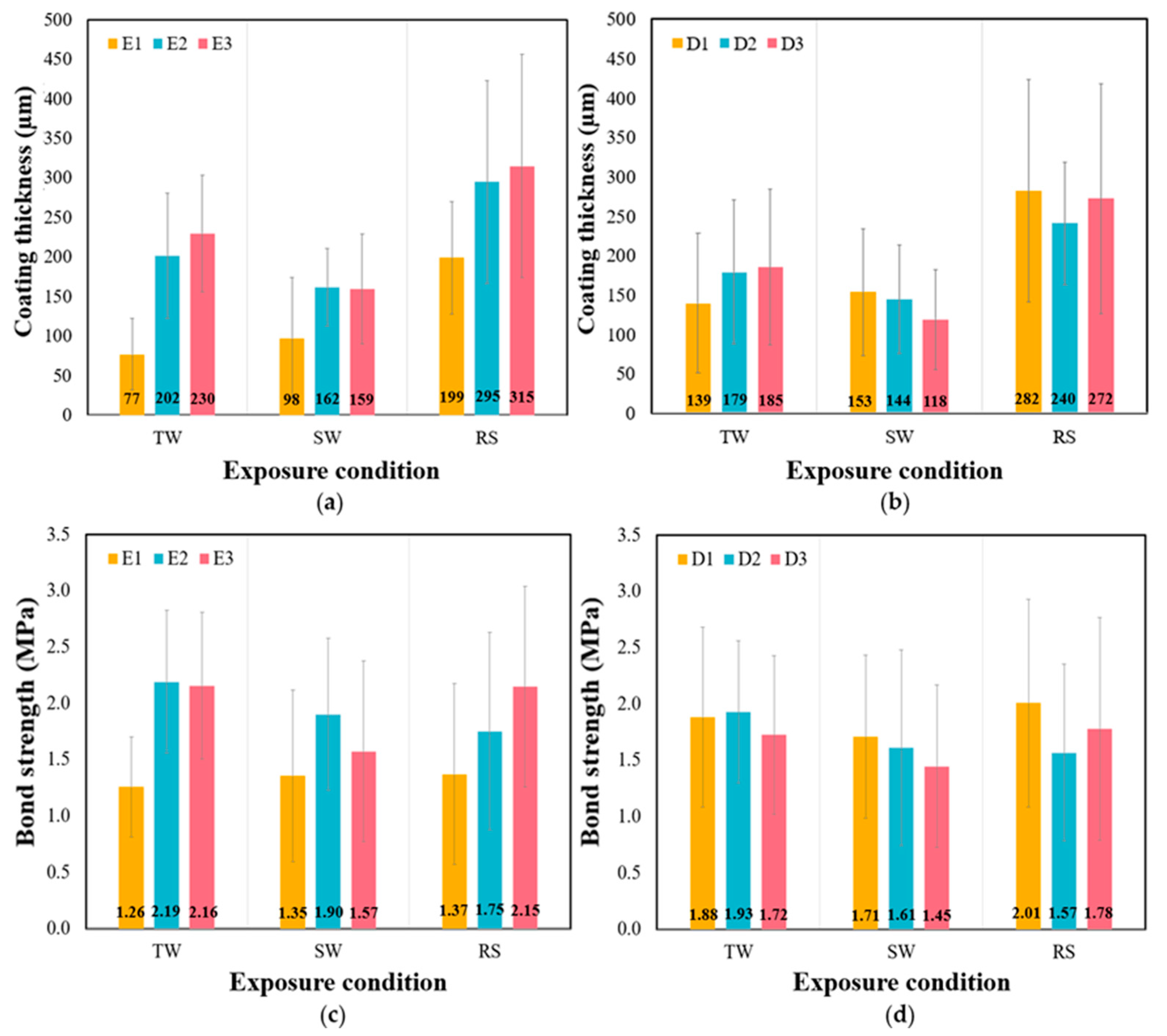
- BPA (E1, control) had the lowest coating thickness and bond strength, whereas BPA with zinc powder (E2) and BPA with cresyl glycidyl ether (E3) demonstrated similar values.
- The impact of the chosen coating method on the measured thickness and strength values appeared to be minimal, and notably, the conventional roller (D1) had the highest thickness variation. This might be attributed to the possibility that the other factors had a more significant influence than the coating method.
