A Feasible Compatibilization Processing Technique for Improving the Mechanical and Thermal Performance of Rubbery Biopolymer/Graphene Nanocomposites
Abstract
1. Introduction
2. Experiments
2.1. Materials
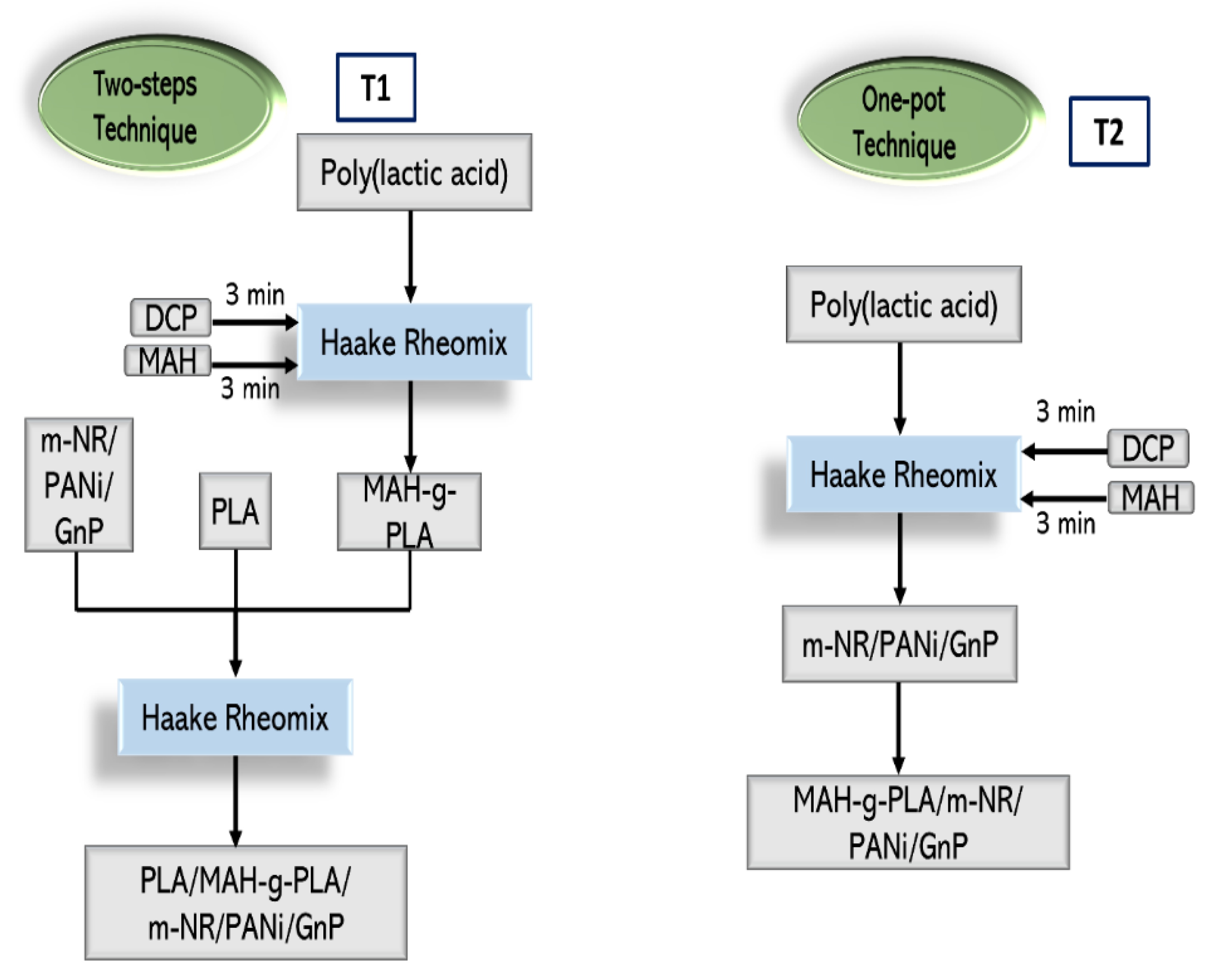
2.2. Nanocomposite Sample Preparation
2.3. Characterization
3. Results and Discussion
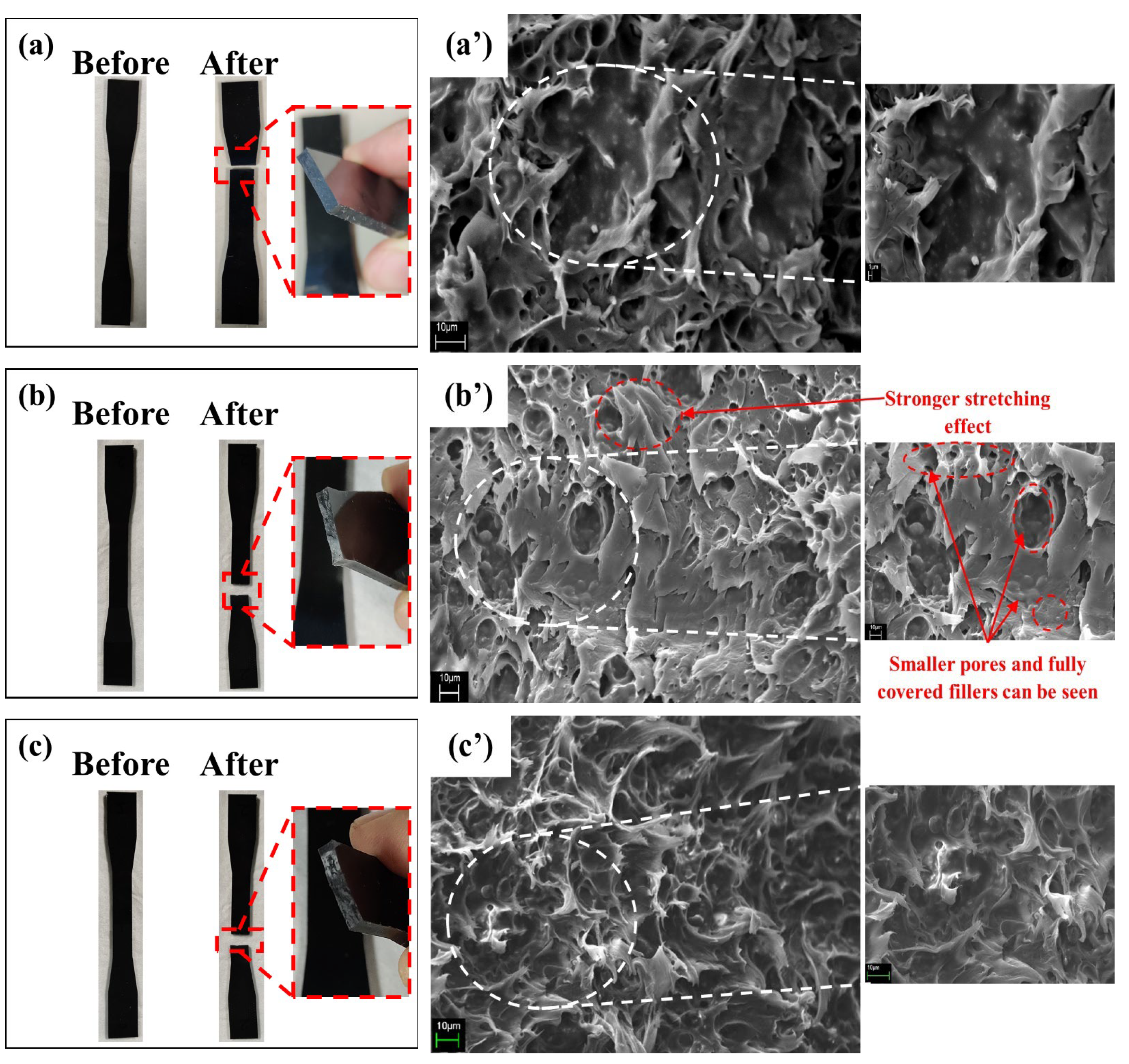
3.1. Tensile Test
3.2. Morphological Analysis
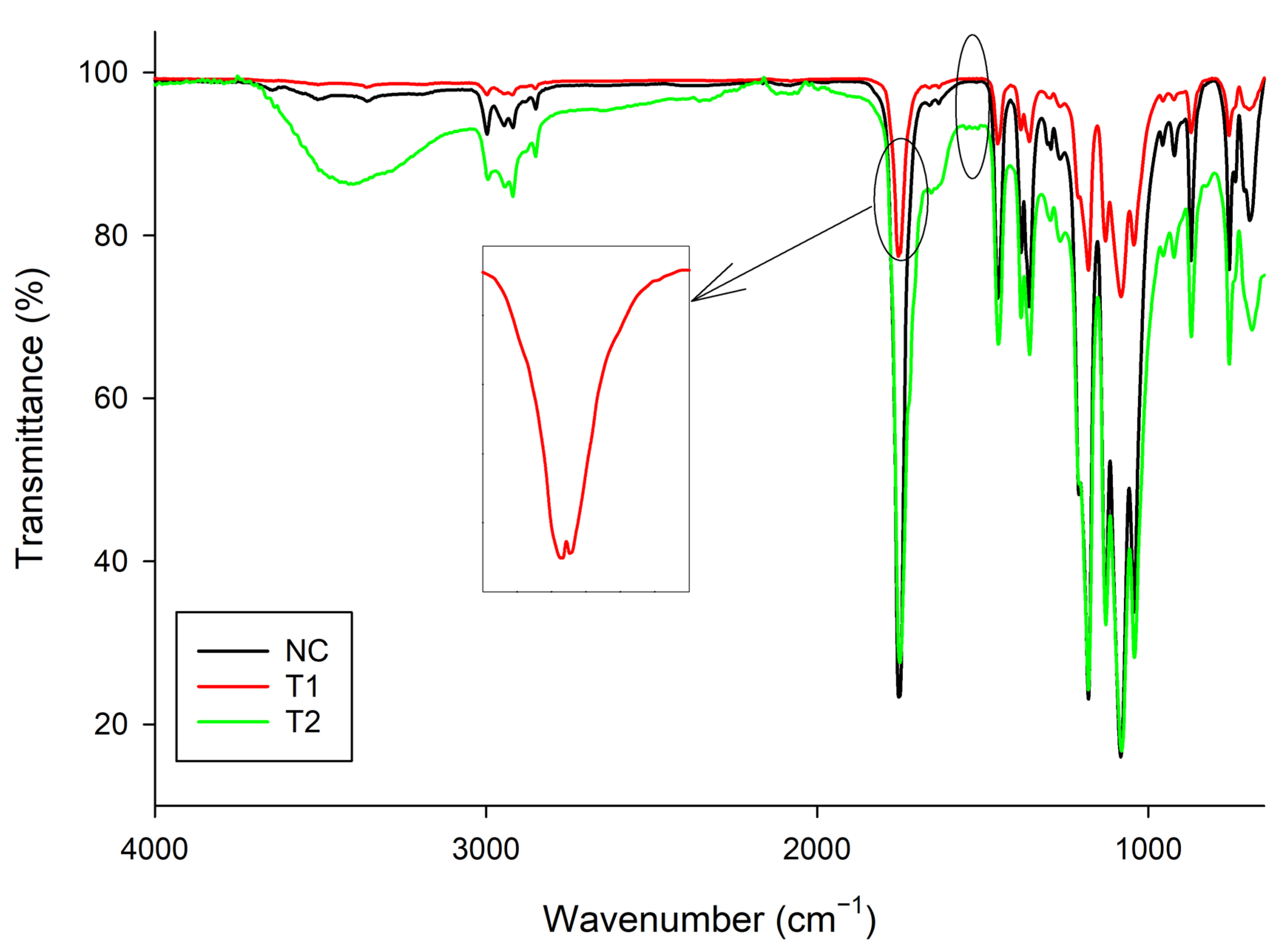
3.3. Spectroscopic Analysis
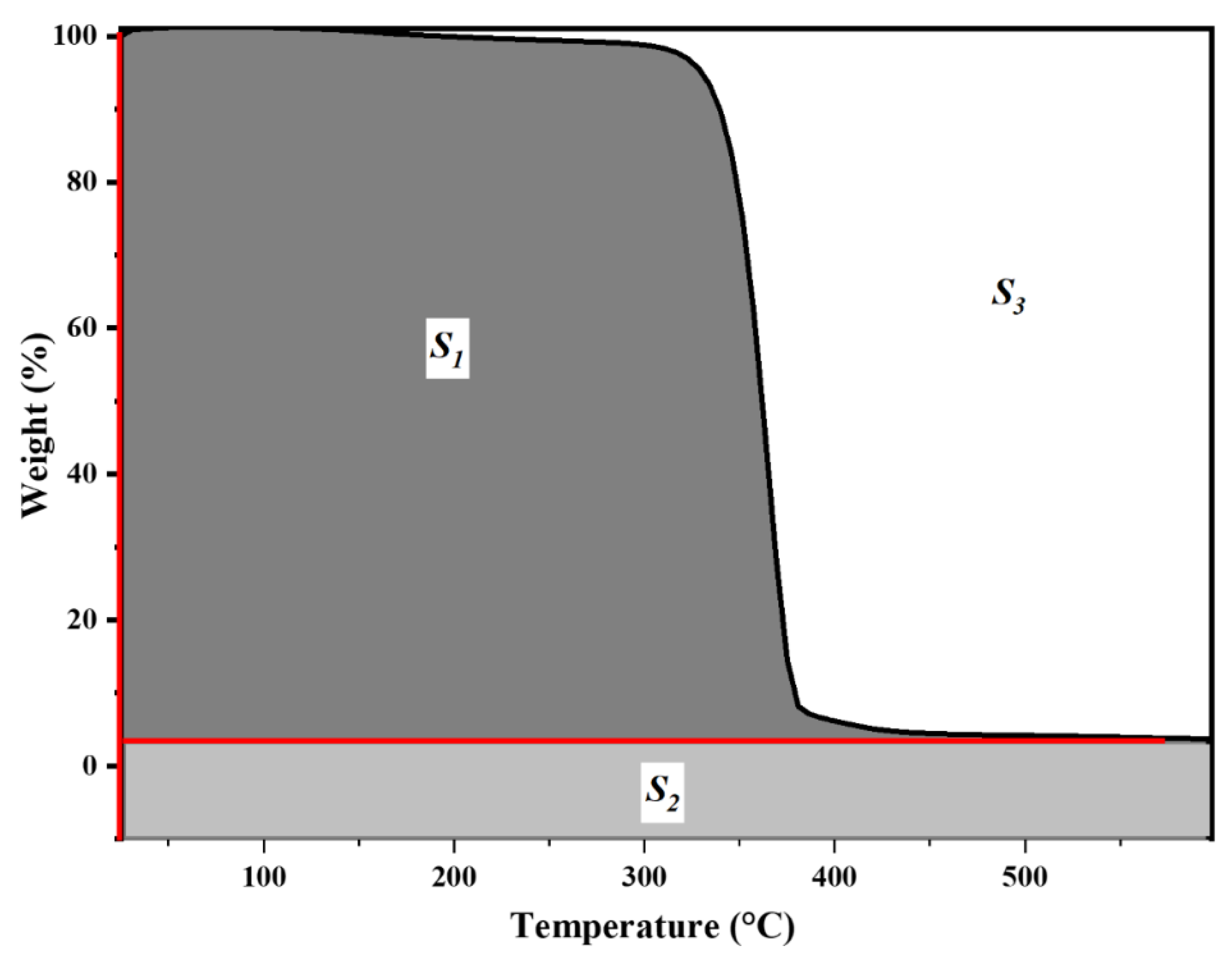
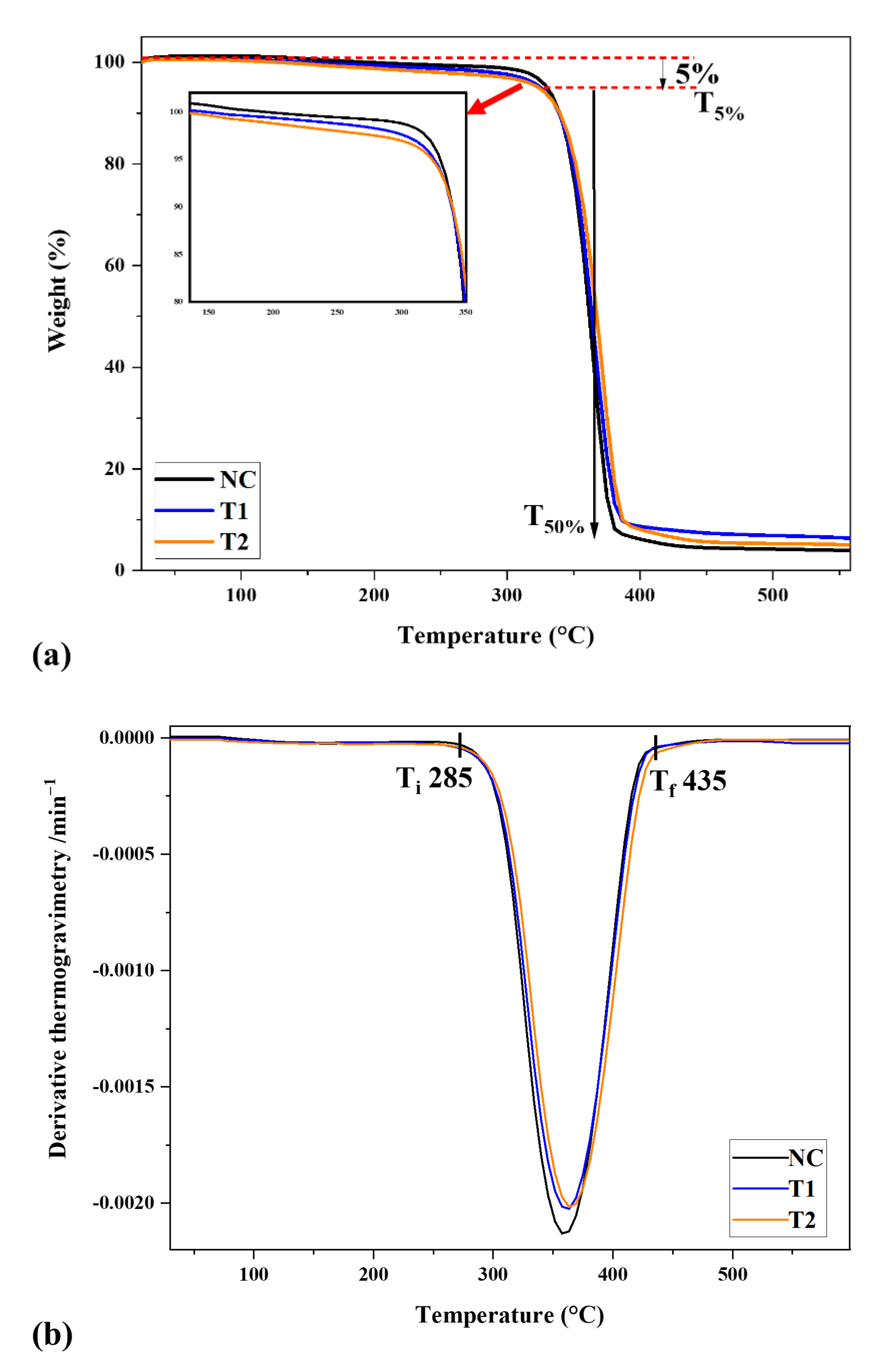
3.4. Thermogravimetric Analysis (TGA)
3.5. Differential Scanning Calorimetry (DSC)
3.6. XRD Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Rehim, H.A.; Kamal, H.; Hegazy, E.-S.A.; Soliman, E.-S.; Sayed, A. Use of gamma rays to improve the mechanical and barrier properties of biodegradable cellulose acetate nanocomposite films. Radiat. Phys. Chem. 2018, 153, 180–187. [Google Scholar] [CrossRef]
- Huang, C.; Qian, X.; Yang, R. Thermal conductivity of polymers and polymer nanocomposites. Mater. Sci. Eng. R Rep. 2018, 132, 1–22. [Google Scholar] [CrossRef]
- Aliabadi, R.S.; Mahmoodi, N.O. Synthesis and characterization of polypyrrole, polyaniline nanoparticles and their nanocomposite for removal of azo dyes; sunset yellow and Congo red. J. Clean. Prod. 2018, 179, 235–245. [Google Scholar] [CrossRef]
- Yousef, S.; Subadra, S.P.; Griškevičius, P.; Varnagiris, S.; Milcius, D.; Makarevicius, V. Superhydrophilic functionalized graphene/fiberglass/epoxy laminates with high mechanical, impact and thermal performance and treated by plasma. Polym. Test. 2020, 90, 106701. [Google Scholar] [CrossRef]
