Synthesis and Analysis of Polymorphic Silver Nanoparticles and Their Incorporation into the Polymer Matrix
Abstract
:1. Introduction
2. Materials and Methods
- An advance prepared AgNO3 solution was vigorously mixed without heating for 5 min on a magnetic stirrer (the weight concentration of Ag in 0.11 mM solution is 12 mg/L);
- Then, sodium citrate (TSC), PVP, and hydrogen peroxide were added in this order;
- After 5 min of stirring, the required amounts of ice-cold, freshly-prepared sodium borohydride were added;
- Solutions were stirred until the color change occurred (10–20 min);
- Prepared solutions were stored at room temperature in daylight;
- The ex-situ method of PVA-AgNPs composite preparation:
- ⚬
- Chosen colloidal solutions were centrifuged (9000 rpm for 20 min) to concentrate the nanoparticles (100 mL of AgNPs colloidal solutions was concentrated to 15 mL);
- ⚬
- Concentrated nanoparticles have been added to the polyvinyl alcohol matrix, an 8% solution of PVA-AgNPs was prepared. PVA-AgNPs solution was stirred at 70 °C for several hours to secure good distribution of AgNPs in matrix;
- ⚬
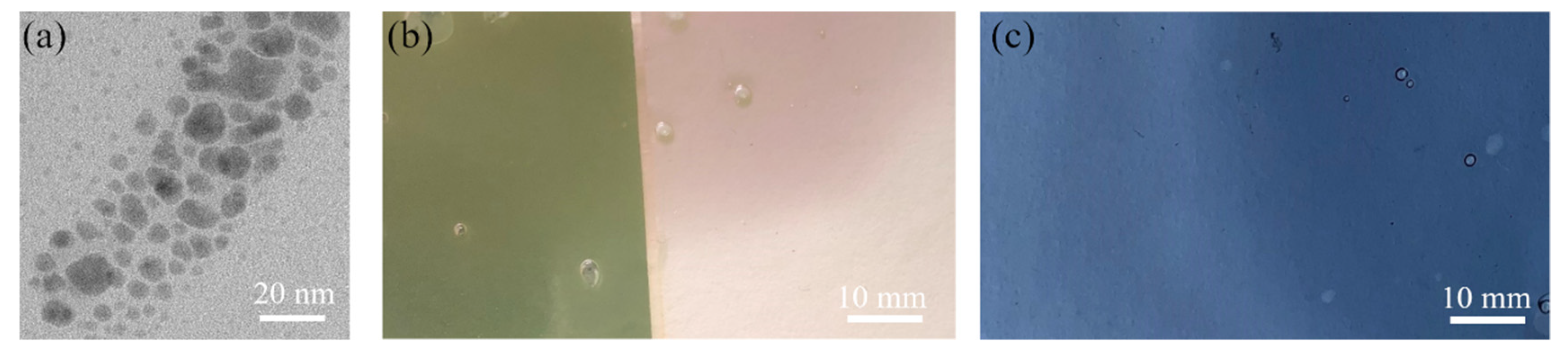
- Subsequently, still-hot solutions of PVA-AgNPs composite were used to prepare the thin layers, the mixture was poured onto the substrate, and spread to a thickness of 0.3 mm; prepared thin layers were allowed to dry at ambient temperature.