- Under RS conditions, there was a noticeable increase in coating thickness compared to the controlled condition. As for bond strength, the exposure conditions had only a limited effect, except for E3.
- Thickness values remained relatively stable regardless of the exposure period, and E3 demonstrated an upward trend in bond strength with increasing exposure time, particularly under the RS conditions.
- The relationship between the measured thickness and bond strength appeared unclear, and this pattern aligns with findings from previous studies.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, X.; Shi, Z.; Shi, C.; Ling, T.; Li, N. A review on concrete surface treatment Part I: Types and mechanisms. Constr. Build. Mater. 2017, 132, 578–590. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Brenna, A.; Bolzoni, F.M.; Berra, M.; Pastore, T.; Ormellese, M. Effect of polymer modified cementitious coatings on water and chloride permeability in concrete. Constr. Build. Mater. 2013, 49, 720–728. [Google Scholar] [CrossRef]
- Almusallam, A.; Khan, F.M.; Maslehuddin, M. Performance of Concrete Coating under Varying Exposure Conditions. Mater. Struct. 2002, 35, 487–494. [Google Scholar] [CrossRef]
- Almusallam, A.A.; Khan, F.M.; Dulaijan, S.U.; Al-Amoudi, O.S.B. Effectiveness of surface coatings in improving concrete durability. Cem. Concr. Compos. 2003, 25, 473–481. [Google Scholar] [CrossRef]
- Aguirre-Guerrero, A.M.; de Gutiérrez, R.M. Alkali-activated protective coatings for reinforced concrete exposed to chlorides. Constr. Build. Mater. 2021, 268, 121098. [Google Scholar] [CrossRef]
- Kang, Y.; Chen, X.; Song, S.; Yu, L.; Zhang, P. Friction and wear behavior of nanosilica-filled epoxy resin composite coatings. Appl. Surf. Sci. 2012, 258, 6384–6390. [Google Scholar] [CrossRef]
- Hang, Z.; He, K.; Zhao, W.; Yu, Y. Fracture properties of a concrete-epoxy mortar interface with precoating treatment applied through image correlation technology. Constr. Build. Mater. 2023, 370, 130640. [Google Scholar] [CrossRef]
- Chruściel, J.J.; Leśniak, E. Modification of epoxy resins with functional silanes, polysiloxanes, silsesquioxanes, silica and silicates. Prog. Polym. Sci. 2015, 41, 67–121. [Google Scholar] [CrossRef]
- Moradllo, M.K.; Shekarchi, M.; Hoseini, M. Time-dependent performance of concrete surface coatings in tidal zone of marine environment. Constr. Build. Mater. 2012, 30, 198–205. [Google Scholar] [CrossRef]
- Won, B.; Kim, M.O.; Park, S.; Yi, J.H. Effects of Water Exposure on the Interfacial Bond between an Epoxy Resin Coating and a Concrete Substrate. Materials 2019, 12, 3715. [Google Scholar] [CrossRef]
- Abdelkader, A.F.; White, J.R. Water absorption in epoxy resins: The effects of the crosslinking agent and curing temperature. J. Appl. Polym. Sci. 2005, 98, 2544–2549. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, R.; Liu, C. An evaluation method of underwater ocean environment safety situation based on D-S evidence theory. Adv. Meteorol. 2015, 2015, 207656. [Google Scholar] [CrossRef]
- Rajasärkkä, J.; Pernica, M.; Kuta, J.; Lašňák, J.; Šimek, Z.; Bláha, L. Drinking water contaminants from epoxy resin-coated pipes: A field study. Water Res. 2016, 103, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Abdur Rahman, A.R.; Bhat, S.; Bhansali, S. Design, fabrication, and impedance characterization of a capacitance-based salinity sensor for marine applications. J. Electrochem. Soc. 2008, 155, J355. [Google Scholar] [CrossRef][Green Version]
