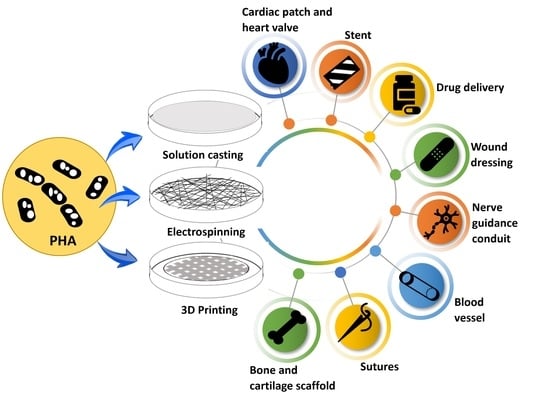
Biomedical Applications of Polyhydroxyalkanoate in Tissue Engineering
Abstract
:1. Introduction
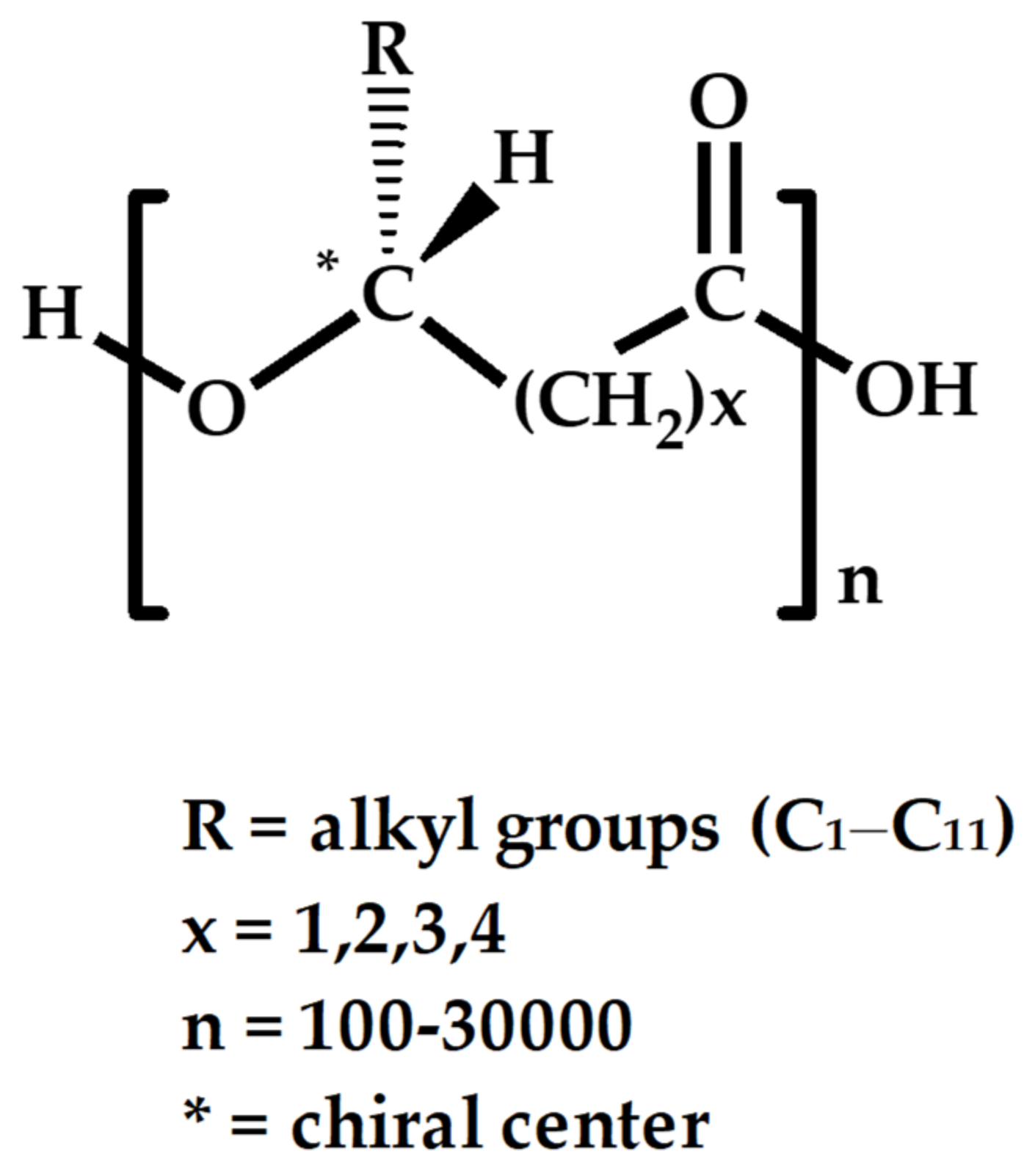
2. Common types of PHA Used in Tissue Repair and Engineering
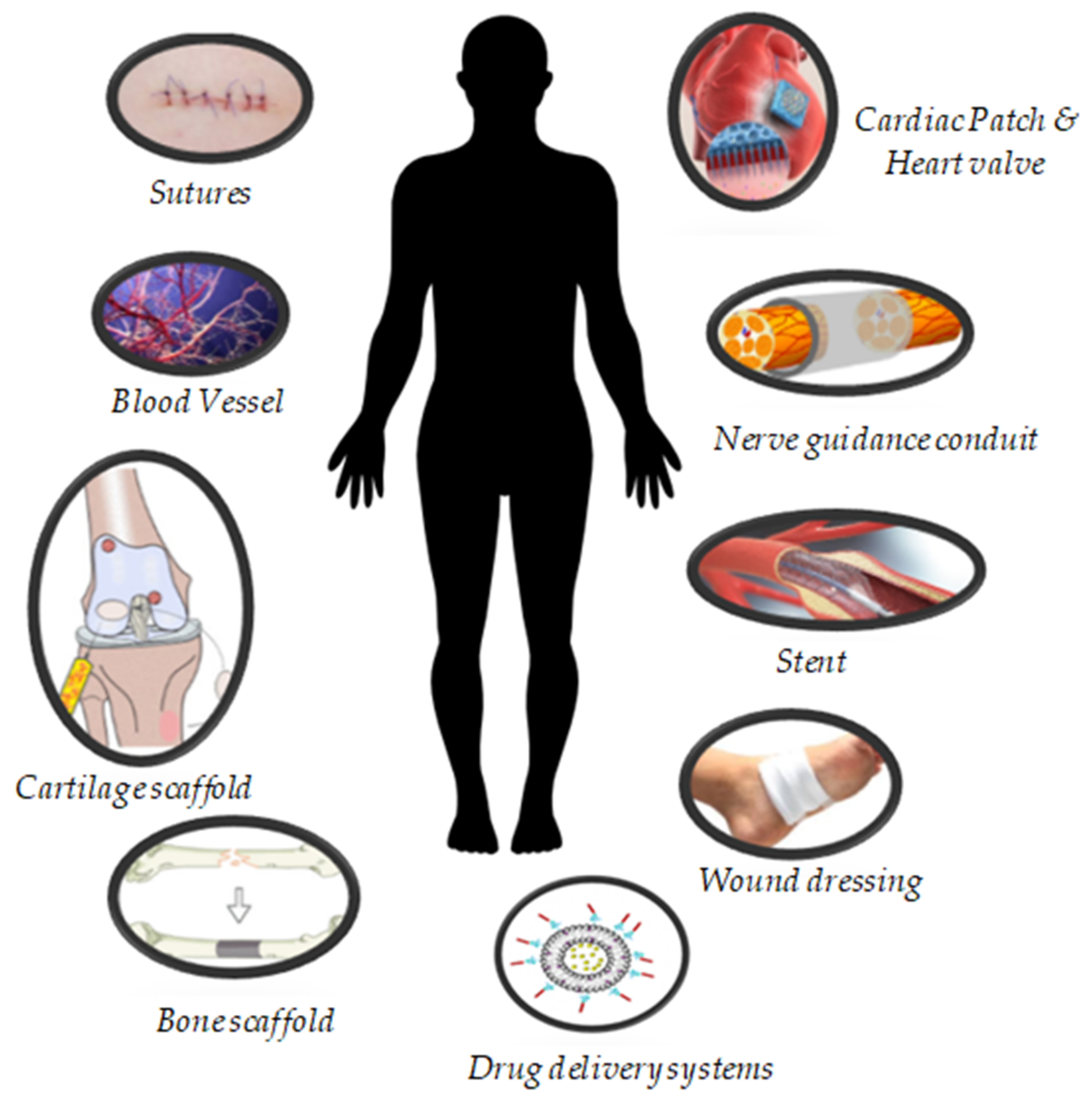
3. Biomedical Applications of PHA
3.1. Soft Tissue Engineering
3.1.1. Sutures
3.1.2. Wound Dressing
3.1.3. Cardiac Patch
3.1.4. Blood Vessel
3.2. Hard Tissue Engineering
3.2.1. Bone Scaffold
3.2.2. Cartilage Scaffold
3.3. Implantable Devices
3.3.1. Heart Valve
3.3.2. Stent
3.3.3. Nerve Guidance Conduit
3.4. Drug Delivery Systems
Nanoparticles
4. Biodegradability of PHA Used in the Medical Sector
4.1. In Vivo and In Vitro Biodegradation of PHA
4.2. Biodegradability of PHA-Based Implants and Biocompatibility of Their In Vivo Degradation Products
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A.M. Recent Advances and Challenges towards Sustainable Polyhydroxyalkanoate (PHA) Production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, S.; Sami, N.; Yasin, D.; Ahmad, N.; Fatma, T. Biomedical applications of environmental friendly poly-hydroxyalkanoates. Int. J. Biol. Macromol. 2021, 183, 549–563. [Google Scholar] [CrossRef] [PubMed]
- Valappil, S.P.; Misra, S.K.; Boccaccini, A.R.; Roy, I. Biomedical applications of polyhydroxyalkanoates, an overview of animal testing and in vivo responses. Expert Rev. Med. Devices 2006, 3, 853–868. [Google Scholar] [CrossRef] [PubMed]
- Williamson, D.; Wilkinson, J. The isolation and estimation of the poly-β-hydroxy-butyrate inclusions of Bacillus species. Microbiology 1958, 19, 198–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinbüchel, A.; Valentin, H.E. Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol. Lett. 1995, 128, 219–228. [Google Scholar] [CrossRef]
- Jendrossek, D. Polyhydroxyalkanoate granules are complex subcellular organelles (carbonosomes). J. Bacteriol. 2009, 191, 3195–3202. [Google Scholar] [CrossRef] [Green Version]
- Obruca, S.; Sedlacek, P.; Koller, M.; Kucera, D.; Pernicova, I. Involvement of polyhydroxyalkanoates in stress resistance of microbial cells: Biotechnological consequences and applications. Biotechnol. Adv. 2018, 36, 856–870. [Google Scholar] [CrossRef]
- Slaninova, E.; Sedlacek, P.; Mravec, F.; Mullerova, L.; Samek, O.; Koller, M.; Hesko, O.; Kucera, D.; Marova, I.; Obruca, S. Light scattering on PHA granules protects bacterial cells against the harmful effects of UV radiation. Appl. Microbiol. Biotechnol. 2018, 102, 1923–1931. [Google Scholar] [CrossRef]
- Anderson, A.J.; Dawes, E. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol. Rev. 1990, 54, 450–472. [Google Scholar] [CrossRef]
- Martínez, V.; Herencias, C.; Jurkevitch, E.; Prieto, M.A. Engineering a predatory bacterium as a proficient killer agent for intracellular bio-products recovery: The case of the polyhydroxyalkanoates. Sci. Rep. 2016, 6, 24381. [Google Scholar] [CrossRef] [Green Version]
- Gregory, D.A.; Taylor, C.S.; Fricker, A.T.; Asare, E.; Tetali, S.S.; Haycock, J.W.; Roy, I. Polyhydroxyalkanoates and their advances for biomedical applications. Trends Mol. Med. 2022, 28, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y. Bacterial polyhydroxyalkanoates. Biotechnol. Bioeng. 1996, 49, 1–14. [Google Scholar] [CrossRef]
- Ali, I.; Jamil, N. Polyhydroxyalkanoates: Current applications in the medical field. Front. Biol. 2016, 11, 19–27. [Google Scholar] [CrossRef]
- Możejko-Ciesielska, J.; Kiewisz, R. Bacterial polyhydroxyalkanoates: Still fabulous? Microbiol. Res. 2016, 192, 271–282. [Google Scholar] [CrossRef]
- Lizarraga-Valderrama, L.; Thomas, C.; Cadiz-Miranda, J.; Roy, I. Tissue engineering: Polyhydroxyalkanoate-based materials and composites. In Encyclopaedia of Polymer Applications; Taylor & Francis Group: Boca Raton, FL, USA, 2018; pp. 2652–2675. [Google Scholar]
- Chanprateep, S. Current trends in biodegradable polyhydroxyalkanoates. J. Biosci. Bioeng. 2010, 110, 621–632. [Google Scholar] [CrossRef]
- Mukai, K.; Yamada, K.; Doi, Y. Efficient hydrolysis of polyhydroxyalkanoates by Pseudomonas stutzeri YM1414 isolated from lake water. Polym. Degrad. Stab. 1994, 43, 319–327. [Google Scholar] [CrossRef]
- Saito, Y.; Nakamura, S.; Hiramitsu, M.; Doi, Y. Microbial synthesis and properties of poly (3-hydroxybutyrate-co-4-hydroxybutyrate). Polym. Int. 1996, 39, 169–174. [Google Scholar] [CrossRef]
- Lemoigne, M. Produits de deshydration et de polymerisation de l’acide β = oxybutyrique. Bull. Soc. Chim. Biol. 1926, 8, 770–782. [Google Scholar]
- Sevastianov, V.; Perova, N.; Shishatskaya, E.; Kalacheva, G.; Volova, T. Production of purified polyhydroxyalkanoates (PHAs) for applications in contact with blood. J. Biomater. Sci. Polym. Ed. 2003, 14, 1029–1042. [Google Scholar] [CrossRef]
- Murueva, A.V.; Shershneva, A.M.; Abanina, K.V.; Prudnikova, S.V.; Shishatskaya, E.I. Development and characterization of ceftriaxone-loaded P3HB-based microparticles for drug delivery. Dry. Technol. 2019, 37, 1131–1142. [Google Scholar] [CrossRef] [Green Version]
- Naveen, S.V.; Tan, I.K.P.; Goh, Y.S.; Raghavendran, H.R.B.; Murali, M.R.; Kamarul, T. Unmodified medium chain length polyhydroxyalkanoate (uMCL-PHA) as a thin film for tissue engineering application–characterization and in vitro biocompatibility. Mater. Lett. 2015, 141, 55–58. [Google Scholar] [CrossRef]
- Tan, D.; Yin, J.; Chen, G.Q. 29—Production of Polyhydroxyalkanoates. In Current Developments in Biotechnology and Bioengineering; Pandey, A., Negi, S., Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 655–692. [Google Scholar]
- Pérez Amaro, L.; Chen, H.; Barghini, A.; Corti, A.; Chiellini, E. High performance compostable biocomposites based on bacterial polyesters suitable for injection molding and blow extrusion. Chem. Biochem. Eng. Q. 2015, 29, 261–274. [Google Scholar] [CrossRef]
- Pietrini, M.; Roes, L.; Patel, M.K.; Chiellini, E. Comparative life cycle studies on poly (3-hydroxybutyrate)-based composites as potential replacement for conventional petrochemical plastics. Biomacromolecules 2007, 8, 2210–2218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinbüchel, A.; Doi, Y.; Steinbüchel, E. Biopolymers: Polyesters III—Applications and Commercial Products; Wiley-Vch: Weinheim, Germany, 2001. [Google Scholar]
- Zhang, J.; Shishatskaya, E.I.; Volova, T.G.; da Silva, L.F.; Chen, G.-Q. Polyhydroxyalkanoates (PHA) for therapeutic applications. Mater. Sci. Eng. C 2018, 86, 144–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmowafy, E.; Abdal-Hay, A.; Skouras, A.; Tiboni, M.; Casettari, L.; Guarino, V. Polyhydroxyalkanoate (PHA): Applications in drug delivery and tissue engineering. Expert Rev. Med. Devices 2019, 16, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Contreras, A. Recent Advances in the Use of Polyhydroyalkanoates in Biomedicine. Bioengineering 2019, 6, 82. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, R.; Pandey, R.; Kumar, S.; Mehrotra, D. Poly hydroxyalkanoates (PHA): Role in bone scaffolds. J. Oral Biol. Craniofac. Res. 2020, 10, 389–392. [Google Scholar] [CrossRef]
