Effect of Copolymer on the Wrinkle Structure Formation and Gloss of a Phase-Separated Ternary Free-Radical/Cationic Hybrid System for the Application of Self-Matting Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Determination of the Phase Diagram for the Ternary Mixture and Solubility Parameters
2.2. Formulation and UV-Curing Processes
2.3. Dynamic Mechanical Analysis (DMA)
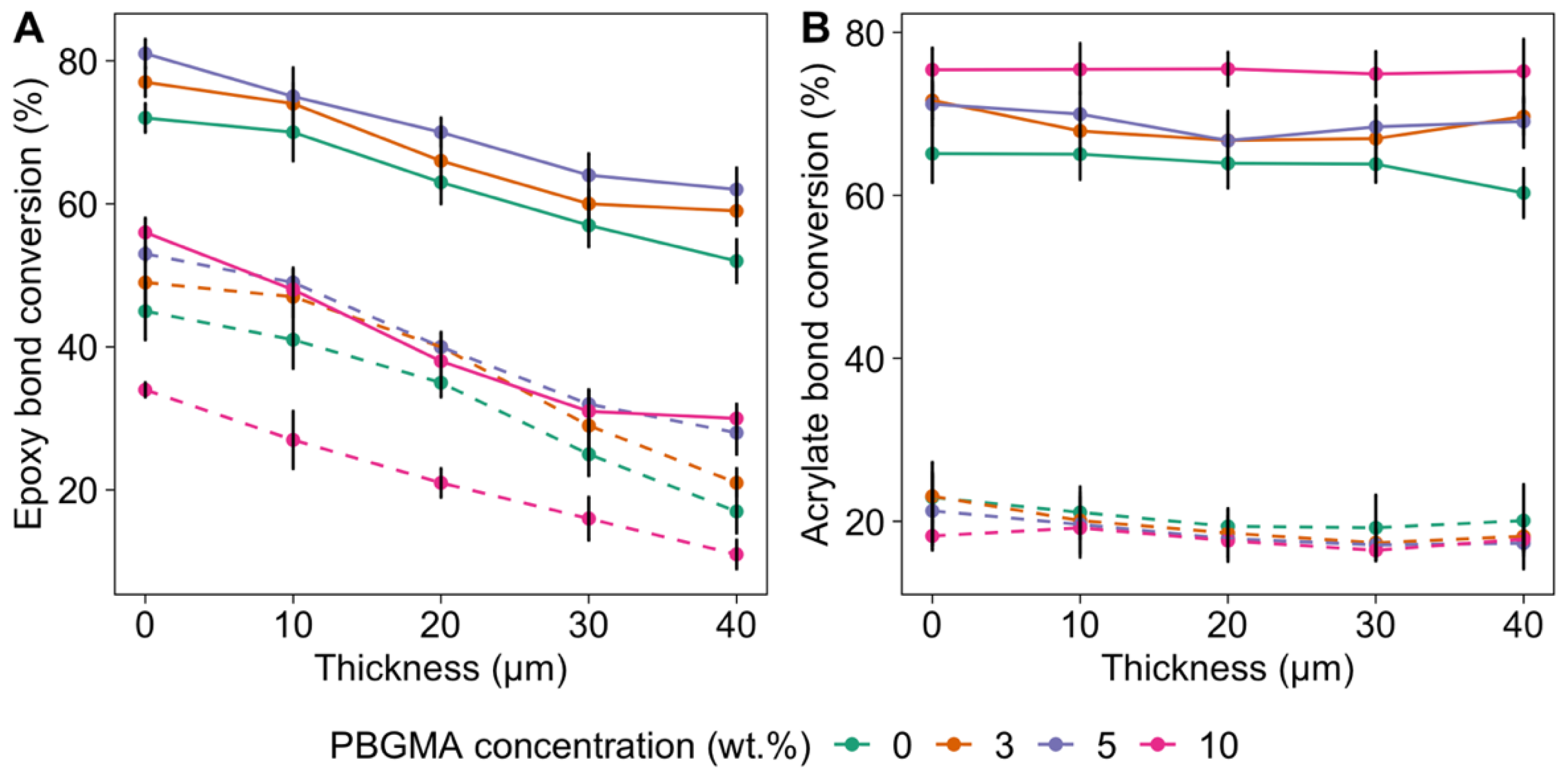
2.4. Confocal Raman Microspectroscopy (CRM)
2.5. Surface Characterization
3. Results and Discussion
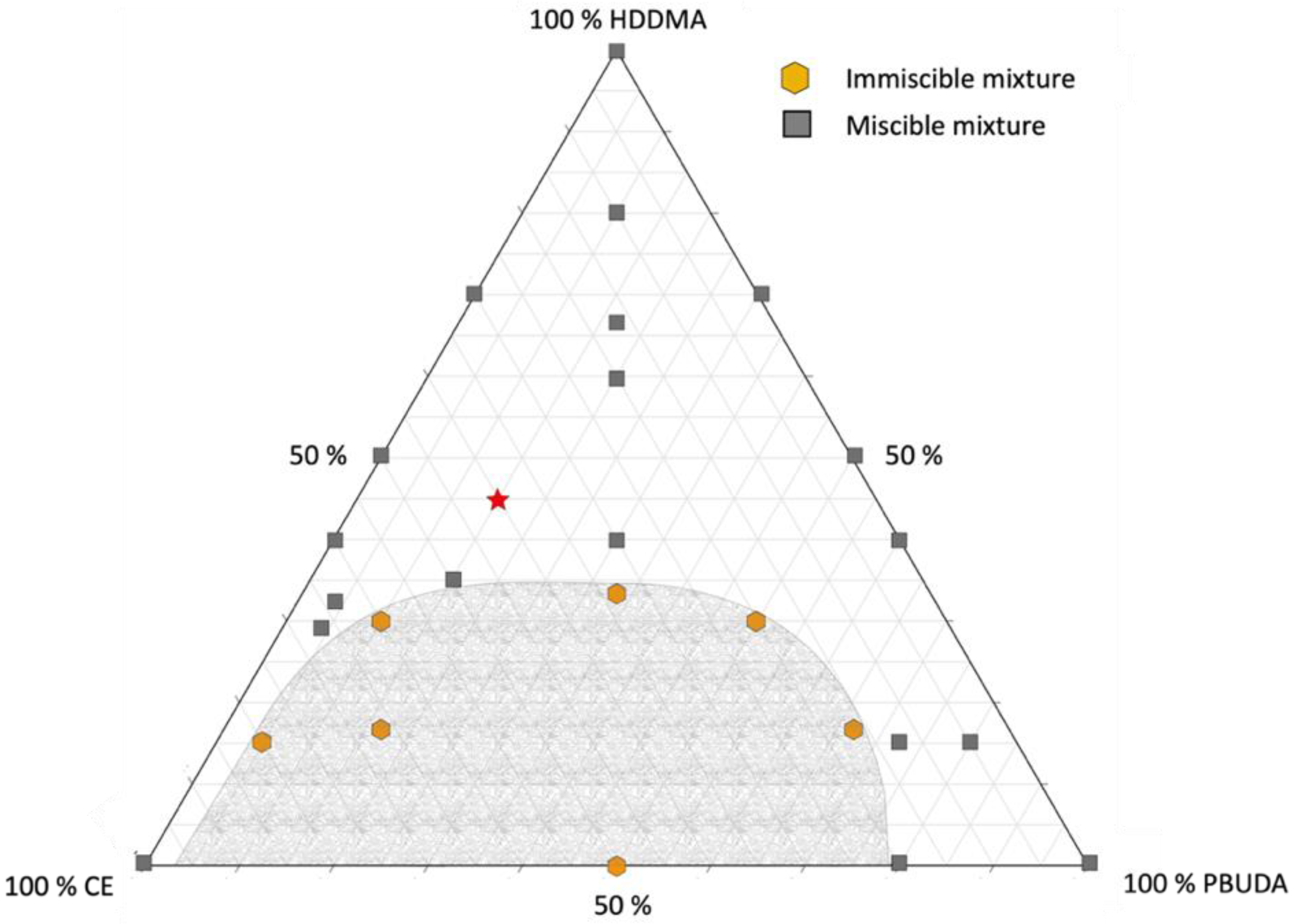
3.1. Miscibility Studies of a Ternary Free-Radical/Cationic Mixture
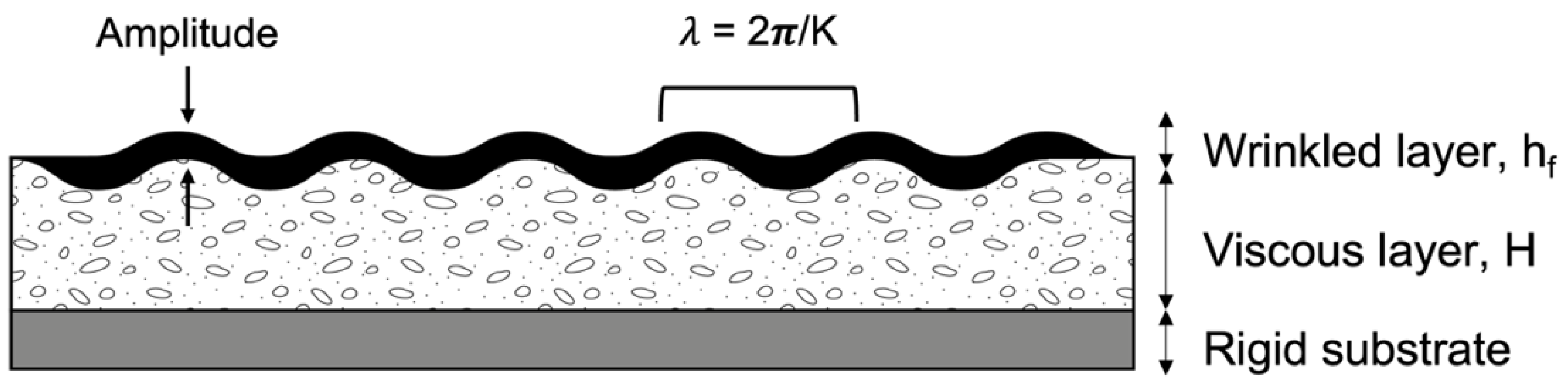
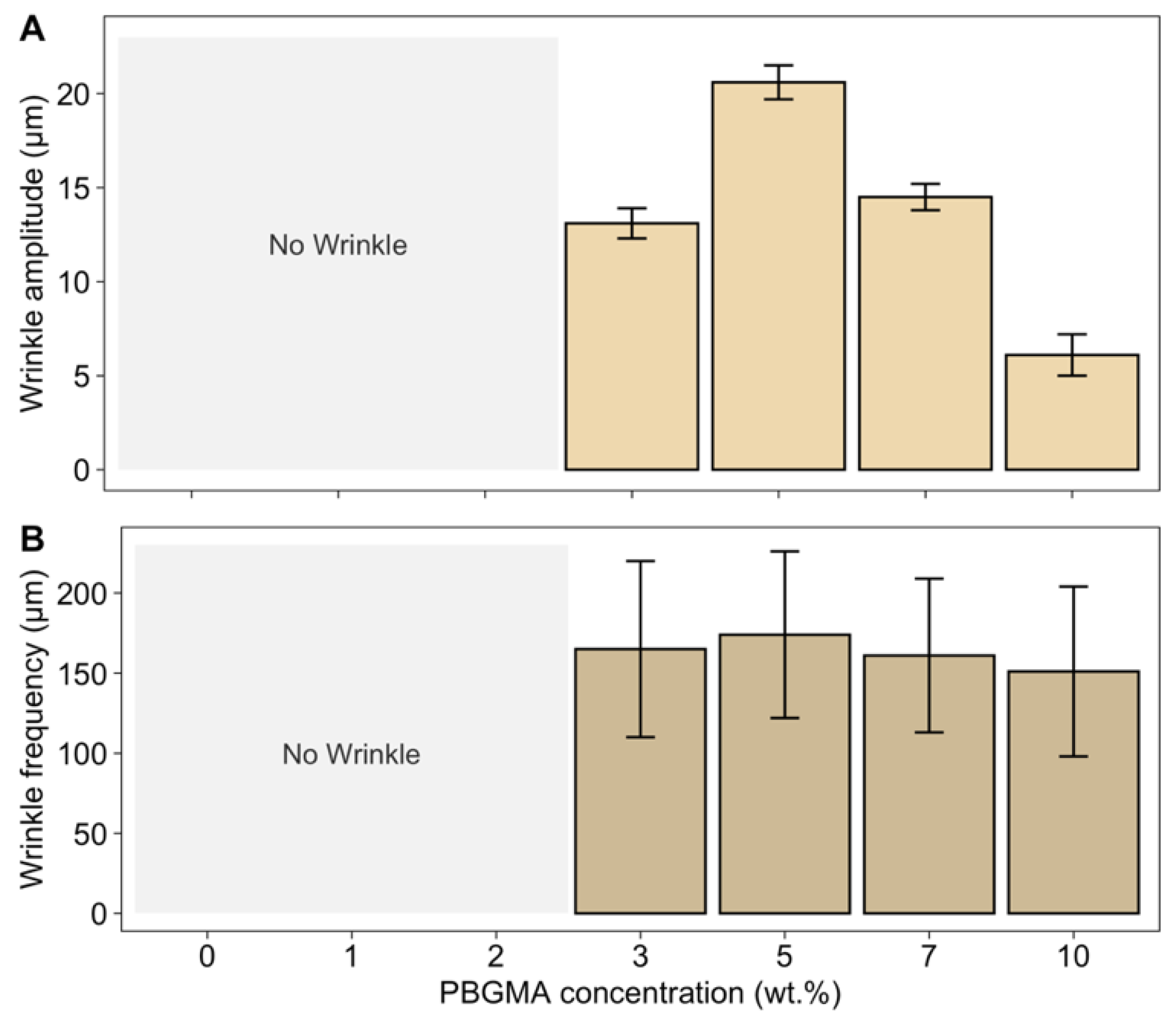
3.2. Wrinkling of Structures by Skin Formation on a Viscous Sublayer
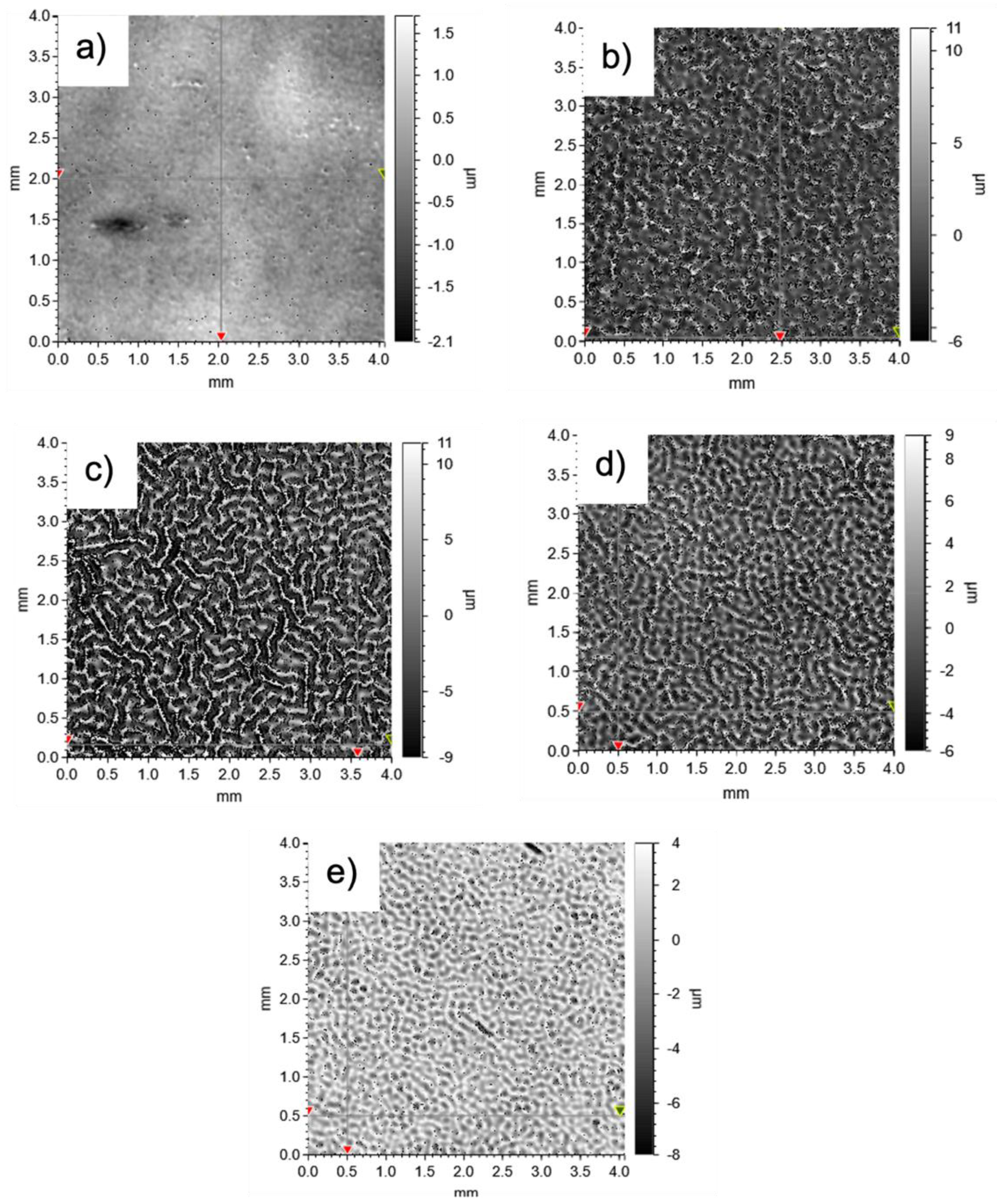
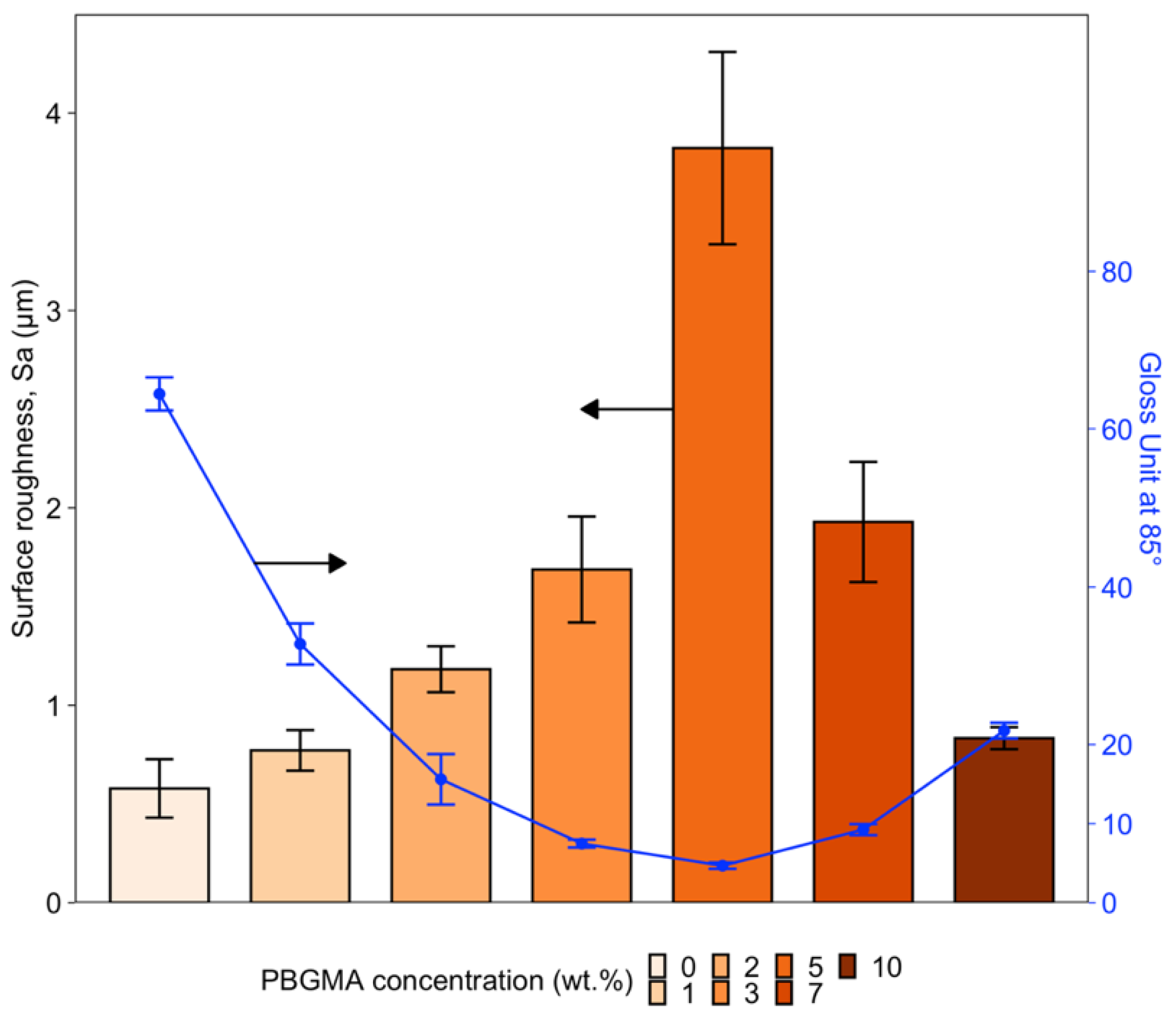
3.3. Gloss and Surface Wrinkling/Roughness
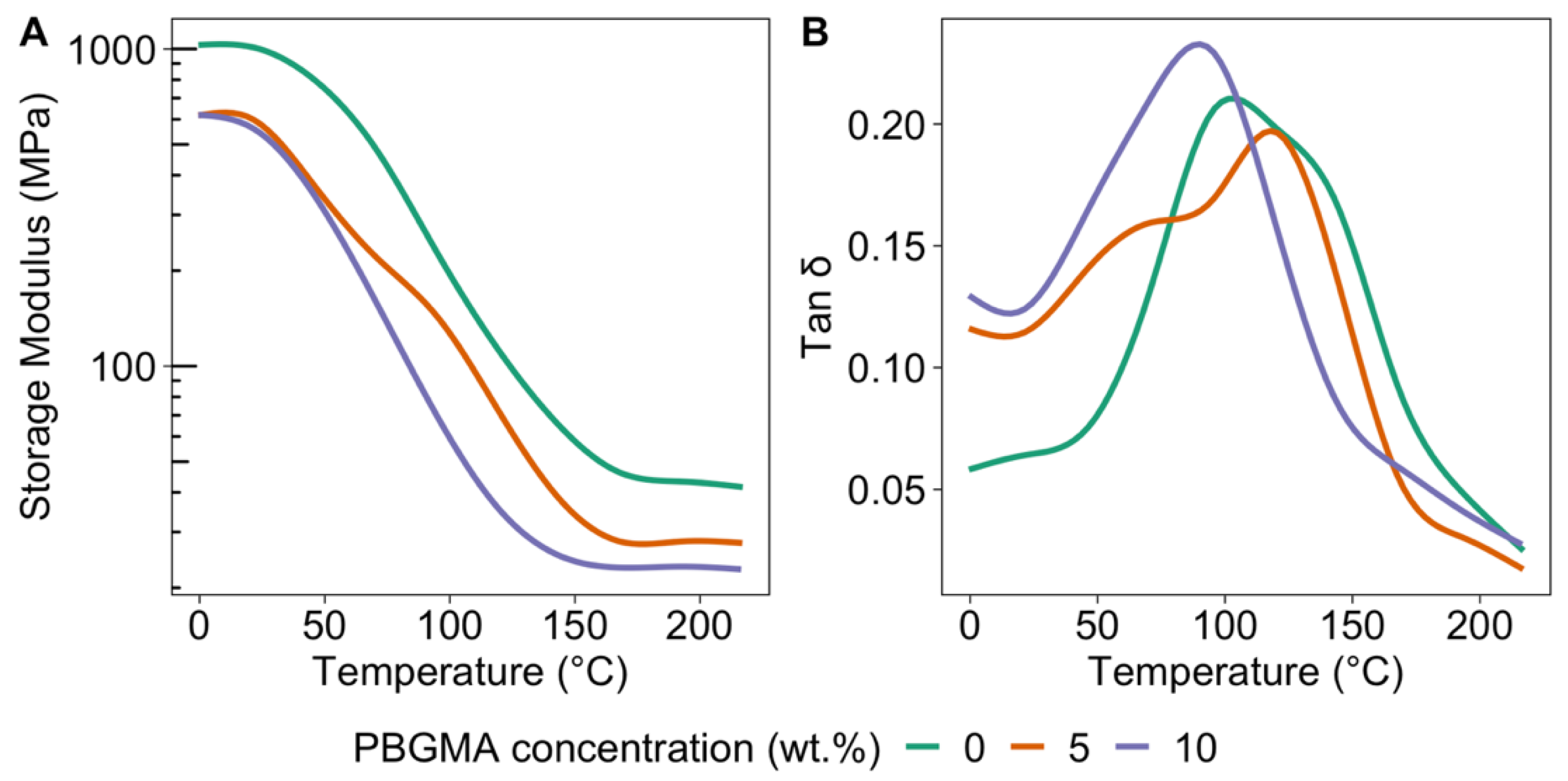
3.4. Mechanical Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bauer, F.; Flyunt, R.; Czihal, K.; Langguth, H.; Mehnert, R.; Schubert, R.; Buchmeiser, M.R. UV curing and matting of acrylate coatings reinforced by nano-silica and micro-corundum particles. Prog. Org. Coat. 2007, 60, 121–126. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, R.; Li, P.; Ma, G.; Hou, C.; Zhang, H. Synthesis of fluorinated polyacrylic acrylate oligomer for the UV-curable coatings. J. Coat. Technol. Res. 2019, 16, 681–688. [Google Scholar] [CrossRef]
- Luciani, A.; Plummer, C.J.G.; Gensler, R.; Månson, J.-A.E. Surface pattern formation in UV-curable coatings. J. Coat. Technol. 2000, 72, 161–163. [Google Scholar] [CrossRef]
- Rocco, C.; Karasu, F.; Croutxé-Barghorn, C.; Allonas, X.; Lecompère, M.; Riess, G.; Zhang, Y.; Esteves, A.C.C.; van der Ven, L.G.J.; van Benthem, R.A.T.M.; et al. Highly-interpenetrated and phase-separated UV-cured interpenetrating methacrylate–epoxide polymer networks: Influence of the composition on properties and microstructure. Mater. Today Commun. 2016, 6, 17–27. [Google Scholar] [CrossRef]
- Oxman, J.D.; Jacobs, D.W.; Trom, M.C.; Sipani, V.; Ficek, B.; Scranton, A.B. Evaluation of initiator systems for controlled and sequentially curable free-radical/cationic hybrid photopolymerizations. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 1747–1756. [Google Scholar] [CrossRef]
