Physical, Mechanical, and Water Vapor Barrier Properties of Starch/Cellulose Nanofiber/Thymol Bionanocomposite Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
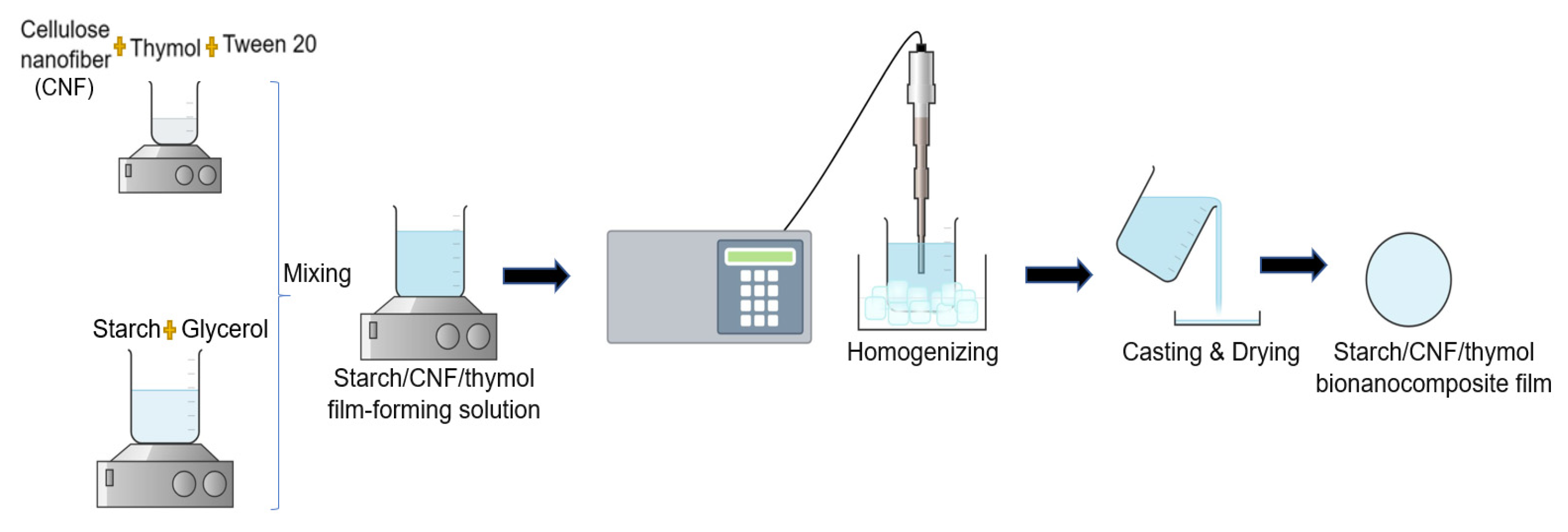
2.2. Preparation of the Films
2.3. Characterization of the Films
2.3.1. Physical Properties
2.3.2. Mechanical Properties
2.3.3. Water Vapor Barrier
2.4. Antibacterial Activity of the Films
2.5. Statistical Analysis
3. Results and Discussion
3.1. Physical Properties
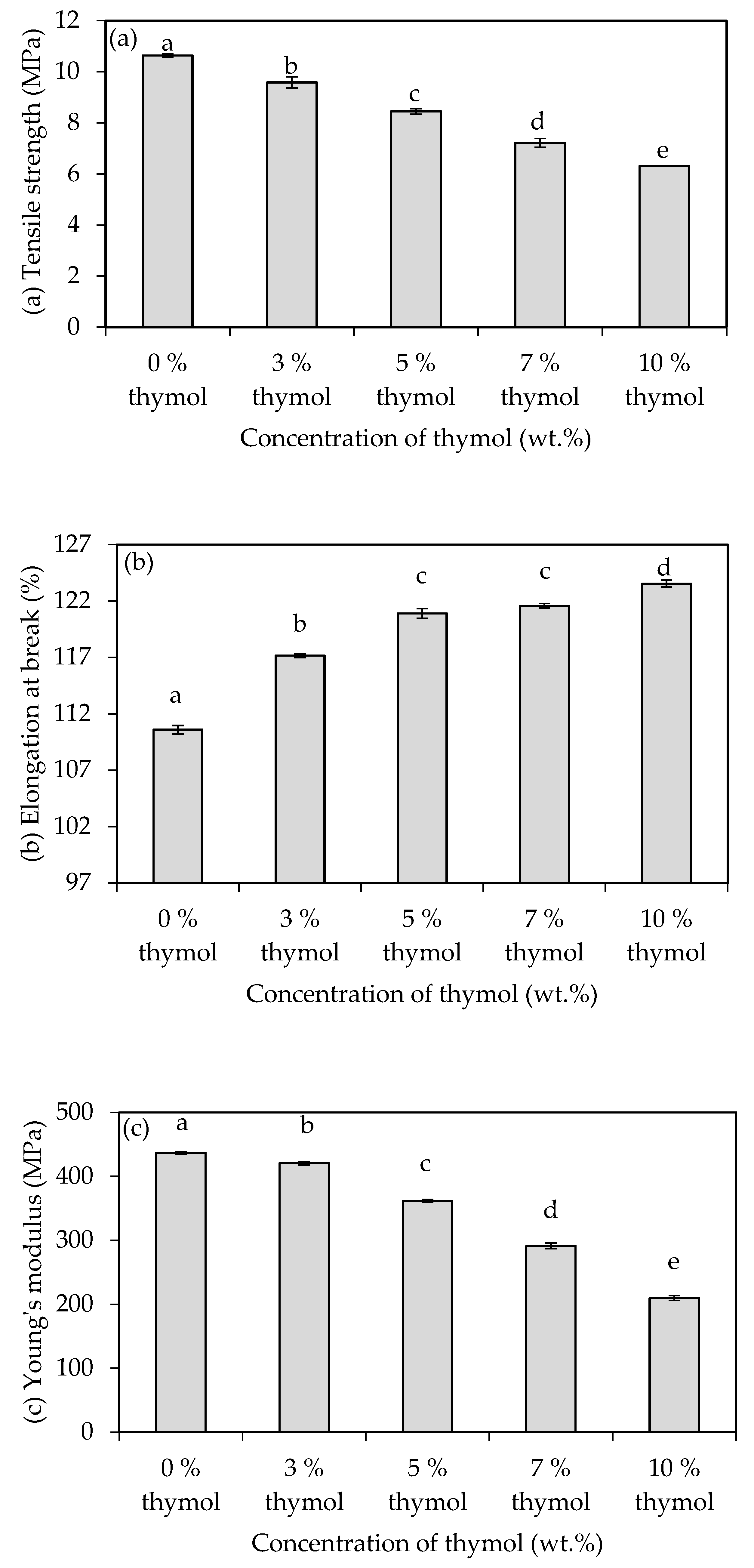
3.2. Mechanical Properties
3.3. Water Vapor Barrier
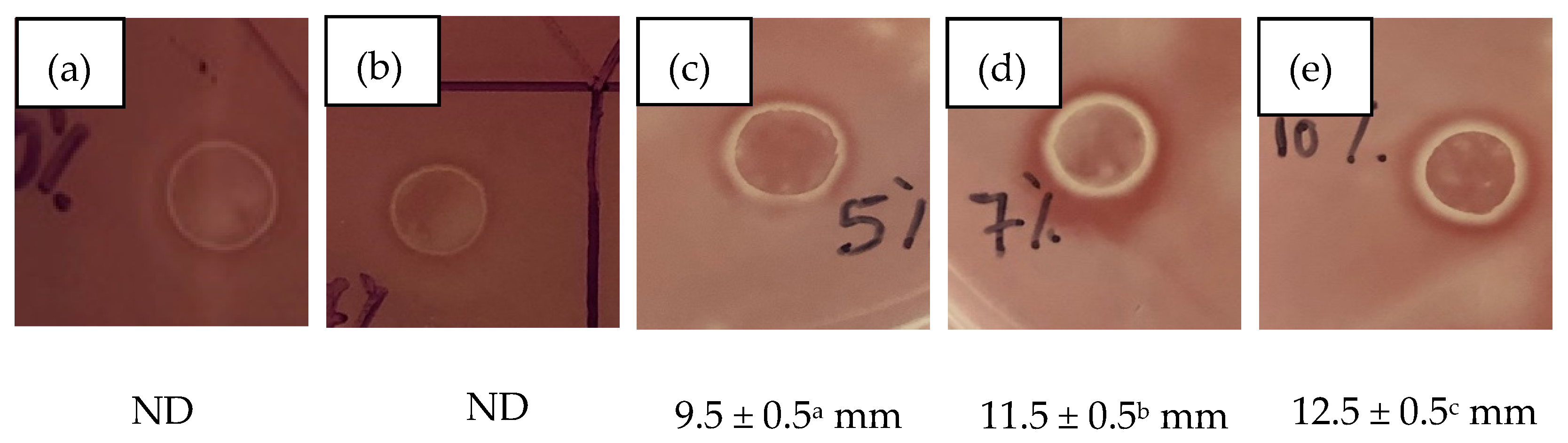
3.4. Antibacterial Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rhim, J.W.; Park, H.M.; Ha, C.S. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Jiang, T.; Duan, Q.; Zhu, J.; Liu, H.; Yu, L. Starch-based biodegradable materials: Challenges and opportunities. Adv. Ind. Eng. Polym. Res. 2020, 3, 8–18. [Google Scholar] [CrossRef]
- Davoodi, M.; Kavoosia, G.; Shakeri, R. Preparation and characterization of potato starch-thymol dispersion and film as potential antioxidant and antibacterial materials. Int. J. Biol. Macromol. 