Chitosan-Enriched Salicylic Acid Nanoparticles Enhanced Anthocyanin Content in Grape (Vitis vinifera L. cv. Red Sultana) Berries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description, Plant Materials, Experimental Design and Treatments
2.2. Chitosan-Salicylic Acid Nanoparticles Characterization
2.3. Fresh and Dry Weights of Berries, Titratable Acidity, Total Soluble Solids and pH
2.4. Vitamin C, Total Carbohydrates and Carotenoids
2.5. Total Phenolic and Flavonoid Content
2.6. Total Anthocyanin Content and Anthocyanin Analysis
2.7. Antioxidant Capacity
2.8. PAL Enzyme Activity
2.9. Statistical Analyses
3. Results
3.1. Characterization of CTS-SA NPs
3.2. FW and DW of Berries, TA, TSS, TSS/TA Ratio, and pH
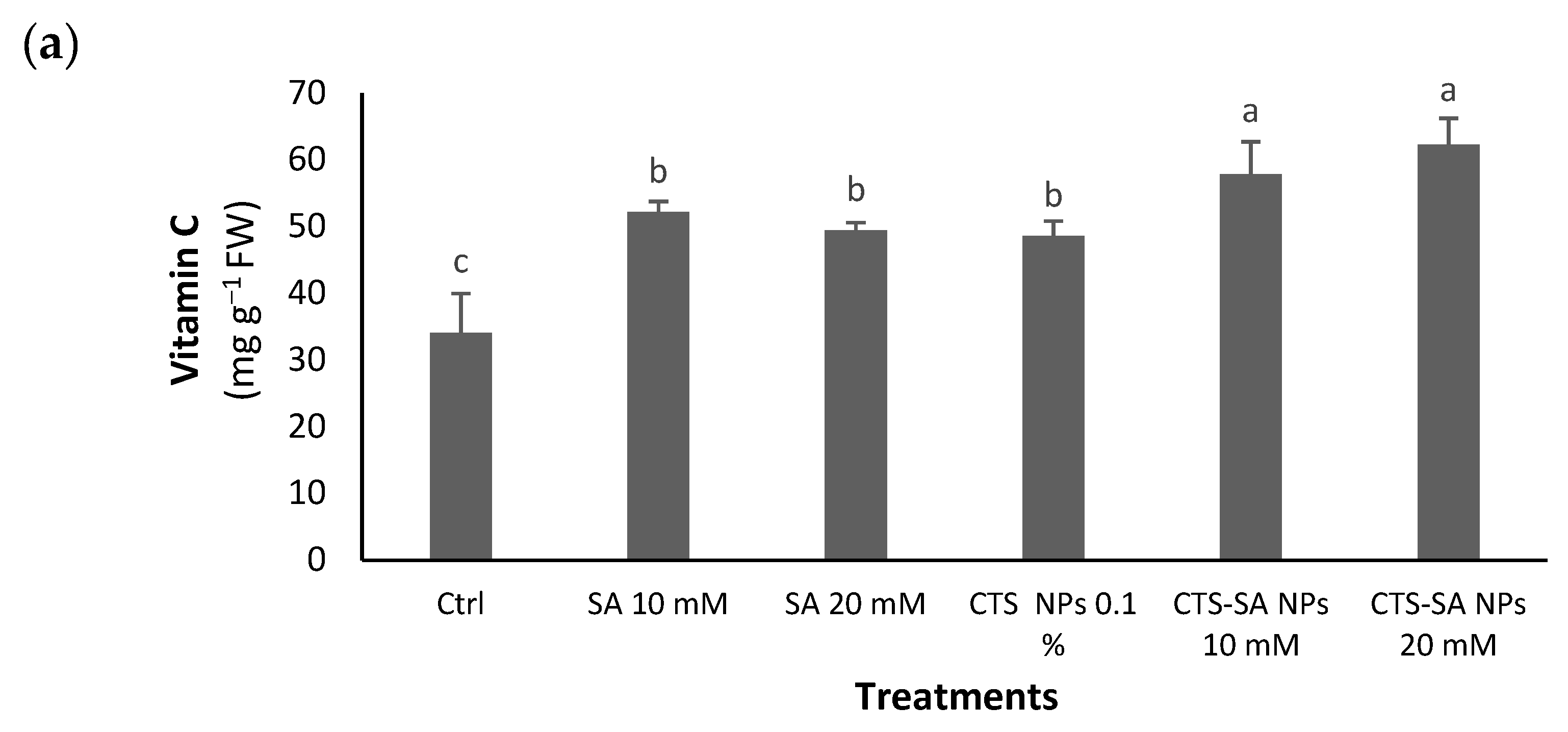
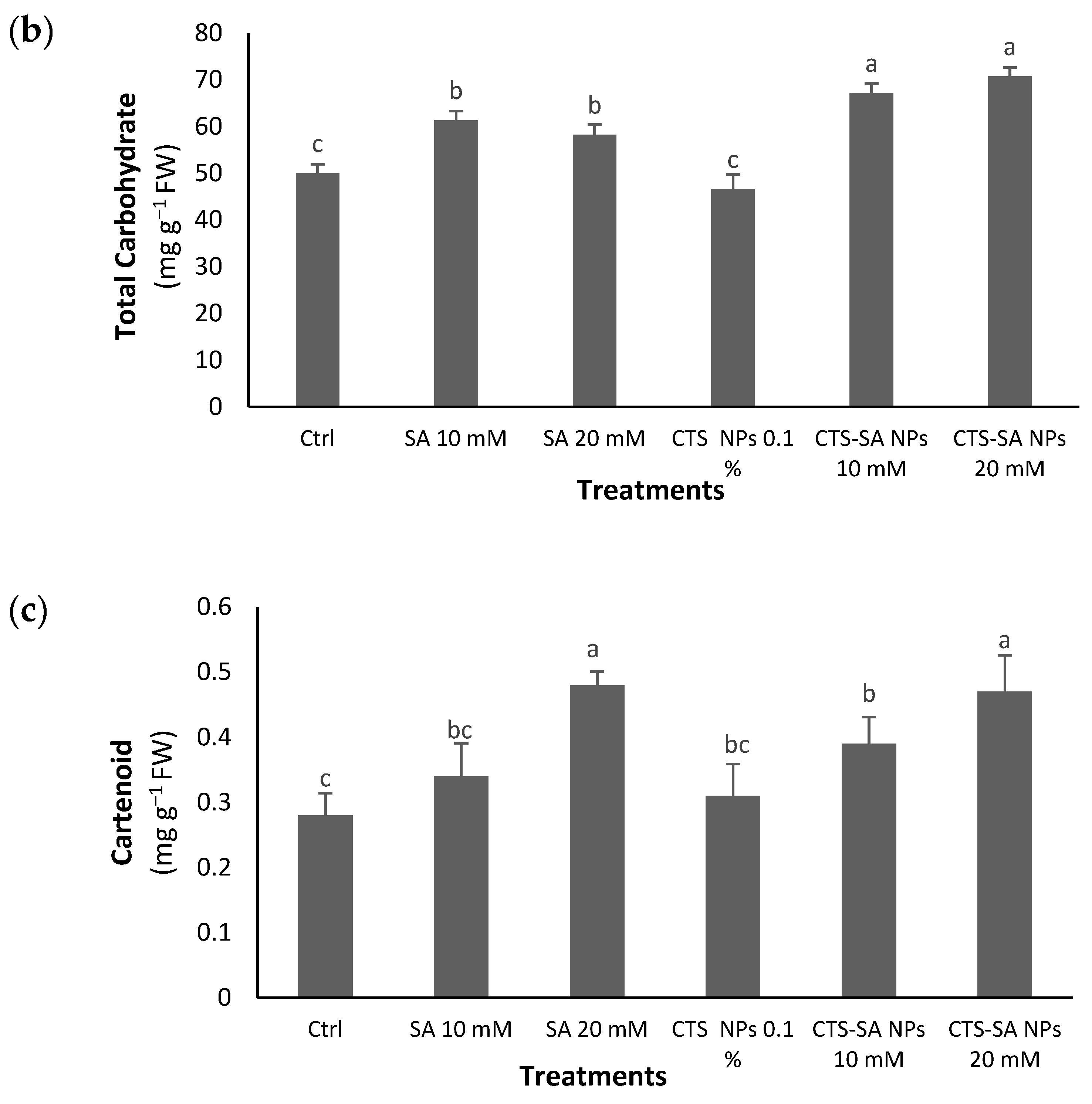
3.3. Vit C, Total Carbohydrates and Carotenoids
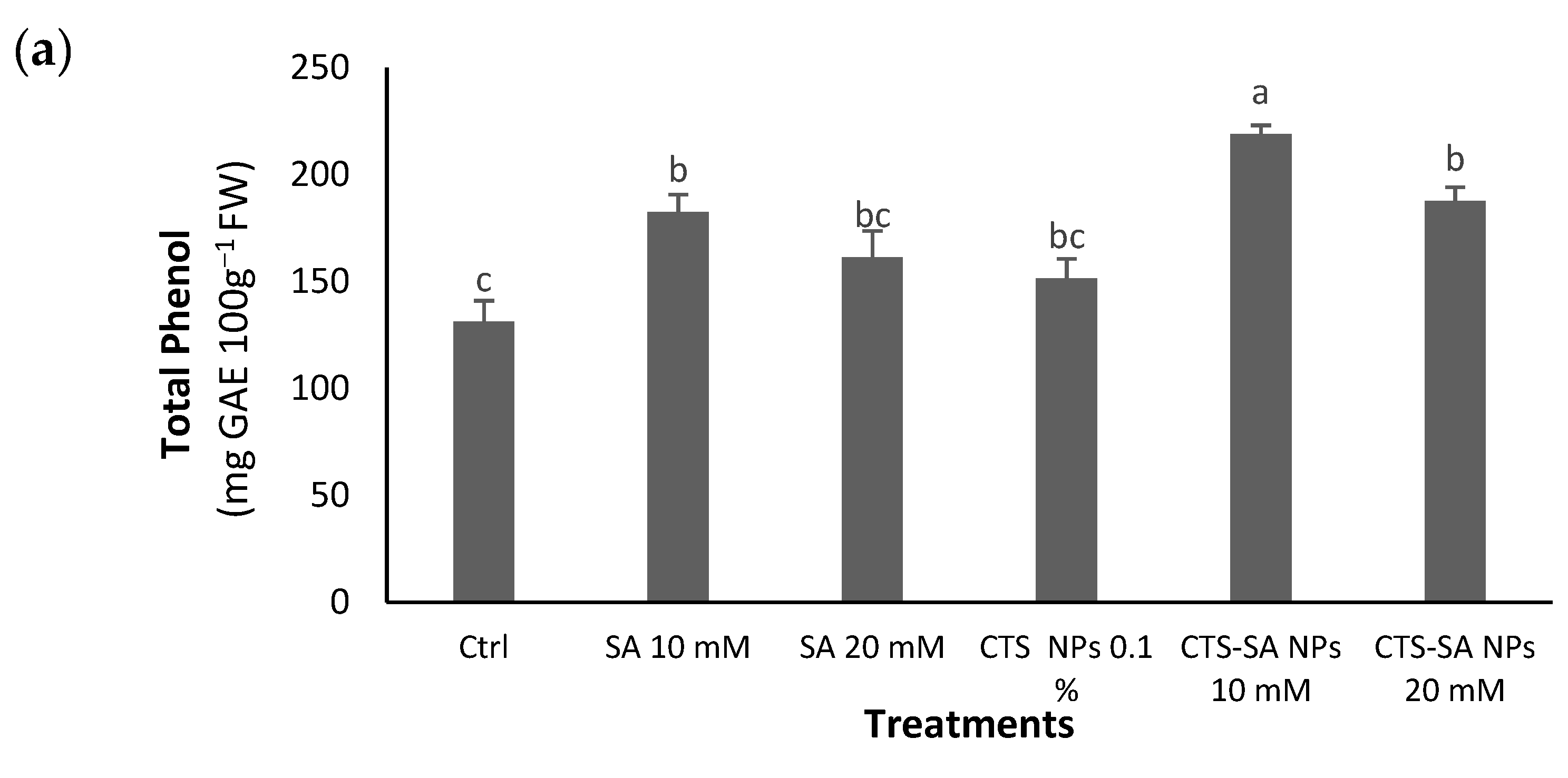
3.4. Total Phenolic Compounds, Flavonoid and Anthocyanin Contents, and Anthocyanin Analysis
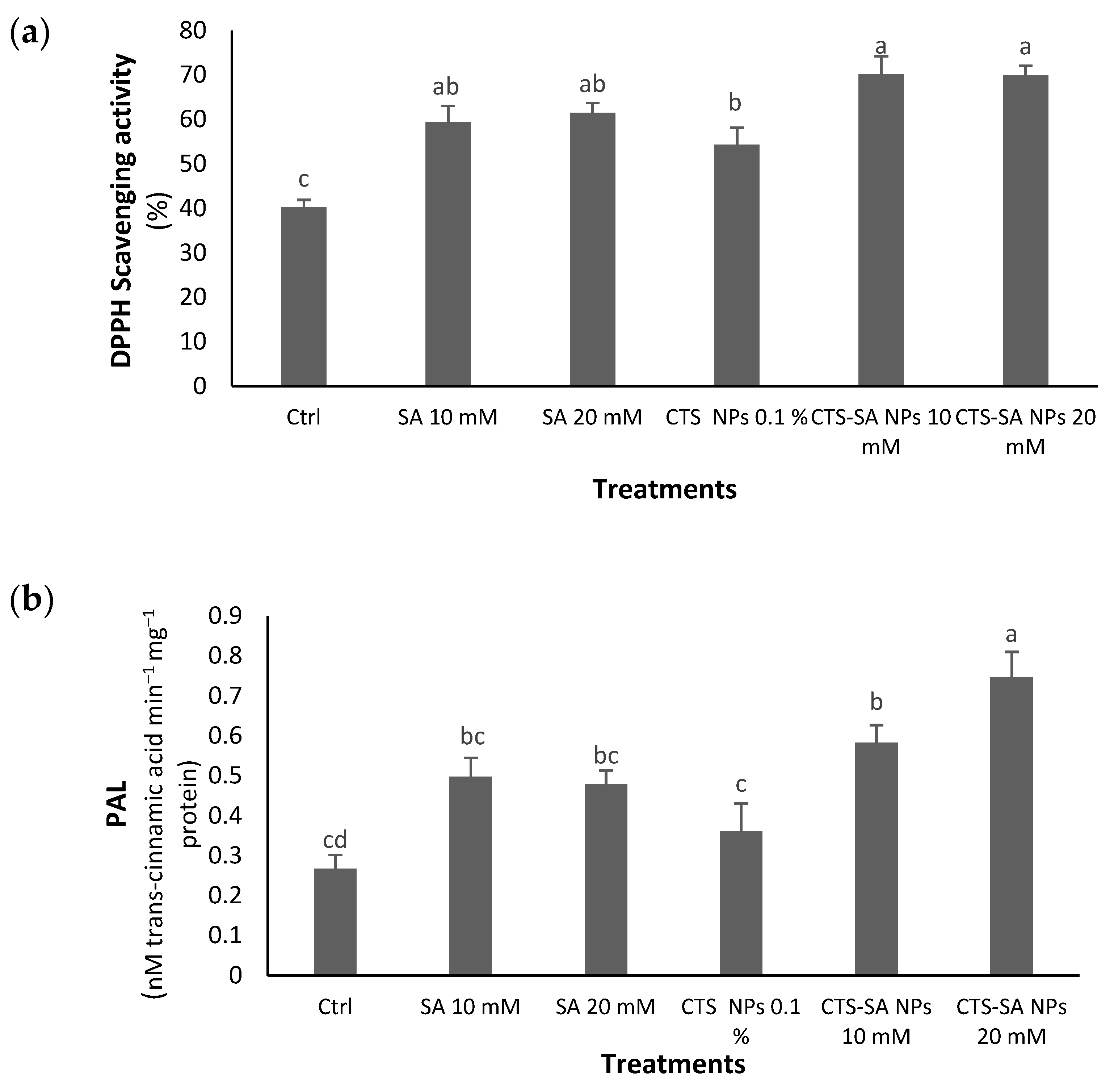
3.5. Antioxidant Capacity Based on DPPH and PAL Enzyme Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.; Barros, L.; Ferreira, I.C. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Oraei, M.; Panahirad, S.; Zaare-Nahandi, F.; Gohari, G. Pre-véraison treatment of salicylic acid to enhance anthocyanin content of grape (Vitis vinifera L.) berries. J. Sci. Food Agric. 2019, 99, 5946–5952. [Google Scholar] [CrossRef] [PubMed]
- Averilla, J.N.; Oh, J.; Kim, H.J.; Kim, J.S.; Kim, J.S. Potential health benefits of phenolic compounds in grape processing by-products. Food Sci. Biotechnol. 2019, 28, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Anna Malinowska, M.; Billet, K.; Drouet, S.; Munsch, T.; Unlubayir, M.; Tungmunnithum, D.; Giglioli-Guivarc’h, N.; Hano, C.; Lanoue, A. Grape cane extracts as multifunctional rejuvenating cosmetic ingredient: Evaluation of sirtuin activity, tyrosinase inhibition and bioavailability potential. Molecules 2020, 25, 2203. [Google Scholar] [CrossRef] [PubMed]