3. Results and Discussion
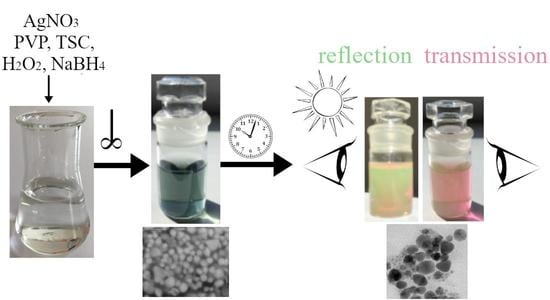
3.1. Synthesis Process
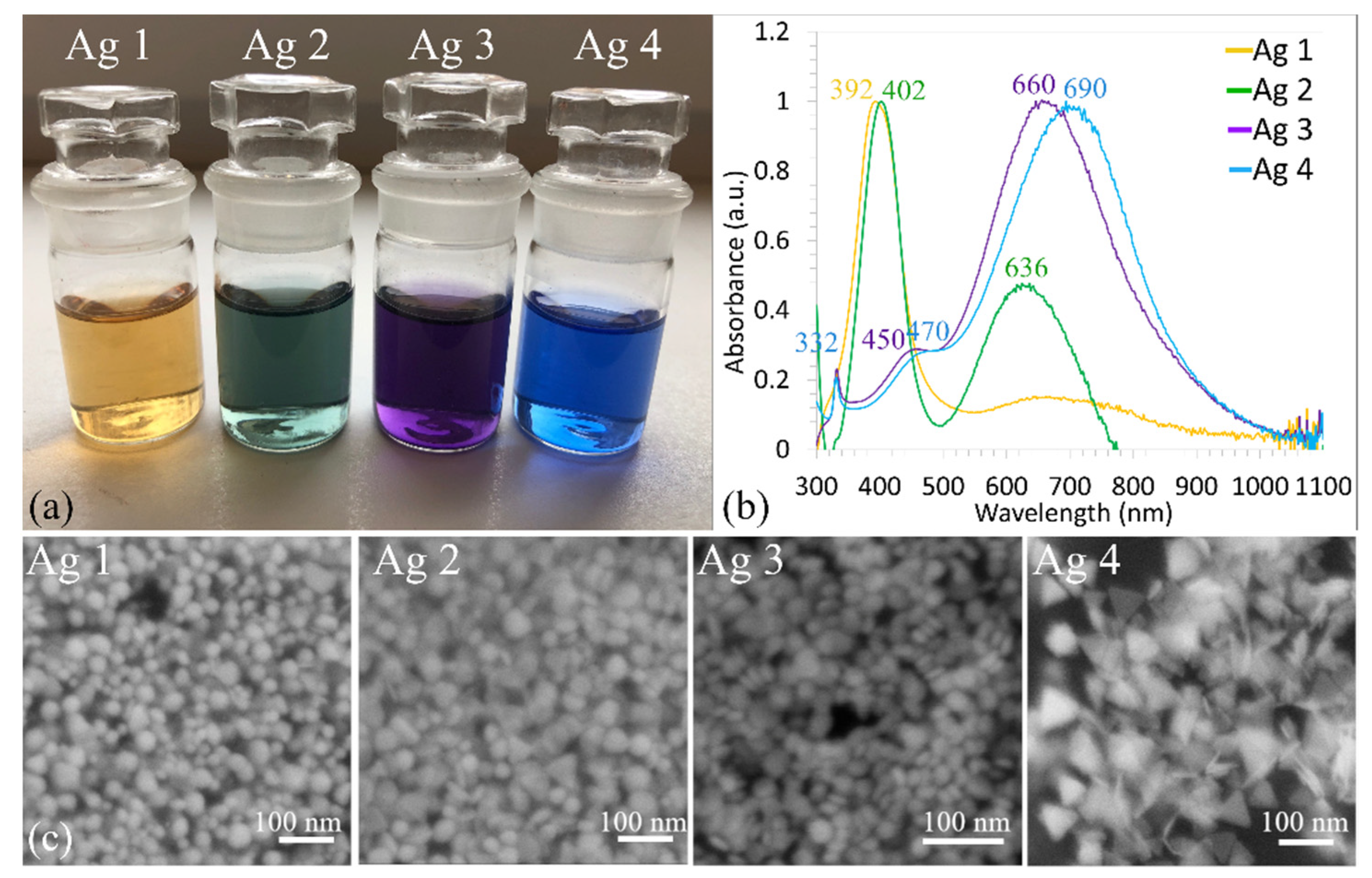