- Jančovičová, V.; Kindernay, J.; Jakubíková, Z.; Mrlláková, I. Influence of photoinitiator and curing conditions on polymerization kinetics and gloss of UV-cured coatings. Chem. Pap. 2007, 61, 383–390. [Google Scholar] [CrossRef]
- Sangermano, M.; Razza, N.; Crivello, J.V. Cationic UV-Curing: Technology and Applications. Macromol. Mater. Eng. 2014, 299, 775–793. [Google Scholar] [CrossRef]
- Decker, C.; Viet, T.N.T.; Thi, H.P. Photoinitiated cationic polymerization of epoxides. Polym. Int. 2001, 50, 986–997. [Google Scholar] [CrossRef]
- Sangermano, M.; Carbonaro, W.; Malucelli, G.; Priola, A. UV-Cured Interpenetrating Acrylic-Epoxy Polymer Networks: Preparation and Characterization. Macromol. Mater. Eng. 2008, 293, 515–520. [Google Scholar] [CrossRef]
- Nowers, J.R.; Narasimhan, B. The effect of interpenetrating polymer network formation on polymerization kinetics in an epoxy-acrylate system. Polymer 2006, 47, 1108–1118. [Google Scholar] [CrossRef]
- Decker, C.; Viet, T.N.T.; Decker, D.; Weber-Koehl, E. UV-radiation curing of acrylate/epoxide systems. Polymer 2001, 42, 5531–5541. [Google Scholar] [CrossRef]
- Ajitha, A.R.; Thomas, S. Chapter 1-Introduction: Polymer Blends, Thermodynamics, Miscibility, Phase Separation, and Compatibilization. Compat. Polym. Blends Micro. Nano. Scale Phase Morphol. Interphase Charact. Prop. 2020, 1–29. [Google Scholar] [CrossRef]
- Brannock, G.R.; Paul, D.R. Phase behavior of ternary polymer blends composed of three miscible binaries. Macromolecules 1990, 23, 5240–5250. [Google Scholar] [CrossRef]
- Kim, C.K.; Paul, D.R. Effects of polycarbonate molecular structure on the miscibility with other polymers. Macromolecules 1992, 25, 3097–3105. [Google Scholar] [CrossRef]
- Rabeony, M.; Siano, D.B.; Peiffer, D.G.; Siakah-Kioulafa, E.; Hadjichristidis, N. Closed-loop immiscibility in a ternary mixture of homopolymers. Polymer 1994, 35, 1033–1037. [Google Scholar] [CrossRef]
- Rigby, D.; Lin, J.L.; Roe, R.J. Compatibilizing effect of random or block copolymer added to binary mixture of homopolymers. Macromolecules 2002, 18, 2269–2273. [Google Scholar] [CrossRef]
- Kobayashi, S.; Cho, J.W.; Miyata, S. Miscibility and Phase Separation Behavior in Ternary Blends of Poly( Vinylidene Fluoride-Hexafluoroacetone)/Poly(Methyl Methacrylate)/Poly( Vinyl Acetate). Polym. J. 1994, 26, 235–242. [Google Scholar] [CrossRef][Green Version]