- Shahdan, D.; Ahmad, S.; Chen, R.S.; Omar, A.; Zailan, F.D.; Abu Hassan, N.A. Mechanical performance, heat transfer and conduction of ultrasonication treated polyaniline bio-based blends. Int. Commun. Heat Mass Transf. 2020, 117, 104742. [Google Scholar] [CrossRef]
- Zahid, M.; Del Río Castillo, A.E.; Thorat, S.B.; Panda, J.K.; Bonaccorso, F.; Athanassiou, A. Graphene morphology effect on the gas barrier, mechanical and thermal properties of thermoplastic polyurethane. Compos. Sci. Technol. 2020, 200, 108461. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.; Hui, D.; Feo, L.; Fraternali, F. Graphene as biomedical sensing element: State of art review and potential engineering applications. Compos. B Eng. 2018, 134, 193–206. [Google Scholar] [CrossRef]
- Cao, C.; Zhu, F.; Yu, X.; Wang, Q.; Wang, C.; Li, B.; Lv, R.; Li, M. Two-step modification of poly(D, L-lactic acid) by ethylenediamine-maleic anhydride. Biomed. Mater. 2007, 3, 015002. [Google Scholar] [CrossRef]
- Song, P.; He, X.; Xie, M.; Tao, J.; Shen, X.; Ji, Z.; Yan, Z.; Zhai, L.; Yuan, A. Polyaniline wrapped graphene functionalized textile with ultrahigh areal capacitance and energy density for high-performance all-solid-state supercapacitors for wearable electronics. Compos. Sci. Technol. 2020, 198, 108305. [Google Scholar] [CrossRef]
- Swaroop, C.; Shukla, M. Development of blown polylactic acid-MgO nanocomposite films for food packaging. Compos. A Appl. Sci. Manuf. 2019, 124, 105482. [Google Scholar] [CrossRef]
- Rosli, N.A.; Ahmad, I.; Anuar, F.H.; Abdullah, I. Mechanical and thermal properties of natural rubber-modified poly (lactic acid) compatibilized with telechelic liquid natural rubber. Polym. Test. 2016, 54, 196–202. [Google Scholar] [CrossRef]