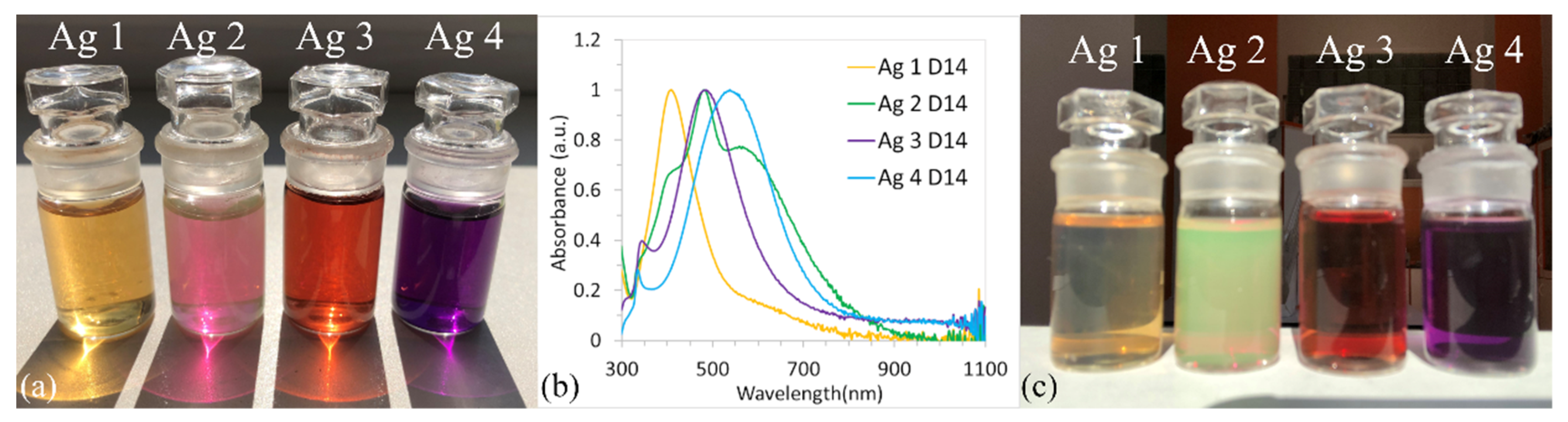
3.2. UV-Vis Spectroscopy
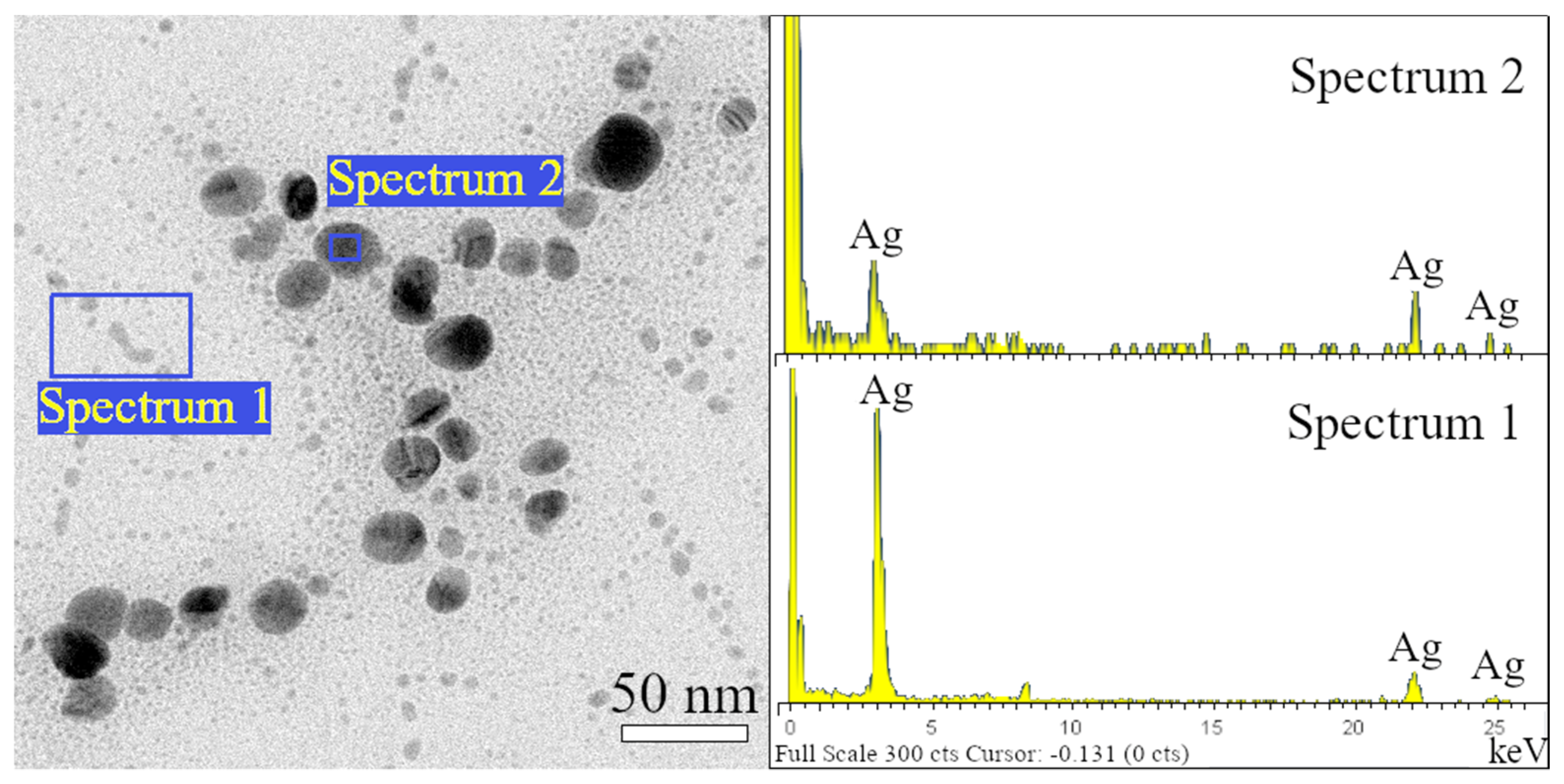
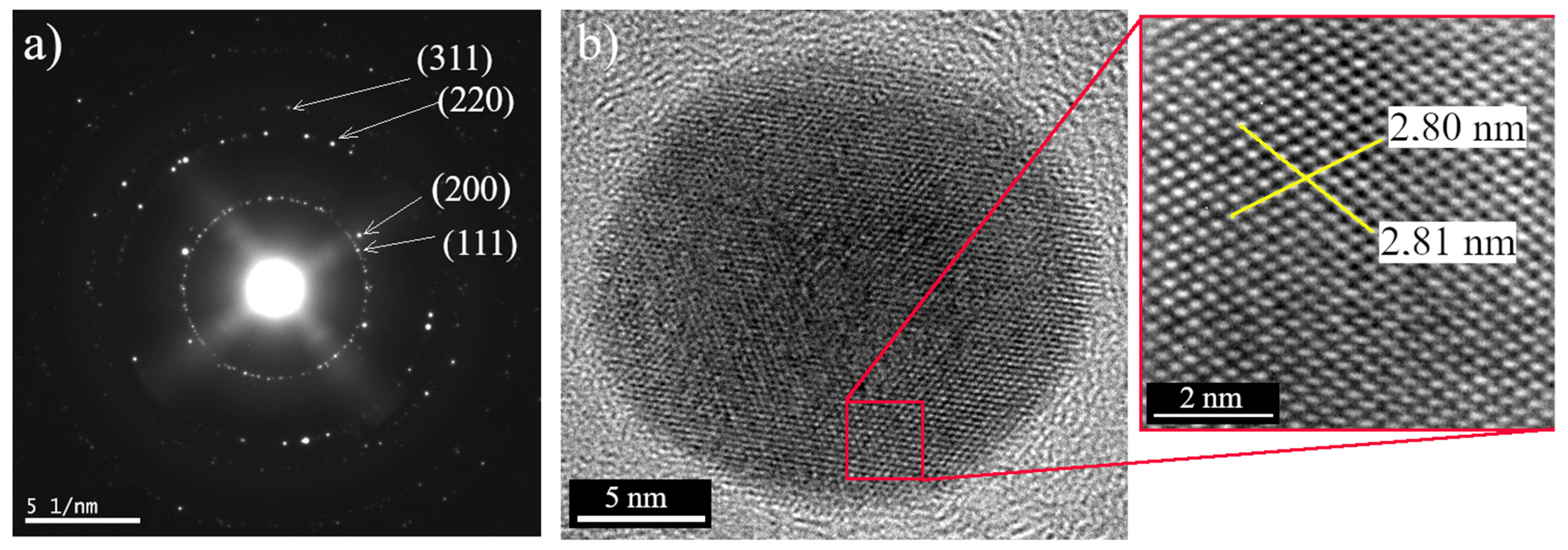
