Humic Acid-Coated Fe3O4 Nanoparticles Confer Resistance to Acremonium Wilt Disease and Improve Physiological and Morphological Attributes of Grain Sorghum
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparations and Characterization of Coated Fe3O4/HA
2.2. Source and Inoculum Preparation of A. strictum
2.3. Effect of Fe3O4/HA Nanoparticles on the Mycelial Growth of A. strictum
2.4. Reaction of Twenty-One Genotypes of Grain Sorghum to A. strictum under Field Conditions
2.5. Effect of Fe3O4/HA Nanoparticels on A. strictum under Field Conditions
2.6. Gibberellic Acid Assay
2.7. Assessment of Growth and Yield Parameters
2.8. Enzyme Assays
2.9. Potential Toxicity of Fe3O4/HA NPs on Rats
2.10. Histopathological Examination
2.11. Data Analysis
3. Results
3.1. Morphology and Size Distribution of Fe3O4/HA NPs
3.2. Inhibitory Effect of Fe3O4/HA NPs on the Mycelial Growth of A. striticum
3.3. Reaction of Grain Sorghum Genotypes to Acremonium Wilt Disease
3.4. Effect of Fe3O4/HA NPs on Controlling Acremonium Wilt under Field Conditions
3.5. Effect of Fe3O4/HA NPs on the Growth and Yield of Grain Sorghum
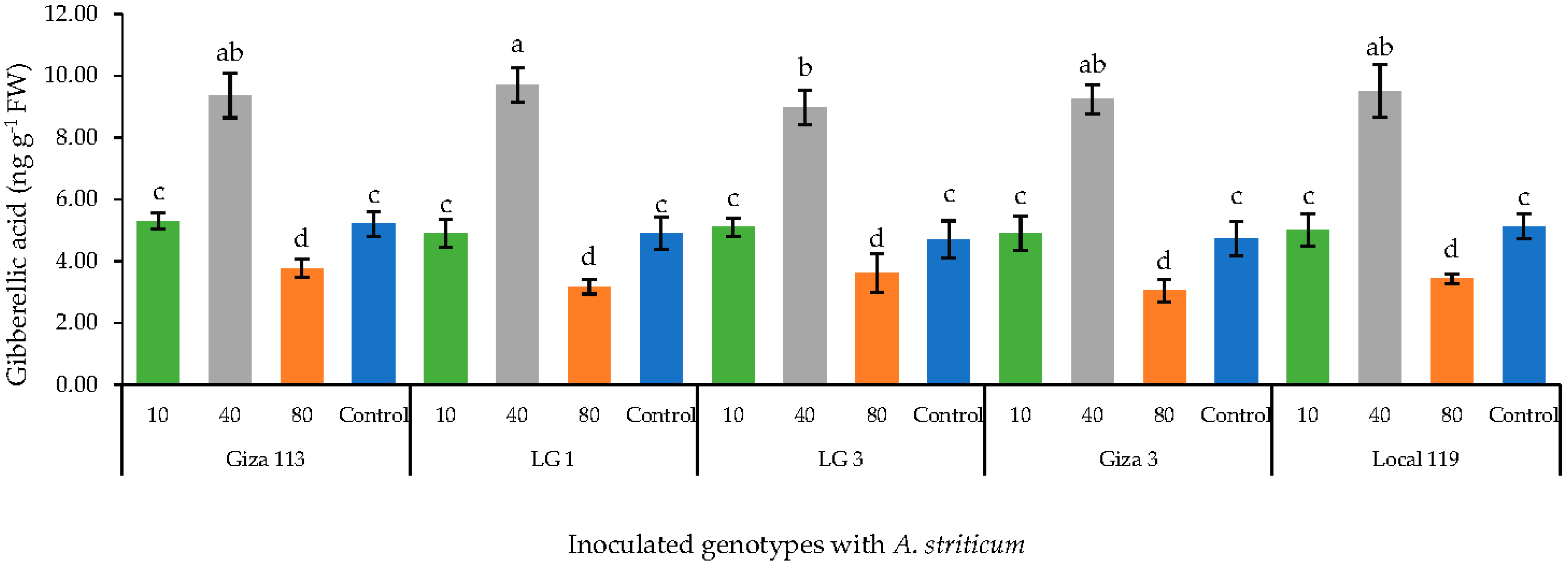
3.6. Effect of Fe3O4/HA NPs on the Level of Gibberillic Acid in Grain Sorghum Genotypes