- Miu, D.-M.; Eremia, M.C.; Moscovici, M. Polyhydroxyalkanoates (PHAs) as Biomaterials in Tissue Engineering: Production, Isolation, Characterization. Materials 2022, 15, 1410. [Google Scholar] [CrossRef]
- Prakash, P.; Lee, W.-H.; Loo, C.-Y.; Wong, H.S.J.; Parumasivam, T. Advances in Polyhydroxyalkanoate Nanocarriers for Effective Drug Delivery: An Overview and Challenges. Nanomaterials 2022, 12, 175. [Google Scholar] [CrossRef]
- Guo, W.; Yang, K.; Qin, X.; Luo, R.; Wang, H.; Huang, R. Polyhydroxyalkanoates in tissue repair and regeneration. Eng. Regen. 2022, 3, 24–40. [Google Scholar] [CrossRef]
- Ang, S.L.; Shaharuddin, B.; Chuah, J.-A.; Sudesh, K. Electrospun poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)/silk fibroin film is a promising scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2020, 145, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Carlson, B. ‘Natural plastics’ ripe with potential. Biotechnol. Healthc. 2007, 4, 11–18. [Google Scholar] [PubMed]
- Van Rooijen, M.M.J.; Jairam, A.P.; Tollens, T.; Jørgensen, L.N.; de Vries Reilingh, T.S.; Piessen, G.; Köckerling, F.; Miserez, M.; Windsor, A.C.J.; Berrevoet, F.; et al. Outcomes of a new slowly resorbable biosynthetic mesh (Phasix™) in potentially contaminated incisional hernias: A prospective, multi-center, single-arm trial. Int. J. Surg. 2020, 83, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Odermatt, E.K.; Funk, L.; Bargon, R.; Martin, D.P.; Rizk, S.; Williams, S.F. MonoMax Suture: A New Long-Term Absorbable Monofilament Suture Made from Poly-4-Hydroxybutyrate. Int. J. Polym. Sci. 2012, 2012, 216137. [Google Scholar] [CrossRef] [Green Version]
- Fu, N.; Meng, Z.; Jiao, T.; Luo, X.; Tang, Z.; Zhu, B.; Sui, L.; Cai, X. P34HB electrospun fibres promote bone regeneration in vivo. Cell Prolif. 2019, 52, e12601. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, R.; Cheng, X.; Lan, Q.; Xie, J.; He, M.; Pang, Y.; Xiong, F.; Lei, D.; Zheng, L.; et al. Osteogenic Potential of Electrospun Poly(3-hydroxybutyrate-co-4-hydroxybutyrate)/ Poly(ethylene glycol) Nanofiber Membranes. J. Biomed. Nanotechnol. 2019, 15, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.G.; Shumilova, A.A.; Nikolaeva, E.D.; Kirichenko, A.K.; Shishatskaya, E.I. Biotechnological wound dressings based on bacterial cellulose and degradable copolymer P(3HB/4HB). Int. J. Biol. Macromol. 2019, 131, 230–240. [Google Scholar] [CrossRef] [Green Version]
- Sanhueza, C.; Hermosilla, J.; Bugallo-Casal, A.; Da Silva-Candal, A.; Taboada, C.; Millán, R.; Concheiro, A.; Alvarez-Lorenzo, C.; Acevedo, F. One-step electrospun scaffold of dual-sized gelatin/poly-3-hydroxybutyrate nano/microfibers for skin regeneration in diabetic wound. Mater. Sci. Eng. C 2021, 119, 111602. [Google Scholar] [CrossRef]
- Zarei, M.; Karbasi, S.; Sari Aslani, F.; Zare, S.; Koohi-Hosseinabad, O.; Tanideh, N. In Vitro and In Vivo Evaluation of Poly (3-hydroxybutyrate)/Carbon Nanotubes Electrospun Scaffolds for Periodontal Ligament Tissue Engineering. J. Dent. 2020, 21, 18–30. [Google Scholar] [CrossRef]
- Khoroushi, M.; Foroughi, M.R.; Karbasi, S.; Hashemibeni, B.; Khademi, A.A. Effect of Polyhydroxybutyrate/Chitosan/Bioglass nanofiber scaffold on proliferation and differentiation of stem cells from human exfoliated deciduous teeth into odontoblast-like cells. Mater. Sci. Eng. C 2018, 89, 128–139. [Google Scholar] [CrossRef]
- Findrik Balogová, A.; Hudák, R.; Tóth, T.; Schnitzer, M.; Feranc, J.; Bakoš, D.; Živčák, J. Determination of geometrical and viscoelastic properties of PLA/PHB samples made by additive manufacturing for urethral substitution. J. Biotechnol. 2018, 284, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Ozer, H.; Bozkurt, H.; Bozkurt, G.; Demirbilek, M. Regenerative potential of chitosan-coated poly-3-hydroxybutyrate conduits seeded with mesenchymal stem cells in a rat sciatic nerve injury model. Int. J. Neurosci. 2018, 128, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Schaakxs, D.; Kalbermatten, D.F.; Pralong, E.; Raffoul, W.; Wiberg, M.; Kingham, P.J. Poly-3-hydroxybutyrate strips seeded with regenerative cells are effective promoters of peripheral nerve repair. J. Tissue Eng. Regen. Med. 2017, 11, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Petrovova, E.; Tomco, M.; Holovska, K.; Danko, J.; Kresakova, L.; Vdoviakova, K.; Simaiova, V.; Kolvek, F.; Hornakova, P.; Toth, T.; et al. PHB/CHIT Scaffold as a Promising Biopolymer in the Treatment of Osteochondral Defects—An Experimental Animal Study. Polymers 2021, 13, 1232. [Google Scholar] [CrossRef] [PubMed]
- Volkov, A.V.; Muraev, A.A.; Zharkova, I.I.; Voinova, V.V.; Akoulina, E.A.; Zhuikov, V.A.; Khaydapova, D.D.; Chesnokova, D.V.; Menshikh, K.A.; Dudun, A.A.; et al. Poly(3-hydroxybutyrate)/hydroxyapatite/alginate scaffolds seeded with mesenchymal stem cells enhance the regeneration of critical-sized bone defect. Mater. Sci. Eng. C 2020, 114, 110991. [Google Scholar] [CrossRef]
- Codreanu, A.; Balta, C.; Herman, H.; Cotoraci, C.; Mihali, C.V.; Zurbau, N.; Zaharia, C.; Rapa, M.; Stanescu, P.; Radu, I.-C.; et al. Bacterial Cellulose-Modified Polyhydroxyalkanoates Scaffolds Promotes Bone Formation in Critical Size Calvarial Defects in Mice. Materials 2020, 13, 1433. [Google Scholar] [CrossRef] [Green Version]
- Goonoo, N.; Khanbabaee, B.; Steuber, M.; Bhaw-Luximon, A.; Jonas, U.; Pietsch, U.; Jhurry, D.; Schönherr, H. κ-Carrageenan Enhances the Biomineralization and Osteogenic Differentiation of Electrospun Polyhydroxybutyrate and Polyhydroxybutyrate Valerate Fibers. Biomacromolecules 2017, 18, 1563–1573. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Z.; Fan, F.; Sun, S.; Wu, X.; Lu, H.; Lu, X. PHBHHx Facilitated the Residence, Survival and Stemness Maintain of Transplanted Neural Stem Cells in Traumatic Brain Injury Rats. Biomacromolecules 2019, 20, 3294–3302. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, S.; Cao, L.; Zhang, X.; Wang, J.; Liu, C. Facilitated vascularization and enhanced bone regeneration by manipulation hierarchical pore structure of scaffolds. Mater. Sci. Eng. C 2020, 110, 110622. [Google Scholar] [CrossRef]
- Xue, Q.; Liu, X.-B.; Lao, Y.-H.; Wu, L.-P.; Wang, D.; Zuo, Z.-Q.; Chen, J.-Y.; Hou, J.; Bei, Y.-Y.; Wu, X.-F.; et al. Anti-infective biomaterials with surface-decorated tachyplesin I. Biomaterials 2018, 178, 351–362. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A.; Patan, N.K.; Dalvi, Y.B.; Varghese, R.; Antony, A.; Unni, R.N.; Sandhyarani, N.; Moustafa, A.-E.A. Cerium Oxide Nanoparticle Incorporated Electrospun Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Membranes for Diabetic Wound Healing Applications. ACS Biomater. Sci. Eng. 2020, 6, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Abazari, M.F.; Zare Karizi, S.; Hajati-Birgani, N.; Norouzi, S.; Khazeni, Z.; Hashemi, J.; Shafaghi, L.; Soleimanifar, F.; Mansour, R.N.; Enderami, S.E. PHBV nanofibers promotes insulin-producing cells differentiation of human induced pluripotent stem cells. Gene 2021, 768, 145333. [Google Scholar] [CrossRef] [PubMed]