- Hano, C.; Tungmunnithum, D. Plant polyphenols, more than just simple natural antioxidants: Oxidative stress, aging and age-related diseases. Medicines 2020, 7, 26. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef]
- Panahirad, S.; Naghshiband-Hassani, R.; Ghanbarzadeh, B.; Zaare-Nahandi, F.; Mahna, N. Shelf life quality of plum fruits (Prunus domestica L.) improves with carboxymethylcellulose-based edible coating. HortScience 2019, 54, 505–510. [Google Scholar] [CrossRef]
- Panahirad, S.; Naghshiband-Hassani, R.; Bergin, S.; Katam, R.; Mahna, N. Improvement of postharvest quality of plum (Prunus domestica L.) using polysaccharide-based edible coatings. Plants 2020, 9, 1148. [Google Scholar] [CrossRef]
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant phenolics: Bioavailability as a key determinant of their potential health-promoting applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef]
- Gomes, E.P.; Borges, C.V.; Monteiro, G.C.; Belin, M.A.F.; Minatel, I.O.; Junior, A.P.; Tecchio, M.A.; Lima, G.P.P. Preharvest salicylic acid treatments improve phenolic compounds and biogenic amines in ‘Niagara Rosada’table grape. Postharvest Biol. Technol. 2021, 176, 111505. [Google Scholar] [CrossRef]
- Jediyi, H.; Naamani, K.; Elkoch, A.A.; Dihazi, A.; El Fels, A.E.A.; Arkize, W. First study on technological maturity and phenols composition during the ripeness of five Vitis vinifera L grape varieties in Morocco. Sci. Hortic. 2019, 246, 390–397. [Google Scholar] [CrossRef]
- Wang, S.; Chu, Z.; Ren, M.; Jia, R.; Zhao, C.; Fei, D.; Su, H.; Fan, X.; Zhang, X.; Li, Y.; et al. Identification of anthocyanin composition and functional analysis of an anthocyanin activator in Solanum nigrum fruits. Molecules 2017, 22, 876. [Google Scholar] [CrossRef] [PubMed]
- Alappat, B.; Alappat, J. Anthocyanin pigments: Beyond aesthetics. Molecules 2020, 25, 5500. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Liu, Y.; Xu, C.; Liu, J. Antiproliferative and proapoptotic activities of anthocyanin and anthocyanidin extracts from blueberry fruits on B16-F10 melanoma cells. Food Nutr. Res. 2017, 61, 1325308. [Google Scholar] [CrossRef]