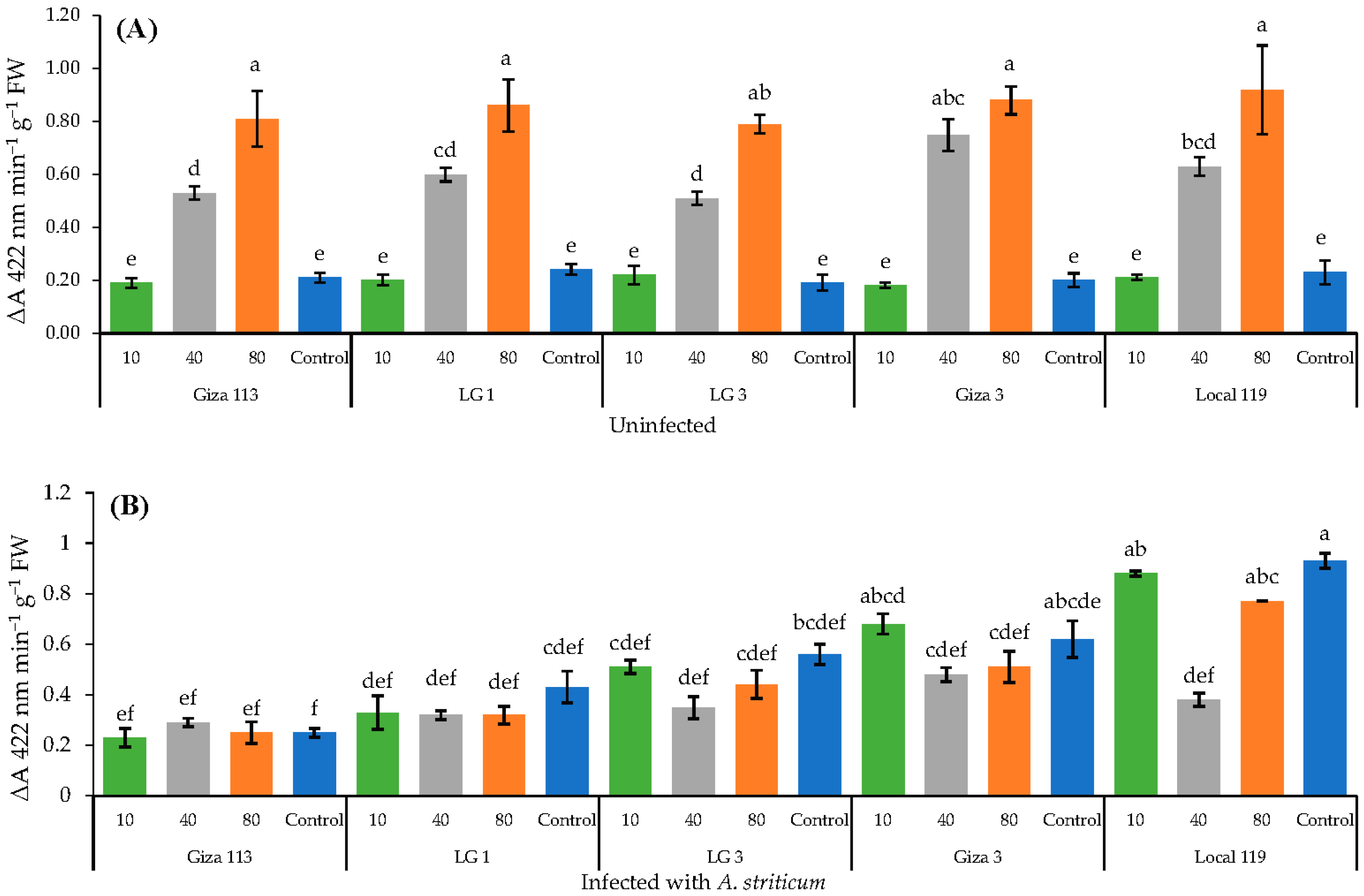
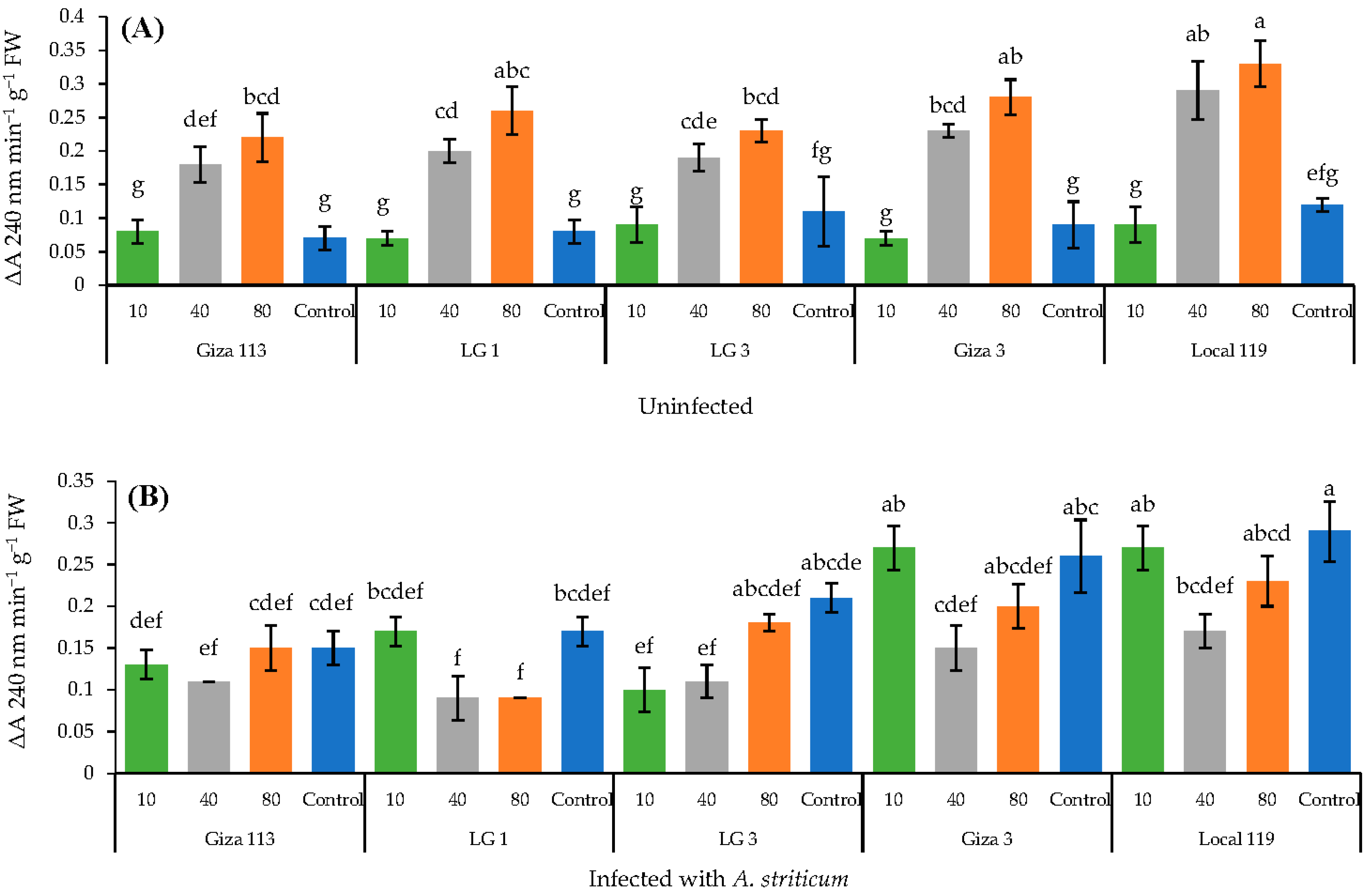
3.7. Effect of Fe3O4/HA NPs on the Activity of Peroxidase and Catalase Enzymes in Grain Sorghum
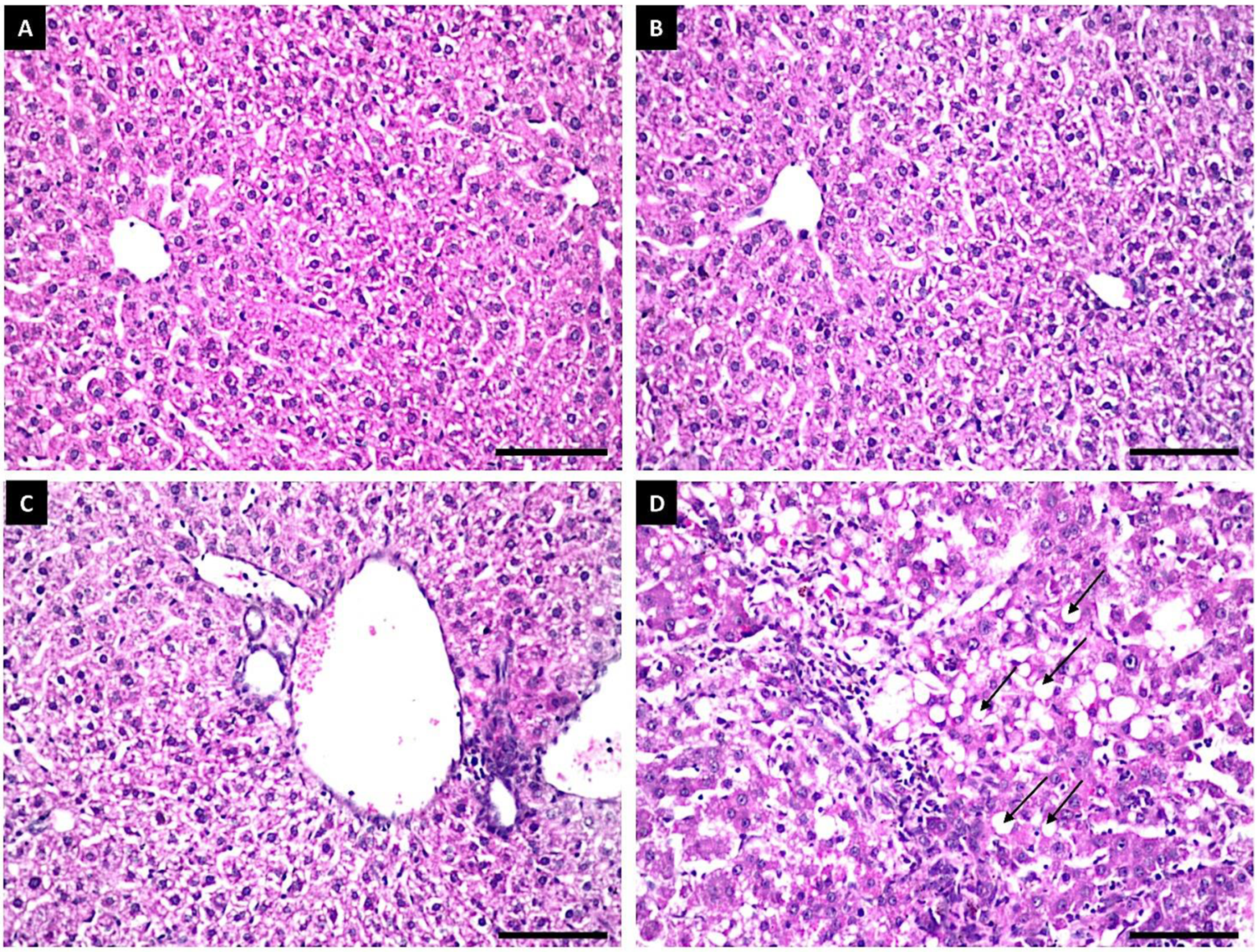
3.8. Potential Toxicity of Fe3O4/HA NPs in Rats
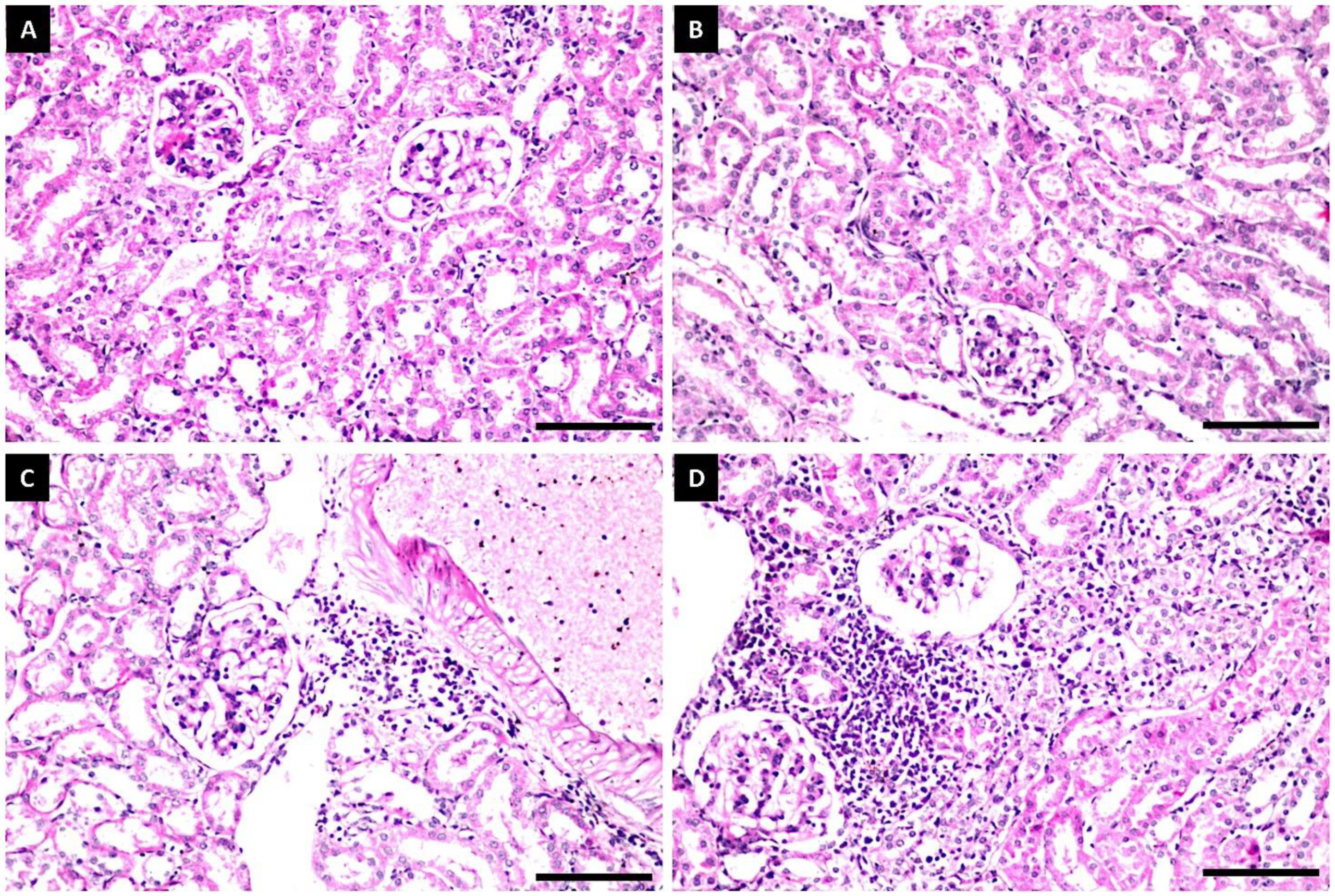
Histopathological Examination
4. Discussion
5. Conclusions and Further Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faostat. Food and Agriculture Organization of the United Nations Statistics Division. Available online: http://faostat3.fao.org/home/E (accessed on 4 May 2022).