- Antonova, L.V.; Krivkina, E.O.; Sevostianova, V.V.; Mironov, A.V.; Rezvova, M.A.; Shabaev, A.R.; Tkachenko, V.O.; Krutitskiy, S.S.; Khanova, M.Y.; Sergeeva, T.Y.; et al. Tissue-Engineered Carotid Artery Interposition Grafts Demonstrate High Primary Patency and Promote Vascular Tissue Regeneration in the Ovine Model. Polymers 2021, 13, 2637. [Google Scholar] [CrossRef] [PubMed]
- Sevostianova, V.V.; Antonova, L.V.; Mironov, A.V.; Yuzhalin, A.E.; Silnikov, V.N.; Glushkova, T.V.; Godovikova, T.S.; Krivkina, E.O.; Bolbasov, E.; Akentyeva, T.N.; et al. Biodegradable Patches for Arterial Reconstruction Modified with RGD Peptides: Results of an Experimental Study. ACS Omega 2020, 5, 21700–21711. [Google Scholar] [CrossRef]
- Deepthi, S.; Nivedhitha Sundaram, M.; Vijayan, P.; Nair, S.V.; Jayakumar, R. Engineering poly(hydroxy butyrate-co-hydroxy valerate) based vascular scaffolds to mimic native artery. Int. J. Biol. Macromol. 2018, 109, 85–98. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, H.X.; Ortiz, L.S.; Xiao, Z.D.; Huang, N.P. Laminin-modified and aligned poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/polyethylene oxide nanofibrous nerve conduits promote peripheral nerve regeneration. J. Tissue Eng. Regen. Med. 2018, 12, e627–e636. [Google Scholar] [CrossRef]
- Chen, W.; Li, Y.; Huang, Y.; Dai, Y.; Xi, T.; Zhou, Z.; Liu, H. Quercetin modified electrospun PHBV fibrous scaffold enhances cartilage regeneration. J. Mater. Sci. Mater. Med. 2021, 32, 92. [Google Scholar] [CrossRef]
- Tahmasebi, A.; Shapouri Moghadam, A.; Enderami, S.E.; Islami, M.; Kaabi, M.; Saburi, E.; Daei Farshchi, A.; Soleimanifar, F.; Mansouri, V. Aloe Vera-Derived Gel-Blended PHBV Nanofibrous Scaffold for Bone Tissue Engineering. Asaio J. 2020, 66, 966–973. [Google Scholar] [CrossRef]
- Zhong, L.; Hu, D.; Qu, Y.; Peng, J.; Huang, K.; Lei, M.; Wu, T.; Xiao, Y.; Gu, Y.; Qian, Z. Preparation of Adenosine-Loaded Electrospun Nanofibers and Their Application in Bone Regeneration. J. Biomed. Nanotechnol. 2019, 15, 857–877. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A.; Dalvi, Y.B.; Rehman, S.R.U.; Varghese, R.; Unni, R.N.; Yalcin, H.C.; Alfkey, R.; Thomas, S.; Al Moustafa, A.-E. Growth factor loaded in situ photocrosslinkable poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/gelatin methacryloyl hybrid patch for diabetic wound healing. Mater. Sci. Eng. C 2021, 118, 111519. [Google Scholar] [CrossRef]
- Ye, J.-P.; Gong, J.-S.; Su, C.; Liu, Y.-G.; Jiang, M.; Pan, H.; Li, R.-Y.; Geng, Y.; Xu, Z.-H.; Shi, J.-S. Fabrication and characterization of high molecular keratin based nanofibrous membranes for wound healing. Colloids Surf. B Biointerfaces 2020, 194, 111158. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Chen, J.; Wu, L.-P.; Wu, J.; Xiang, H.; Leong, K.W.; Han, J. Prevention of excessive scar formation using nanofibrous meshes made of biodegradable elastomer poly(3-hydroxybutyrate-co-3-hydroxyvalerate). J. Tissue Eng. 2020, 11, 2041731420949332. [Google Scholar] [CrossRef] [PubMed]
- Bagdadi, A.V.; Safari, M.; Dubey, P.; Basnett, P.; Sofokleous, P.; Humphrey, E.; Locke, I.; Edirisinghe, M.; Terracciano, C.; Boccaccini, A.R.; et al. Poly(3-hydroxyoctanoate), a promising new material for cardiac tissue engineering. J. Tissue Eng. Regen. Med. 2018, 12, e495–e512. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.; Shishatskaya, E.; Sevastianov, V.; Efremov, S.; Mogilnaya, O. Results of biomedical investigations of PHB and PHB/PHV fibers. Biochem. Eng. J. 2003, 16, 125–133. [Google Scholar] [CrossRef]
- Hazer, D.B.; Hazer, B.; Kaymaz, F. Synthesis of microbial elastomers based on soybean oily acids. Biocompatibility studies. Biomed. Mater. 2009, 4, 035011. [Google Scholar] [CrossRef]
- Rathbone, S.; Furrer, P.; Lübben, J.; Zinn, M.; Cartmell, S. Biocompatibility of polyhydroxyalkanoate as a potential material for ligament and tendon scaffold material. J. Biomed. Mater. Res. Part A 2010, 93A, 1391–1403. [Google Scholar] [CrossRef]
- Mierziak, J.; Burgberger, M.; Wojtasik, W. 3-Hydroxybutyrate as a Metabolite and a Signal Molecule Regulating Processes of Living Organisms. Biomolecules 2021, 11, 402. [Google Scholar] [CrossRef]
- Xiao, X.-Q.; Zhao, Y.; Chen, G.-Q. The effect of 3-hydroxybutyrate and its derivatives on the growth of glial cells. Biomaterials 2007, 28, 3608–3616. [Google Scholar] [CrossRef]
- Kovalcik, A.; Obruca, S.; Kalina, M.; Machovsky, M.; Enev, V.; Jakesova, M.; Sobkova, M.; Marova, I. Enzymatic Hydrolysis of Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) Scaffolds. Materials 2020, 13, 2992. [Google Scholar] [CrossRef]
- Turco, R.; Santagata, G.; Corrado, I.; Pezzella, C.; Di Serio, M. In vivo and Post-synthesis Strategies to Enhance the Properties of PHB-Based Materials: A Review. Front. Bioeng. Biotechnol. 2021, 8, 619266. [Google Scholar] [CrossRef]
- McAdam, B.; Brennan Fournet, M.; McDonald, P.; Mojicevic, M. Production of Polyhydroxybutyrate (PHB) and Factors Impacting Its Chemical and Mechanical Characteristics. Polymers 2020, 12, 2908. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-L.; Chen, P.-Y.; Lan, C.-H.; Sun, Y.-M. Structure, mechanical properties and degradation behaviors of the electrospun fibrous blends of PHBHHx/PDLLA. Polymer 2011, 52, 1391–1401. [Google Scholar] [CrossRef]
- Sultana, N.; Wang, M. PHBV/PLLA-based composite scaffolds containing nano-sized hydroxyapatite particles for bone tissue engineering. J. Exp. Nanosci. 2008, 3, 121–132. [Google Scholar] [CrossRef]
- Berezina, N. Enhancing the 3-hydroxyvalerate component in bioplastic PHBV production by Cupriavidus necator. Biotechnol. J. 2012, 7, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-Q.; Chen, X.-Y.; Wu, F.-Q.; Chen, J.-C. Polyhydroxyalkanoates (PHA) toward cost competitiveness and functionality. Adv. Ind. Eng. Polym. Res. 2020, 3, 1–7. [Google Scholar] [CrossRef]
- Cavalheiro, J.M.B.T.; de Almeida, M.C.M.D.; Grandfils, C.; da Fonseca, M.M.R. Poly(3-hydroxybutyrate) production by Cupriavidus necator using waste glycerol. Process Biochem. 2009, 44, 509–515. [Google Scholar] [CrossRef]
- Verlinden, R.A.J.; Hill, D.J.; Kenward, M.A.; Williams, C.D.; Piotrowska-Seget, Z.; Radecka, I.K. Production of polyhydroxyalkanoates from waste frying oil by Cupriavidus necator. AMB Express 2011, 1, 11. [Google Scholar] [CrossRef] [Green Version]
- Loo, C.-Y.; Sudesh, K. Biosynthesis and native granule characteristics of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) in Delftia acidovorans. Int. J. Biol. Macromol. 2007, 40, 466–471. [Google Scholar] [CrossRef]