2017, 104, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Nordin, N.; Othman, S.H.; Rashid, S.; Basha, R.K. Effects of glycerol and thymol on physical, mechanical, and thermal properties of corn starch films. Food Hydrocoll. 2020, 106, 105884. [Google Scholar] [CrossRef]
- Singh, N.; Singh, J.; Kaur, L.; Sodhi, N.S.; Gill, B.S. Morphological, thermal and rheological properties of starches from different botanical sources. Food Chem. 2003, 81, 219–231. [Google Scholar] [CrossRef]
- Shapi’i, R.A.; Othman, S.H.; Nordin, N.; Basha, R.K.; Naim, M.N. Antimicrobial properties of starch films incorporated with chitosan nanoparticles: In vitro and in vivo evaluation. Carbohydr. Polym. 2020, 230, 115602. [Google Scholar] [CrossRef]
- Othman, S.H.; Nordin, N.; Azman, N.A.A.; Tawakkal, I.S.M.A.; Basha, R.K. Effects of nanocellulose fiber and thymol on mechanical, thermal, and barrier properties of corn starch films. Int. J. Biol. Macromol. 2021, 183, 1352. [Google Scholar] [CrossRef]
- Chen, J.H.; Ren, Y.; Seow, J.; Liu, T.; Bang, W.S.; Yuk, H.G. Intervention technologies for ensuring microbiological safety of meat: Current and future trends. Compr. Rev. Food Sci. Food Saf. 2012, 11, 119–132. [Google Scholar] [CrossRef]