- Cooke, W.B. Cephalosporium-artige schimmelpilze (hyphomycetes). Mycologia 1973, 65, 253–257. [Google Scholar] [CrossRef]
- El-Shafey, H.; Abd-El-Rahim, M.; Refaat, M. A New Cephalosporium-Wilt Disease of Grain Sorghum in Egypt. In Proceedings of the Egyptian Phytopathology Congress, Cairo, Egypt, 26–29 November 1979; The Egyptian Phytopathology Society: Cairo, Egypt, 1980. [Google Scholar]
- Rodrigues, R.T.; de Souza Silva, M.M.; de Oliveira, D.M.; Simplício, J.B.; Costa, C.M.C.; de Siqueira, V.M. Endophytic fungi from Sorghum bicolor (L.) moench: Influence of genotypes and crop systems and evaluation of antimicrobial activity. J. Agric. Sci. Technol. 2018, 8, 267–277. [Google Scholar]
- Natural, M.; Frederiksen, R.; Rosenow, D. Acremonium wilt of sorghum [Sorghum bicolor, Acremonium strictum]. Plant Dis. 1982, 66, 863–865. [Google Scholar] [CrossRef]
- Ali, M.; Osman, E.; Ibrahim, T.; Khalafalla, G.; Amin, G. In vitro antagonism between soilrhizosphere microorganisms and acremonium strictum the causal fungus of acremonium wilt in grain sorghum. J. Agric. Sci. Mansoura Univ. 2004, 29, 5821–5833. [Google Scholar] [CrossRef]
- Salama, S. Investigations of the major stalk, foliar and grain disease of sorghum (Sorghum bicolor) including studies on the genetic mature of resistance. Ann. Rep. Public Law 1976, 63, 1–17. [Google Scholar]
- Prasad, R.; Bhattacharyya, A.; Nguyen, Q.D. Nanotechnology in sustainable agriculture: Recent developments, challenges, and perspectives. Front. Microbiol. 2017, 8, 01014. [Google Scholar] [CrossRef] [PubMed]
- El-Beltagi, H.S.; Ali, M.R.; Ramadan, K.M.A.; Anwar, R.; Shalaby, T.A.; Rezk, A.A.; El-Ganainy, S.M.; Mahmoud, S.F.; Alkafafy, M.; El-Mogy, M.M. Exogenous postharvest application of calcium chloride and salicylic acid to maintain the quality of broccoli florets. Plants 2022, 11, 1513. [Google Scholar] [CrossRef] [PubMed]
- Parisi, C.; Vigani, M.; Rodríguez-Cerezo, E. Agricultural nanotechnologies: What are the current possibilities? Nano Today 2015, 10, 124–127. [Google Scholar] [CrossRef]
- Khatoon, U.T.; Rao, G.N.; Mohan, K.M.; Ramanaviciene, A.; Ramanavicius, A. Antibacterial and antifungal activity of silver nanospheres synthesized by tri-sodium citrate assisted chemical approach. Vacuum 2017, 146, 259–265. [Google Scholar] [CrossRef]
- Khatoon, U.T.; Rao, G.N.; Mohan, M.K.; Ramanaviciene, A.; Ramanavicius, A. Comparative study of antifungal activity of silver and gold nanoparticles synthesized by facile chemical approach. J. Environ. Chem. Eng. 2018, 6, 5837–5844. [Google Scholar] [CrossRef]
- El-Gazzar, N.; Ismail, A.M. The potential use of titanium, silver and selenium nanoparticles in controlling leaf blight of tomato caused by Alternaria alternata. Biocatal. Agric. Biotechnol. 2020, 27, 101708. [Google Scholar] [CrossRef]
- Ismail, A.M. Efficacy of copper oxide and magnesium oxide nanoparticles on controlling black scurf disease on potato. Egypt. J. Phytopathol. 2021, 49, 116–130. [Google Scholar] [CrossRef]
- Ismail, A.M.; El-Gawad, A.; Mona, E. Antifungal activity of mgo and zno nanoparticles against powdery mildew of pepper under greenhouse conditions. Egypt. J. Agric. Res. 2021, 99, 421–434. [Google Scholar]
- Raveendran, P.; Fu, J.; Wallen, S.L. Completely “green” synthesis and stabilization of metal nanoparticles. J. Am. Chem. Soc. 2003, 125, 13940–13941. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Kumar, V.; Prasad, K.S. Nanotechnology in sustainable agriculture: Present concerns and future aspects. Afr. J. Biotechnol. 2014, 13, 705–713. [Google Scholar]
- Prasad, R.; Gupta, N.; Kumar, M.; Kumar, V.; Wang, S.; Abd-Elsalam, K.A. Nanomaterials act as plant defense mechanism. In Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 253–269. [Google Scholar]
- Mohapatra, M.; Anand, S. Synthesis and applications of nano-structured iron oxides/hydroxides—A review. Int. J. Eng. Technol. 2010, 2, 127–146. [Google Scholar] [CrossRef]
- Fang, S.; Bresser, D.; Passerini, S. Transition metal oxide anodes for electrochemical energy storage in lithium- and sodium-ion batteries. Adv. Energy Mater. 2020, 10, 1902485. [Google Scholar] [CrossRef]
- Tuharin, K.; Turek, Z.; Zanáška, M.; Kudrna, P.; Tichý, M. Iron oxide and iron sulfide films prepared for dye-sensitized solar cells. Materials 2020, 13, 1797. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ma, Y.; Liu, L.; Yao, S.; Wu, W.; Wang, Z.; Lv, P.; Zheng, J.; Yu, K.; Wei, W. Plasma enabled Fe2O3/Fe3O4 nano-aggregates anchored on nitrogen-doped graphene as anode for sodium-ion batteries. Nanomaterials 2020, 10, 782. [Google Scholar] [CrossRef]
- Cichello, S.A. Oxygen absorbers in food preservation: A review. J. Food Sci. Technol. 2015, 52, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- Magro, M.; Baratella, D.; Bonaiuto, E.; de A. Roger, J.; Vianello, F. New perspectives on biomedical applications of iron oxide nanoparticles. Curr. Med. Chem. 2018, 25, 540–555. [Google Scholar] [CrossRef]
- Rui, M.; Ma, C.; Hao, Y.; Guo, J.; Rui, Y.; Tang, X.; Zhao, Q.; Fan, X.; Zhang, Z.; Hou, T. Iron oxide nanoparticles as a potential iron fertilizer for peanut (Arachis hypogaea). Front. Plant Sci. 2016, 7, 815. [Google Scholar] [CrossRef] [PubMed]
- Noqta, O.A.; Aziz, A.A.; Usman, I.A.; Bououdina, M. Recent advances in iron oxide nanoparticles (ionps): Synthesis and surface modification for biomedical applications. J. Supercond. Nov. Magn. J. 2019, 32, 779–795. [Google Scholar] [CrossRef]
- Suturin, S.; Kaveev, A.; Korovin, A.; Fedorov, V.; Sawada, M.; Sokolov, N. Structural transformations and interfacial iron reduction in heterostructures with epitaxial layers of 3d metals and ferrimagnetic oxides. J. Appl. Crystallogr. 2018, 51, 1069–1081. [Google Scholar] [CrossRef]