- Policastro, G.; Luongo, V.; Fabbricino, M. Biohydrogen and poly-β-hydroxybutyrate production by winery wastewater photofermentation: Effect of substrate concentration and nitrogen source. J. Environ. Manag. 2020, 271, 111006. [Google Scholar] [CrossRef]
- Hermann-Krauss, C.; Koller, M.; Muhr, A.; Fasl, H.; Stelzer, F.; Braunegg, G. Archaeal Production of Polyhydroxyalkanoate (PHA) Co- and Terpolyesters from Biodiesel Industry-Derived By-Products. Archaea 2013, 2013, 129268. [Google Scholar] [CrossRef] [Green Version]
- Policastro, G.; Panico, A.; Fabbricino, M. Improving biological production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) co-polymer: A critical review. Rev. Environ. Sci. Bio/Technol. 2021, 20, 479–513. [Google Scholar] [CrossRef]
- Dhania, S.; Bernela, M.; Rani, R.; Parsad, M.; Grewal, S.; Kumari, S.; Thakur, R. Scaffolds the backbone of tissue engineering: Advancements in use of polyhydroxyalkanoates (PHA). Int. J. Biol. Macromol. 2022, 208, 243–259. [Google Scholar] [CrossRef]
- Butt, F.I.; Muhammad, N.; Hamid, A.; Moniruzzaman, M.; Sharif, F. Recent progress in the utilization of biosynthesized polyhydroxyalkanoates for biomedical applications–review. Int. J. Biol. Macromol. 2018, 120, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Grigore, M.E.; Grigorescu, R.M.; Iancu, L.; Ion, R.-M.; Zaharia, C.; Andrei, E.R. Methods of synthesis, properties and biomedical applications of polyhydroxyalkanoates: A review. J. Biomater. Sci. Polym. Ed. 2019, 30, 695–712. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Srivastava, J.K.; Chandel, A.K.; Sharma, L.; Mallick, N.; Singh, S.P. Biomedical applications of microbially engineered polyhydroxyalkanoates: An insight into recent advances, bottlenecks, and solutions. Appl. Microbiol. Biotechnol. 2019, 103, 2007–2032. [Google Scholar] [CrossRef] [PubMed]
- Sahana, T.G.; Rekha, P.D. Biopolymers: Applications in wound healing and skin tissue engineering. Mol. Biol. Rep. 2018, 45, 2857–2867. [Google Scholar] [CrossRef] [PubMed]
- Soylemez, S.; Kesika, M.; Toppare, L. Biosensing Devices: Conjugated Polymer Based Scaffolds. In Encyclopedia of Polymer Applications; Taylor & Francis: Abingdon, UK, 2018; pp. 360–386. [Google Scholar]
- He, Y.; Hu, Z.; Ren, M.; Ding, C.; Chen, P.; Gu, Q.; Wu, Q. Evaluation of PHBHHx and PHBV/PLA fibers used as medical sutures. J. Mater. Sci. Mater. Med. 2014, 25, 561–571. [Google Scholar] [CrossRef]
- Bennett, R.G. Selection of wound closure materials. J. Am. Acad. Dermatol. 1988, 18, 619–637. [Google Scholar] [CrossRef]
- Moy, R.L.; Waldman, B.; Hein, D.W. A review of sutures and suturing techniques. J. Dermatol. Surg. Oncol. 1992, 18, 785–795. [Google Scholar] [CrossRef]
- Baptist, J.N.; Ziegler, J.B. Method of Making Absorbable Surgical Sutures from Poly Beta Hydroxy Acids. US3225766A, 28 December 1965. [Google Scholar]
- Shishatskaya, E.; Volova, T.; Puzyr, A.; Mogilnaya, O.; Efremov, S. Tissue response to the implantation of biodegradable polyhydroxyalkanoate sutures. J. Mater. Sci. Mater. Med. 2004, 15, 719–728. [Google Scholar] [CrossRef]
- Chu, C. Materials for absorbable and nonabsorbable surgical sutures. In Biotextiles as Medical Implants; Elsevier: Amsterdam, The Netherlands, 2013; pp. 275–334. [Google Scholar]
- Rajaratanam, D.D.; Ariffin, H.; Hassan, M.A.; Nik Abd Rahman, N.M.A.; Nishida, H. In vitro cytotoxicity of superheated steam hydrolyzed oligo ((R)-3-hydroxybutyrate-co-(R)-3-hydroxyhexanoate) and characteristics of its blend with poly (L-lactic acid) for biomaterial applications. PLoS ONE 2018, 13, e0199742. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-W.; Wu, Q.; Chen, G.-Q. Attachment, proliferation and differentiation of osteoblasts on random biopolyester poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) scaffolds. Biomaterials 2004, 25, 669–675. [Google Scholar] [CrossRef]
- Yan, C.; Wang, Y.; Shen, X.-Y.; Yang, G.; Jian, J.; Wang, H.-S.; Chen, G.-Q.; Wu, Q. MicroRNA regulation associated chondrogenesis of mouse MSCs grown on polyhydroxyalkanoates. Biomaterials 2011, 32, 6435–6444. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Hu, Y.J.; Xie, W.P.; Lin, R.L.; Chen, G.Q. Influence of poly (3-hydroxybutyrate-co-4-hydroxybutyrate-co-3-hydroxyhexanoate) on growth and osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. J. Biomed. Mater. Res. Part A 2009, 90, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Lomas, A.J.; Webb, W.R.; Han, J.; Chen, G.-Q.; Sun, X.; Zhang, Z.; El Haj, A.J.; Forsyth, N.R. Poly (3-hydroxybutyrate-co-3-hydroxyhexanoate)/collagen hybrid scaffolds for tissue engineering applications. Tissue Eng. Part C Methods 2013, 19, 577–585. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Jiang, X.-L.; Peng, S.-W.; Guo, X.-Y.; Shang, G.-G.; Chen, J.-C.; Wu, Q.; Chen, G.-Q. Induced apoptosis of osteoblasts proliferating on polyhydroxyalkanoates. Biomaterials 2013, 34, 3737–3746. [Google Scholar] [CrossRef]
- Azimi, B.; Maleki, H.; Zavagna, L.; De la Ossa, J.G.; Linari, S.; Lazzeri, A.; Danti, S. Bio-based electrospun fibers for wound healing. J. Funct. Biomater. 2020, 11, 67. [Google Scholar] [CrossRef]
- Shishatskaya, E.; Volova, T. A comparative investigation of biodegradable polyhydroxyalkanoate films as matrices for in vitro cell cultures. J. Mater. Sci. Mater. Med. 2004, 15, 915–923. [Google Scholar] [CrossRef]
- Vigneswari, S.; Murugaiyah, V.; Kaur, G.; Abdul Khalil, H.P.S.; Amirul, A.A. Simultaneous dual syringe electrospinning system using benign solvent to fabricate nanofibrous P(3HB-co-4HB)/collagen peptides construct as potential leave-on wound dressing. Mater. Sci. Eng. C 2016, 66, 147–155. [Google Scholar] [CrossRef]
- Yuan, J.; Xing, Z.-C.; Park, S.-W.; Geng, J.; Kang, I.-K.; Shen, J.; Meng, W.; Shim, K.-J.; Han, I.-S.; Kim, J.-C. Fabrication of PHBV/keratin composite nanofibrous mats for biomedical applications. Macromol. Res. 2009, 17, 850–855. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Yuan, J.; Shen, J. Differences in cytocompatibility between collagen, gelatin and keratin. Mater. Sci. Eng. C 2016, 59, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Shishatskaya, E.I.; Nikolaeva, E.D.; Vinogradova, O.N.; Volova, T.G. Experimental wound dressings of degradable PHA for skin defect repair. J. Mater. Sci. Mater. Med. 2016, 27, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duvernoy, O.; Malm, T.; Ramström, J.; Bowald, S. A biodegradable patch used as a pericardial substitute after cardiac surgery: 6-and 24-month evaluation with CT. Thorac. Cardiovasc. Surg. 1995, 43, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Malm, T.; Bowald, S.; Bylock, A.; Saldeen, T.; Busch, C. Regeneration of pericardial tissue on absorbable polymer patches implanted into the pericardial sac: An immunohistochemical, ultrastructural and biochemical study in the sheep. Scand. J. Thorac. Cardiovasc. Surg. 1992, 26, 15–21. [Google Scholar] [CrossRef]
