Development of Biocompatible Polyhydroxyalkanoate/Chitosan-Tungsten Disulphide Nanocomposite for Antibacterial and Biological Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
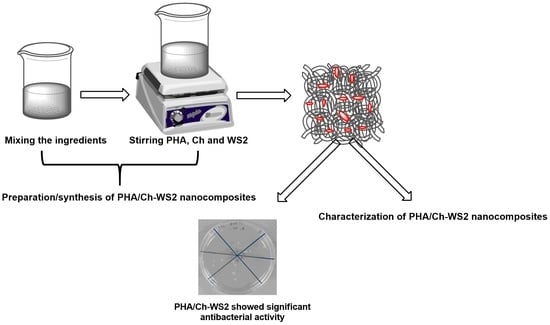
2.2. Preparation of Precursor Solution
2.3. Casting Film
2.4. Antibacterial Assay
2.5. Lactate Dehydrogenase Assays
2.6. Characterization
3. Results
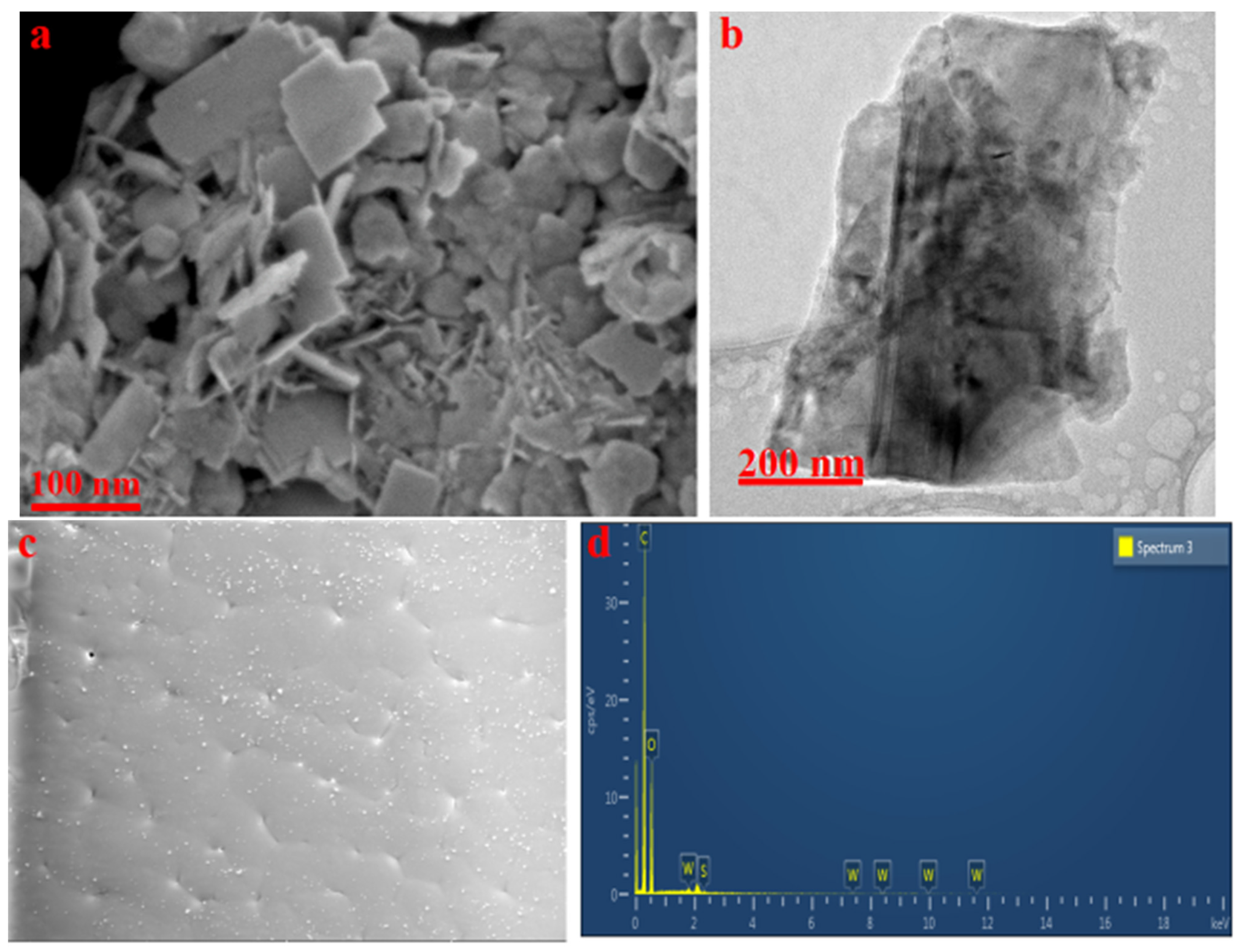
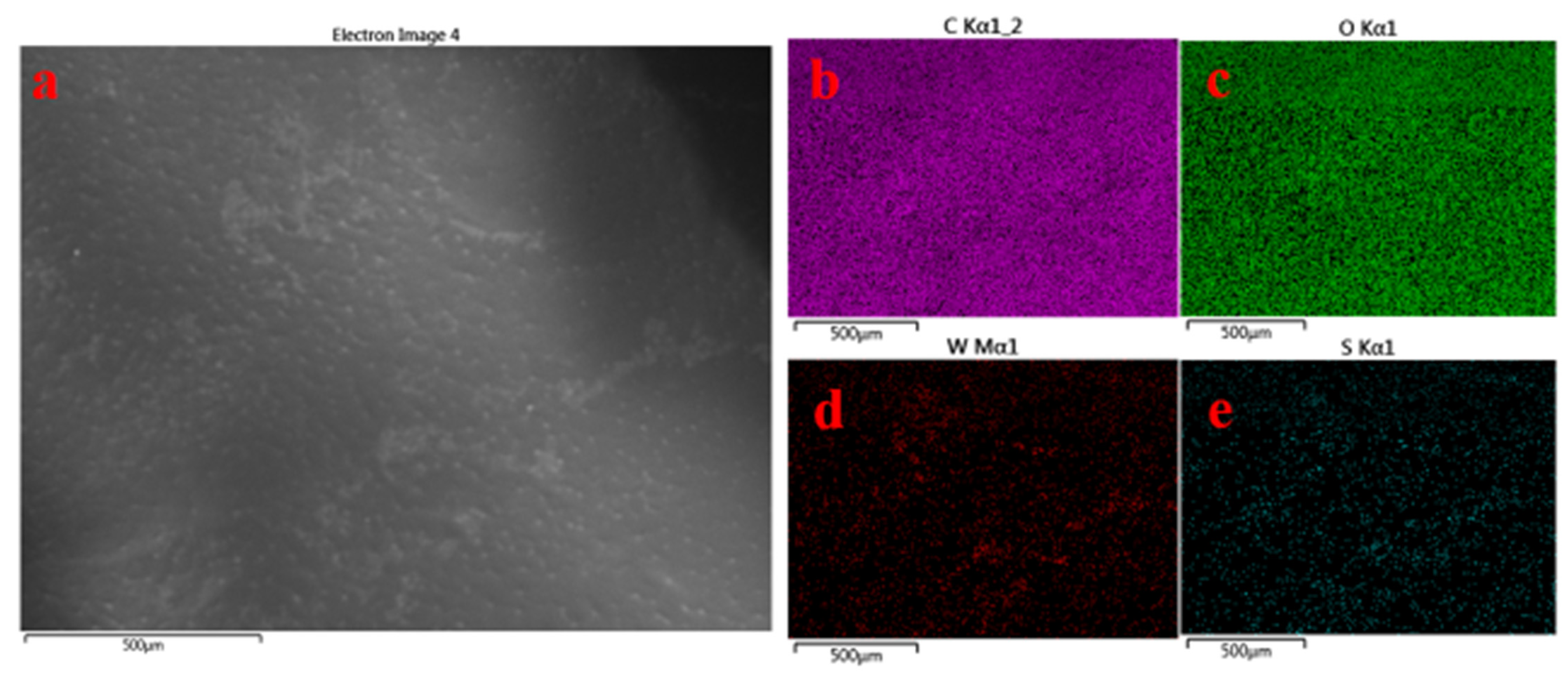
3.1. Surface Morphology
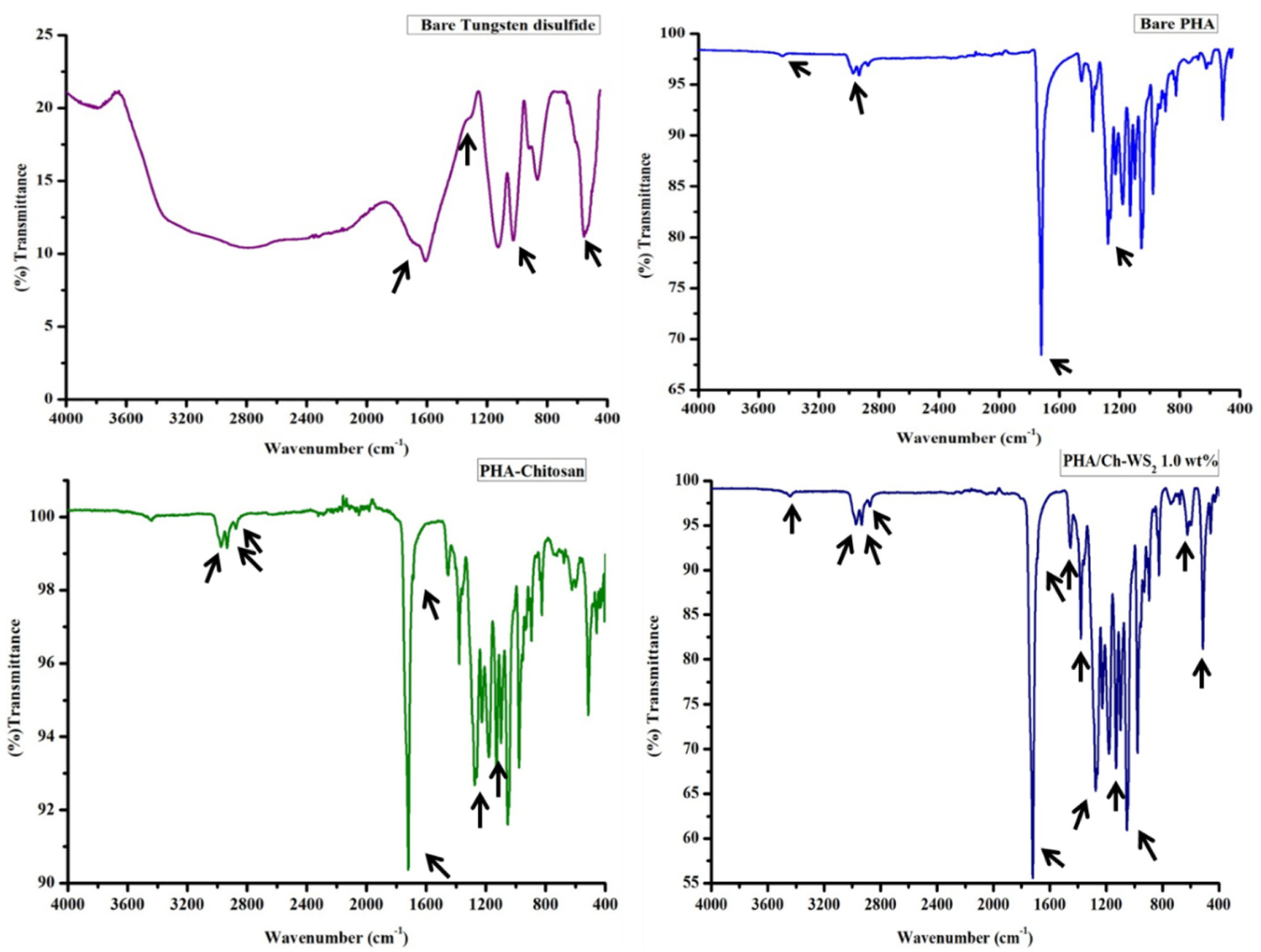
3.2. FT-IR
3.3. TGA
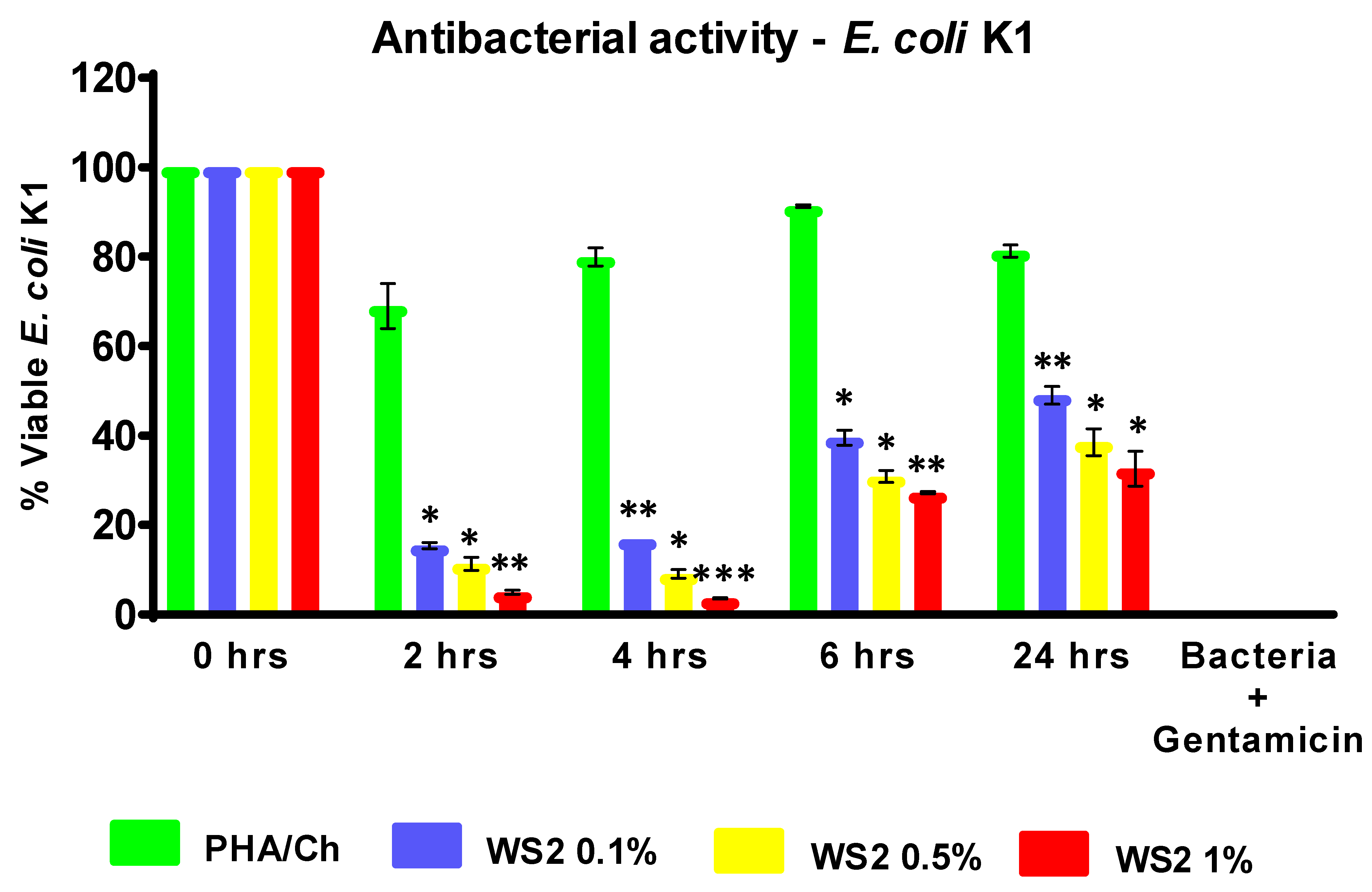
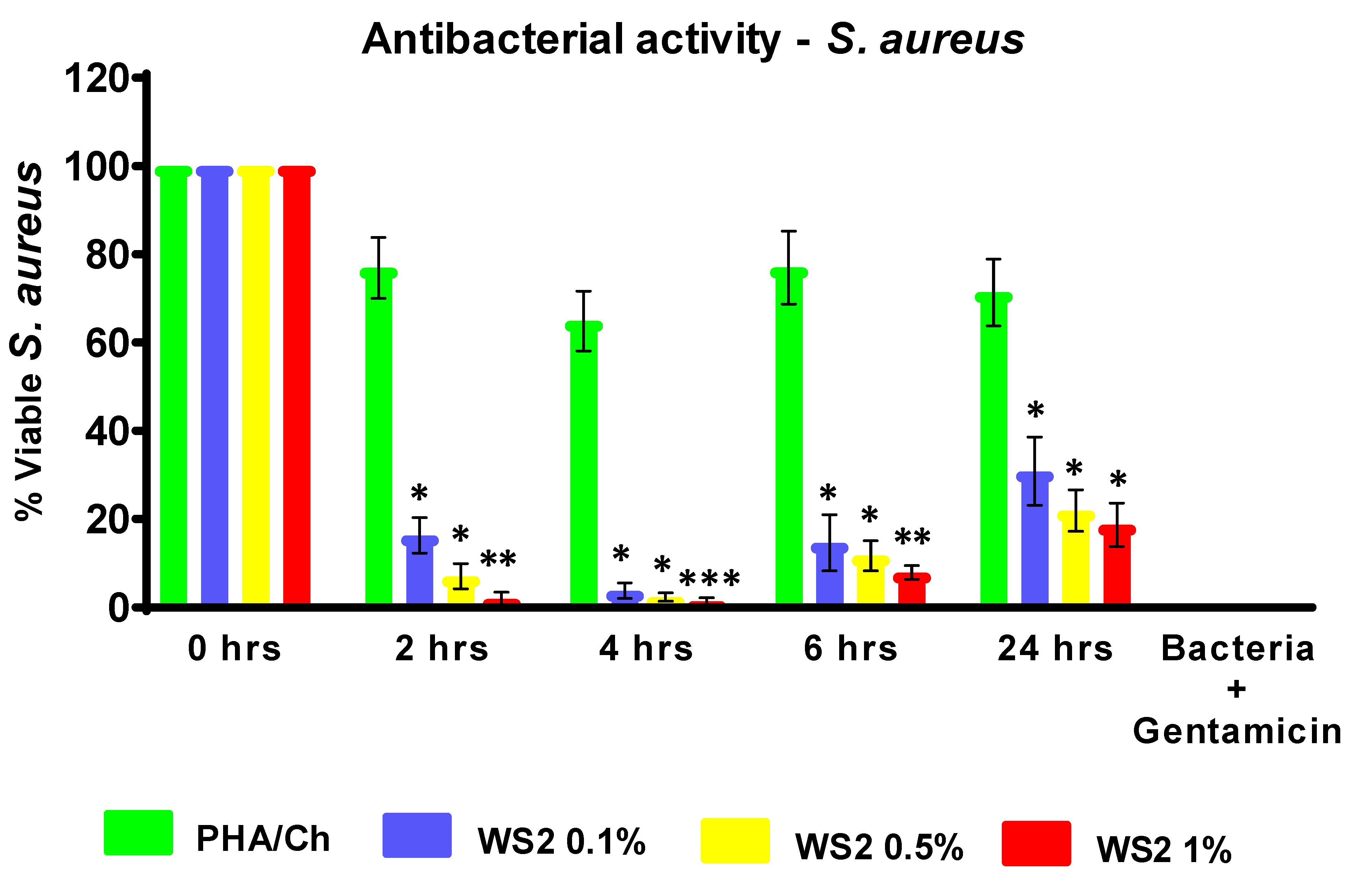
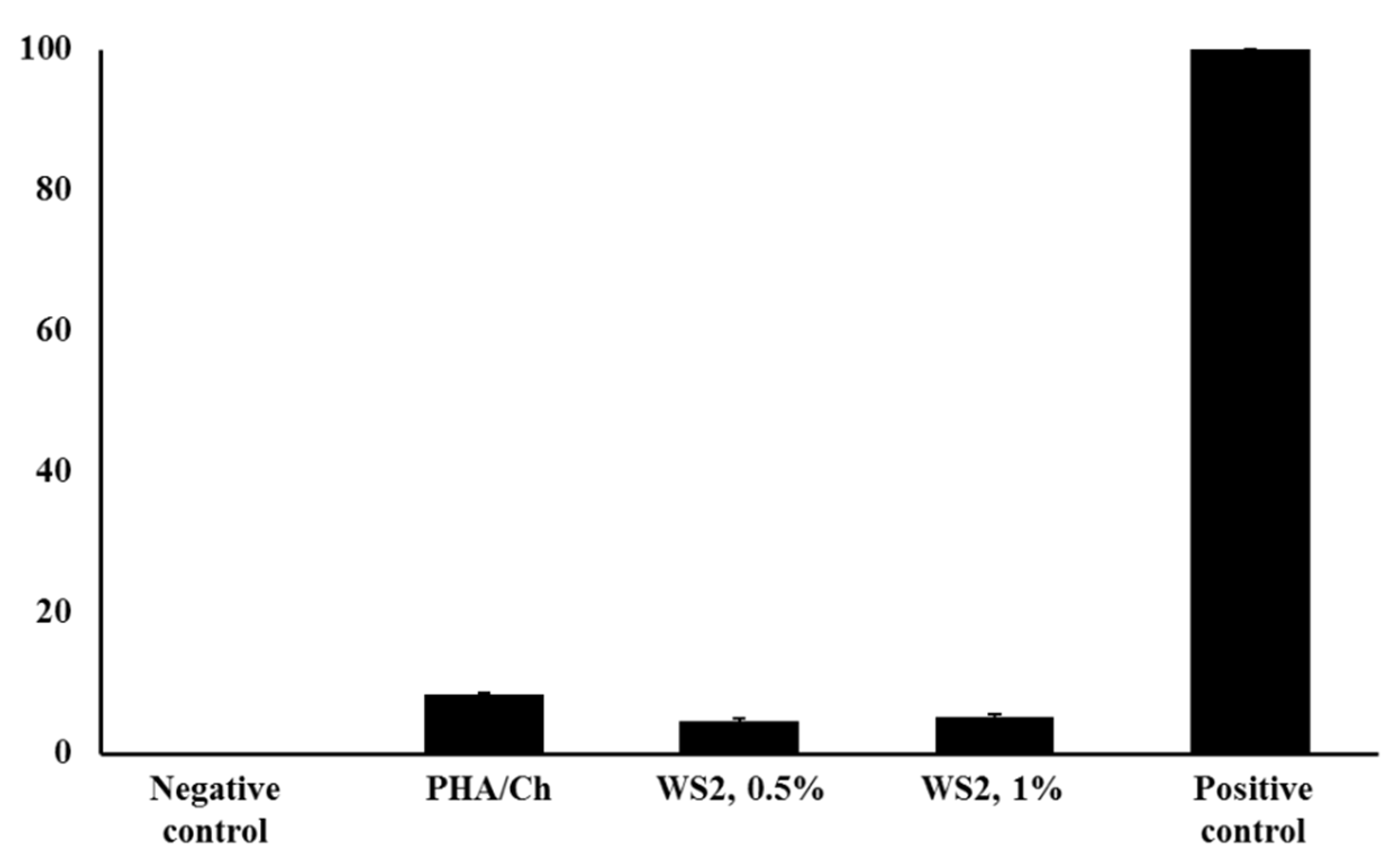
3.4. Antibacterial and LDH Assays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novoselov, K.S.; Colombo, L.; Gellert, P.; Schwab, M.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Young, R.J.; Kinloch, I.A.; Gong, L.; Novoselov, K.S. The mechanics of graphene nanocomposites: A review. Compos. Sci. Technol. 2012, 72, 1459–1476. [Google Scholar] [CrossRef]
- Rapoport, L.; Bilik, Y.; Feldman, Y.; Homyonfer, M.; Cohen, S.; Tenne, R. Hollow nanoparticles of WS2 as potential solid-state lubricants. Nature 1997, 387, 791–793. [Google Scholar] [CrossRef]