- Tong, Z.; Guo, H.; Di, Z.; Sheng, Y.; Song, L.; Hu, J.; Gao, X.; Hou, Y.; Zhan, X.; Zhang, Q. Squid inspired elastomer marine coating with efficient antifouling strategies: Hydrophilized defensive surface and lower modulus. Colloids Surf. B Biointerfaces 2022, 213, 112392. [Google Scholar] [CrossRef]
- Hussain, A.K.; Seetharamaiah, N.; Pichumani, M.; Chakra, C.S. Research progress in organic zinc rich primer coatings for cathodic protection of metals—A comprehensive review. Prog. Org. Coat. 2021, 153, 106040. [Google Scholar] [CrossRef]
- Sharma, J.; Mishra, I.M.; Kumar, V. Degradation and mineralization of Bisphenol A (BPA) in aqueous solution using advanced oxidation processes: UV/H2O2 and UV/S2O82− oxidation systems. J. Environ. Manag. 2015, 156, 266–275. [Google Scholar] [CrossRef]
- Liu, Y.L. Flame-retardant epoxy resins from novel phosphorus-containing novolac. Polymer 2001, 42, 3445–3454. [Google Scholar] [CrossRef]
- Park, S.; Shon, M. Effects of multi-walled carbo nano tubes on corrosion protection of zinc rich epoxy resin coating. J. Ind. Eng. Chem. 2015, 21, 1258–1264. [Google Scholar] [CrossRef]
- Sadowski, Ł.; Czarnecki, S.; Hoła, J. Evaluation of the height 3D roughness parameters of concrete substrate and the adhesion to epoxy resin. Int. J. Adhes. Adhes. 2016, 67, 3–13. [Google Scholar] [CrossRef]
- Kim, S.; Hong, H.; Han, T.H.; Kim, M.O. Early-age tensile bond characteristics of epoxy coatings for underwater applications. Coatings 2019, 9, 757. [Google Scholar] [CrossRef]
- Kim, S.; Hong, H.; Park, J.K.; Park, S.; Choi, S.I.; Kim, M.O. Effect of exposure conditions on the interfacial bond properties of SS400 plate coated with various epoxy resins. Coatings 2020, 10, 1159. [Google Scholar] [CrossRef]
- Al-Kheetan, M.J.; Rahman, M.M.; Chamberlain, D.A. Influence of early water exposure on modified cementitious coating. Constr. Build. Mater. 2017, 141, 64–71. [Google Scholar] [CrossRef]
- Sadati, S.; Arezoumandi, M.; Shekarchi, M. Long-term performance of concrete surface coatings in soil exposure of marine environments. Constr. Build. Mater. 2015, 94, 656–663. [Google Scholar] [CrossRef]
- Basheer, P.; Basheer, L.; Cleland, D.; Long, A. Surface treatments for concrete: Assessment methods and reported performance. Constr. Build. Mater. 1997, 11, 413–429. [Google Scholar] [CrossRef]
- Kim, M.O.; Jeong, Y.; Kang, S.H.; Moon, J.; Yi, J.H. Tensile bond characteristics between underwater coating materials and concrete substrate. J. Korean Soc. Coast. Ocean. Eng. 2018, 30, 298–305. [Google Scholar] [CrossRef]
- Dolan, C.W.; Ahearn, E.B.; Deng, J.; Tanner, J.E.; Mukai, D. Durability and accelerated aging testing of CFRP repair systems. In Proceedings of the CICE 2008: 4th International Conference on FRP Composites in Civil Engineering, Zurich, Switzerland, 22 July 2008; pp. 22–24. [Google Scholar]
- Toutanji, H.A.; Choi, H.; Wong, D.; Gilbert, J.A.; Alldredge, D.J. Applying a polyurea coating to high-performance organic cementitious materials. Constr. Build. Mater. 2013, 38, 1170–1179. [Google Scholar] [CrossRef]
- Yang, F.; Liu, T.; Li, J.; Qiu, S.; Zhao, H. Anticorrosive behavior of a zinc-rich epoxy coating containing sulfonated polyaniline in 3.5% NaCl solution. RSC Adv. 2018, 8, 13237–13247. [Google Scholar] [CrossRef]
- ASTM C39/C39M; Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens. ASTM International: West Conshohocken, PA, USA, 2005.
- ASTM C496; Standard Test Method for Splitting Tensile Strength of cylindrical Concrete Specimens. American Society for Testing and Materials: West Conshohocken, PA, USA, 1996; p. 496.