- Malm, T.; Bowald, S.; Bylock, A.; Busch, C.; Saldeen, T. Enlargement of the right ventricular outflow tract and the pulmonary artery with a new biodegradable patch in transannular position. Eur. Surg. Res. 1994, 26, 298–308. [Google Scholar] [CrossRef]
- Castellano, D.; Blanes, M.; Marco, B.; Cerrada, I.; Ruiz-Saurí, A.; Pelacho, B.; Arana, M.; Montero, J.A.; Cambra, V.; Prosper, F. A comparison of electrospun polymers reveals poly (3-hydroxybutyrate) fiber as a superior scaffold for cardiac repair. Stem Cells Dev. 2014, 23, 1479–1490. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.-T.; Chen, Z.-F.; Chen, G.-Q. The expression of cross-linked elastin by rabbit blood vessel smooth muscle cells cultured in polyhydroxyalkanoate scaffolds. Biomaterials 2008, 29, 4187–4194. [Google Scholar] [CrossRef]
- Opitz, F.; Schenke-Layland, K.; Cohnert, T.U.; Starcher, B.; Halbhuber, K.J.; Martin, D.P.; Stock, U.A. Tissue engineering of aortic tissue: Dire consequence of suboptimal elastic fiber synthesis in vivo. Cardiovasc. Res. 2004, 63, 719–730. [Google Scholar] [CrossRef] [Green Version]
- Opitz, F.; Schenke-Layland, K.; Richter, W.; Martin, D.; Degenkolbe, I.; Wahlers, T.; Stock, U. Tissue engineering of ovine aortic blood vessel substitutes using applied shear stress and enzymatically derived vascular smooth muscle cells. Ann. Biomed. Eng. 2004, 32, 212–222. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Lupescu, I.; Frone, A.N.; Chiulan, I.; Nicolae, C.A.; Tofan, V.; Stefaniu, A.; Somoghi, R.; Trusca, R. Medium Chain-Length Polyhydroxyalkanoate Copolymer Modified by Bacterial Cellulose for Medical Devices. Biomacromolecules 2017, 18, 3222–3232. [Google Scholar] [CrossRef]
- Lim, J.; You, M.; Li, J.; Li, Z. Emerging bone tissue engineering via Polyhydroxyalkanoate (PHA)-based scaffolds. Mater. Sci. Eng. C 2017, 79, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.; Cipitria, A.; Epari, D.; Saifzadeh, S.; Krishnakanth, P.; Berner, A.; Woodruff, M.; Schell, H.; Mehta, M.; Schuetz, M. A tissue engineering solution for segmental defect regeneration in load-bearing long bones. Sci. Transl. Med. 2012, 4, 141ra93. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Li, W.; Müller, T.; Schubert, D.W.; Boccaccini, A.R.; Yao, Q.; Roether, J.A. Electrospun polyhydroxybutyrate/poly (ε-caprolactone)/58S sol–gel bioactive glass hybrid scaffolds with highly improved osteogenic potential for bone tissue engineering. ACS Appl. Mater. Interfaces 2016, 8, 17098–17108. [Google Scholar] [CrossRef] [PubMed]
- Sakar, M.; Korkusuz, P.; Demirbilek, M.; Çetinkaya, D.U.; Arslan, S.; Denkbaş, E.B.; Temuçin, Ç.M.; Bilgiç, E.; Hazer, D.B.; Bozkurt, G. The effect of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate)(PHBHHx) and human mesenchymal stem cell (hMSC) on axonal regeneration in experimental sciatic nerve damage. Int. J. Neurosci. 2014, 124, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Parvizifard, M.; Karbasi, S. Physical, mechanical and biological performance of PHB-Chitosan/MWCNTs nanocomposite coating deposited on bioglass based scaffold: Potential application in bone tissue engineering. Int. J. Biol. Macromol. 2020, 152, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.L.; Choo, E.S.G.; Wong, S.Y.; Li, X.; He, C.B.; Wang, J.; Li, J. Designing poly [(R)-3-hydroxybutyrate]-based polyurethane block copolymers for electrospun nanofiber scaffolds with improved mechanical properties and enhanced mineralization capability. J. Phys. Chem. B 2010, 114, 7489–7498. [Google Scholar] [CrossRef]
- Mohammadalizadeh, Z.; Karbasi, S.; Arasteh, S. Physical, mechanical and biological evaluation of poly (3-hydroxybutyrate)-chitosan/MWNTs as a novel electrospun scaffold for cartilage tissue engineering applications. Polym. Plast. Technol. Mater. 2020, 59, 417–429. [Google Scholar] [CrossRef]
- Ching, K.Y.; Andriotis, O.G.; Li, S.; Basnett, P.; Su, B.; Roy, I.; Tare, R.S.; Sengers, B.G.; Stolz, M. Nanofibrous poly (3-hydroxybutyrate)/poly (3-hydroxyoctanoate) scaffolds provide a functional microenvironment for cartilage repair. J. Biomater. Appl. 2016, 31, 77–91. [Google Scholar] [CrossRef] [Green Version]
- De Pascale, C.; Marcello, E.; Getting, S.; Roy, I.; Locke, I. Populated collagen hydrogel and polyhydroxyalkanoate composites: Novel matrices for cartilage repair and regeneration? Osteoarthr. Cartil. 2019, 27, S432–S433. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Wang, Y.; Chen, G.-Q. Medical application of microbial biopolyesters polyhydroxyalkanoates. Artif. Cells Blood Substit. Biotechnol. 2009, 37, 1–12. [Google Scholar] [CrossRef]
- Engelmayr, G.C.; Hildebrand, D.K.; Sutherland, F.W.H.; Mayer, J.E.; Sacks, M.S. A novel bioreactor for the dynamic flexural stimulation of tissue engineered heart valve biomaterials. Biomaterials 2003, 24, 2523–2532. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Schantz, T.; Zein, I.; Ng, K.W.; Teoh, S.H.; Tan, K.C. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J. Biomed. Mater. Res. 2001, 55, 203–216. [Google Scholar] [CrossRef]
- Sodian, R.; Hoerstrup, S.P.; Sperling, J.S.; Daebritz, S.; Martin, D.P.; Moran, A.M.; Kim, B.S.; Schoen, F.J.; Vacanti, J.P.; Mayer, J.E., Jr. Early in vivo experience with tissue-engineered trileaflet heart valves. Circulation 2000, 102, III22–III29. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.F.; Martin, D.P. Applications of PHAs in medicine and pharmacy. Biopolymers 2002, 4, 91–127. [Google Scholar]
- Hoerstrup, S.P.; Sodian, R.; Daebritz, S.; Wang, J.; Bacha, E.A.; Martin, D.P.; Moran, A.M.; Guleserian, K.J.; Sperling, J.S.; Kaushal, S. Functional living trileaflet heart valves grown in vitro. Circulation 2000, 102, III44–III49. [Google Scholar] [CrossRef]
- Stock, U.A.; Nagashima, M.; Khalil, P.N.; Nollert, G.D.; Herdena, T.; Sperling, J.S.; Moran, A.; Lien, J.; Martin, D.P.; Schoen, F.J. Tissue-engineered valved conduits in the pulmonary circulation. J. Thorac. Cardiovasc. Surg. 2000, 119, 732–740. [Google Scholar] [CrossRef] [Green Version]
- Sodian, R.; Hoerstrup, S.P.; Sperling, J.S.; Martin, D.P.; Daebritz, S.; Mayer, J.E., Jr.; Vacanti, J.P. Evaluation of biodegradable, three-dimensional matrices for tissue engineering of heart valves. Asaio J. 2000, 46, 107–110. [Google Scholar] [CrossRef]
- Steinbüchel, A.; Lütke-Eversloh, T. Metabolic engineering and pathway construction for biotechnological production of relevant polyhydroxyalkanoates in microorganisms. Biochem. Eng. J. 2003, 16, 81–96. [Google Scholar] [CrossRef]
- Kamath, K.R.; Barry, J.J.; Miller, K.M. The Taxus™ drug-eluting stent: A new paradigm in controlled drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 412–436. [Google Scholar] [CrossRef]