- Rosentsveig, R.; Gorodnev, A.; Feuerstein, N.; Friedman, H.; Zak, A.; Fleischer, N.; Tannous, J.; Dassenoy, F.; Tenne, R. Fullerene-like MoS2 nanoparticles and their tribological behavior. Tribol. Lett. 2009, 36, 175–182. [Google Scholar] [CrossRef]
- Feng, C.; Huang, L.; Guo, Z.; Liu, H. Synthesis of tungsten disulfide (WS2) nanoflakes for lithium ion battery application. Electrochem. Commun. 2007, 9, 119–122. [Google Scholar] [CrossRef]
- Gong, C.; Zhang, H.; Wang, W.; Colombo, L.; Wallace, R.M.; Cho, K. Band alignment of two-dimensional transition metal dichalcogenides: Application in tunnel field effect transistors. Appl. Phys. Lett. 2013, 103, 053513. [Google Scholar] [CrossRef]
- Wu, K.; Guo, C.; Wang, H.; Zhang, X.; Wang, J.; Chen, J. Two-dimension Nanomaterial Tungsten Disulfide (WS2) Integrated Fiber Device as All Optical Phase Shifter, Switch and Modulator Near 1550 nm. In Proceedings of the CLEO: Science and Innovations, San Jose, CA, USA, 14–19 May 2017; Volume SF2L, p. 7. [Google Scholar]
- Yang, Y.; Unalan, H.E.; Hiralal, P.; Chremmou, K.; Teh, A.; Alexandrou, I.; Tenne, R.; Amaratunga, G.A. Phototransistors utilizing individual WS2 nanotubes. In Proceedings of the 2008 8th IEEE Conference on Nanotechnology, Arlington, TX, USA, 18–21 August 2008; pp. 85–87. [Google Scholar]