- Nematollahi, M.; Jalali-Arani, A.; Modarress, H. High-performance bio-based poly (lactic acid)/natural rubber/epoxidized natural rubber blends: Effect of epoxidized natural rubber on microstructure, toughness and static and dynamic mechanical properties. Polym. Int. 2019, 68, 439–446. [Google Scholar] [CrossRef]
- Batakliev, T.; Petrova-Doycheva, I.; Angelov, V.; Georgiev, V.; Ivanov, E.; Kotsilkova, R.; Casa, M.; Cirillo, C.; Adami, R.; Sarno, M.; et al. Effects of graphene nanoplatelets and multiwall carbon nanotubes on the structure and mechanical properties of poly (lactic acid) composites: A comparative study. Appl. Sci. 2019, 9, 469. [Google Scholar] [CrossRef]
- Shahdan, D.; Flaifel, M.H.; Chen, R.S.; Ahmad, S.; Hassan, A. Role of sonication time on thermal behaviour and dynamic mechanical analysis of NiZn ferrite incorporated PLA/LNR nanocomposite. Bull. Mater. Sci. 2019, 42, 78. [Google Scholar] [CrossRef]
- Bhasney, S.M.; Bhagabati, P.; Kumar, A.; Katiyar, V. Morphology and crystalline characteristics of polylactic acid [PLA]/linear low density polyethylene [LLDPE]/microcrystalline cellulose [MCC] fiber composite. Compos. Sci. Technol. 2019, 171, 54–61. [Google Scholar] [CrossRef]
- Shahdan, D.; Chen, R.S.; Ahmad, S.; Zailan, F.D.; Mat Ali, A. Assessment of mechanical performance, thermal stability and water resistance of novel conductive poly (lactic acid)/modified natural rubber blends with low loading of polyaniline. Polym. Int. 2018, 67, 1070–1080. [Google Scholar] [CrossRef]
- Shahdan, D.; Chen, R.S.; Ahmad, S. Optimization of graphene nanoplatelets dispersion and nano-filler loading in bio-based polymer nanocomposites based on tensile and thermogravimetry analysis. J. Mater. Res. Technol. 2021, 15, 1284–1299. [Google Scholar] [CrossRef]