- Wang, D.; Xie, X.-M. Novel strategy for ternary polymer blend compatibilization. Polymer 2006, 47, 7859–7863. [Google Scholar] [CrossRef]
- Dadmun, M. Effect of Copolymer Architecture on the Interfacial Structure and Miscibility of a Ternary Polymer Blend Containing a Copolymer and Two Homopolymers. Macromolecules 1996, 29, 3868–3874. [Google Scholar] [CrossRef]
- De Brito, M.; Allonas, X.; Croutxé-Barghorn, C.; Palmieri, M.; Dietlin, C.; Agarwal, S.; Lellinger, D.; Alig, I. Kinetic study of photoinduced quasi-simultaneous interpenetrating polymer networks. Prog. Org. Coat. 2012, 73, 186–193. [Google Scholar] [CrossRef]
- Sangermano, M.; Malucelli, G.; Priola, A.; Manea, M. Synthesis and characterization of acrylate–oxetane interpenetrating polymer networks through a thermal-UV dual cure process. Prog. Org. Coat. 2006, 55, 225–230. [Google Scholar] [CrossRef]
- Chen, F.; Cook, W.D. Curing kinetics and morphology of IPNs from a flexible dimethacrylate and a rigid epoxy via sequential photo and thermal polymerization. Eur. Polym. J. 2008, 44, 1796–1813. [Google Scholar] [CrossRef]
- Gan, Y.; Yin, J.; Jiang, X. Self-wrinkling induced by the photopolymerization and self-assembly of fluorinated polymer at air/liquid interface. J. Mater. Chem. A 2014, 2, 18574–18582. [Google Scholar] [CrossRef]
- Chan, E.P.; Crosby, A.J. Spontaneous formation of stable aligned wrinkling patterns. Soft Matter 2006, 2, 324–328. [Google Scholar] [CrossRef]
- Song, J.-O.; McCormick, A.V.; Francis, L.F. Depthwise Viscosity Gradients in UV-Cured Epoxy Coatings. Macromol. Mater. Eng. 2013, 298, 145–152. [Google Scholar] [CrossRef]
- Park, S.K.; Kwark, Y.-J.; Nam, S.; Park, S.; Park, B.; Yun, S.; Moon, J.; Lee, J.-I.; Yu, B.; Kyung, K.-U. Wrinkle structures formed by formulating UV-crosslinkable liquid prepolymers. Polymer 2016, 99, 447–452. [Google Scholar] [CrossRef]
- Ebata, Y.; Croll, A.B.; Crosby, A.J. Wrinkling and strain localizations in polymer thin films. Soft Matter 2012, 8, 9086–9091. [Google Scholar] [CrossRef]
- Chandra, D.; Crosby, A.J. Self-Wrinkling of UV-Cured Polymer Films. Adv. Mater. 2011, 23, 3441–3445. [Google Scholar] [CrossRef]
- Basu, S.K.; Chung, J.A.; Francis, L.F.; McCormick, A.V.; Scriven, L.E. Modeling the Depthwise Gradient in Curing and Skin Formation in Wrinkling Coatings. Ind. Eng. Chem. Res. 2007, 46, 3358–3365. [Google Scholar] [CrossRef]