- ASTM D6132-13; Standard Test Method for Nondestructive Measurement of Dry Film Thickness of Applied Organic Coatings Using an Ultrasonic Coating Thickness Gage. ASTM International: West Conshohocken, PA, USA, 2013.
- ASTM C1583-13; Standard Test Method for Tensile Strength of Concrete Surfaces and the Bond Strength or Tensile Strength of Concrete Repair and Overlay Materials by Direct Tension (Pull-Off Method). ASTM International: West Conshohocken, PA, USA, 2013.
- Liu, Y.; Li, C.X.; Huang, X.F.; Ma, K.; Luo, X.T.; Li, C.J. Effect of water environment on particle deposition of underwater cold spray. Appl. Surf. Sci. 2020, 506, 144542. [Google Scholar] [CrossRef]
- Jones, M.; Dhir, R.; Gill, J. Concrete surface treatment: Effect of exposure temperature on chloride diffusion resistance. Cem. Concr. Res. 1995, 25, 197–208. [Google Scholar] [CrossRef]
- Medeiros, M.H.; Helene, P. Surface treatment of reinforced concrete in marine environment: Influence on chloride diffusion coefficient and capillary water absorption. Constr. Build. Mater. 2009, 23, 1476–1484. [Google Scholar] [CrossRef]
- Bao, J.; Wei, J.; Zhang, P.; Zhuang, Z.; Zhao, T. Experimental and theoretical investigation of chloride ingress into concrete exposed to real marine environment. Cem. Concr. Compos. 2022, 130, 104511. [Google Scholar] [CrossRef]
- Mujahid, M.; Chen, C.; Hu, W.; Wang, Z.-K.; Duan, Y. Progress of high-throughput and low-cost flexible perovskite solar cells. Sol. RRL 2020, 4, 1900556. [Google Scholar] [CrossRef]
- Butt, M.A.; Tyszkiewicz, C.; Karasinski, P.; Zieba, M.; Kazmierczak, A.; Zdonczyk, M.; Duda, L.; Guzik, M.; Olszewski, J.; Martynkien, T.; et al. Optical thin films fabrication techniques—Towards a low-cost solution for the integrated photonic platform: A review of the current status. Materials 2022, 15, 4591. [Google Scholar] [CrossRef]
- Utne-Palm, A.C. Visual feeding of fish in a turbid environment: Physical and behavioural aspects. Mar. Freshw. Behav. Physiol. 2002, 35, 111–128. [Google Scholar] [CrossRef]
- Cheng, H.; Chu, J.; Zhang, R.; Zhang, P. Simulation and measurement of the effect of various factors on underwater polarization patterns. Optik 2021, 237, 166637. [Google Scholar] [CrossRef]
- Matos, T.; Faria, C.L.; Martins, M.S.S.; Henriques, R.; Gomes, P.A.A.; Goncalves, L.M.M. Development of a Cost-Effective Optical Sensor for Continuous Monitoring of Turbidity and Suspended Particulate Matter in Marine Environment. Sensors 2019, 19, 4439. [Google Scholar] [CrossRef]
- Malikov, A.K.U.; Kim, Y.H.; Yi, J.-H.; Kim, J.; Zhang, J.; Cho, Y. Neural-Network-Based Ultrasonic Inspection of Offshore Coated Concrete Specimens. Coatings 2022, 12, 773. [Google Scholar] [CrossRef]
- Zhang, J.; Cho, Y.; Kim, J.; Malikov, A.K.U.; Kim, Y.H.; Yi, J.-H. Nondestructive Inspection of Underwater Coating Layers Using Ultrasonic Lamb Waves. Coatings 2023, 4, 728. [Google Scholar] [CrossRef]