- Kischkel, S.; Grabow, N.; Püschel, A.; Erdle, B.; Kabelitz, M.; Martin, D.P.; Williams, S.F.; Bombor, I.; Sternberg, K.; Schmitz, K.P.; et al. Biodegradable polymeric stents for vascular application in a porcine carotid artery model. Gefässchirurgie 2016, 21, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Unverdorben, M.; Spielberger, A.; Schywalsky, M.; Labahn, D.; Hartwig, S.; Schneider, M.; Lootz, D.; Behrend, D.; Schmitz, K.; Degenhardt, R. A polyhydroxybutyrate biodegradable stent: Preliminary experience in the rabbit. Cardiovasc. Interv. Radiol. 2002, 25, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Markelova, N.; Shishatskaya, E.; Vinnik, Y.; Cherdantsev, D.; Beletskiy, I.; Kuznetsov, M.; Zykova, L. In Vivo Justification of Using Endobiliary Stents Made of Polyhydroxyalkanoates. Macromol. Symp. 2008, 269, 82–91. [Google Scholar] [CrossRef]
- Babu, P.; Behl, A.; Chakravarty, B.; Bhandari, P.S.; Bhatti, T.S.; Maurya, S. Entubulation techniques in peripheral nerve repair. Indian J. Neurotrauma 2008, 5, 15–20. [Google Scholar] [CrossRef]
- Bell, J.H.; Haycock, J.W. Next generation nerve guides: Materials, fabrication, growth factors, and cell delivery. Tissue Eng. Part B Rev. 2012, 18, 116–128. [Google Scholar] [CrossRef]
- Yang, Y.; De Laporte, L.; Rives, C.B.; Jang, J.-H.; Lin, W.-C.; Shull, K.R.; Shea, L.D. Neurotrophin releasing single and multiple lumen nerve conduits. J. Control. Release 2005, 104, 433–446. [Google Scholar] [CrossRef] [Green Version]
- Daly, W.; Yao, L.; Zeugolis, D.; Windebank, A.; Pandit, A. A biomaterials approach to peripheral nerve regeneration: Bridging the peripheral nerve gap and enhancing functional recovery. J. R. Soc. Interface 2012, 9, 202–221. [Google Scholar] [CrossRef] [Green Version]
- Taylor, C.S.; Chen, R.; D’Sa, R.; Hunt, J.A.; Curran, J.M.; Haycock, J.W. Cost effective optimised synthetic surface modification strategies for enhanced control of neuronal cell differentiation and supporting neuronal and Schwann cell viability. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1713–1723. [Google Scholar] [CrossRef]
- Hazari, A.; Johansson-Ruden, G.; Junemo-Bostrom, K.; Ljungberg, C.; Terenghi, G.; Green, C.; Wiberg, M. A new resorbable wrap-around implant as an alternative nerve repair technique. J. Hand Surg. Br. Eur. Vol. 1999, 24, 291–295. [Google Scholar] [CrossRef]
- Ljungberg, C.; Johansson-Ruden, G.; Boström, K.J.; Novikov, L.; Wiberg, M. Neuronal survival using a resorbable synthetic conduit as an alternative to primary nerve repair. Microsurg. Off. J. Int. Microsurg. Soc. Eur. Fed. Soc. Microsurg. 1999, 19, 259–264. [Google Scholar] [CrossRef]
- Hazari, A.; Wiberg, M.; Johansson-Ruden, G.; Green, C.; Terenghi, G. A resorbable nerve conduit as an alternative to nerve autograft in nerve gap repair. Br. J. Plast. Surg. 1999, 52, 653–657. [Google Scholar] [CrossRef]
- Taylor, C.S.; Haycock, J.W. Biomaterials and Scaffolds for Repair of the Peripheral Nervous System. In Peripheral Nerve Tissue Engineering and Regeneration; Phillips, J., Hercher, D., Hausner, T., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–35. [Google Scholar]
- Lizarraga-Valderrama, L.R.; Taylor, C.S.; Claeyssens, F.; Haycock, J.W.; Knowles, J.C.; Roy, I. Unidirectional neuronal cell growth and differentiation on aligned polyhydroxyalkanoate blend microfibres with varying diameters. J. Tissue Eng. Regen. Med. 2019, 13, 1581–1594. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, G.; Liu, X.; Yang, M.; Xing, S.; Du, Y.; Xiong, X. Synthesis of an electrospun PHA/RGO/Au scaffold for peripheral nerve regeneration: An in vitro study. Appl. Nanosci. 2020, 10, 687–694. [Google Scholar] [CrossRef]
- Pouton, C.W. Polymeric materials for advanced drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 1–3. [Google Scholar] [PubMed]
- Basnett, P.; Ching, K.Y.; Stolz, M.; Knowles, J.C.; Boccaccini, A.R.; Smith, C.; Locke, I.C.; Roy, I. Aspirin-loaded P (3HO)/P (3HB) blend films: Potential materials for biodegradable drug-eluting stents. Bioinspired Biomim. Nanobiomater. 2013, 2, 141–153. [Google Scholar] [CrossRef]
- Kassab, A.C.; Xu, K.; Denkbas, E.; Dou, Y.; Zhao, S.; Piskin, E. Rifampicin carrying polyhydroxybutyrate microspheres as a potential chemoembolization agent. J. Biomater. Sci. Polym. Ed. 1997, 8, 947–961. [Google Scholar] [CrossRef]
- Li, H.; Chang, J. Preparation, characterization and in vitro release of gentamicin from PHBV/wollastonite composite microspheres. J. Control. Release 2005, 107, 463–473. [Google Scholar] [CrossRef]
- Yagmurlu, M.F.; Korkusuz, F.; Gürsel, I.; Korkusuz, P.; Örs, Ü.; Hasirci, V. Sulbactam-cefoperazone polyhydroxybutyrate-co-hydroxyvalerate (PHBV) local antibiotic delivery system: In vivo effectiveness and biocompatibility in the treatment of implant-related experimental osteomyelitis. J. Biomed. Mater. Res. 1999, 46, 494–503. [Google Scholar] [CrossRef]
- Yao, Y.-C.; Zhan, X.-Y.; Zhang, J.; Zou, X.-H.; Wang, Z.-H.; Xiong, Y.-C.; Chen, J.; Chen, G.-Q. A specific drug targeting system based on polyhydroxyalkanoate granule binding protein PhaP fused with targeted cell ligands. Biomaterials 2008, 29, 4823–4830. [Google Scholar] [CrossRef]
- Fenton, O.S.; Olafson, K.N.; Pillai, P.S.; Mitchell, M.J.; Langer, R. Advances in biomaterials for drug delivery. Adv. Mater. 2018, 30, 1705328. [Google Scholar] [CrossRef]
- Wu, Y.L.; Wang, H.; Qiu, Y.K.; Liow, S.S.; Li, Z.; Loh, X.J. PHB-Based Gels as Delivery Agents of Chemotherapeutics for the Effective Shrinkage of Tumors. Adv. Healthc. Mater. 2016, 5, 2679–2685. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, Z.; Chi, W.; Yang, X.; Wang, W.; Zhang, B. Mono-methoxy-poly(3-hydroxybutyrate-co-4-hydroxybutyrate)-graft-hyper-branched polyethylenimine copolymers for siRNA delivery. Biomaterials 2012, 33, 2334–2344. [Google Scholar] [CrossRef] [PubMed]
- Skibiński, S.; Cichoń, E.; Haraźna, K.; Marcello, E.; Roy, I.; Witko, M.; Ślósarczyk, A.; Czechowska, J.; Guzik, M.; Zima, A. Functionalized tricalcium phosphate and poly(3-hydroxyoctanoate) derived composite scaffolds as platforms for the controlled release of diclofenac. Ceram. Int. 2021, 47, 3876–3883. [Google Scholar] [CrossRef]
- Cao, K.; Liu, Y.; Olkhov, A.A.; Siracusa, V.; Iordanskii, A.L. PLLA-PHB fiber membranes obtained by solvent-free electrospinning for short-time drug delivery. Drug Deliv. Transl. Res. 2018, 8, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S. Biodegradable Polymers: Present Opportunities and Challenges in Providing a Microplastic-Free Environment. Macromol. Chem. Phys. 2020, 221, 2000017. [Google Scholar] [CrossRef] [Green Version]
- Qu, X.H.; Wu, Q.; Zhang, K.Y.; Chen, G.Q. In vivo studies of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) based polymers: Biodegradation and tissue reactions. Biomaterials 2006, 27, 3540–3548. [Google Scholar] [CrossRef] [PubMed]
- Brigham, C.J.; Sinskey, A.J. Applications of Polyhydroxyalkanoates in the Medical Industry. Int. J. Biotechnol. Wellness Ind. 2012, 1, 53–60. [Google Scholar] [CrossRef]
- Grande, D.; Ramier, J.; Versace, D.L.; Renard, E.; Langlois, V. Design of functionalized biodegradable PHA-based electrospun scaffolds meant for tissue engineering applications. New Biotechnol. 2017, 37, 129–137. [Google Scholar] [CrossRef]