- Taguchi, K.; Hirose, S.; Abe, Y. Photo-curing composite paint containing urushi (Oriental lacquer), and wrinkled coating caused by phase separation. Prog. Org. Coat. 2007, 58, 290–295. [Google Scholar] [CrossRef]
- Basu, S.K.; Scriven, L.E.; Francis, L.F.; McCormick, A.V. Mechanism of wrinkle formation in curing coatings. Prog. Org. Coat. 2005, 53, 1–16. [Google Scholar] [CrossRef]
- Payne, J.A.; Francis, L.F.; Mccormick, A. V The Effects of Processing Variables on Stress Development in Ultraviolet-Cured Coatings. J. Appl. Polym. Sci. 1997, 66, 1267–1277. [Google Scholar] [CrossRef]
- Rodriguez-Hernandez, J. Wrinkled interfaces: Taking advantage of surface instabilities to pattern polymer surfaces. Prog. Polym. Sci. 2015, 42, 1–41. [Google Scholar] [CrossRef]
- González-Henríquez, C.M.; Sarabia Vallejos, M.A.; Rodríguez-Hernández, J. Strategies for the Fabrication of Wrinkled Polymer Surfaces. In Wrinkled Polymer Surfaces; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 9783030051235. [Google Scholar]
- Calvez, I.; Szczepanski, C.R.; Landry, V. Hybrid Free-Radical/Cationic Phase-Separated UV-Curable System: Impact of Photoinitiator Content and Monomer Fraction on Surface Morphologies and Gloss Appearance. Macromolecules 2022, 55, 3129–3139. [Google Scholar] [CrossRef] [PubMed]
- Van Krevelen, D.W.; Te Nijenhuis, K. Properties of Polymers; Elsevier Inc.: Amsterdam, The Netherlands, 2009; ISBN 9780080548197. [Google Scholar]
- McAninch, I.M.; Palmese, G.R.; Lenhart, J.L.; La Scala, J.J. DMA testing of epoxy resins: The importance of dimensions. Polym. Eng. Sci. 2015, 55, 2761–2774. [Google Scholar] [CrossRef]
- Asmussen, S.; Schroeder, W.; Dell’Erba, I.; Vallo, C. Monitoring of visible light photopolymerization of an epoxy/dimethacrylate hybrid system by Raman and near-infrared spectroscopies. Polym. Test. 2013, 32, 1283–1289. [Google Scholar] [CrossRef]
- Courtecuisse, F.; Dietlin, C.; Croutxé-Barghorn, C.; Van Der Ven, L.G.J. Depth Characterization of Photopolymerized Films by Confocal Raman Microscopy Using an Immersion Objective. Appl. Spectrosc. 2011, 65, 1126–1132. [Google Scholar] [CrossRef]
- Hancock, M.; Hawes, E.; Yang, F.; Grulke, E. Crosslinking gradients of a photopolymerized multifunctional acrylate film control mechanical properties. J. Coat. Technol. Res. 2019, 16, 1153–1163. [Google Scholar] [CrossRef]