- Mohammad, N.; Arsad, A.; Sani, N.; Basri, M. Effect of compatibilisers on thermal and morphological properties of polylactic acid/natural rubber blends. Chem. Eng. Trans. 2017, 56, 1027–1032. [Google Scholar]
- Chauhan, S.; Raghu, N.; Raj, A. Effect of maleic anhydride grafted polylactic acid concentration on mechanical and thermal properties of thermoplasticized starch filled polylactic acid blends. Polym. Polym. Compos. 2021, 29, S400–S410. [Google Scholar] [CrossRef]
- Coudane, J.; Van Den Berghe, H.; Mouton, J.; Garric, X.; Nottelet, B. Poly (lactic acid)-based graft copolymers: Syntheses strategies and improvement of properties for biomedical and environmentally friendly applications: A review. Molecules 2022, 27, 4135. [Google Scholar] [CrossRef]
- Oromiehie, A.; Ebadi-Dehaghani, H.; Mirbagheri, S. Chemical modification of polypropylene by maleic anhydride: Melt grafting, characterization and mechanism. Int. J. Chem. Eng. Appl. 2014, 5, 117–122. [Google Scholar]
- Verginio, G.E.A.; Montanheiro, T.L.d.A.; Montagna, L.S.; Marini, J.; Passador, F.R. Effectiveness of the preparation of maleic anhydride grafted poly (lactic acid) by reactive processing for poly (lactic acid)/carbon nanotubes nanocomposites. J. Appl. Polym. Sci. 2021, 138, 50087. [Google Scholar] [CrossRef]
- Jang, H.; Kwon, S.; Kim, S.J.; Park, S.-I. Maleic anhydride-grafted PLA preparation and characteristics of compatibilized PLA/PBSeT blend films. Int. J. Mol. Sci. 2022, 23, 7166. [Google Scholar] [CrossRef] [PubMed]