- Serga, V.; Burve, R.; Maiorov, M.; Krumina, A.; Skaudžius, R.; Zarkov, A.; Kareiva, A.; Popov, A.I. Impact of gadolinium on the structure and magnetic properties of nanocrystalline powders of iron oxides produced by the extraction-pyrolytic method. Materials 2020, 13, 4147. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Wang, J.; Wu, C.; Meng, F.; Shi, Z.; Lian, J.; Feng, J.; Meng, J. Novel complex-coprecipitation route to form high quality triethanolamine-coated Fe3O4 nanocrystals: Their high saturation magnetizations and excellent water treatment properties. CrystEngComm 2012, 14, 5741–5744. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Mosleh, A.M.; Abd Elghany, A.; Shams-Eldin, E.; Serea, E.S.A.; Ali, S.A.; Shalan, A.E. Coated silver nanoparticles: Synthesis, cytotoxicity, and optical properties. RSC Adv. 2019, 9, 20118–20136. [Google Scholar] [CrossRef]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; Lima, T.M.T.d.; Delbem, A.C.B.; Monteiro, D.R. Iron oxide nanoparticles for biomedical applications: A perspective on synthesis, drugs, antimicrobial activity, and toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-F.; Zhao, Z.-S.; Jiang, G.-B. Coating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water. Environ. Sci. Technol. 2008, 42, 6949–6954. [Google Scholar] [CrossRef] [PubMed]
- Illés, E.; Tombácz, E. The effect of humic acid adsorption on ph-dependent surface charging and aggregation of magnetite nanoparticles. J. Colloid Interface Sci. 2006, 295, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Chekli, L.; Phuntsho, S.; Roy, M.; Shon, H.K. Characterisation of Fe-oxide nanoparticles coated with humic acid and suwannee river natural organic matter. Sci. Total Environ. 2013, 461, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Cuenya, B.R. Synthesis and catalytic properties of metal nanoparticles: Size, shape, support, composition, and oxidation state effects. Thin Solid Films 2010, 518, 3127–3150. [Google Scholar] [CrossRef]
- Wu, S.; Sun, A.; Zhai, F.; Wang, J.; Xu, W.; Zhang, Q.; Volinsky, A.A. Fe3O4 magnetic nanoparticles synthesis from tailings by ultrasonic chemical co-precipitation. Mater. Lett. 2011, 65, 1882–1884. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Sakthivel, N. Biological synthesis of metal nanoparticles by microbes. Adv. Colloid. Interface Sci. 2010, 156, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Zafar, H.; Zia, M.; ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Koesnarpadi, S.; Santosa, S.J.; Siswanta, D.; Rusdiarso, B. Synthesis and characterizatation of magnetite nanoparticle coated humic acid (Fe3O4/ha). Procedia Environ. Sci. 2015, 30, 103–108. [Google Scholar] [CrossRef]
- Gonçalves, L.; Seabra, A.B.; Pelegrino, M.T.; De Araujo, D.; Bernardes, J.S.; Haddad, P.S. Superparamagnetic iron oxide nanoparticles dispersed in pluronic f127 hydrogel: Potential uses in topical applications. RSC Adv. 2017, 7, 14496–14503. [Google Scholar] [CrossRef]
- Enneffati, M.; Rasheed, M.; Louati, B.; Guidara, K.; Barillé, R. Morphology, uv–visible and ellipsometric studies of sodium lithium orthovanadate. Opt. Quant. Electron. 2019, 51, 1–19. [Google Scholar] [CrossRef]
- Grover, R.; Moore, J. Toximetric studies of fungicides against brown rot organisms, Sclerotinia fructicola and S. Laxa. Phytopathology 1962, 52, 876–879. [Google Scholar]
- Karunakar, R.; Pande, S.; Satyaprasad, K.; Ramarao, P. Varietal reaction of non-senescent sorghums to the pathogens causing root and stalk rot of sorghum in india. Int. J. Pest Manag. 1993, 39, 343–346. [Google Scholar] [CrossRef][Green Version]
- Azzam, C.R.; Atta, A.; Ismail, M. Development of molecular genetic markers for acremonium wilt disease resistance in grain sorghum. Egypt. J. Plant Breed. 2010, 14, 299–319. [Google Scholar]
- Okamoto, M.; Hanada, A.; Kamiya, Y.; Yamaguchi, S.; Nambara, E. Measurement of abscisic acid and gibberellins by gas chromatography/mass spectrometry. In Plant Hormones; Springer: Berlin/Heidelberg, Germany, 2009; pp. 53–60. [Google Scholar]
- Allam, A.; Hollis, J. Sulfide inhibition of oxidases in rice roots. Phytopathology 1972, 62, 634–639. [Google Scholar] [CrossRef]
- Pereira, G.; Molina, S.; Lea, P.; Azevedo, R. Activity of antioxidant enzymes in response to cadmium in Crotalaria juncea. Plant Soil 2002, 239, 123–132. [Google Scholar] [CrossRef]
- Mills, K.L.; Morton, R.; Page, G. Textbook of Clinical Pathology; Williams & Wilkins: Philadelphia, PA, USA, 1971. [Google Scholar]
- Jain, N.C. Schalm’s Veterinary Hematology; Lea and Febiger: Palo Alto, CA, USA, 1986. [Google Scholar]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.A.; Coresh, J.; Greene, T.; Levey, A.S. Assessing kidney function—Measured and estimated glomerular filtration rate. N. Engl. J. Med. 2006, 354, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Snedecor, G.; Cochran, W. Statistical Methods, 7th ed.; Iowa State University Press: Ames, IA, USA, 1980. [Google Scholar]
- Haydar, M.S.; Ghosh, S.; Mandal, P. Application of iron oxide nanoparticles as micronutrient fertilizer in mulberry propagation. J. Plant Growth Regul. 2021, 41, 1726–1746. [Google Scholar] [CrossRef]
- Chandrasekar, N.; Kumar, K.; Balasubramnian, K.S.; Karunamurthy, K.; Varadharajan, R. Facile synthesis of iron oxide, iron-cobalt and zero valent iron nanoparticles and evaluation of their anti microbial activity, free radicle scavenginging activity and antioxidant assay. Dig. J. Namomater. Bios. 2013, 8, 765–775. [Google Scholar]
- Parveen, S.; Wani, A.H.; Shah, M.A.; Devi, H.S.; Bhat, M.Y.; Koka, J.A. Preparation, characterization and antifungal activity of iron oxide nanoparticles. Microb. Pathog. 2018, 115, 287–292. [Google Scholar] [CrossRef]
- Nehra, P.; Chauhan, R.; Garg, N.; Verma, K. Antibacterial and antifungal activity of chitosan coated iron oxide nanoparticles. Br. J. Biomed. Sci. 2018, 75, 13–18. [Google Scholar] [CrossRef]