- Cai, Y.; Jessop, J. Effect of water concentration on photopolymerized acrylate/epoxide hybrid polymer coatings as demonstrated by Raman spectroscopy. Polymer 2009, 50, 5406–5413. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, E.J.; Roy, S.; Sharbirin, A.S.; Ranz, L.-G.; Dieing, T.; Kim, J. Measurement of lateral and axial resolution of confocal Raman microscope using dispersed carbon nanotubes and suspended graphene. Curr. Appl. Phys. 2020, 20, 71–77. [Google Scholar] [CrossRef]
- Adar, F.; Mamedov, S.; Whitely, A.; Yvon, H.J. Limits of Spatial Resolution of a Raman Microscope. Microsc. Microanal. 2005, 11, 728–729. [Google Scholar] [CrossRef]
- Krizbergs, J.; Kromanis, A. Methods for Predication of the Surface Roughness 3D Parameters According to Technological Parameters. In Proceedings of the 5th International DAAAM Baltic Conference, Tallinn, Estonia, 20–22 April 2006. [Google Scholar]
- Yang, S.; Qu, J. Computing thermomechanical properties of crosslinked epoxy by molecular dynamic simulations. Polymer 2012, 53, 4806–4817. [Google Scholar] [CrossRef]
- ASTM D523-14; Standard Test Method for Specular Gloss. 2018. [CrossRef]
- ASTM D6736-08; Standard Test Method for Burnish Resistance of Latex Paints. 2019. [CrossRef]
- ASTM D2794-93; Standard Test Method for Resistance of Organic Coatings to the Effects of Rapid Deformation (Impact). 2019. [CrossRef]
- Szczepanski, C.R.; Pfeifer, C.S.; Stansbury, J.W. A new approach to network heterogeneity: Polymerization induced phase separation in photo-initiated, free-radical methacrylic systems. Polymer 2012, 53, 4694–4701. [Google Scholar] [CrossRef] [PubMed]
- Hasa, E. Nano/Microstructured Materials Obtained Using Photopolymerization-Induced Phase Separation. Ph.D. Thesis, University of Iowa, Iowa City, IA, USA, 2020. [Google Scholar]
- Li, X.; Ni, C.; Ma, F.; Yao, B.; Zhu, C. Preparation of poly(N-Butyl Acrylate-Co-Glycidyl Methacrylate) and its Application in Enhancement of Epoxy Resin. Polym. Plast. Technol. Eng. 2014, 53, 262–267. [Google Scholar] [CrossRef]
- Liu, J.; Sue, H.J.; Thompson, Z.J.; Bates, F.S.; Dettloff, M.; Jacob, G.; Verghese, N.; Pham, H. Effect of crosslink density on fracture behavior of model epoxies containing block copolymer nanoparticles. Polymer 2009, 50, 4683–4689. [Google Scholar] [CrossRef]
- Pirman, T.; Ocepek, M.; Likozar, B. Radical Polymerization of Acrylates, Methacrylates, and Styrene: Biobased Approaches, Mechanism, Kinetics, Secondary Reactions, and Modeling. Ind. Eng. Chem. Res. 2021, 60, 9347–9367. [Google Scholar] [CrossRef]