- Malikov, A.K.U.; Cho, Y.; Kim, Y.H.; Kim, J.; Park, J.; Yi, J.-H. Ultrasonic assessment of thickness and bonded zone quality of coating layer based on short-time fourier transform and convolutional neural networks. Coatings 2021, 11, 909. [Google Scholar] [CrossRef]
- Zhang, J.; Cho, Y.; Kim, J.; Malikov, A.K.u.; Kim, Y.H.; Yi, J.-H.; Li, W. Non-destructive evaluation of coating thickness using water immersion ultrasonic testing. Coatings 2021, 11, 1421. [Google Scholar] [CrossRef]
- Park, J.K.; Kim, M.O. The effect of different exposure conditions on the pull-off strength of various epoxy resins. J. Build. Eng. 2021, 38, 102223. [Google Scholar] [CrossRef]
- Roghanian, N.; Banthia, N. Development of a sustainable coating and repair material to prevent bio-corrosion in concrete sewer and waste-water pipes. Cem. Concr. Compos. 2019, 100, 99–107. [Google Scholar] [CrossRef]
- Vaidya, S.; Allouche, E.N. Electrokinetically deposited coating for increasing the service life of partially deteriorated concrete sewers. Constr. Build. Mater. 2010, 24, 2164–2170. [Google Scholar] [CrossRef]
- Haile, T.; Nakhla, G.; Allouche, E.; Vaidya, S. Evaluation of the bactericidal characteristics of nano-copper oxide or functionalized zeolite coating for bio-corrosion control in concrete sewer pipes. Corros. Sci. 2010, 52, 45–53. [Google Scholar] [CrossRef]
- Merachtsaki, D.; Tsardaka, E.-C.; Anastasiou, E.; Zouboulis, A. Anti-corrosion properties of magnesium oxide/magnesium hydroxide coatings for application on concrete surfaces (sewerage network pipes). Constr. Build. Mater. 2021, 312, 125441. [Google Scholar] [CrossRef]
- Vincke, E.; Wanseele, E.V.; Monteny, J.; Beeldens, A.; Belie, N.D.; Taerwe, L.; Gemert, D.V.; Verstraete, W. Influence of polymer addition on biogenic sulfuric acid attack of concrete. Int. Biodeterior. Biodegrad. 2002, 49, 283–292. [Google Scholar] [CrossRef]
- Hayek, M.; Salgues, M.; Souche, J.-C.; Cunge, E.; Giraudel, C.; Paireau, O. Influence of the Intrinsic Characteristics of Cementitious Materials on Biofouling in the Marine Environment. Sustainability 2021, 13, 2625. [Google Scholar] [CrossRef]
- Bank, L.C.; Gentry, T.R.; Barkatt, A. Accelerated Test Methods to Determine the Long-Term Behavior of FRP Composite Structures: Environmental Effects. J. Reinf. Plast. Compos. 1995, 14, 559–587. [Google Scholar] [CrossRef]
- Sethi, S.; Ray, B.C. Environmental Effects on Fibre Reinforced Polymeric Composites: Evolving Reasons and Remarks on Interfacial Strength and Stability. Adv. Colloid Interface Sci. 2015, 217, 43–67. [Google Scholar] [CrossRef]
- Liu, T.Q.; Liu, X.; Feng, P. A Comprehensive Review on Mechanical Properties of Pultruded FRP Composites Subjected to Long-Term Environmental Effects. Compos. B Eng. 2020, 191, 107958. [Google Scholar] [CrossRef]