3.3. Electron Microscopy Analysis
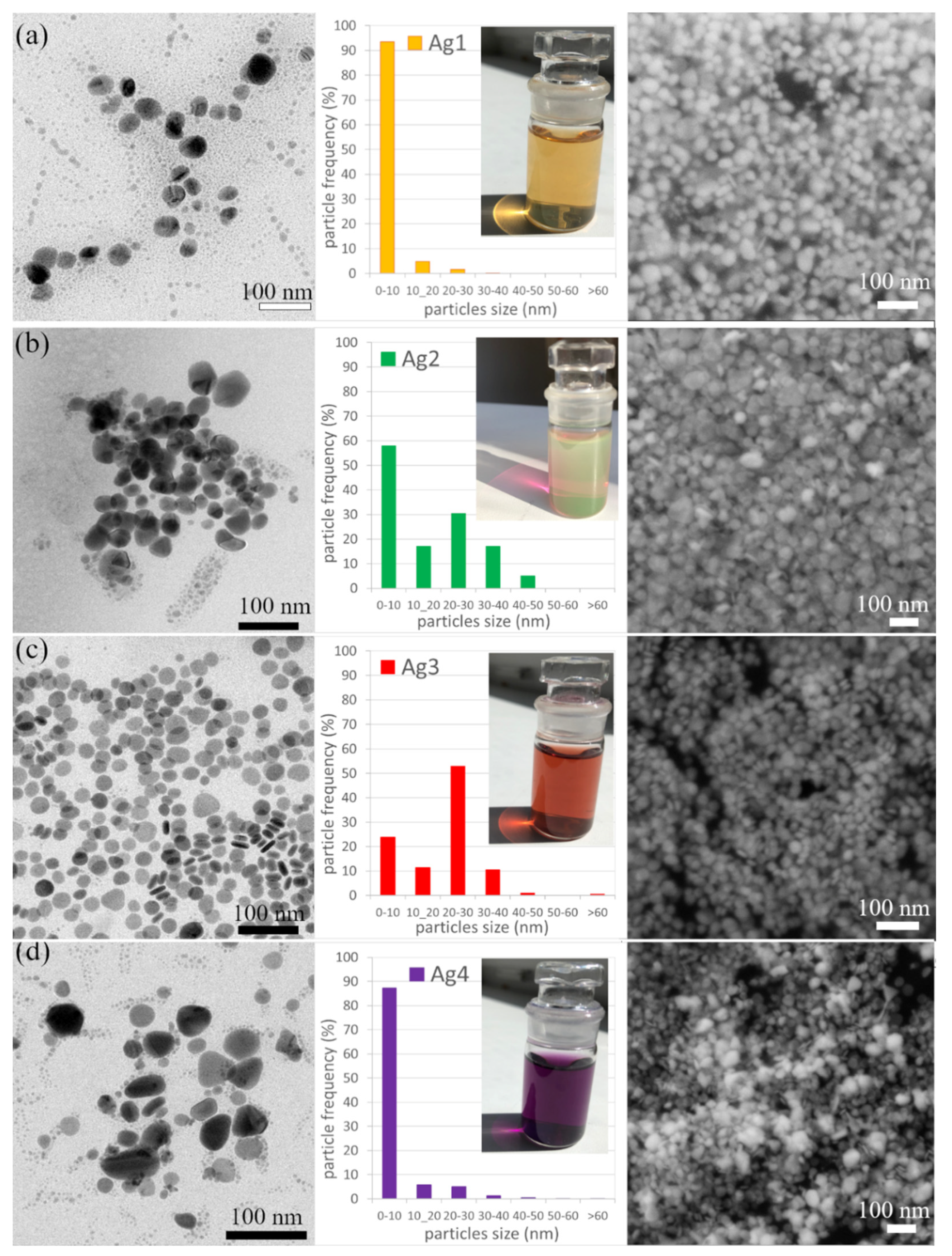
- The sample Ag 1 contains 94% of nanoparticles with a mean diameter up to 10 nm. The max particle diameter was 30 nm (6%). The TEM and SEM images confirmed that all nanoparticles are spherical or near-spherical.