- Oraei, M.; Gohari, G.; Panahirad, S.; Zareei, E.; Zaare-Nahandi, F. Effect of salicylic acid foliar application on Vitis vinifera L. cv.‘sultana’under salinity stress. Acta Sci. Pol.-Hortorum Cultus 2019, 18, 159–169. [Google Scholar] [CrossRef]
- Yanik, F.; Aytürk, Ö.; Çetinbaş-Genç, A.; Vardar, F. Salicylic acid-induced germination, biochemical and developmental alterations in rye (Secale cereale L.). Acta Bot. Croat. 2018, 77, 45–50. [Google Scholar] [CrossRef]
- Dong, J.; Wan, G.; Liang, Z. Accumulation of salicylic acid-induced phenolic compounds and raised activities of secondary metabolic and antioxidative enzymes in Salvia miltiorrhiza cell culture. J. Biotechnol. 2010, 148, 99–104. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Taher, M.A. Influence of edible coatings chitosan/PVP blending with salicylic acid on biochemical fruit skin browning incidence and shelf life of guava fruits cv.‘Banati’. Sci. Hortic. 2018, 235, 424–436. [Google Scholar] [CrossRef]
- Nadeem, M.; Ahmad, W.; Zahir, A.; Hano, C.; Abbasi, B.H. Salicylic acid-enhanced biosynthesis of pharmacologically important lignans and neo lignans in cell suspension culture of Linum ussitatsimum L. Eng. Life Sci. 2019, 19, 168–174. [Google Scholar] [CrossRef]
- Khan, T.; Khan, T.; Hano, C.; Abbasi, B.H. Effects of chitosan and salicylic acid on the production of pharmacologically attractive secondary metabolites in callus cultures of Fagonia indica. Ind. Crops Prod. 2019, 129, 525–535. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Ullah, M.A.; Nadeem, M.; Tungmunnithum, D.; Hano, C. Exogenous application of salicylic acid and gibberellic acid on biomass accumulation, antioxidant and anti-inflammatory secondary metabolites production in multiple shoot culture of Ajuga integrifolia Buch. Ham. ex D. Don. Ind. Crops Prod. 2020, 145, 112098. [Google Scholar] [CrossRef]