- Issa, A.; Ibrahim, S.A.; Tahergorabi, R. Impact of sweet potato starch-based nanocomposite films activated with thyme essential oil on the shelf-life of baby spinach leaves. Foods 2017, 6, 43. [Google Scholar] [CrossRef] [Green Version]
- Chagnot, C.; Venien, A.; Renier, S.; Caccia, N.; Talon, R.; Astruc, T.; Desvaux, M. Colonisation of meat by Escherichia coli O157: H7: Investigating bacterial tropism with respect to the different types of skeletal muscles, subtypes of myofibres, and postmortem time. Front. Microbiol. 2017, 8, 1366. [Google Scholar] [CrossRef] [Green Version]
- Yamaji, R.; Friedman, C.R.; Rubin, J.; Suh, J.; Thys, E.; McDermott, P.; Hung-Fan, M.; Riley, L.W. A population-based surveillance study of shared genotypes of Escherichia coli isolates from retail meat and suspected cases of urinary tract infections. Msphere 2018, 3, e00179-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wattanasatcha, A.; Rengpipat, S.; Wanichwecharungruang, S. Thymol nanospheres as an effective anti-bacterial agent. Int. J. Pharm. 2012, 434, 360–365. [Google Scholar] [CrossRef]
- ASTM D882. In Standard Test Method Tensile Properties of Thin Plastic Sheeting; ASTM International: Pennsylvania, PA, USA, 2018; Available online: www.astm.org (accessed on 23 August 2021).