- The sample Ag 2 contains 58% of nanoparticles with a mean diameter up to 10 nm, where even the nanoparticles in the size of quantum dots (<5 nm, 40%) were observed Figure 7a. Only 0.6% of the particles were >50 nm in diameter (max diameter 57 nm). The TEM and SEM images confirmed that nanoparticles are polydisperse, near-spherical, and irregular. Nanoparticles with a mean diameter up to 10 nm are non-symmetrical (58%) Figure 7a. We assume that such shape distribution (irregularity and the presence of small nanoparticles in the range of up to 5 nm) caused a dichroic effect Figure 6b. There are several works dealing with the explanation of the dichroic effect. In his work, Caseri analyzed several works from which, it is clear, that there are discrepancies in the explanation of the dichroic effect, and there are several hypotheses about what this effect (in the case of colloidal solutions) causes [30]. Most authors agree that anisotropic metal particles are responsible for the dichroic effect. However, other authors argue that the particles must be arranged in a particular system in order to achieve this effect. Others argue that dichroism does not come from anisotropic crystal modification of metal particles but rather from rod-shaped metal particles that are able to arrange in a certain spatial arrangement. Based on our observations and TEM photographs, we conclude that anisotropic nanoparticles measuring about 5 nm are the main reason for the dichroic effect, but at the same time, larger nanoparticles of irregular shapes (~30–40 nm) must be present in the solution. No other regular shapes were observed in our dichroic solution, e.g., rod-like or triangles. These are present in combination with quasi-spherical nanoparticles in Ag 3 and Ag 4 solutions, but these solutions are not dichroic.
- The sample Ag 3 contains only 24% of nanoparticles with a mean diameter up to 10 nm and a larger number of nanoparticles with a diameter up to 30 nm (64%). There were also observed particles with a mean diameter >60 nm (0.4%). The TEM and SEM images confirmed that the mix of spherical and rod-like nanoparticles is present (the ratio spherical:rodlike = 3:1).
- The sample Ag 4 contains the largest amount (87%) of nanoparticles with a mean diameter up to 10 nm and only 13% of larger nanoparticles. The TEM and SEM images confirmed that small nanoparticles are all spherical, larger nanoparticles have mostly irregular, quasi-spherical shapes, and a small number of triangular nanoparticles were also observed.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Wet chemical synthesis of silver nanorods and nanowires of controllable aspect ratio. Chem. Commun. 2001, 7, 617–618. [Google Scholar] [CrossRef]
- Huang, T.; Xu, X.-H.N. Synthesis and Characterization of Tunable Rainbow Colored Colloidal Silver Nanoparticles Using Single-Nanoparticle Plasmonic Microscopy and Spectroscopy. Mater. Chem. 2010, 20, 9867–9876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Sciau, P. Nanoparticles in Ancient Materials: The Metallic Lustre Decorations of Medieval Ceramics. Deliv. Nanoparticles 2012, 25, 525–540. [Google Scholar] [CrossRef] [Green Version]
- Dekker, F.; Kool, L.; Bunschoten, A.; Velders, A.H.; Saggiomo, V. Syntheses of gold and silver dichroic nanoparticles; looking at the Lycurgus cup colors. Chem. Teach. Int. 2020, 3, 20190011. [Google Scholar] [CrossRef]
- Magruder, R.H.; Robinson, S.J.; Smith, C.; Meldrum, A.; Halabica, A.; Haglund, R.F. Dichroism in Ag nanoparticle composites with bimodal size distribution. J. Appl. Phys. 2009, 105, 024303. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, N.; Goebl, J.; Lu, Z.; Yin, Y. Systematic Study of the Synthesis of Silver Nanoplates: Is Citrate a “Magic” Reagent? J. Am. Chem. Soc. 2011, 133, 18931–18939. [Google Scholar] [CrossRef]