- Alikarami, N.; Abrisham, M.; Huang, X.; Panahi-Sarmad, M.; Zhang, K.; Dong, K.; Xiao, X. Compatibilization of PLA grafted maleic anhydrate through blending of thermoplastic starch (TPS) and nanoclay nanocomposites for the reduction of gas permeability. Int. J. Smart Nano Mater. 2022, 13, 130–151. [Google Scholar] [CrossRef]
- Shi, D.; Li, R.K.Y.; Zhu, Y.; Ke, Z.; Yin, J.; Jiang, W.; Hu, G.-H. Nano-reactors for controlling the selectivity of the free radical grafting of maleic anhydride onto polypropylene in the melt. Polym. Eng. Sci. 2006, 46, 1443–1454. [Google Scholar] [CrossRef]
- Fowlks, A.C.; Narayan, R. The effect of maleated polylactic acid (PLA) as an interfacial modifier in PLA-talc composites. J. Appl. Polym. Sci. 2010, 118, 2810–2820. [Google Scholar] [CrossRef]
- Chen, R.S.; Amran, N.A.M.; Ahmad, S. Reinforcement effect of nanocomposites with single/hybrid graphene nanoplatelets and magnesium hydroxide. J. Therm. Anal. Calorim. 2019, 137, 79–92. [Google Scholar] [CrossRef]
- Patel, A.K.; Bajpai, R.; Keller, J.M. On the crystallinity of PVA/palm leaf biocomposite using DSC and XRD techniques. Microsyst. Technol. 2014, 20, 41–49. [Google Scholar] [CrossRef]
- Nyambo, C.; Mohanty, A.K.; Misra, M. Effect of maleated compatibilizer on performance of PLA/wheat straw-based green composites. Macromol. Mater. Eng. 2011, 296, 710–718. [Google Scholar] [CrossRef]
- Tham, W.L.; Mohd Ishak, Z.A.; Chow, W.S. Mechanical and Thermal Properties Enhancement of Poly (Lactic Acid)/Halloysite Nanocomposites by Maleic-Anhydride Functionalized Rubber. J. Macromol. Sci. B 2014, 53, 371–382. [Google Scholar] [CrossRef]