- Win, T.T.; Khan, S.; Bo, B.; Zada, S.; Fu, P. Green synthesis and characterization of Fe3O4 nanoparticles using chlorella-k01 extract for potential enhancement of plant growth stimulating and antifungal activity. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.-H.; Tsai, P.-H.; Lin, K.-S.; Ke, W.-J.; Chiang, C.-L. Antimicrobial effects of zero-valent iron nanoparticles on gram-positive bacillus strains and gram-negative Escherichia coli strains. J. Nanobiotechnol. 2017, 15, 1–12. [Google Scholar] [CrossRef]
- Jindo, K.; Olivares, F.L.; Malcher, D.J.d.P.; Sánchez-Monedero, M.A.; Kempenaar, C.; Canellas, L.P. From lab to field: Role of humic substances under open-field and greenhouse conditions as biostimulant and biocontrol agent. Front. Plant Sci. 2020, 11, 426. [Google Scholar] [CrossRef] [PubMed]
- Tomke, P.D.; Rathod, V.K. Facile fabrication of silver on magnetic nanocomposite (Fe3O4@ chitosan–agnp nanocomposite) for catalytic reduction of anthropogenic pollutant and agricultural pathogens. Int. J. Biol. Macromol. 2020, 149, 989–999. [Google Scholar] [CrossRef]
- Ali, M.; Haroon, U.; Khizar, M.; Chaudhary, H.J.; Munis, M.F.H. Facile single step preparations of phyto-nanoparticles of iron in Calotropis procera leaf extract to evaluate their antifungal potential against Alternaria alternata. Curr. Plant Biol. 2020, 23, 100157. [Google Scholar] [CrossRef]
- Devi, H.S.; Boda, M.A.; Shah, M.A.; Parveen, S.; Wani, A.H. Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity. Green Process. Synth. 2019, 8, 38–45. [Google Scholar] [CrossRef]
- Crecchio, C.; Stotzky, G. Insecticidal activity and biodegradation of the toxin from Bacillus thuringiensis subsp. Kurstaki bound to humic acids from soil. Soil Biol. Biochem. 1998, 30, 463–470. [Google Scholar]
- Wu, M.; Song, M.; Liu, M.; Jiang, C.; Li, Z. Fungicidal activities of soil humic/fulvic acids as related to their chemical structures in greenhouse vegetable fields with cultivation chronosequence. Sci. Rep. 2016, 6, 32858. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, Y.; Cui, Z.; Beattie, D.; Gu, Y.; Wang, Q. Design, synthesis, and biological activities of arylmethylamine substituted chlorotriazine and methylthiotriazine compounds. J. Agric. Food Chem. 2011, 59, 11711–11717. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, S.; Isaac, R.R.; Geo, S.; Sornalekshmi, S.; Rose, A.; Praseetha, P. Evaluation of antimicrobial activity of biosynthesized iron and silver nanoparticles using the fungi Fusarium oxysporum and Actinomycetes sp. on human pathogens. Nano Biomed. Eng. 2013, 5, 39–45. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.Y.; Lee, W.I.; Nelson, K.L.; Yoon, J.; Sedlak, D.L. Bactericidal effect of zero-valent iron nanoparticles on Escherichia coli. Environ. Sci. Technol. 2008, 42, 4927–4933. [Google Scholar] [CrossRef]
- Behera, S.S.; Patra, J.K.; Pramanik, K.; Panda, N.; Thatoi, H. Characterization and evaluation of antibacterial activities of chemically synthesized iron oxide nanoparticles. World J. Nano Sci. Eng. 2012, 2, 196–200. [Google Scholar] [CrossRef]
- Park, H.-J.; Kim, J.Y.; Kim, J.; Lee, J.-H.; Hahn, J.-S.; Gu, M.B.; Yoon, J. Silver-ion-mediated reactive oxygen species generation affecting bactericidal activity. Water Res. 2009, 43, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Keenan, C.R.; Sedlak, D.L. Factors affecting the yield of oxidants from the reaction of nanoparticulate zero-valent iron and oxygen. Environ. Sci. Technol. 2008, 42, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dick, W.; Hoitink, H. Compost-induced systemic acquired resistance in cucumber to pythium root rot and anthracnose. Phytopathology 1996, 86, 1066–1070. [Google Scholar] [CrossRef]
- Loffredo, E.; Berloco, M.; Casulli, F.; Senesi, N. In vitro assessment of the inhibition of humic substances on the growth of two strains of Fusarium oxysporum. Biol. Fertil. Soils 2007, 43, 759–769. [Google Scholar] [CrossRef]
- Ali, M.; Osman, E.A.; Dawoud, E.I.; Ibrahim, T.F.; Amin, G. Optimization of the bioagent Bacillus subtilis biomass production and antibosis against Acremonium strictum. J. Soil Sci. Agric. Eng. 2007, 32, 4075–4090. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, Y.; Li, H.; Lu, J.; Zhao, H.; Liu, M.; Nechitaylo, G.S.; Glushchenko, N.N. New insights into the cellular responses to iron nanoparticles in Capsicum annuum. Sci. Rep. 2018, 8, 3228. [Google Scholar] [CrossRef] [PubMed]
- Elanchezhian, R.; Kumar, D.; Ramesh, K.; Biswas, A.K.; Guhey, A.; Patra, A.K. Morpho-physiological and biochemical response of maize (Zea mays L.) plants fertilized with nano-iron (Fe3O4) micronutrient. J. Plant Nutr. 2017, 40, 1969–1977. [Google Scholar] [CrossRef]
- Li, J.; Ma, Y.; Xie, Y. Stimulatory effect of Fe3O4 nanoparticles on the growth and yield of Pseudostellaria heterophylla via improved photosynthetic performance. HortScience 2021, 56, 753–761. [Google Scholar] [CrossRef]
- Bystrzejewska-Piotrowska, G.; Asztemborska, M.; Stęborowski, R.; Polkowska-Motrenko, H.; Danko, B.; Ryniewicz, J. Application of neutron activation for investigation of Fe3O4 nanoparticles accumulation by plants. Nukleonika 2012, 57, 427–430. [Google Scholar]
- Yigit, V.; Dikilitaş, M. Effect of humic acid applications on the root-rot diseases caused by Fusarium spp. on tomato plants. Plant Pathol. J. 2008, 7, 179–182. [Google Scholar] [CrossRef]
- Abd-El-Kareem, F.; Abd-El-Latif, F.M.; Fotouh, Y. Integrated treatments between humic acid and sulfur for controlling early blight disease of potato plants under field infection. Res. J. Agric. Biol. Sci. 2009, 5, 1039–1045. [Google Scholar]
- Ali, E.; Abd El-Rahman, K.; El-Far, I.; Mohamed, A. Response of some grain sorghum genotypes to foliar spray by humic acid. Assiut J. Agric. Sci. 2020, 51, 54–63. [Google Scholar]