- Wen, P.F.; Chen, J.Y.; Kong, W.F.; Pan, Q.H.; Wan, S.B.; Huang, W.D. Salicylic acid induced the expression of phenylalanine ammonia-lyase gene in grape berry. Plant Sci. 2005, 169, 928–934. [Google Scholar] [CrossRef]
- Mendoza, D.; Cuaspud, O.; Arias, J.P.; Ruiz, O.; Arias, M. Effect of salicylic acid and methyl jasmonate in the production of phenolic compounds in plant cell suspension cultures of Thevetia peruviana. Biotechnol. Rep. 2018, 19, e00273. [Google Scholar] [CrossRef] [PubMed]
- Azimi, F.; Oraei, M.; Gohari, G.; Panahirad, S.; Farmarzi, A. Chitosan-selenium nanoparticles (Cs–Se NPs) modulate the photosynthesis parameters, antioxidant enzymes activities and essential oils in Dracocephalum moldavica L. under cadmium toxicity stress. Plant Physiol. Biochem. 2021, 167, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, M.; Hasanuzzaman, M.; Rahman, M.; Khan, M.; Rahman, A.; Bhowmik, P.; Mahmud, N.U.; Tanveer, M.; Islam, T. Mechanism of plant growth promotion and disease suppression by chitosan biopolymer. Agriculture 2020, 10, 624. [Google Scholar] [CrossRef]
- Mujtaba, M.; Khawar, K.M.; Camara, M.C.; Carvalho, L.B.; Fraceto, L.F.; Morsi, R.E.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Ullah, H.; et al. Chitosan-based delivery systems for plants: A brief overview of recent advances and future directions. Int. J. Biol. Macromol. 2020, 154, 683–697. [Google Scholar] [CrossRef]
- Ahmad, W.; Zahir, A.; Nadeem, M.; Garros, L.; Drouet, S.; Renouard, S.; Doussot, J.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Enhanced production of lignans and neolignans in chitosan-treated flax (Linum usitatissimum L.) cell cultures. Process Biochem. 2019, 79, 155–165. [Google Scholar] [CrossRef]
- Shah, M.; Jan, H.; Drouet, S.; Tungmunnithum, D.; Shirazi, J.H.; Hano, C.; Abbasi, B.H. Chitosan elicitation impacts flavonolignan biosynthesis in Silybum marianum (L.) Gaertn cell suspension and enhances antioxidant and anti-inflammatory activities of cell extracts. Molecules 2021, 26, 791. [Google Scholar] [CrossRef]