- Bonartsev, A.P.; Bonartseva, G.A.; Reshetov, I.V.; Kirpichnikov, M.P.; Shaitan, K.V. Application of polyhydroxyalkanoates in medicine and the biological activity of natural poly(3-hydroxybutyrate). Acta Nat. 2019, 11, 4–16. [Google Scholar] [CrossRef]
- Saad, B.; Ciardelli, G.; Matter, S.; Welti, M.; Uhlschmid, G.K.; Neuenschwander, P.; Suter, U.W. Characterization of the cell response of cultured macrophages and fibroblasts to particles of short-chain poly[(R)-3-hydroxybutyric acid]. J. Biomed. Mater. Res. 1996, 30, 429–439. [Google Scholar] [CrossRef]
- Wu, A.C.K.; Grøndahl, L.; Jack, K.S.; Foo, M.X.; Trau, M.; Hume, D.A.; Cassady, A.I. Reduction of the in vitro pro-inflammatory response by macrophages to poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Biomaterials 2006, 27, 4715–4725. [Google Scholar] [CrossRef]
- Bat, E.; van Kooten, T.G.; Feijen, J.; Grijpma, D.W. Macrophage-mediated erosion of gamma irradiated poly(trimethylene carbonate) films. Biomaterials 2009, 30, 3652–3661. [Google Scholar] [CrossRef] [PubMed]
- Shishatskaya, E.I.; Volova, T.G.; Gordeev, S.A.; Puzyr, A.P. Degradation of P(3HB) and P(3HB-co-3HV) in biological media. J. Biomater. Sci. Polym. Ed. 2005, 16, 643–657. [Google Scholar] [CrossRef] [PubMed]
- Gogolewski, S.; Jovanovic, M.; Perren, S.M.; Dillon, J.G.; Hughes, M.K. Tissue response and in vivo degradation of selected polyhydroxyacids: Polylactides (PLA), poly(3-hydroxybutyrate) (PHB), and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHB/VA). J. Biomed. Mater. Res. 1993, 27, 1135–1148. [Google Scholar] [CrossRef] [PubMed]
- Freier, T.; Kunze, C.; Nischan, C.; Kramer, S.; Sternberg, K.; Saß, M.; Hopt, U.T.; Schmitz, K.P. In vitro and in vivo degradation studies for development of a biodegradable patch based on poly(3-hydroxybutyrate). Biomaterials 2002, 23, 2649–2657. [Google Scholar] [CrossRef]
- Zhuikov, V.A.; Akoulina, E.A.; Chesnokova, D.V.; Wenhao, Y.; Makhina, T.K.; Demyanova, I.V.; Zhuikova, Y.V.; Voinova, V.V.; Belishev, N.V.; Surmenev, R.A.; et al. The growth of 3t3 fibroblasts on phb, pla and phb/pla blend films at different stages of their biodegradation in vitro. Polymers 2021, 13, 108. [Google Scholar] [CrossRef]
- Volova, T.G.; Shishatskaya, E.I.; Nikolaeva, E.D.; Sinskey, A.J. In vivo study of 2D PHA matrices of different chemical compositions: Tissue reactions and biodegradations. Mater. Sci. Technol. 2014, 30, 549–557. [Google Scholar] [CrossRef]
- Ong, S.Y.; Chee, J.Y.; Sudesh, K. Degradation of Polyhydroxyalkanoate (PHA): A Review. J. Sib. Fed. Univ. Biol. 2017, 10, 21–225. [Google Scholar] [CrossRef]
- Zhao, K.; Deng, Y.; Chen, J.C.; Chen, G.Q. Polyhydroxyalkanoate (PHA) scaffolds with good mechanical properties and biocompatibility. Biomaterials 2003, 24, 1041–1045. [Google Scholar] [CrossRef]
- Shishatskaya, E.I.; Voinova, O.N.; Goreva, A.V.; Mogilnaya, O.A.; Volova, T.G. Biocompatibility of polyhydroxybutyrate microspheres: In vitro and in vivo evaluation. J. Mater. Sci. Mater. Med. 2008, 19, 2493–2502. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Wu, L.P.; Liu, X.B.; Hou, J.; Zhao, L.L.; Chen, J.Y.; Zhao, D.H.; Xiang, H. Biodegradation and biocompatibility of haloarchaea-produced poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymers. Biomaterials 2017, 139, 172–186. [Google Scholar] [CrossRef]
- Wang, Y.W.; Wu, Q.; Chen, J.; Chen, G.Q. Evaluation of three-dimensional scaffolds made of blends of hydroxyapatite and poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) for bone reconstruction. Biomaterials 2005, 26, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Matthews, B.; Deeken, C.R. Characterization of the Mechanical Strength, Resorption Properties, and Histologic Characteristics of a Fully Absorbable Material (Poly-4-hydroxybutyrate-PHASIX Mesh) in a Porcine Model of Hernia Repair. Int. Sch. Res. Not. 2013, 2013, 238067. [Google Scholar]
- Deng, Y.; Zhao, K.; Zhang, X.F.; Hu, P.; Chen, G.Q. Study on the three-dimensional proliferation of rabbit articular cartilage-derived chondrocytes on polyhydroxyalkanoate scaffolds. Biomaterials 2002, 23, 4049–4056. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zou, B.; Shi, Z.; Wu, Q.; Chen, G.Q. The effect of 3-hydroxybutyrate on the in vitro differentiation of murine osteoblast MC3T3-E1 and in vivo bone formation in ovariectomized rats. Biomaterials 2007, 28, 3063–3073. [Google Scholar] [CrossRef]
- Robinson, A.M.; Williamson, D.H. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol. Rev. 1980, 60, 143–187. [Google Scholar] [CrossRef]
- Cheng, S.; Wu, Q.; Yang, F.; Xu, M.; Leski, M.; Chen, G.Q. Influence of DL-β-hydroxybutyric acid on cell proliferation and calcium influx. Biomacromolecules 2005, 6, 593–597. [Google Scholar] [CrossRef]
- Kehail, A.A.; Boominathan, V.; Fodor, K.; Chalivendra, V.; Ferreira, T.; Brigham, C.J. In Vivo and In Vitro Degradation Studies for Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) Biopolymer. J. Polym. Environ. 2017, 25, 296–307. [Google Scholar] [CrossRef]
- Meischel, M.; Eichler, J.; Martinelli, E.; Karr, U.; Weigel, J.; Schmöller, G.; Tschegg, E.K.; Fischerauer, S.; Weinberg, A.M.; Stanzl-Tschegg, S.E. Adhesive strength of bone-implant interfaces and in-vivo degradation of PHB composites for load-bearing applications. J. Mech. Behav. Biomed. Mater. 2016, 53, 104–118. [Google Scholar] [CrossRef]
- Tarazona, N.A.; Machatschek, R.; Lendlein, A. Influence of Depolymerases and Lipases on the Degradation of Polyhydroxyalkanoates Determined in Langmuir Degradation Studies. Adv. Mater. Interfaces 2020, 7, 2000872. [Google Scholar] [CrossRef]
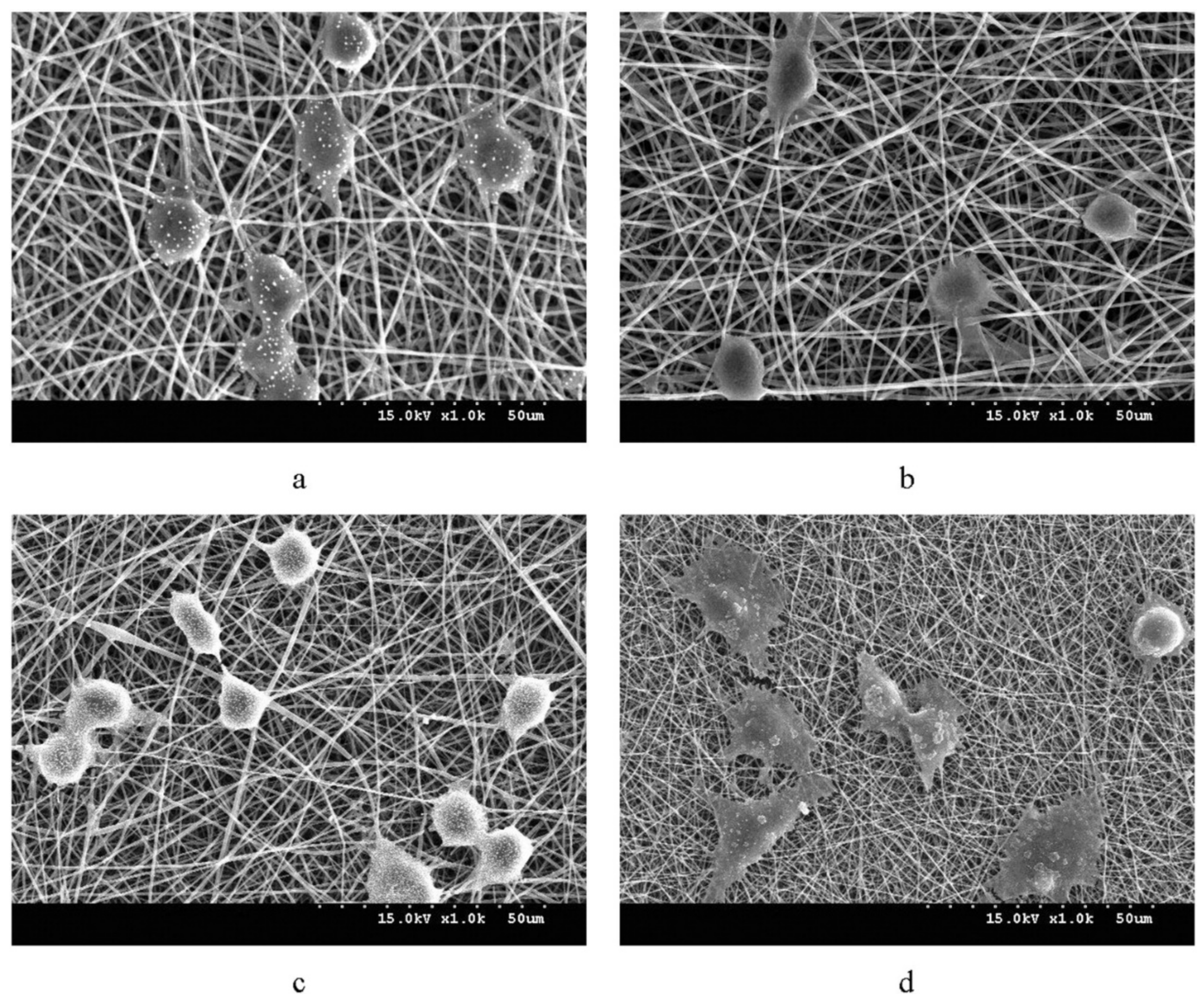
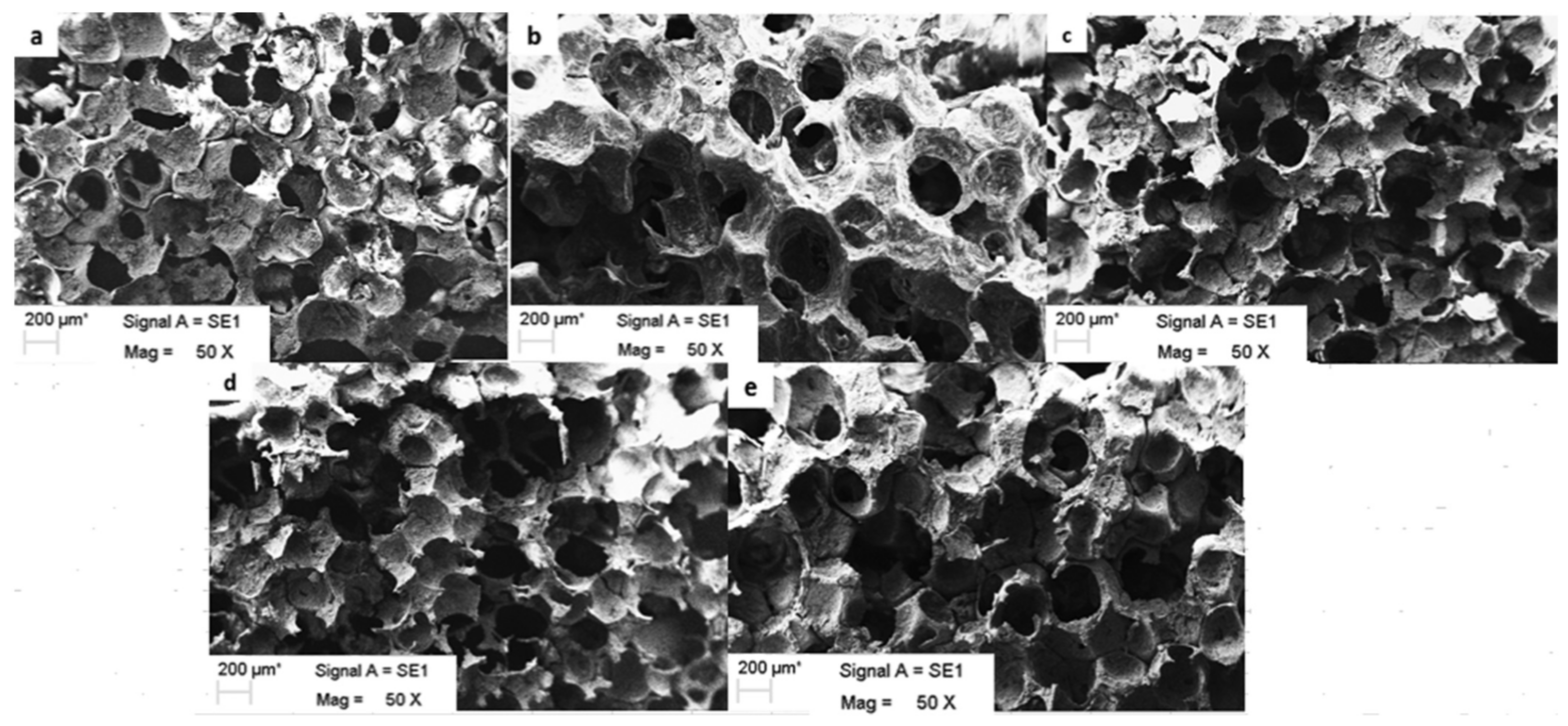
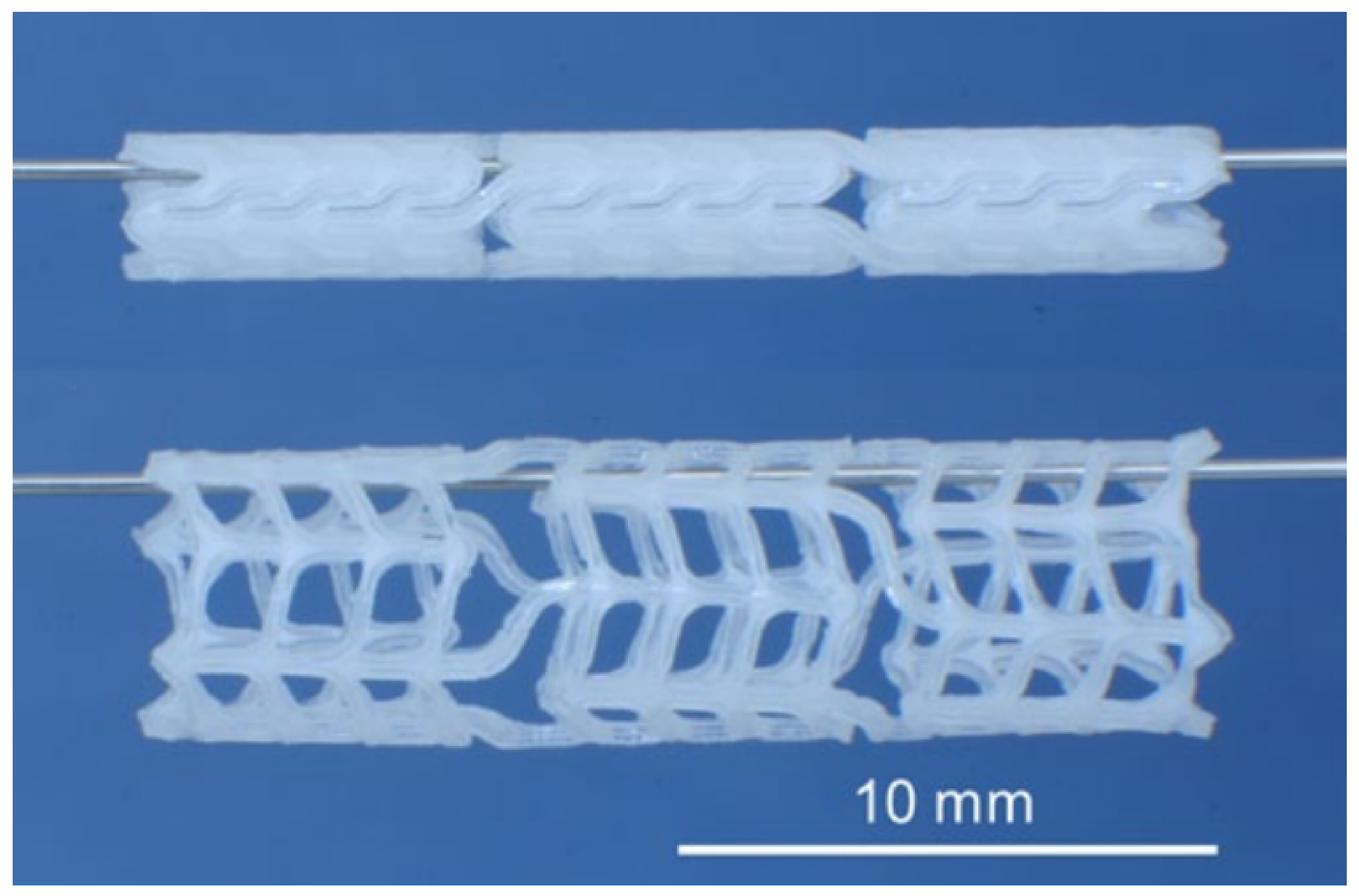
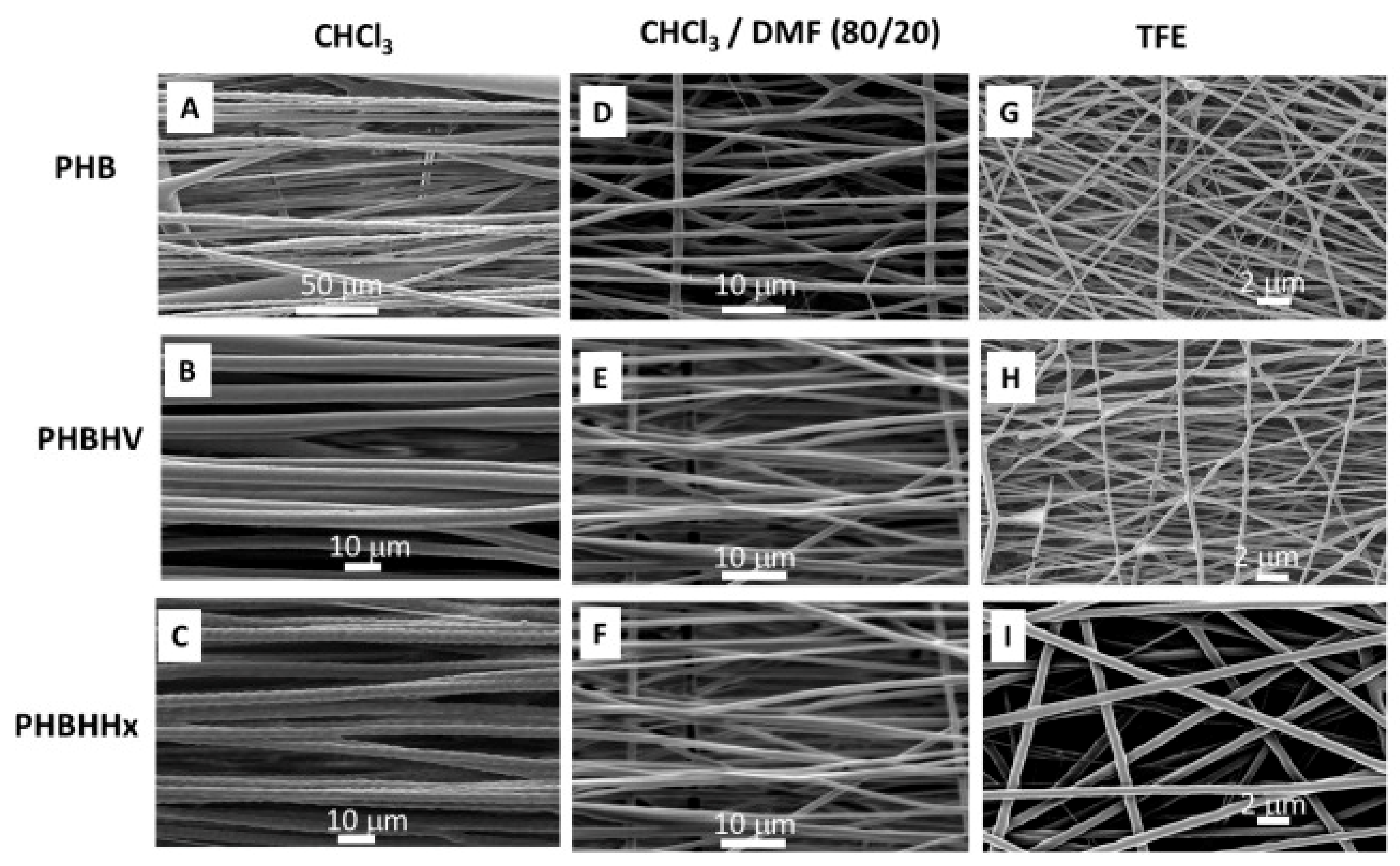
| Type of PHA * | Therapeutic Agent | Combination with | Formulation Description | Technique | Key Finding | Ref |
|---|---|---|---|---|---|---|
| P34HB | - | - | Fibre scaffold | Electrospinning | The scaffold was interwoven with fibres and had good physical and chemical properties as well as induced cell adhesion and proliferation without cytotoxicity. | Fu, et al. [38] |
| P34HB | - | Poly(ethylene glycol) | Nanofiber membrane | Electrospinning | The nanofiber membrane supported cell adhesion, spreading, and proliferation and promoted osteoinduction capacity in vitro. | Wang, et al. [39] |
| P34HB | Actovegin or fibroblast cells | Bacterial cellulose | Film | Solvent evaporation | Bacterial cellulose and P3HB/4HB in combination with actovegin and fibroblast are more effective than a commercial wound dressing. | Volova, et al. [40] |
| PHB | - | Gelatin | Microfibers and nanofibers | Electrospinning | The combination fiber scaffolds were biocompatible and promoted fibroblast attachment and skin regeneration which makes it suitable for wound healing. | Sanhueza, et al. [41] |