- Yong, Y.; Cheng, X.; Bao, T.; Zu, M.; Yan, L.; Yin, W.; Ge, C.; Wang, D.; Gu, Z.; Zhao, Y. Tungsten sulfide quantum dots as multifunctional nanotheranostics for in vivo dual-modal image-guided photothermal/radiotherapy synergistic therapy. ACS Nano 2015, 9, 12451–12463. [Google Scholar] [CrossRef]
- Goldman, E.B.; Zak, A.; Tenne, R.; Kartvelishvily, E.; Levin-Zaidman, S.; Neumann, Y.; Stiubea-Cohen, R.; Palmon, A.; Hovav, A.-H.; Aframian, D.J. Biocompatibility of tungsten disulfide inorganic nanotubes and fullerene-like nanoparticles with salivary gland cells. Tissue Eng. Part A 2015, 21, 1013–1023. [Google Scholar] [CrossRef]
- Samorodnitzky-Naveh, G.R.; Redlich, M.; Rapoport, L.; Feldman, Y.; Tenne, R. Inorganic fullerene-like tungsten disulfide nanocoating for friction reduction of nickel–titanium alloys. Nanomedicine 2009, 4, 943–950. [Google Scholar] [CrossRef]
- Goldbart, O.; Sedova, A.; Yadgarov, L.; Rosentsveig, R.; Shumalinsky, D.; Lobik, L.; Wagner, H.D.; Tenne, R. Lubricating medical devices with fullerene-like nanoparticles. Tribol. Lett. 2014, 55, 103–109. [Google Scholar] [CrossRef]