- Singh, R.K.; Soares, B.; Goufo, P.; Castro, I.; Cosme, F.; Pinto-Sintra, A.L.; Inês, A.; Oliveira, A.A.; Falco, V. Chitosan upregulates the genes of the ROS pathway and enhances the antioxidant potential of grape (Vitis vinifera L. ‘Touriga Franca’ and ’Tinto Cão’) tissues. Antioxidants 2019, 8, 525. [Google Scholar] [CrossRef]
- Gohari, G.; Zareei, E.; Kulak, M.; Labib, P.; Mahmoudi, R.; Panahirad, S.; Jafari, H.; Mahdavinia, G.; Juárez-Maldonado, A.; Lorenzo, J.M. Improving the berry quality and antioxidant potential of flame seedless grapes by foliar application of chitosan–phenylalanine nanocomposites (CS–Phe NCs). Nanomaterials 2021, 11, 2287. [Google Scholar] [CrossRef]
- Barrientos Carvacho, H.; Pérez, C.; Zúñiga, G.; Mahn, A. Effect of methyl jasmonate, sodium selenate and chitosan as exogenous elicitors on the phenolic compounds profile of broccoli sprouts. J. Sci. Food Agric. 2014, 94, 2555–2561. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Mukta, J.A.; Sabir, A.A.; Gupta, D.R.; Mohi-Ud-Din, M.; Hasanuzzaman, M.; Miah, M.G.; Rahman, M.; Islam, M.T. Chitosan biopolymer promotes yield and stimulates accumulation of antioxidants in strawberry fruit. PLoS ONE 2018, 13, e0203769. [Google Scholar] [CrossRef] [PubMed]
- Ishkeh, S.R.; Shirzad, H.; Asghari, M.R.; Alirezalu, A.; Pateiro, M.; Lorenzo, J.M. Effect of chitosan nanoemulsion on enhancing the phytochemical contents, health-promoting components, and shelf life of raspberry (Rubus sanctus Schreber). Appl. Sci. 2021, 11, 2224. [Google Scholar] [CrossRef]
- Anjum, S.; Anjum, I.; Hano, C.; Kousar, S. Advances in nanomaterials as novel elicitors of pharmacologically active plant specialized metabolites: Current status and future outlooks. RSC Adv. 2019, 9, 40404–40423. [Google Scholar] [CrossRef] [PubMed]
- Sheikhalipour, M.; Esmaielpour, B.; Behnamian, M.; Gohari, G.; Giglou, M.T.; Vachova, P.; Rastogi, A.; Brestic, M.; Skalicky, M. Chitosan–selenium nanoparticle (Cs–Se NP) foliar spray alleviates salt stress in bitter melon. Nanomaterials 2021, 11, 684. [Google Scholar] [CrossRef]