- Millstone, J.E.; Hurst, S.J.; Métraux, G.S.; Cutler, J.I.; Mirkin, C.A. Colloidal Gold and Silver Triangular Nanoprisms. Small 2009, 5, 646–664. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, Y.; Guo, S.; Goebl, J.; Yin, Y. Seeded Growth of Uniform Ag Nanoplates with High Aspect Ratio and Widely Tunable Surface Plasmon Bands. Nano Lett. 2010, 10, 5037–5042. [Google Scholar] [CrossRef]
- Tao, A.R.; Habas, S.; Yang, P. Shape Control of Colloidal Metal Nanocrystals. Small 2008, 4, 310–325. [Google Scholar] [CrossRef]
- Rashid, M.U.; Bhuiyan, M.K.H.; Quayum, M.E. Synthesis of Silver Nano Particles (Ag-NPs) and their uses for Quantitative Analysis of Vitamin C Tablets, Dhaka Univ. J. Pharm. Sci. 2013, 12, 29–33. [Google Scholar]
- Xu, X.; Wang, Y.; Wang, H.; Su, H.; Mao, X.; Jiang, L.; Liu, M.; Sun, D.; Hou, S. Synthesis of triangular silver nanoprisms and studies on the interactions with human serum albumin. J. Mol. Liq. 2016, 220, 14–20. [Google Scholar] [CrossRef]
- Van Dong, P.; Ha, C.H.; Binh, L.T.; Kasbohm, J. Chemical synthesis and antibacterial activity of novel-shaped silver nanoparticles. Int. Nano Lett. 2012, 2, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Zannotti, M.; Vicomandi, V.; Rossi, A.; Minicucci, M.; Ferraro, S.; Petetta, L.; Giovannetti, R. Tuning of hydrogen peroxide etching during the synthesis of silver nanoparticles. An application of triangular nanoplates as plasmon sensors for Hg2+ in aqueous solution. J. Mol. Liq. 2020, 309, 113238. [Google Scholar] [CrossRef]
- Nishimoto, M.; Abe, S.; Yonezawa, T. Preparation of Ag nanoparticles using hydrogen peroxide as a reducing agent. New J. Chem. 2018, 42, 14493–14501. [Google Scholar] [CrossRef]
- Miesen, T.J.; Engstrom, A.M.; Frost, D.C.; Ajjarapu, R.; Ajjarapu, R.; Lira, C.N.; Mackiewicz, M.R. A hybrid lipid membrane coating “shape-locks” silver nanoparticles to prevent surface oxidation and silver ion dissolution. RSC Adv. 2020, 10, 15677–15693. [Google Scholar] [CrossRef] [Green Version]
- Thiele, H.; von Levern, H. Synthetic protective colloids. J. Colloid Sci. 1965, 20, 679–694. [Google Scholar] [CrossRef]
- Gatemala, H.; Pienpinijtham, P.; Thammacharoen, C.; Ekgasit, S. Rapid fabrication of silver microplates under an oxidative etching environment consisting of O2/Cl−, NH4OH/H2O2, and H2O2. CrystEngComm 2015, 17, 5530–5537. [Google Scholar] [CrossRef]
- Gatemala, H.; Ekgasit, S.; Wongravee, K. High purity silver microcrystals recovered from silver wastes by eco-friendly process using hydrogen peroxide. Chemosphere 2017, 178, 249–258. [Google Scholar] [CrossRef]
- Oh, J.-H.; Kim, B.C.; Lee, J.-S. Colorimetric detection of acetylcholine with plasmonic nanomaterials signaling. Anal. Bioanal. Chem. 2014, 406, 7591–7600. [Google Scholar] [CrossRef]
- Parnklang, T.; Lamlua, B.; Gatemala, H.; Thammacharoen, C.; Kuimalee, S.; Lohwongwatana, B.; Ekgasit, S. Shape transformation of silver nanospheres to silver nanoplates induced by redox reaction of hydrogen peroxide. Mater. Chem. Phys. 2015, 153, 127–134. [Google Scholar] [CrossRef]