- Adini, A.; Redlich, M.; Tenne, R. Medical applications of inorganic fullerene-like nanoparticles. J. Mater. Chem. 2011, 21, 15121–15131. [Google Scholar] [CrossRef]
- Redlich, M.; Katz, A.; Rapoport, L.; Wagner, H.; Feldman, Y.; Tenne, R. Improved orthodontic stainless steel wires coated with inorganic fullerene-like nanoparticles of WS2 impregnated in electroless nickel–phosphorous film. Dent. Mater. 2008, 24, 1640–1646. [Google Scholar] [CrossRef] [PubMed]
- Appel, J.H.; Li, D.O.; Podlevsky, J.D.; Debnath, A.; Green, A.A.; Wang, Q.H.; Chae, J. Low cytotoxicity and genotoxicity of two-dimensional MoS2 and WS2. ACS Biomater. Sci. Eng. 2016, 2, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Duan, G.; Li, W.; Zhou, Z.; Zhou, R. Membrane destruction-mediated antibacterial activity of tungsten disulfide (WS2). Rsc. Adv. 2017, 7, 37873–37880. [Google Scholar] [CrossRef] [Green Version]
- Ke, Y.; Zhang, X.; Ramakrishna, S.; He, L.; Wu, G. Reactive blends based on polyhydroxyalkanoates: Preparation and biomedical application. Mater. Sci. Eng. C 2017, 70, 1107–1119. [Google Scholar] [CrossRef]