- Khan, A.K.; Kousar, S.; Tungmunnithum, D.; Hano, C.; Abbasi, B.H.; Anjum, S. Nano-elicitation as an effective and emerging strategy for in vitro production of industrially important flavonoids. Appl. Sci. 2021, 11, 1694. [Google Scholar] [CrossRef]
- Blanch, G.P.; Gómez-Jiménez, M.C.; Del Castillo, M.L.R. Exogenous salicylic acid improves phenolic content and antioxidant activity in table grapes. Plant Foods Hum. Nutr. 2020, 75, 177–183. [Google Scholar] [CrossRef]
- Cioroi, M. Study on L-ascorbic acid contents from exotic fruits. Cercet. Agron. Mold. 2007, 1, 23–27. [Google Scholar]
- Kumar, V.P.; Madhu, C.; Mannem, K.; Asha, V.S.; Rao, A.S.; Prasad, M.S. Quantitative evaluation of carbohydrate levels in fruits by UV-visible Spectrophotometer. AJP Technol. 2012, 2, 99–100. [Google Scholar]
- Arnon, A. Method of extraction of chlorophyll in the plants. J. Agron. 1967, 23, 112–121. [Google Scholar]
- Giusti, M.M.; Wrolstad, R.E. Characterization of red radish anthocyanins. J. Food Sci. 1996, 61, 322–326. [Google Scholar] [CrossRef]
- Roggero, J.P.; Coen, S.; Ragonnet, B. High performance liquid chromatography survey on changes in pigment content in ripening grapes of Syrah. An approach to anthocyanin metabolism. Am. J. Enol. Vitic. 1986, 37, 77–83. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Hassanpour, M.; Jafari, H.; Sharifi, S.; Rezaie, J.; Lighvan, Z.M.; Mahdavinia, G.R.; Gohari, G.; Akbari, A. Salicylic acid-loaded chitosan nanoparticles (SA/CTS NPs) for breast cancer targeting: Synthesis, characterization and controlled release kinetics. J. Mol. Struct. 2021, 1245, 131040. [Google Scholar] [CrossRef]
- Chandra, A.; Anand, A.; Dubey, A. Effect of salicylic acid on morphological and biochemical attributes in cowpea. J. Environ. Biol. 2007, 28, 193–196. [Google Scholar] [PubMed]
- Panahirad, S.; Naghshiband-Hassani, R.; Mahna, N. Pectin-based edible coating preserves antioxidative capacity of plum fruit during shelf life. Food Sci. Technol. Int. 2020, 26, 583–592. [Google Scholar] [CrossRef]
- Hazarika, T.K.; Marak, T. Salicylic acid and oxalic acid in enhancing the quality and extending the shelf life of grape cv. Thompson seedless. Food Sci. Technol. Int. 2021, 10820132211020612. [Google Scholar] [CrossRef]