- Pirmohamed, T.; Dowding, J.M.; Singh, S.; Wasserman, B.; Heckert, E.; Karakoti, A.S.; King, J.E.; Seal, S.; Self, W.T. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem. Commun. 2010, 46, 2736–2738. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef]
- Penel, C.; Gaspar, T.; Greppin, H. Plant Peroxidases, 1980–1990: Topics and Detailed Literature on Molecular, Biochemical, and Physiological Aspects; University of Geneva: Geneva, Switzerland, 1992. [Google Scholar]
- Ramanaviciene, A.; Acaite, J.; Ramanavicius, A.; Ramanavicius, A. Chronic caffeine intake affects lysozyme activity and immune cells in mice. J. Pharm. Pharmocol. 2004, 56, 671–676. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Liu, R. Superparamagnetic α-Fe2O3/Fe3O4 heterogeneous nanoparticles with enhanced biocompatibility. Nanomaterials 2021, 11, 834. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; Kausaite, A.; Tautkus, S.; Ramanavicius, A. Biocompatibility of polypyrrole particles: An in-vivo study in mice. J. Pharm. Pharmocol. 2007, 59, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Parivar, K.; Malekvand Fard, F.; Bayat, M.; Alavian, S.M.; Motavaf, M. Evaluation of iron oxide nanoparticles toxicity on liver cells of balb/c rats. Iran. Red Crescent Med. J. 2016, 18, e28939. [Google Scholar] [CrossRef]
- Kim, J.S.; Yoon, T.-J.; Yu, K.N.; Kim, B.G.; Park, S.J.; Kim, H.W.; Lee, K.H.; Park, S.B.; Lee, J.-K.; Cho, M.H. Toxicity and tissue distribution of magnetic nanoparticles in mice. Toxicol. Sci. 2006, 89, 338–347. [Google Scholar] [CrossRef]
- Salehi, M.; Fatahian, S.; Shahanipour, K. Effect of iron oxide nanoparticles coated with chitosan on renal functional indeces in rats. J. Gorgan. Univ. Med. Sci. 2017, 19, 14–19. [Google Scholar]
- Battis, G.N., Jr. The enigma of liver enzymes: Transferases. J. Insur. Med. 1988, 20, 17–20. [Google Scholar]
- Vozarova, B.; Stefan, N.; Lindsay, R.S.; Saremi, A.; Pratley, R.E.; Bogardus, C.; Tataranni, P.A. High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes 2002, 51, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, L.; Tanwir, F.; Babadi, V.Y. In vitro toxicity of iron oxide nanoparticle: Oxidative damages on hep g2 cells. Exp. Toxicol. Pathol. 2015, 67, 197–203. [Google Scholar] [CrossRef] [PubMed]


| Numerical Grade | Degree of Infection |
|---|---|
| 0.1 | Minimal reaction, indistinguishable from that to a sterile toothpick. |
| 0.2 | Discoloration centered around the wound, progressing in the superficial parts of the stalk, but not reaching either node. |
| 0.5 | Extensive discoloration progressing in the central part of the stalk. |
| 0.8 | Discoloration reaching one or both nodes superficially or forming a cylinder. |
| 1.0 | Most or all of one internode discolored with no penetration of nodal areas. |
| 1.1 | Slight penetration of one or both nodes. |
| 1.2 | Nearly complete penetration of one or both nodes, |
| 1.5 | Penetration of one node and slight invasion of next internode. |
| 2.0 | More than one internode but not more than two affected; infection must have spread through at least one internode. |
| 2.5 | Penetration of two nodes and slight invasion of distal internode. |
| 3.0 | Infection passed through two or more internodes. |
| 4.0 | Extensive invasion of plant but not killed. |
| 5.0 | Death of plant due to stalk-rot. |
| No. | Category Numerical | Level of Infection |
|---|---|---|
| 1 | 0.0–0.5 | Highly resistant |
| 2 | 0.6–1.0 | Resistant |
| 3 | 1.1–1.5 | Moderately resistant |
| 4 | 1.6–3.0 | Moderately susceptible |
| 5 | 3.1–4.0 | Susceptible |
| 6 | 4.1–5.0 | Highly Susceptible |
| No. | Genotypes | DR ± St.Dev. | Group Type |
|---|---|---|---|
| 1 | LG 13 | 0.17 ± 0.03 | HR |
| 2 | Assuit 14 | 0.67 ± 0.10 | R |
| 3 | Line c | 1.11 ± 0.10 | MR |
| 4 | Local 119 | 0.52 ± 0.08 | HR |
| 5 | Local 245 | 0.72 ± 0.04 | R |
| 6 | Dorado | 0.39 ± 0.01 | HR |
| 7 | LG 23 | 0.46 ± 0.07 | HR |
| 8 | Sel 1007 | 0.83 ± 0.04 | R |
| 9 | LG 35 | 0.89 ± 0.10 | R |
| 10 | LG 47 | 0.73 ± 0.03 | R |
| 11 | H sh 1 | 0.72 ± 0.05 | R |
| 12 | H 301 | 1.25 ± 0.06 | MR |
| 13 | H 305 | 0.90 ± 0.04 | R |
| 14 | H 306 | 0.93 ± 0.02 | R |
| 15 | LG 1 | 4.12 ± 0.11 | HS |
| 16 | Giza 3 | 3.45 ± 0.22 | S |
| 17 | Giza 113 | 3.06 ± 0.13 | S |
| 18 | Local 129 | 1.29 ± 0.08 | MR |
| 19 | ICSR 92003 | 1.02 ± 0.08 | R |
| 20 | ICSR 93002 | 1.08 ±0.08 | MR |
| 21 | LG 3 | 2.03 ± 0.06 | MS |
| Average | 1.25 | ||
| LSD 0.05 | 0.14 | ||
| Genotypes | Concentration (mg L−1) | Season 2020 | Season 2021 | ||
|---|---|---|---|---|---|
| DR ± St.Dev. | Efficacy (%) | DR ± St.Dev. | Efficacy (%) | ||
| Giza 113 | 10 | 3.2 ± 0.20 | 17.3 | 3.53 ± 0.15 | 7.8 |
| 40 | 1.8 ± 0.10 | 53.49 | 1.87 ± 0.25 | 51.17 | |
| 80 | 1.53 ±0.06 | 60.47 | 1.5 ± 0.30 | 60.84 | |
| Control | 3.87 ± 0.15 | - | 3.83 ± 0.21 | - | |
| LG 1 | 10 | 3.3 ± 0.20 | 22.72 | 3.37 ± 0.31 | 22.25 |
| 40 | 1.73 ± 0.15 | 59.48 | 1.83 ± 0.06 | 57.74 | |
| 80 | 1.2 ± 0.10 | 71.9 | 1.23 ± 0.06 | 71.59 | |
| Control | 4.27 ± 0.06 | - | 4.33 ± 0.25 | - | |
| LG 3 | 10 | 2.03 ± 0.06 | 2.4 | 2.5 ± 0.20 | 8.42 |
| 40 | 1.3 ± 0.10 | 37.2 | 1.23 ±0.06 | 54.95 | |
| 80 | 0.97 ± 0.21 | 53.14 | 0.9 ± 0.17 | 67.03 | |
| Control | 2.07 ± 0.31 | - | 2.73 ± 0.31 | - | |
| Giza 3 | 10 | 2.93 ± 0.12 | 14.0 | 2.77 ± 0.21 | 20.17 |
| 40 | 1.47 ± 0.06 | 58.82 | 1.4 ± 0.10 | 59.65 | |
| 80 | 1.1 ± 0.20 | 69.19 | 0.97 ± 0.12 | 72.04 | |
| Control | 3.57 ± 0.25 | - | 3.47 ± 0.15 | - | |
| Local 119 | 10 | 0.37 ± 0.06 | 26.0 | 0.4 ± 0.10 | 20.0 |
| 40 | 0.13 ± 0.06 | 74.0 | 0.13 ± 0.06 | 74.0 | |
| 80 | 0.1 ± 0.00 | 80.0 | 0.1 ± 0.00 | 80.0 | |
| Control | 0.5 ± 0.10 | - | 0.5 ± 0.00 | - | |
| LSD of treatments | 0.11 | 0.48 | |||
| LSD of genotypes | 0.18 | 0.53 | |||