- Rasool, S.; Raza, M.A.; Manzoor, F.; Kanwal, Z.; Riaz, S.; Iqbal, M.J.; Naseem, S. Biosynthesis, characterization and anti-dengue vector activity of silver nanoparticles prepared from Azadirachta indica and Citrullus colocynthis. R. Soc. Open Sci. 2020, 7, 200540. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S.; Bharath, L.V. Mechanism of plant-mediated synthesis of silver nanoparticles—A review on biomolecules involved, characterization and antibacterial activity. Chem. Biol. Interact. 2017, 273, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, Y.N.; Kim, J.; Choi, W.M.; Park, S.H.; Kim, M.H. A systematic study of triangular silver nanoplates: One-pot green synthesis, chemical stability, and sensing application. Nanoscale 2017, 9, 11705–11712. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.; Popa, M.; Crespo, D.; Moreno, J.M. Silver nanoprism coatings on optical glass substrates. Microelectron. Eng. 2007, 84, 1665–1668. [Google Scholar] [CrossRef]
- Raza, M.A.; Kanwal, Z.; Rauf, A.; Sabri, A.N.; Riaz, R.; Naseem, S. Size- and shape-dependent antibacterial studies of silver nanoparticles synthesized by wet chemical routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Ye, J.; Tan, K.; Wang, J.; Yang, G. Colorimetric visualization of glucose at the submicromole level in serum by a homogenous silver nanoprism–glucose oxidase system. Anal. Chem. 2013, 85, 6241–6247. [Google Scholar] [CrossRef]
- Tan, K.; Yang, G.; Chen, H.; Shen, P.; Huang, Y.; Xia, Y. Facet dependent binding and etching: Ultra-sensitive colorimetric visualization of blood uric acid by unmodified silver nanoprisms. Biosens. Bioelectron. 2014, 59, 227–232. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Gao, Z. A highly sensitive plasmonic DNA assay based on triangular silver nanoprism etching. ACS Nano 2014, 8, 4902–4907. [Google Scholar] [CrossRef]
- Caseri, W.R. Dichroic nanocomposites based on polymers and metallic particles: From biology to materials science. Polym. Int. 2017, 67, 46–54. [Google Scholar] [CrossRef]
- Jakhmola, A.; Vecchione, R.; Onesto, V.; Gentile, F.; Celentano, M.; Netti, P. Experimental and Theoretical Studies on Sustainable Synthesis of Gold Sol Displaying Dichroic Effect. Nanomaterials 2021, 11, 236. [Google Scholar] [CrossRef] [PubMed]
- Kool, L.; Bunschoten, A.; Velders, A.; Saggiomo, V. Gold nanoparticles embedded in a polymer as a 3D-printable dichroic nanocomposite material. Beilstein J. Nanotechnol. 2019, 10, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Some, S.; Bulut, O.; Biswas, K.; Kumar, A.; Roy, A.; Sen, I.K.; Mandal, A.; Franco, O.L.; İnce, İ.A.; Neog, K.; et al. Effect of feed supplementation with biosynthesized silver nanoparticles using leaf extract of Morus indica L. V1 on Bombyx mori L. (Lepidoptera: Bombycidae). Sci. Rep. 2019, 9, 14839. [Google Scholar] [CrossRef] [PubMed]

| Reagent | Amount (mL) | Concentration |
|---|---|---|
| Silver nitrate | 130 | 0.11 mM |
| Sodium citrate | 11 | 30 mM |
| Polyvinylpyrrolidone | 11 | 2% w/w |
| Hydrogen peroxide | 0.36 | 30% w/w |
| Sodium borohydride | 0.45; 0.84; 1; 1.5 | 0.1 M |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velgosova, O.; Mačák, L.; Lisnichuk, M.; Vojtko, M. Synthesis and Analysis of Polymorphic Silver Nanoparticles and Their Incorporation into the Polymer Matrix. Polymers 2022, 14, 2666. https://doi.org/10.3390/polym14132666
Velgosova O, Mačák L, Lisnichuk M, Vojtko M. Synthesis and Analysis of Polymorphic Silver Nanoparticles and Their Incorporation into the Polymer Matrix. Polymers. 2022; 14(13):2666. https://doi.org/10.3390/polym14132666
Chicago/Turabian StyleVelgosova, Oksana, Livia Mačák, Maksym Lisnichuk, and Marek Vojtko. 2022. "Synthesis and Analysis of Polymorphic Silver Nanoparticles and Their Incorporation into the Polymer Matrix" Polymers 14, no. 13: 2666. https://doi.org/10.3390/polym14132666
APA StyleVelgosova, O., Mačák, L., Lisnichuk, M., & Vojtko, M. (2022). Synthesis and Analysis of Polymorphic Silver Nanoparticles and Their Incorporation into the Polymer Matrix. Polymers, 14(13), 2666. https://doi.org/10.3390/polym14132666