- ASTM E96. In Standard Test Method for Water Vapor Transmission of Materials; ASTM International: Pennsylvania, PA, USA, 2016; Available online: www.astm.org (accessed on 23 August 2021).
- Guo, M.; Jin, T.Z.; Yadav, M.P.; Yang, R. Antimicrobial property and microstructure of micro-emulsion edible composite films against Listeria. Int. J. Food Microbiol. 2015, 208, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Tawakkal, I.S.M.A.; Cran, M.J.; Bigger, S.W. Effect of poly(lactic acid)/kenaf composites incorporated with thymol on the antimicrobial activity of processed meat. J. Food Process. Preserv. 2017, 41, 1745–4549. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Deng, H.; Guo, Y.; Xue, J. Gelatin films incorporated with thymol nanoemulsions: Physical properties and antimicrobial activities. Int. J. Biol. Macromol. 2020, 150, 161–168. [Google Scholar] [CrossRef]
- Lozano-Navarro, J.I.; Dìaz-Zavala, N.P.; Velasco-Santos, C.; Martìnez-Hermández, A.L.; Tijerina-Ramos, B.I.; Garcìa-Hermández, M.; Rivera-Armenta, J.L.; Páramo-García, U.; Reyes-de la Torre, A.I. Antimicrobial, optical and mechanical properties of chitosan-starch films with natural extracts. Int. J. Mol. Sci. 2017, 18, 997. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, M.H.; Razavi, S.H.; Mousavi, M.A. Antimicrobial, physical and mechanical properties of chitosan-based films incorporated with thyme, clove and cinnamon essential oils. J. Food Process. Preserv. 2009, 33, 727–743. [Google Scholar] [CrossRef]
- Wang, C.; Chang, T.; Dong, S.; Zhang, D.; Ma, C.; Chen, S.; Li, H. Biopolymer films based on chitosan/potato protein/linseed oil/ZnO NPs to maintain the storage quality of raw meat. Food Chem. 2020, 332, 127–375. [Google Scholar] [CrossRef]
- Garavand, F.; Rouhi, M.; Razavi, S.H.; Cacciotti, I.; Mohammadi, R. Improving the integrity of natural biopolymer films used in food packaging by crosslinking approach: A review. Int. J. Biol. Macromol. 2017, 104, 687–707. [Google Scholar] [CrossRef]
- Zhong, T.; Liang, Y.; Jiang, S.; Yang, L.; Shi, Y.; Guo, S.; Zhang, C. Physical, antioxidant and antimicrobial properties of modifies peanut protein isolate based films incorporated with thymol. R. Soc. Chem. 2017, 7, 41610–41618. [Google Scholar]
- Chin, A.W. Polymers for Innovative Food Packaging. 2010. Available online: https://web.wpi.edu/Pubs/E-project/Available/E-project-050610-124822/unrestricted/Polymers_for_Innovative_Food_Packaging.pdf (accessed on 23 August 2021).