| Plant Height (cm) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (mg L−1) | Season 2020 | Season 2021 | ||||||||||
| Giza 113 | LG 1 | LG 3 | Giza 3 | Local119 | Mean | Giza 113 | LG 1 | LG 3 | Giza 3 | Local119 | Mean | |
| 10 | 370.33 | 363.00 | 355.33 | 375.00 | 376.67 | 368.07 | 371.67 | 362.67 | 353.50 | 373.44 | 375.67 | 367.39 |
| 40 | 386.00 | 377.00 | 371.33 | 388.67 | 390.33 | 382.67 | 382.67 | 370.00 | 360.00 | 381.33 | 392.33 | 377.27 |
| 80 | 311.00 | 307.33 | 297.33 | 318.67 | 325.67 | 312.00 | 309.37 | 300.14 | 291.33 | 315.47 | 324.67 | 308.20 |
| Control | 370.33 | 360.33 | 353.33 | 371.33 | 375.33 | 366.13 | 370.67 | 361.44 | 350.00 | 372.00 | 375.00 | 365.82 |
| Mean | 359.42 | 351.92 | 344.33 | 363.42 | 367.00 | 357.22 | 358.59 | 348.56 | 338.71 | 360.56 | 366.92 | 354.67 |
| F test | LSD at 0.05 | F test | LSD at 0.05 | |||||||||
| Treatments | ** | 3.17 | ** | 3.57 | ||||||||
| Genotypes | ** | 2.00 | ** | 2.71 | ||||||||
| Interaction | NS | NS | ||||||||||
| 1000-Grain Weight (g) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (mg L−1) | Season 2020 | Season 2021 | ||||||||||
| Giza 113 | LG 1 | LG 3 | Giza 3 | Local119 | Mean | Giza 113 | LG 1 | LG 3 | Giza 3 | Local119 | Mean | |
| 10 | 42.45 | 36.60 | 38.55 | 40.47 | 41.74 | 39.96 | 40.78 | 36.00 | 39.89 | 42.89 | 41.90 | 40.29 |
| 40 | 45.31 | 38.07 | 40.55 | 43.27 | 44.32 | 42.31 | 43.35 | 38.30 | 42.60 | 44.34 | 45.08 | 42.73 |
| 80 | 32.86 | 27.82 | 30.39 | 31.69 | 34.91 | 31.53 | 32.29 | 26.43 | 30.26 | 31.29 | 33.05 | 30.66 |
| Control | 42.22 | 36.20 | 38.27 | 40.26 | 41.50 | 39.69 | 40.50 | 35.73 | 39.03 | 42.75 | 41.34 | 39.87 |
| Mean | 40.71 | 34.67 | 36.94 | 38.92 | 40.62 | 38.37 | 39.23 | 34.12 | 37.95 | 40.32 | 40.34 | 38.39 |
| F test | LSD at 0.05 | F test | LSD at 0.05 | |||||||||
| Treatments | ** | 1.3 | ** | 0.84 | ||||||||
| Genotypes | ** | 1.08 | ** | 0.6 | ||||||||
| Interaction | NS | 1.2 | ||||||||||
| Grain Yield/Plant (g) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (mg L−1) | Season 2020 | Season 2021 | ||||||||||
| Giza 113 | LG 1 | LG 3 | Giza 3 | Local119 | Mean | Giza 113 | LG 1 | LG 3 | Giza 3 | Local119 | Mean | |
| 10 | 79.41 | 71.51 | 73.05 | 74.81 | 80.93 | 75.94 | 78.71 | 71.89 | 72.68 | 75.51 | 81.86 | 76.13 |
| 40 | 84.10 | 74.44 | 75.64 | 76.59 | 83.96 | 78.95 | 83.25 | 73.67 | 75.91 | 79.04 | 85.54 | 79.48 |
| 80 | 70.81 | 62.54 | 60.88 | 65.01 | 71.82 | 66.21 | 67.72 | 62.16 | 59.61 | 61.69 | 70.57 | 64.35 |
| Control | 79.09 | 71.30 | 73.17 | 74.18 | 80.29 | 75.60 | 78.36 | 71.67 | 72.49 | 75.16 | 81.48 | 75.83 |
| Mean | 78.35 | 69.95 | 70.68 | 72.65 | 79.25 | 74.18 | 77.01 | 69.85 | 70.17 | 72.85 | 79.86 | 73.95 |
| F test | LSD at 0.05 | F test | LSD at 0.05 | |||||||||
| Treatments | ** | 0.77 | ** | 0.98 | ||||||||
| Genotypes | ** | 1.11 | ** | 0.62 | ||||||||
| Interaction | NS | 1.25 | ||||||||||
| Days after Feeding | Concentration (mg L−1) | CBC Analysis | ||
|---|---|---|---|---|
| RBCs (106) | WBCs (103) | Hb (mg dL−1) | ||
| 21 | 10 | 7.63 | 6.83 | 14. 2 |
| 40 | 7.47 | 7.43 | 14. 1 | |
| 80 | 7.5 | 7.6 | 14.5 | |
| Control | 7.42 | 7.57 | 14.1 | |
| 36 | 10 | 7.52 | 7.32 | 14.63 |
| 40 | 7.72 | 7.83 | 14.78 | |
| 80 | 7.8 | 7.6 | 15 | |
| Control | 7.38 | 7.08 | 14.5 | |
| 51 | 10 | 7.87 | 7.15 | 14.49 |
| 40 | 8.29 | 7 | 15.3 | |
| 80 | 8.88 | 7.5 | 16.1 | |
| Control | 7.49 | 7.25 | 14.4 | |
| LSD 0.05 Treatment | 0.54 | 0.52 | 0.4 | |
| LSD 0.05 Days of feed | 0.47 | 0.45 | 0.35 | |
| Days after Feeding | Concentration (mg L−1) | Blood Chemistry | ||
|---|---|---|---|---|
| AST (u L−1) | ALT (u L−1) | Creatinine (mg dL−1) | ||
| 21 | 10 | 79 | 34.4 | 0.57 |
| 40 | 83 | 36.7 | 0.57 | |
| 80 | 86 | 57.2 | 0.6 | |
| Control | 80 | 33.5 | 0.58 | |
| 36 | 10 | 85 | 35 | 0.6 |
| 40 | 84.6 | 38 | 0.59 | |
| 80 | 86 | 65.7 | 0.61 | |
| Control | 84 | 34.5 | 0.6 | |
| 51 | 10 | 83.8 | 35 | 0.67 |
| 40 | 84.3 | 40.7 | 0.69 | |
| 80 | 85 | 80.3 | 0.88 | |
| Control | 83 | 35 | 0.66 | |
| LSD 0.05 Treatment | 0.88 | 0.88 | 0.07 | |
| LSD 0.05 Days of feed | 0.76 | 0.76 | 0.06 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Ganainy, S.M.; El-Bakery, A.M.; Hafez, H.M.; Ismail, A.M.; El-Abdeen, A.Z.; Ata, A.A.E.; Elraheem, O.A.Y.A.; El Kady, Y.M.Y.; Hamouda, A.F.; El-Beltagi, H.S.; et al. Humic Acid-Coated Fe3O4 Nanoparticles Confer Resistance to Acremonium Wilt Disease and Improve Physiological and Morphological Attributes of Grain Sorghum. Polymers 2022, 14, 3099. https://doi.org/10.3390/polym14153099
El-Ganainy SM, El-Bakery AM, Hafez HM, Ismail AM, El-Abdeen AZ, Ata AAE, Elraheem OAYA, El Kady YMY, Hamouda AF, El-Beltagi HS, et al. Humic Acid-Coated Fe3O4 Nanoparticles Confer Resistance to Acremonium Wilt Disease and Improve Physiological and Morphological Attributes of Grain Sorghum. Polymers. 2022; 14(15):3099. https://doi.org/10.3390/polym14153099
Chicago/Turabian StyleEl-Ganainy, Sherif Mohamed, Amal M. El-Bakery, Heba M. Hafez, Ahmed Mahmoud Ismail, Ali Zein El-Abdeen, Abed Abd Elgalel Ata, Omar A. Y. Abd Elraheem, Yousef M. Y. El Kady, Ahlam F. Hamouda, Hossam S. El-Beltagi, and et al. 2022. "Humic Acid-Coated Fe3O4 Nanoparticles Confer Resistance to Acremonium Wilt Disease and Improve Physiological and Morphological Attributes of Grain Sorghum" Polymers 14, no. 15: 3099. https://doi.org/10.3390/polym14153099
APA StyleEl-Ganainy, S. M., El-Bakery, A. M., Hafez, H. M., Ismail, A. M., El-Abdeen, A. Z., Ata, A. A. E., Elraheem, O. A. Y. A., El Kady, Y. M. Y., Hamouda, A. F., El-Beltagi, H. S., Shehata, W. F., Shalaby, T. A., Abbas, A. O., Almaghsla, M. I., Sattar, M. N., & Iqbal, Z. (2022). Humic Acid-Coated Fe3O4 Nanoparticles Confer Resistance to Acremonium Wilt Disease and Improve Physiological and Morphological Attributes of Grain Sorghum. Polymers, 14(15), 3099. https://doi.org/10.3390/polym14153099