- Gorni, P.H.; da Silva Cornelissen, B.; Pereira, A.A. Exogenous salicylic acid and ferulic acid improve growth, phenolic and carotenoid content in tomato. Adv. Hortic. Sci. 2021, 35, 335–341. [Google Scholar] [CrossRef]
- Hano, C.; Addi, M.; Bensaddek, L.; Crônier, D.; Baltora-Rosset, S.; Doussot, J.; Maury, S.; Mesnard, F.; Chabbert, B.; Hawkins, S.; et al. Differential accumulation of monolignol-derived compounds in elicited flax (Linum usitatissimum) cell suspension cultures. Planta 2006, 223, 975–989. [Google Scholar] [CrossRef]
- Kiselev, K.V.; Dubrovina, A.S.; Isaeva, G.A.; Zhuravlev, Y.N. The effect of salicylic acid on phenylalanine ammonia-lyase and stilbene synthase gene expression in Vitis amurensis cell culture. Russ. J. Plant Physiol. 2010, 57, 415–421. [Google Scholar] [CrossRef]


| Treatment | Berry FW (g) | Berry DW (g) | TA (%) | TSS (%) | TSS/TA | pH |
|---|---|---|---|---|---|---|
| Control | 3.056 ± 0.45 c | 0.26 ± 0.031 de | 0.386 ± 0.024 a | 11.304 ± 1.55 e | 29.28 ± 1.24 e | 3.62 ± 0.4 c |
| SA 10 mM | 4.108 ± 0.37 b | 0.31 ± 0.018 bc | 0.334 ± 0.024 bc | 13.27 ± 0.88 cd | 39.73 ± 2.24 c | 4.27 ± 0.1 bc |
| SA 20 mM | 4.566 ± 0.67 ab | 0.33 ± 0.034 b | 0.323 ± 0.061 bc | 15.288 ± 1.31 b | 47.32 ± 3.2 b | 4.41 ± 0.15 b |
| CTS NPs 0.1% | 4.305 ± 0.41 b | 0.28 ± 0.028 d | 0.385 ± 0.059 a | 13.841 ± 0.95 c | 35.95 ± 3.29 d | 3.96 ± 0.22 b |
| CTS-SA NPs 10 mM | 4.187 ± 0.28 b | 0.39 ± 0.045 a | 0.323 ± 0.048 bc | 16.46 ± 0.67 a | 50.95 ± 3.24 a | 4.65 ± 0.38 a |
| CTS-SA NPs 20 mM | 4.891 ± 0.31 a | 0.41 ± 0.018 a | 0.343 ± 0.036 b | 17.183 ± 1.2 ab | 50.096 ± 6.2 a | 4.77 ± 0.24 a |
| Treatments | Control | SA 10 mM | SA 20 mM | CTS NPs 0.1% | CTS-SA NPs 10 mM | CTS-SA NPs 20 mM |
|---|---|---|---|---|---|---|
| Delphinidin-3-O-monoglucoside (mg Mlv g−1 FW) | 2.45 ± 0.89 d | 6.71 ± 0.67 c | 7.94 ± 0.91 bc | 6.37 ± 0.39 c | 8.75 ± 0.42 b | 10.89 ± 0.38 a |