| PHB | - | Carbon nanotubes | Nanotubes scaffolds | Electrospinning | The PHB and nanotubes composite caused mild foreign body type giant cell reaction, moderate vascularization, and mild inflammation. | Zarei, et al. [42] |
| PHB | - | Chitosan and nano-bioglass | Nanofiber scaffold | Electrospinning | The nanofiber scaffold showed significantly greater expression of dentin sialophosphoprotein, collagen type-I, and ALP making it suitable for dentin tissue engineering. | Khoroushi, et al. [43] |
| PHB | - | Polylactic acid | - | 3D printing | The blending of polylactic acid and PHB can produce a stable tubular substitute for urethra replacement. | Findrik Balogová, et al. [44] |
| PHB | Bone marrow-derived mesenchymal stem cells | Chitosan | Conduit | Electrospinning | The conduit caused damage to the axons. The incorporation of chitosan with PHB resulted in a stronger and biodegradable nerve conduit. | Ozer, et al. [45] |
| PHB | Primary Schwann cells (SCs) or SC-like differentiated adipose-derived stem cells | - | Strips | - | PHB strip seeded with cells provides less muscle atrophy and greater axon myelination, which is beneficial for nerve regeneration. | Schaakxs, et al. [46] |
| PHB | - | Chitosan | Implant | Co-precipitation | The implant supported osteochondral regeneration and could improve cartilage tissue regeneration. | Petrovova, et al. [47] |
| PHB | Hydroxyapatite and mesenchymal stem cells | Alginate hydrogel | Bioactive biopolymer/mineral/hydrogel scaffold | Salt leaching technique and 3D- printing | The scaffold induced the osteogenic differentiation of mesenchymal stem cells. | Volkov, et al. [48] |
| PHB | - | Bacterial cellulose | Bone grafts | Salt leaching | The scaffolds supported 3T3-L1 preadipocyte viability and proliferation without toxicity and showed promising biocompatibility. | Codreanu, et al. [49] |
| PHB and PHBV | - | Anionic sulfated polysaccharide κ-carrageenan (κ-CG) | Fiber | Electrospinning | κ-CG loaded PHBV fibers showed good bioactive and osteogenic properties. | Goonoo, et al. [50] |
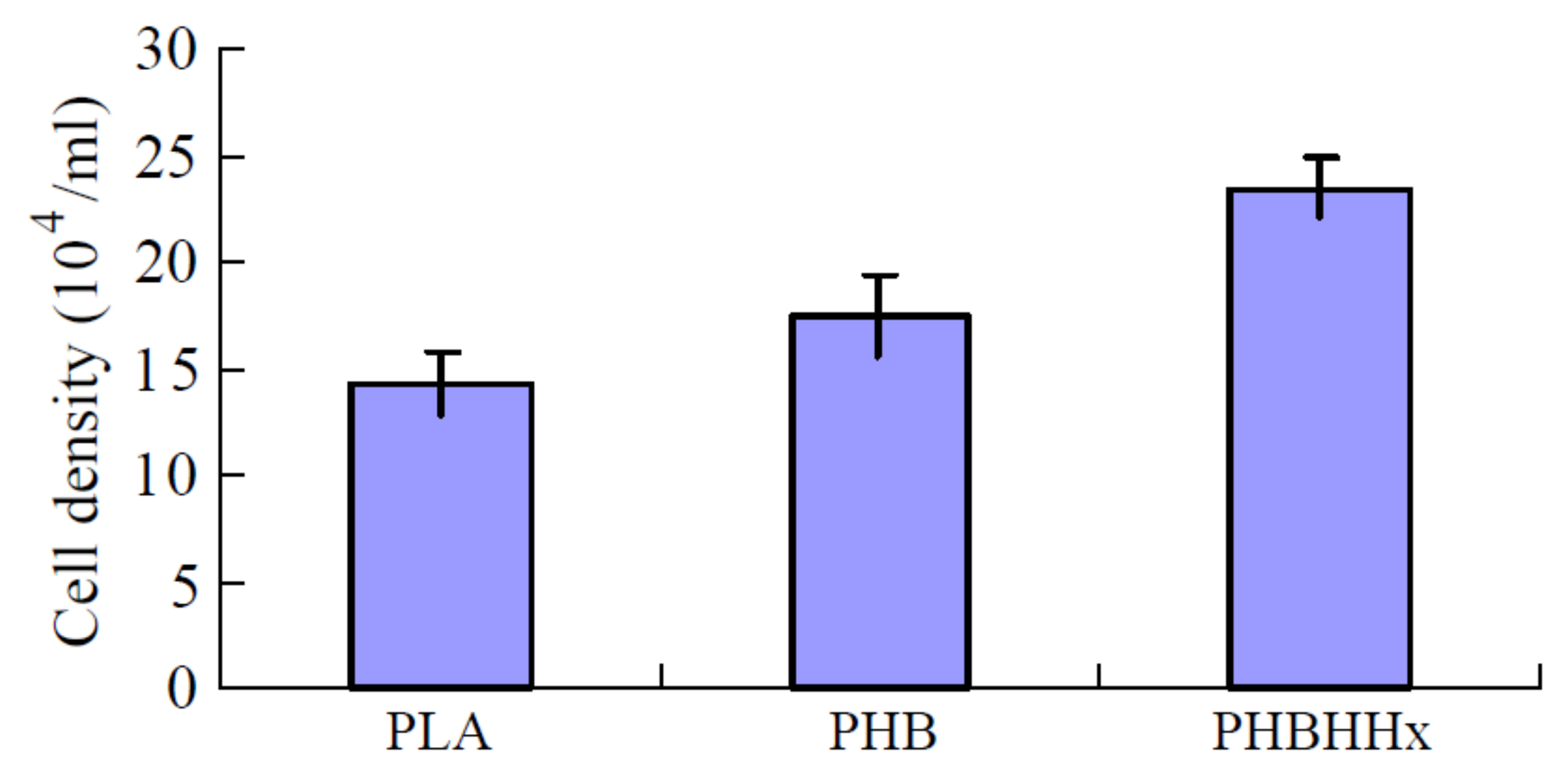
| PHBHHx | Neural stem cells | - | Film | Solution casting | PHBHHx did not trigger reactive gliosis as well as survival and growth of the transplanted stem cells in a rat traumatic brain injury model | Wang, et al. [51] |
| PHBHHx | Recombinant BMP-2 proteins | - | Porous structured scaffold | Solvent casting-particulate leaching | The porous biocompatible scaffolds successfully formed a network of blood vessels and promoted bone regeneration in rabbit radius. | Liu, et al. [52] |
| PHBV | Tachyplesin I (Tac) peptide | - | Film | Solution casting | The surface functionalization of PHBHV with Tac improved antibacterial and fibroblast proliferation. | Xue, et al. [53] |
| PHBV | Cerium oxide nanoparticles | - | Membrane | Electrospinning | The cerium oxide nanoparticles loaded PHBV membranes enhanced cell proliferation, vascularization and promoted the healing of diabetic wounds. | Augustine, et al. [54] |
| PHBV | Insulin-producing cells | - | Nanofibers | Electrospinning | PHBV was found to increase the survival rate of insulin-producing cells. Insulin-producing cells in combination with PHBV is a promising cell-copolymer construct that could be used for pancreatic tissue engineering applications. | Abazari, et al. [55] |
| PHBV | Vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) and stromal cell-derived factor 1α | Poly(caprolactone) | tissue-engineered vascular graft | Electrospinning | PHBV based graft showed high biocompatibility and calcification resistance as well as a moderate haemocompatibility but was prone to aneurysmatic dilation. | Antonova, et al. [56] |
| PHBV | Arg-Gly-Asp peptide | Poly(caprolactone) | Patches | Electrospinning | The PHBV based patches showed neointima formation and continuous endothelial lining on their surface. | Sevostianova, et al. [57] |
| PHBV | Vascular endothelial growth factor and platelet factor concentrate | Poly (vinyl alcohol) and elastin nanofiber | Fibrous scaffold | Electrospinning | The tri-layered scaffold was compatible to blood and promising for small diameter vascular grafting. | Deepthi, et al. [58] |
| PHBV | - | Polyethylene oxide | Nanofiber film | Electrospinning | When tested in a nerve rat model, the PHBV incorporated with polyethylene oxide promoted peripheral nerve regeneration. | Zhang, et al. [59] |
| PHBV | Quercetin | - | Fibrous scaffolds | Electrospinning | The scaffolds facilitated growth of chondrocytes and maintained chondrocyte phenotype and inhibited apoptosis and reduced oxidative stress of chondrocytes. | Chen, et al. [60] |
| PHBV | - | Aloe vera gel | Nanofibrous scaffold | electrospinning | The aloe vera gel-blended PHBV scaffold showed promising osteoinductive potential with complete degradation without harmful products. | Tahmasebi, et al. [61] |
| PHBV | Adenosine | - | Composite nanofiber | Electrospinning | The composite nanofiber showed good tissue biocompatibility and promoted bone regeneration capacity in vitro and in vivo. | Zhong, et al. [62] |
| PHBV | Epidermal growth factor | Gelatin-methacryloyl | Hydrogel patches | Electrospinning | The drug-loaded patches provided promising cellular response, angiogenesis and wound healing. | Augustine, et al. [63] |
| PHBV | Silver nanoparticles | High molecular weight keratin | Nanofibrous mat | Electrospinning | The nanofibrous mat demonstrated favourable mechanical and antibacterial properties with good biocompatibility, makes it suitable for wound healing. | Ye, et al. [64] |
| PHBV | - | - | Nanofibrous meshes or film | Electrospinning or solution casting | The electrospun nanofibrous meshes were better in mitigating excessive scar formation by regulating myofibroblast formation through downregulation of α-SMA and TGF-β1, and upregulation of TGF-β3 as compared to the solution-cast films. | Kim, et al. [65] |
| PHO | - | - | Patch | Electrospinning | The PHO patches were as good as collagen in cell viability, proliferation, and adhesion with enhanced cell adhesion and proliferation. | Bagdadi, et al. [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pulingam, T.; Appaturi, J.N.; Parumasivam, T.; Ahmad, A.; Sudesh, K. Biomedical Applications of Polyhydroxyalkanoate in Tissue Engineering. Polymers 2022, 14, 2141. https://doi.org/10.3390/polym14112141
Pulingam T, Appaturi JN, Parumasivam T, Ahmad A, Sudesh K. Biomedical Applications of Polyhydroxyalkanoate in Tissue Engineering. Polymers. 2022; 14(11):2141. https://doi.org/10.3390/polym14112141
Chicago/Turabian StylePulingam, Thiruchelvi, Jimmy Nelson Appaturi, Thaigarajan Parumasivam, Azura Ahmad, and Kumar Sudesh. 2022. "Biomedical Applications of Polyhydroxyalkanoate in Tissue Engineering" Polymers 14, no. 11: 2141. https://doi.org/10.3390/polym14112141
APA StylePulingam, T., Appaturi, J. N., Parumasivam, T., Ahmad, A., & Sudesh, K. (2022). Biomedical Applications of Polyhydroxyalkanoate in Tissue Engineering. Polymers, 14(11), 2141. https://doi.org/10.3390/polym14112141