- Cammas, S.; Bear, M.-M.; Moine, L.; Escalup, R.; Ponchel, G.; Kataoka, K.; Guérin, P. Polymers of malic acid and 3-alkylmalic acid as synthetic PHAs in the design of biocompatible hydrolyzable devices. Int. J. Biol. Macromol. 1999, 25, 273–282. [Google Scholar] [CrossRef]
- Doyle, V.; Pearson, R.; Lee, D.; Wolowacz, S.; Mc Taggart, S. An investigation of the growth of human dermal fibroblasts on poly-L-lactic acid in vitro. J. Mater. Sci. Mater. Med. 1996, 7, 381–385. [Google Scholar] [CrossRef]
- Witholt, B.; Kessler, B. Perspectives of medium chain length poly (hydroxyalkanoates), a versatile set of bacterial bioplastics. Curr. Opin. Biotechnol. 1999, 10, 279–285. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011, 2011, 1687–9422. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An update on potential biomedical and pharmaceutical applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.; Kim, Y.; Kim, Y.-G.; Lee, J.-H.; Lee, J. Chitosan-gum arabic embedded alizarin nanocarriers inhibit biofilm formation of multispecies microorganisms. Carbohydr. Polym. 2022, 284, 118959. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.; Lee, J.-H.; Shim, J.-J.; Lee, J. Recent findings and future directions of grafted gum karaya polysaccharides and their various applications: A review. Carbohydr. Polym. 