- Chen, T.; Lin, Y.; Chien, C.; Chen, Y.; Yang, Y.; Wang, W.; Chien, L.; Hsueh, H. Fabrication of Frog-Skin-Inspired Slippery Antibiofouling Coatings Through Degradable Block Copolymer Wrinkling. Adv. Funct. Mater. 2021, 31, 2104173. [Google Scholar] [CrossRef]
- Francos, X.F.; Kazarian, S.G.; Ramis, X.; Serra, A. Simultaneous Monitoring of Curing Shrinkage and Degree of Cure of Thermosets by Attenuated Total Reflection Fourier Transform Infrared (ATR FT-IR) Spectroscopy. Appl. Spectrosc. 2013, 67, 1427–1436. [Google Scholar] [CrossRef]
- Pfeifer, C.S.; Shelton, Z.R.; Szczpanski, C.R.; Barros, M.D.; Wilson, N.D.; Stansbury, J.W. Tailoring heterogeneous polymer networks through polymerization-induced phase separation: Influence of composition and processing conditions on reaction kinetics and optical properties. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 1796–1806. [Google Scholar] [CrossRef]
- Gu, Y.; Zhou, S.; Luo, H.; Wu, L.; Gao, W.; Yang, J. Temperature-dependent phase-segregation behavior and antifouling performance of UV-curable methacrylated PDMS/PEG coatings. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1612–1623. [Google Scholar] [CrossRef]
- Yazdani-Ahmadabadi, H.; Rastegar, S.; Ranjbar, Z. Slip-stick diffusion behavior caused by photo-polymerization-induced nano-gelation in highly heterogeneous photo-polymeric coatings. Prog. Org. Coat. 2016, 97, 184–193. [Google Scholar] [CrossRef]
- Huang, R.; Suo, Z. Instability of a compressed elastic film on a viscous layer. Int. J. Solids Struct. 2002, 39, 1791–1802. [Google Scholar] [CrossRef]
- Basu, S.K.; McCormick, A.V.; Scriven, L.E. Stress Generation by Solvent Absorption and Wrinkling of a Cross-Linked Coating atop a Viscous or Elastic Base. Langmuir 2006, 22, 5916–5924. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, N.; Srolovitz, D.J.; Suo, Z. Kinetics of buckling of a compressed film on a viscous substrate. Appl. Phys. Lett. 2001, 78, 2482–2484. [Google Scholar] [CrossRef]
- Chen, D.; Yoon, J.; Chandra, D.; Crosby, A.J.; Hayward, R.C. Stimuli-responsive buckling mechanics of polymer films. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 1441–1461. [Google Scholar] [CrossRef]
- Reichert, V.; Basu, S.K.; Francis, L.F.; McCormick, A.V.; Scriven, L. An analysis of starburst defects in wrinkled powder coatings. Prog. Org. Coat. 2008, 63, 33–37. [Google Scholar] [CrossRef]