- Butt, M.; Tyszkiewicz, C.; Wojtasik, K.; Karasinski, P.; Kazmierczak, A.; Piramidowicz, R. Subwavelength grating waveguide structures proposed on the low-cost silica-titania platform for optical filtering and refractive index sensing applications. Int. J. Mol. Sci. 2022, 23, 6614. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.; Tyszkiewicz, C.; Karasinski, P.; Zieba, M.; Hlushchenko, D.; Baraniecki, T.; Kazmierczak, A.; Piramidowicz, R.; Guzik, M.; Bachmatiuk, A. Development of a low-cost silica-titania optical platform for integrated photonics applications. Opt. Express 2022, 30, 23678–23694. [Google Scholar] [CrossRef] [PubMed]




| Nomenclature | Epoxy Resin | Hardener | Mixing Ratio (Resin:Hardener) | Density (g/cm3) | Product |
|---|---|---|---|---|---|
| E1 | Bisphenol A (C15H16O2) | Isophorone diamine (C10H22N2) | 5.1:1.0 | 1.60 | RS500P |
| E2 | Bisphenol A with zinc powder | Isophorone diamine (C10H22N2) | 5.0:1.0 | 1.82 | Alocit 28.14 |
| E3 | Bisphenol A with cresyl glycidyl ether (C10H12O2) | Isophorone diamine (C10H22N2) | 5.0:1.0 | 1.55 | Alocit 28.15 |
| Ion | Seawater (mg/L) | Tap Water (mg/L) |
|---|---|---|
| Chloride (Cl−) | 19,000 | 39.1 |
| Sodium (Na+) | 7500 | 86.2 |
| Sulfate (SO42−) | 3300 | 58.8 |
| Magnesium (Mg2+) | 880 | - |
| Calcium (Ca2+) | 400 | - |
| Potassium (K+) | 490 | - |
| Nitrate (NO3−) | - | 16.1 |
| Type of Water | pH | Salinity (%) | Temperature (°C) | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| TW in the laboratory | 7.15 | 1.35 | 0.05 | - | 20.70 | 0.58 |
| SW in the laboratory | 8.17 | 0.17 | 3.26 | 0.18 | 19.50 | 0.45 |
| SW in the real sea environment | 8.14 | 0.16 | 3.27 | 0.19 | 24.62 | 1.87 |
| Exposure Condition | Epoxy Type | E1 | E2 | E3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure Period | Coating Device | T (µm) | S.D. (µm) | fbond (MPa) | S.D. (MPa) | T (µm) | S.D. (µm) | fbond (MPa) | S.D. (MPa) | T (µm) | S.D. (µm) | fbond (MPa) | S.D. (MPa) | |
| Tap Water (TW) | 7D | D1 | 27.78 | 5.17 | 0.87 | 0.32 | 162.22 | 15.71 | 2.27 | 0.29 | 146.67 | 26.25 | 3.04 | 0.26 |
| D2 | 82.89 | 4.94 | 1.89 | 0.34 | 310.00 | 58.94 | 2.29 | 0.27 | 291.11 | 38.62 | 1.64 | 0.37 | ||
| D3 | 42.22 | 4.23 | 0.91 | 0.17 | 128.89 | 3.14 | 1.81 | 0.22 | 304.44 | 20.43 | 1.75 | 0.23 | ||
| 28D | D1 | 30.67 | 3.81 | 1.74 | 0.40 | 176.67 | 14.40 | 2.52 | 0.13 | 200.00 | 38.39 | 1.24 | 0.19 | |
| D2 | 54.67 | 3.31 | 1.15 | 0.19 | 224.44 | 20.61 | 2.22 | 0.26 | 156.67 | 36.67 | 2.79 | 0.34 | ||
| D3 | 91.33 | 7.91 | 1.41 | 0.33 | 143.33 | 20.00 | 1.89 | 0.51 | 324.44 | 43.40 | 2.12 | 0.47 | ||
| 56D | D1 | 30.00 | 2.37 | 1.16 | 0.20 | 313.33 | 125.11 | 3.02 | 0.12 | 166.67 | 9.81 | 2.33 | 0.08 | |