- Petersson, L.; Oksman, K.; Mathew, A.P. Using maleic anhydride grafted poly(lactic acid) as a compatibilizer in poly(lactic acid)/layered-silicate nanocomposites. J. Appl. Polym. Sci. 2006, 102, 1852–1862. [Google Scholar] [CrossRef]
- Ma, P.; Jiang, L.; Ye, T.; Dong, W.; Chen, M. Melt free-radical grafting of maleic anhydride onto biodegradable poly (lactic acid) by using styrene as a comonomer. Polymers 2014, 6, 1528–1543. [Google Scholar] [CrossRef]
- Hong, S.-G.; Lin, Y.-C.; Lin, C.-H. Improvement of the thermal stability of polyhydroxybutyrates by grafting with maleic anhydride by different methods: Differential scanning calorimetry, thermogravimetric analysis, and gel permeation chromatography. J. Appl. Polym. Sci. 2008, 110, 2718–2726. [Google Scholar] [CrossRef]
- Deka, B.K.; Maji, T.K. Study on the properties of nanocomposite based on high density polyethylene, polypropylene, polyvinyl chloride and wood. Compos. A Appl. Sci. Manuf. 2011, 42, 686–693. [Google Scholar] [CrossRef]
- Arsad, A.; Rahmat, A.; Sani, N.; Mohammad, N. Effects of Compatibilizer on Thermal and Mechanical Properties of PLA/NR Blends. Mater. Sci. Forum 2015, 819, 241–245. [Google Scholar]
- Chen, R.S.; Ahmad, S.; Gan, S.; Salleh, M.N.; Ab Ghani, M.H.; Tarawneh, M.A. Effect of polymer blend matrix compatibility and fibre reinforcement content on thermal stability and flammability of ecocomposites made from waste materials. Thermochim. Acta 2016, 640, 52–61. [Google Scholar] [CrossRef]
- Khan, B.A.; Na, H.; Chevali, V.; Warner, P.; Zhu, J.; Wang, H. Glycidyl methacrylate-compatibilized poly (lactic acid)/hemp hurd biocomposites: Processing, crystallization, and thermo-mechanical response. J. Mater. Sci. Technol. 2018, 34, 387–397. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Effect of functionalized graphene on the physical properties of linear low density polyethylene nanocomposites. Polym. Test. 2012, 31, 31–38. [Google Scholar] [CrossRef]