| Cyanidin-3-O-monoglucoside (mg Mlv g−1 FW) | 0.94 ± 0.12 d | 1.59 ± 0.18 bc | 1.88 ± 0.18 b | 1.07 ± 0.13 c | 2.51 ± 0.19 a | 2.54 ± 0.27 a |
| Petunidin-3-O-monoglucoside (mg Mlv g−1 FW) | 1.81 ± 0.89 c | 2.19 ± 0.37 b | 3.87 ± 0.68 a | 1.47 ± 0.27 cd | 2.08 ± 0.6 b | 3.08 ± 0.28 b |
| Peonidin-3-O-monoglucoside (mg Mlv g−1 FW) | 3.67 ± 0.98 d | 4.29 ± 0.67 cd | 6.04 ± 0.99 b | 4.67 ± 1.48 c | 6.98 ± 0.29 ab | 7.08 ± 0.84 a |
| Malvidin-3-O-monoglucoside (Oenin) (mg Mlv g−1 FW) | 12.41 ± 1.38 d | 17.36 ± 2.45 c | 19.89 ± 2.84 b | 14.25 ± 3.05 cd | 21.27 ± 1.95 ab | 23.37 ± 2.07 a |
| Malvidin-3-O-acetylmonoglucoside (mg Mlv g−1 FW) | 5.23 ± 1.08 cd | 6.36 ± 2.36 c | 10.91 ± 3.07 ab | 8.04 ± 2.59 bc | 9.07 ± 1.97 b | 12.91 ± 2.19 a |
| Malvidin-3-(6-O-p-coumaroyl) monoglucoside (mg Mlv g−1 FW) | 4.04 ± 0.98 bc | 8.38 ± 1.38 a | 6.45 ± 0.81 ab | 5.17 ± 1.04 b | 7.08 ± 1.54 ab | 8.79 ± 0.87 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalili, N.; Oraei, M.; Gohari, G.; Panahirad, S.; Nourafcan, H.; Hano, C. Chitosan-Enriched Salicylic Acid Nanoparticles Enhanced Anthocyanin Content in Grape (Vitis vinifera L. cv. Red Sultana) Berries. Polymers 2022, 14, 3349. https://doi.org/10.3390/polym14163349
Khalili N, Oraei M, Gohari G, Panahirad S, Nourafcan H, Hano C. Chitosan-Enriched Salicylic Acid Nanoparticles Enhanced Anthocyanin Content in Grape (Vitis vinifera L. cv. Red Sultana) Berries. Polymers. 2022; 14(16):3349. https://doi.org/10.3390/polym14163349
Chicago/Turabian StyleKhalili, Naser, Mehdi Oraei, Gholamreza Gohari, Sima Panahirad, Hassan Nourafcan, and Christophe Hano. 2022. "Chitosan-Enriched Salicylic Acid Nanoparticles Enhanced Anthocyanin Content in Grape (Vitis vinifera L. cv. Red Sultana) Berries" Polymers 14, no. 16: 3349. https://doi.org/10.3390/polym14163349
APA StyleKhalili, N., Oraei, M., Gohari, G., Panahirad, S., Nourafcan, H., & Hano, C. (2022). Chitosan-Enriched Salicylic Acid Nanoparticles Enhanced Anthocyanin Content in Grape (Vitis vinifera L. cv. Red Sultana) Berries. Polymers, 14(16), 3349. https://doi.org/10.3390/polym14163349