- Huang, R.; Im, S.H. Dynamics of wrinkle growth and coarsening in stressed thin films. Phys. Rev. E—Stat. Nonlinear Soft Matter Phys. 2006, 74, 026214. [Google Scholar] [CrossRef]
- Kim, S.J.; Yoon, J.-I.; Moon, M.-W.; Lee, K.-R. Frictional behavior on wrinkle patterns of diamond-like carbon films on soft polymer. Diam. Relat. Mater. 2012, 23, 61–65. [Google Scholar] [CrossRef]
- Rand, C.J.; Crosby, A.J. Friction of soft elastomeric wrinkled surfaces. J. Appl. Phys. 2009, 106, 64913. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, C.; Du, Z.; Li, H.; Zhang, L.; Zou, W. Synthesis of Poly(n-Butyl Methacrylate)-(Glycidyl Methacrylate) Block Copolymer and Its Compatibilization at the Interface of the QD/Epoxy Nanocomposite for White LED Encapsulation †. RSC Adv. 2015, 5, 65184–65191. [Google Scholar] [CrossRef]
- Bakhshi, H.; Zohuriaan-Mehr, M.J.; Bouhendi, H.; Kabiri, K. Analysis Method Spectral and Chemical Determination of Copolymer Composition of Poly (Butyl Acrylate-Co-Glycidyl Methacrylate) from Emulsion Polymerization. Polym. Test. 2009, 28, 730–736. [Google Scholar] [CrossRef]



| Chemical and Commercial Name | Chemical Structure | Supplier |
|---|---|---|
| Polybutadiene urethane diacrylate (PBUDA)—Dymax BR-640D | N.A. | EMCO-Inortech (Terrebonne, Canada) |
| 1,6-hexanediol dimethacrylate (HDDMA)—Miwon Miramer M201 | 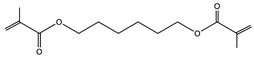 | EMCO-Inortech (Terrebonne, Canada) |
| Epoxycyclohexylmethyl 3,4-epoxycyclohexanecarboxylate (CE)—Omnilane 1005 | 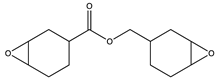 | MarkChem Inc. (Mirabel, Canada) |
| Poly(butyl acrylate-co-glycidyl methacrylate) (PBGMA) | 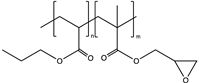 | Information in the Supplementary Materials |
| PBUDA 1 | HDDMA | CE | |
|---|---|---|---|
| V (cm3·mol−1) | 507 | 255 | 215 |
| δd (J1/2·cm−3/2) | 12.7 | 15.8 | 20.8 |
| δp (J1/2·cm−3/2) | 5.0 | 3.8 | 3.5 |
| δh (J1/2·cm−3/2) | 9.3 | 7.4 | 7.8 |
| δt (J1/2·cm−3/2) | 16.5 | 17.8 | 22.5 |
| CE | PBUDA | HDDMA | PBGMA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Composition (wt.%) | 40 | 15 | 45 | 0 | 1 | 2 | 3 | 5 | 7 | 10 |
| Viscosity (mpa·s) at 25 °C | 180–450 | 6000 at 60 °C | 1–10 | 51 ± 2 | 59 ± 1 | 76 ± 5 | 104 ± 6 | 139 ± 3 | 207 ± 4 | 234 ± 5 |
| PBGMA (wt.%) | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 5 | 7 | 10 | |