| D2 | 61.33 | 22.65 | 1.39 | 0.20 | 237.78 | 26.43 | 2.75 | 0.24 | 187.78 | 29.10 | 2.14 | 0.65 | ||
| D3 | 123.33 | 26.73 | 0.87 | 0.29 | 140.00 | 19.63 | 1.95 | 0.67 | 313.33 | 47.69 | 1.67 | 0.17 | ||
| 91D | D1 | 112.67 | 9.54 | 1.05 | 0.10 | 160.00 | 9.43 | 0.97 | 0.27 | 154.44 | 11.33 | 2.05 | 0.17 | |
| D2 | 91.33 | 10.93 | 1.10 | 0.30 | 227.78 | 9.56 | 2.06 | 0.28 | 234.44 | 20.06 | 2.14 | 0.32 | ||
| D3 | 174.89 | 24.08 | 1.46 | 0.47 | 126.67 | 0.00 | 2.53 | 0.68 | 252.22 | 69.99 | 3.15 | 0.15 | ||
| Sea Water (SW) | 7D | D1 | 97.56 | 26.96 | 2.62 | 0.43 | 102.22 | 31.54 | 1.33 | 0.20 | 92.00 | 18.06 | 1.86 | 0.57 |
| D2 | 54.67 | 9.49 | 0.99 | 0.44 | 181.11 | 30.10 | 2.16 | 0.21 | 182.22 | 41.84 | 1.39 | 0.72 | ||
| D3 | 42.44 | 14.73 | 1.10 | 0.51 | 173.33 | 26.81 | 2.48 | 0.06 | 243.33 | 21.26 | 2.32 | 0.14 | ||
| 28D | D1 | 241.11 | 12.57 | 2.61 | 0.26 | 142.22 | 35.52 | 1.40 | 0.13 | 263.33 | 57.35 | 1.32 | 0.39 | |
| D2 | 115.56 | 11.00 | 2.25 | 0.42 | 222.22 | 40.40 | 2.70 | 0.37 | 100.00 | 11.86 | 0.45 | 0.12 | ||
| D3 | 51.56 | 6.31 | 0.74 | 0.05 | 151.11 | 32.81 | 0.98 | 0.24 | 164.44 | 60.63 | 1.30 | 0.65 | ||
| 56D | D1 | 253.33 | 98.99 | 1.39 | 0.33 | 196.67 | 43.20 | 1.16 | 0.38 | 105.56 | 32.81 | 1.02 | 0.05 | |
| D2 | 47.56 | 6.56 | 0.57 | 0.13 | 197.78 | 10.30 | 2.18 | 0.55 | 238.89 | 39.85 | 1.10 | 0.32 | ||
| D3 | 58.89 | 10.90 | 0.66 | 0.10 | 92.22 | 8.31 | 1.50 | 0.40 | 116.67 | 35.59 | 1.03 | 0.15 | ||
| 91D | D1 | 62.22 | 10.42 | 0.98 | 0.17 | 183.33 | 18.86 | 2.25 | 0.25 | 100.22 | 8.02 | 2.74 | 0.20 | |
| D2 | 65.78 | 12.54 | 1.16 | 0.33 | 160.00 | 15.15 | 2.49 | 0.62 | 171.11 | 29.98 | 2.34 | 0.53 | ||
| D3 | 81.11 | 11.70 | 1.71 | 0.56 | 131.67 | 11.67 | 2.01 | 0.31 | 118.89 | 9.56 | 1.98 | 0.76 | ||
| Real Sea (RS) | 7D | D1 | 183.33 | 62.78 | 1.00 | 0.02 | 294.44 | 131.38 | 2.20 | 0.46 | Not measured | |||
| D2 | 236.67 | 67.71 | 0.74 | 0.24 | 214.44 | 10.30 | 1.34 | 0.41 | 372.22 | 21.83 | 2.20 | 0.05 | ||
| D3 | 230.00 | 82.78 | 1.14 | 0.26 | 425.56 | 64.54 | 0.90 | 0.22 | 167.78 | 51.74 | 1.31 | 0.16 | ||
| 28D | D1 | 198.89 | 50.36 | 1.89 | 0.15 | 476.67 | 151.51 | 2.36 | 0.09 | Not measured | ||||
| D2 | 191.33 | 24.99 | 1.44 | 0.42 | 240.00 | 89.57 | 2.23 | 0.41 | 352.22 | 5.67 | 0.90 | 0.44 | ||
| D3 | 230.00 | 17.85 | 0.67 | 0.41 | 206.67 | 56.83 | 2.63 | 0.49 | 635.56 | 99.23 | 3.21 | 0.18 | ||
| 56D | D1 | 93.33 | 11.86 | 0.49 | 0.12 | 431.11 | 15.71 | 2.22 | 0.93 | 348.89 | 36.24 | 1.94 | 0.85 | |
| D2 | 164.44 | 31.54 | 1.61 | 0.19 | 285.56 | 32.47 | 1.85 | 0.21 | 233.33 | 20.55 | 2.40 | 0.81 | ||
| D3 | 276.56 | 19.12 | 0.64 | 0.21 | 198.89 | 20.61 | 1.15 | 0.32 | 287.78 | 39.09 | 2.37 | 0.26 | ||
| 91D | D1 | 253.33 | 47.69 | 3.02 | 0.09 | 262.22 | 58.20 | 2.72 | 0.49 | Not measured | ||||
| D2 | 226.67 | 51.93 | 1.25 | 0.31 | 141.11 | 26.15 | 0.09 | 0.03 | 202.22 | 33.26 | 2.27 | 0.71 | ||