2021, 258, 117687. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.; Raorane, C.J.; Lee, J.-H.; Lee, J. Appraisal of Chitosan-Gum Arabic-Coated Bipolymeric Nanocarriers for Efficient Dye Removal and Eradication of the Plant Pathogen Botrytis cinerea. ACS Appl. Mater. Interfaces 2021, 13, 47354–47370. [Google Scholar] [CrossRef]
- Sunilkumar, M.; Francis, T.; Thachil, E.T.; Sujith, A. Low density polyethylene–chitosan composites: A study based on biodegradation. Chem. Eng. J. 2012, 204, 114–124. [Google Scholar] [CrossRef]
- Alves, N.M.; Mano, J.F. Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications. Int. J. Biol. Macromol. 2008, 43, 401–414. [Google Scholar] [CrossRef] [Green Version]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Bergmann, A.; Owen, A. Hydroxyapatite as a filler for biosynthetic PHB homopolymer and P (HB–HV) copolymers. Polym. Int. 2003, 52, 1145–1152. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Syed, S. Polyaniline based nanocomposites as adsorbents and photocatalysts in the removal of organic dyes/Syed Shahabuddin. Ph.D. Thesis, University of Malaya, Kuala Lumpur, Malaysia, 2016. [Google Scholar]
- Mukheem, A.; Shahabuddin, S.; Akbar, N.; Miskon, A.; Muhamad Sarih, N.; Sudesh, K.; Ahmed Khan, N.; Saidur, R.; Sridewi, N. Boron nitride doped polyhydroxyalkanoate/chitosan nanocomposite for antibacterial and biological applications. Nanomaterials 2019, 9, 645. [Google Scholar] [CrossRef] [Green Version]
- Salim, Y.S.; Chan, C.H.; Sudesh, K.; Gan, S.N. Influence of thermal treatment on the molecular weights of polyhydroxyalkanoate containing 3-hydroxyhexanoate. In Advanced Materials Research; Trans Tech Publications Ltd.: Zurich, Switzerland, 2013; pp. 250–253. [Google Scholar]
- Mukheem, A.; Muthoosamy, K.; Manickam, S.; Sudesh, K.; Shahabuddin, S.; Saidur, R.; Akbar, N.; Sridewi, N. Fabrication and characterization of an electrospun PHA/graphene silver nanocomposite scaffold for antibacterial applications. Materials 2018, 11, 1673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kisku, S.K.; Swain, S.K. Synthesis and characterization of chitosan/boron nitride composites. J. Am. Ceram. Soc. 2012, 95, 2753–2757. [Google Scholar] [CrossRef]
- Hazarika, S.J.; Mohanta, D. Inorganic fullerene-type WS2 nanoparticles: Processing, characterization and its photocatalytic performance on malachite green. Appl. Phys. A 2017, 123, 381. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukheem, A.; Shahabuddin, S.; Akbar, N.; Ahmad, I.; Sudesh, K.; Sridewi, N. Development of Biocompatible Polyhydroxyalkanoate/Chitosan-Tungsten Disulphide Nanocomposite for Antibacterial and Biological Applications. Polymers 2022, 14, 2224. https://doi.org/10.3390/polym14112224
Mukheem A, Shahabuddin S, Akbar N, Ahmad I, Sudesh K, Sridewi N. Development of Biocompatible Polyhydroxyalkanoate/Chitosan-Tungsten Disulphide Nanocomposite for Antibacterial and Biological Applications. Polymers. 2022; 14(11):2224. https://doi.org/10.3390/polym14112224
Chicago/Turabian StyleMukheem, Abdul, Syed Shahabuddin, Noor Akbar, Irfan Ahmad, Kumar Sudesh, and Nanthini Sridewi. 2022. "Development of Biocompatible Polyhydroxyalkanoate/Chitosan-Tungsten Disulphide Nanocomposite for Antibacterial and Biological Applications" Polymers 14, no. 11: 2224. https://doi.org/10.3390/polym14112224
APA StyleMukheem, A., Shahabuddin, S., Akbar, N., Ahmad, I., Sudesh, K., & Sridewi, N. (2022). Development of Biocompatible Polyhydroxyalkanoate/Chitosan-Tungsten Disulphide Nanocomposite for Antibacterial and Biological Applications. Polymers, 14(11), 2224. https://doi.org/10.3390/polym14112224