| Tg1 (°C) Acrylate part | 95 ± 1 | 88 ± 2 | 82 ± 1 | 70 ± 4 | 65 ± 1 | 68 ± 3 | 91 ± 1 |
| Tg2 (°C) Epoxy part | 129 ± 1 | 126 ± 3 | 132 ± 1 | 130 ± 1 | 124 ± 2 | 120 ± 2 | |
| νXL (mol·m−3) | 3690 ± 110 | 3420 ± 50 | 3260 ± 50 | 2830 ± 110 | 2560 ± 90 | 2310 ± 90 | 1980 ± 60 |
| PBGMA (wt.%) | 0 | 1 | 2 | 3 | 5 | 7 | 10 |
|---|---|---|---|---|---|---|---|
| λmeasured | / | / | / | 165 | 174 | 161 | 151 |
| E (MPa) | 8.4 | 8.1 | 7.5 | 6.9 | 5.7 | 5.1 | 4.2 |
| hf (μm) | 6 | 10 | 10 | 12 | 20 | 14 | 6 |
| σ0 (MPa) | / | / | / | 0.14 | 0.29 | 0.15 | 0.03 |
| PBGMA Concentration (wt.%) | Reverse Impact (kg/cm) | Direct Impact (kg/cm) | Gloss before Burnishing (at 85°) | Gloss after Burnishing (at 85°) | Difference in Gloss |
|---|---|---|---|---|---|
| 0 | 8.0 ± 0.0 | 43.0 ± 1.0 | 62.4 | 64.3 | 1.9 ± 0.9 |
| 1 | 8.0 ± 0.5 | 43.0 ± 1.0 | 25.7 | 27.3 | 1.6 ± 0.4 |
| 2 | 8.0 ± 0.5 | 33.0 ± 1.0 | 12.4 | 14.9 | 2.5 ± 0.3 |
| 3 | 8.0 ± 0.5 | 31.0 ± 1.0 | 7.0 | 8.7 | 1.7 ± 0.3 |
| 5 | 8.0 ± 0.5 | 33.0 ± 1.0 | 4.3 | 4.9 | 0.6 ± 0.2 |
| 7 | 8.0 ± 0.5 | 33.0 ± 1.0 | 8.6 | 9.0 | 0.4 ± 0.5 |
| 10 | 9.0 ± 0.0 | 32.0 ± 1.0 | 20.9 | 22.8 | 1.9 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvez, I.; Szczepanski, C.R.; Landry, V. Effect of Copolymer on the Wrinkle Structure Formation and Gloss of a Phase-Separated Ternary Free-Radical/Cationic Hybrid System for the Application of Self-Matting Coatings. Polymers 2022, 14, 2371. https://doi.org/10.3390/polym14122371
Calvez I, Szczepanski CR, Landry V. Effect of Copolymer on the Wrinkle Structure Formation and Gloss of a Phase-Separated Ternary Free-Radical/Cationic Hybrid System for the Application of Self-Matting Coatings. Polymers. 2022; 14(12):2371. https://doi.org/10.3390/polym14122371
Chicago/Turabian StyleCalvez, Ingrid, Caroline R. Szczepanski, and Véronic Landry. 2022. "Effect of Copolymer on the Wrinkle Structure Formation and Gloss of a Phase-Separated Ternary Free-Radical/Cationic Hybrid System for the Application of Self-Matting Coatings" Polymers 14, no. 12: 2371. https://doi.org/10.3390/polym14122371
APA StyleCalvez, I., Szczepanski, C. R., & Landry, V. (2022). Effect of Copolymer on the Wrinkle Structure Formation and Gloss of a Phase-Separated Ternary Free-Radical/Cationic Hybrid System for the Application of Self-Matting Coatings. Polymers, 14(12), 2371. https://doi.org/10.3390/polym14122371