| D3 | 104.00 | 4.25 | 2.44 | 0.14 | 253.33 | 52.49 | 2.20 | 0.78 | 251.11 | 46.93 | 3.14 | 0.16 | ||
| Coating Thickness (µm) | ||||||
| Groups | Count | Sum | Average | Variance | ||
| E1 | 95 | 11,166.7 | 117.54 | 6979.596 | ||
| E2 | 95 | 19,436.7 | 204.60 | 7972.383 | ||
| E3 | 95 | 21,603.3 | 227.40 | 13,266.681 | ||
| Source of variation | SS | df | MS | F | p-value | F crit. |
| Between groups | 638,636.3 | 2 | 319,318.17 | 33.9476 | 6.1643 × 10−14 | 3.02778 |
| Within groups | 2,652,553.9 | 282 | 9406.22 | |||
| Total | 3,291,190.3 | 284 | ||||
| Bond Strength (MPa) | ||||||
| Groups | Count | Sum | Average | Variance | ||
| E1 | 93 | 116.9 | 1.26 | 0.421 | ||
| E2 | 93 | 179.8 | 1.93 | 0.591 | ||
| E3 | 93 | 178.9 | 1.92 | 0.679 | ||
| Source of variation | SS | df | MS | F | p-value | F crit. |
| Between groups | 27.9 | 2 | 13.94 | 24.7317 | 1.32 × 10−10 | 9.52465 |
| Within groups | 155.5 | 276 | 0.56 | |||
| Total | 183.4 | 278 | ||||
| Coating Thickness (µm) | |||||||
| Groups | Count | Sum | Average | Variance | |||
| D1 | 90 | 16,406.0 | 182.29 | 15,184.540 | |||
| D2 | 90 | 15,775.3 | 175.28 | 7009.231 | |||
| D3 | 90 | 15,469.3 | 171.88 | 9557.819 | |||
| Source of variation | SS | df | MS | F | p-value | F crit. | |
| Between groups | 5069.3 | 2 | 2534.67 | 0.2395 | 0.7872 | 3.02960 | |
| Within groups | 2,825,891.4 | 267 | 10,583.86 | ||||
| Total | 2,830,960.8 | 269 | |||||
| Bond Strength (MPa) | |||||||
| Groups | Count | Sum | Average | Variance | |||
| D1 | 90 | 168.2 | 1.87 | 0.665 | |||
| D2 | 90 | 152.7 | 1.70 | 0.622 | |||
| D3 | 90 | 142.3 | 1.58 | 0.596 | |||
| Source of variation | SS | df | MS | F | p-value | F crit. | |
| Between groups | 3.8 | 2 | 1.89 | 3.0090 | 0.0510 | 3.02960 | |
| Within groups | 167.6 | 267 | 0.63 | ||||
| Total | 171.3 | 269 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Yi, J.-H.; Hong, H.; Choi, S.I.; Kim, D.; Kim, M.O. Interfacial Bond Properties of Underwater Concrete Coated with Bisphenol A Epoxy Resins. Polymers 2023, 15, 4290. https://doi.org/10.3390/polym15214290
Kim S, Yi J-H, Hong H, Choi SI, Kim D, Kim MO. Interfacial Bond Properties of Underwater Concrete Coated with Bisphenol A Epoxy Resins. Polymers. 2023; 15(21):4290. https://doi.org/10.3390/polym15214290
Chicago/Turabian StyleKim, Sungwon, Jin-Hak Yi, Hyemin Hong, Seoung Ik Choi, Dongchan Kim, and Min Ook Kim. 2023. "Interfacial Bond Properties of Underwater Concrete Coated with Bisphenol A Epoxy Resins" Polymers 15, no. 21: 4290. https://doi.org/10.3390/polym15214290
APA StyleKim, S., Yi, J.-H., Hong, H., Choi, S. I., Kim, D., & Kim, M. O. (2023). Interfacial Bond Properties of Underwater Concrete Coated with Bisphenol A Epoxy Resins. Polymers, 15(21), 4290. https://doi.org/10.3390/polym15214290


.png)