- Modak, P.; Kondawar, S.B.; Nandanwar, D.V. Synthesis and characterization of conducting polyaniline/graphene nanocomposites for electromagnetic interference shielding. Proc. Mater. Sci. 2015, 10, 588–594. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; Shao, W.; Ren, Y.; Dong, W.; Zhang, F.; Liu, L.-Z. Crystallization, structures, and properties of different polyolefins with similar grafting degree of maleic anhydride. Polymers 2020, 12, 675. [Google Scholar] [CrossRef]
- Chen, R.S.; Ruf, M.F.H.M.; Shahdan, D.; Ahmad, S. Enhanced mechanical and thermal properties of electrically conductive TPNR/GnP nanocomposites assisted with ultrasonication. PLoS ONE 2019, 14, e0222662. [Google Scholar] [CrossRef] [PubMed]

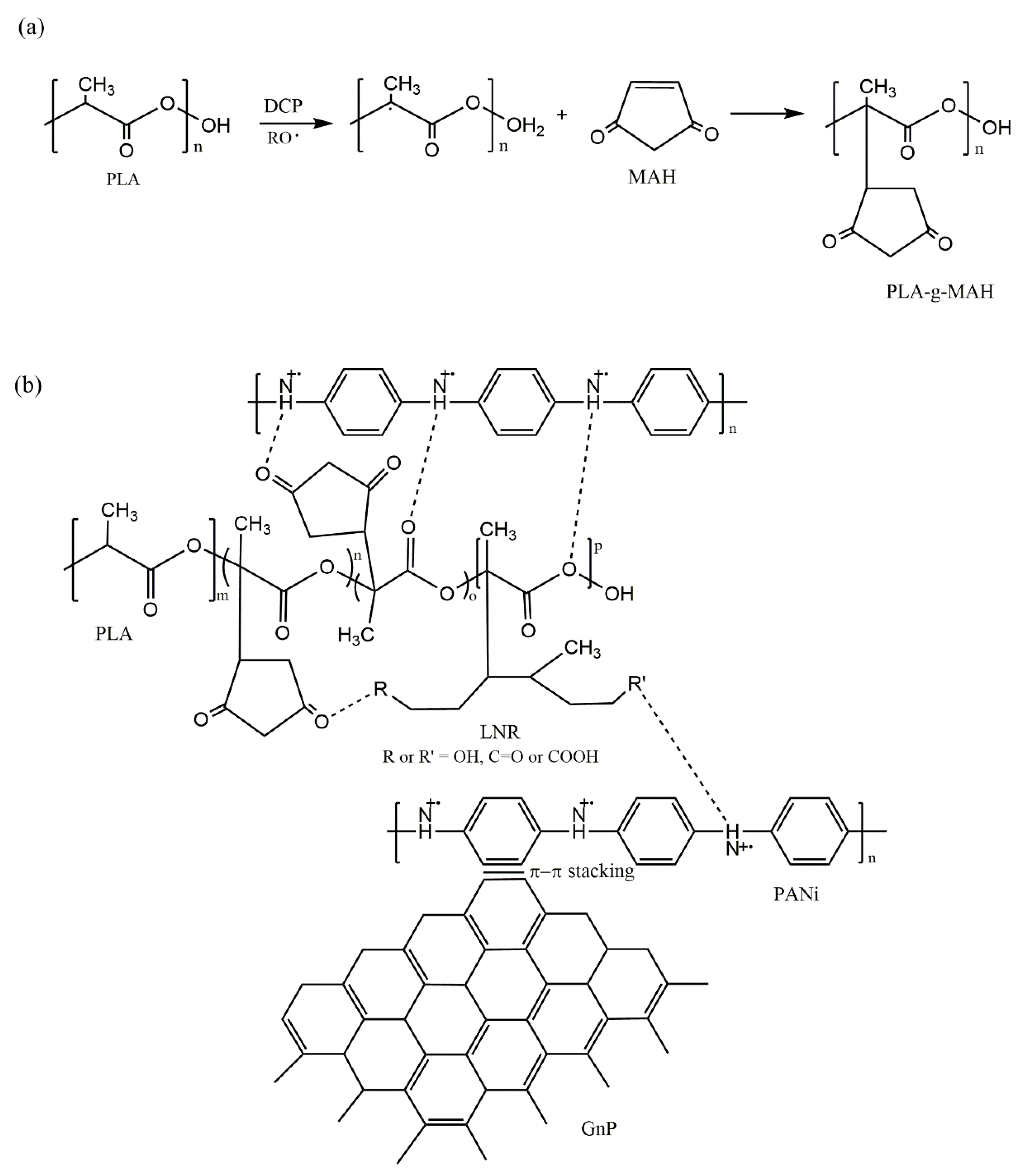
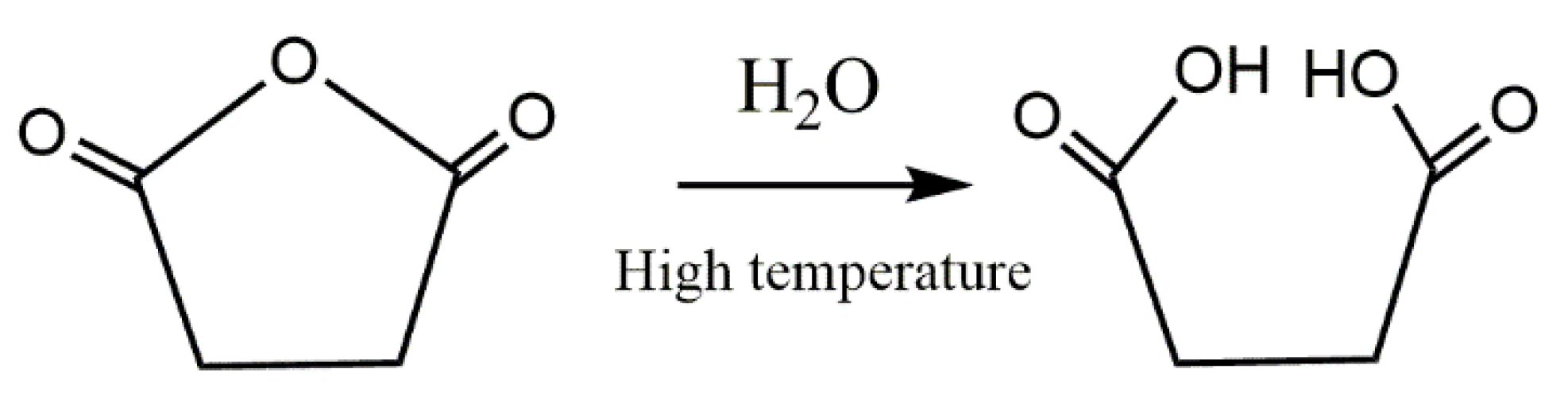


| Sample | PLA | LNR | PANi | GnP |
|---|---|---|---|---|
| Melting temperature (°C) | 155–170 | Not applicable | Not applicable | >3600 |
| Degradation temperature (°C) | Not applicable | 300 | 250 | Not applicable |
| Tensile strength (GPa) | 9 | Not applicable | Not applicable | ~1000 |
| Strain at break (%) | 3.5 | Not applicable | Not applicable | Not applicable |
| Average molecular weight (g/mol) | 55,000 | ~50,000 | Not applicable | Not applicable |
| Form and dimension of particles | Pellet | Liquid | Powder with L/D: 10~25 (Average diameter of 8 μm and length of 100 Å) | Powder with thickness: 5–15 nm Diameter: 5 μm |
| Appearance | White | Honey color | Black-green powder | Shiny black powder |
| Sample | S1 | S2 | S3 | A* | K* | IPDT/°C |
|---|---|---|---|---|---|---|
| NC | 32,388.8 | 7855.9 | 22,602.7 | 0.64036 | 1.24255 | 482.5 |
| T1 | 31,839.2 | 8919.3 | 23,460.8 | 0.63468 | 1.28014 | 492.2 |
| T2 | 32,010.8 | 8575.1 | 22,012.2 | 0.64836 | 1.26788 | 497.7 |
| Nanocomposite System | Tg (°C) | Tc (°C) | T’m (°C) | Tm (°C) | χcDSC (%) | χcXRD (%) |
|---|---|---|---|---|---|---|
| NC | 61.0 | 112.0 | - | 169.7 | 38.4 | 49.4 |
| T1 | 59.9 | 111.6 | - | 167.3 | 36.1 | 42.9 |
| T2 | 59.9 | 110.0 | 160.7 | 167.1 | 38.1 | 48.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahdan, D.; Rosli, N.A.; Chen, R.S.; Ahmad, S. A Feasible Compatibilization Processing Technique for Improving the Mechanical and Thermal Performance of Rubbery Biopolymer/Graphene Nanocomposites. Polymers 2022, 14, 5009. https://doi.org/10.3390/polym14225009
Shahdan D, Rosli NA, Chen RS, Ahmad S. A Feasible Compatibilization Processing Technique for Improving the Mechanical and Thermal Performance of Rubbery Biopolymer/Graphene Nanocomposites. Polymers. 2022; 14(22):5009. https://doi.org/10.3390/polym14225009
Chicago/Turabian StyleShahdan, Dalila, Noor Afizah Rosli, Ruey Shan Chen, and Sahrim Ahmad. 2022. "A Feasible Compatibilization Processing Technique for Improving the Mechanical and Thermal Performance of Rubbery Biopolymer/Graphene Nanocomposites" Polymers 14, no. 22: 5009. https://doi.org/10.3390/polym14225009
APA StyleShahdan, D., Rosli, N. A., Chen, R. S., & Ahmad, S. (2022). A Feasible Compatibilization Processing Technique for Improving the Mechanical and Thermal Performance of Rubbery Biopolymer/Graphene Nanocomposites. Polymers, 14(22), 5009. https://doi.org/10.3390/polym14225009