- Kavoosi, G.; Dadfar, S.M.M.; Purfard, A.M. Mechanical, physical, antioxidant, and antimicrobial properties of gelatin films incorporated with thymol for potential use as nano wound dressing. J. Food Sci. 2013, 78, E244–E250. [Google Scholar] [CrossRef]
- Petchwattana, N.; Naknaen, P. Utilization of thymol as an antimicrobial agent for biodegradable poly (butylene succinate). Mater. Chem. Phys. 2015, 163, 369–375. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Benjakul, S.; Prodpran, T.; Sumpavapol, P.; Songtipya, P. Properties and antimicrobial activity of fish protein isolate/fish skin gelatin film containing basil leaf essential oil and zinc oxide nanoparticles. Food Hydrocoll. 2014, 41, 265–273. [Google Scholar] [CrossRef]
- Ansorena, M.R.; Zubeldía, F.; Marcovich, N.E. Active wheat gluten films obtained by thermoplastic processing. LWT Food Sci. Technol. 2016, 69, 47–54. [Google Scholar] [CrossRef]
- Wu, H.; Lu, J.; Xiao, D.; Yan, Z.; Li, S.; Li, T.; Wan, X.; Zhang, Z.; Liu, Y.; Shen, G.; et al. Development and characterization of antimicrobial protein films based on soybean protein isolate incorporating diatomite/thymol complex. Food Hydrocoll. 2021, 110, 106–138. [Google Scholar] [CrossRef]
- Ramos, M.; Jiménez, A.; Peltzer, M.; Garrigós, M.C. Characterization and antimicrobial activity studies of polypropylene films with carvacrol and thymol for active packaging. J. Food Eng. 2012, 109, 513–519. [Google Scholar] [CrossRef]
- Khairuddin, N.; Muhamad, I.I.; Wan Abd Rahman, W.A.; Siddique, B.M. Physiochemical and thermal characterization of hydroxyethyl cellulose-wheat starch-based films incorporated thymol intended for active packaging. Sains Malays. 2020, 49, 323–333. [Google Scholar] [CrossRef]
- Vahedikia, N.; Garavand, F.; Tajeddin, B.; Cacciotti, I.; Jafari, S.M.; Omidi, T.; Zahedi, Z. Biodegradable zein film composites reinforced with chitosan nanoparticles and cinnamon essential oil: Physical, mechanical, structural and antimicrobial attributes. Colloids Surf. B Biointerfaces 2019, 177, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Almasi, H.; Azizi, S.; Amjadi, S. Development and characterization of pectin films activated by nanoemulsion and Pickering emulsion stabilized marjoram (Origanum majorana L.) essential oil. Food Hydrocoll. 2020, 99, 105338. [Google Scholar] [CrossRef]
- Kalateh-Seifari, F.; Yousefi, S.; Ahari, H.; Hosseini, S.H. Corn starch-chitosan nanocomposite film containing nettle essential oil nanoemulsions and starch nanocrystals: Optimization and characterization. Polymers 2021, 13, 2113. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Lee, E.S.; Han, J. Enhancement of the water-resistance properties of an edible film prepared from mung bean starch via the incorporation of sunflower seed oil. Sci. Rep. 2020, 10, 1–5. [Google Scholar]
- Abdollahi, M.; Damirchi, S.; Shafafi, M.; Rezaei, M.; Ariaii, P. Carboxymethyl cellulose-agar biocomposite film activated with summer savory essential oil as an antimicrobial agent. Int. J. Biol. Macromol. 2019, 126, 561–568. [Google Scholar] [CrossRef] [PubMed]
- López-Mata, M.A.; Ruiz-Cruz, S.; Silva-Beltrán, N.P.; de Jésus Ornelas-Paz, J.; Zamudio-Flores, P.B.; Burruel-Ibarra, S.E. Physiochemical, antimicrobial and antioxidant properties of chitosan films incorporated with carvacrol. Molecules 2013, 18, 13735–13753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syafiq, R.; Sapuan, S.M.; Zuhri, M.R. Antimicrobial activity, physical, mechanical and barrier properties of sugar palm based nanocellulose/starch biocomposite films incorporated with cinnamon essential oil. J. Mat. Res. Tech. 2021, 11, 144–157. [Google Scholar] [CrossRef]
- Sun, H.; Li, S.; Chen, S.; Wang, C.; Liu, D.; Li, X. Antibacterial and antioxidant activities of sodium starch octenylsuccinate-based Pickering emulsion films incorporated with cinnamon essential oil. Int. J. Biol. Macromol. 2020, 159, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Edible films and coatings to prevent the detrimental effect of oxygen on food quality: Possibilities and limitations. J. Food Eng. 2012, 110, 208–213. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Kang, D. Susceptibility of Escherichia coli O157:H7 grown at low temperatures to the krypton-chlorine excilamp. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhou, L.; Zhang, C.; Show, P.L.; Du, A.; Fu, J.; Ashokkumar, V. Preparation and characterization of curdlan/polyvinyl alcohol/thyme essential oil blending film and its application to chilled meat preservation. Carbohydr. Polym. 2020, 247, 116670. [Google Scholar] [CrossRef] [PubMed]
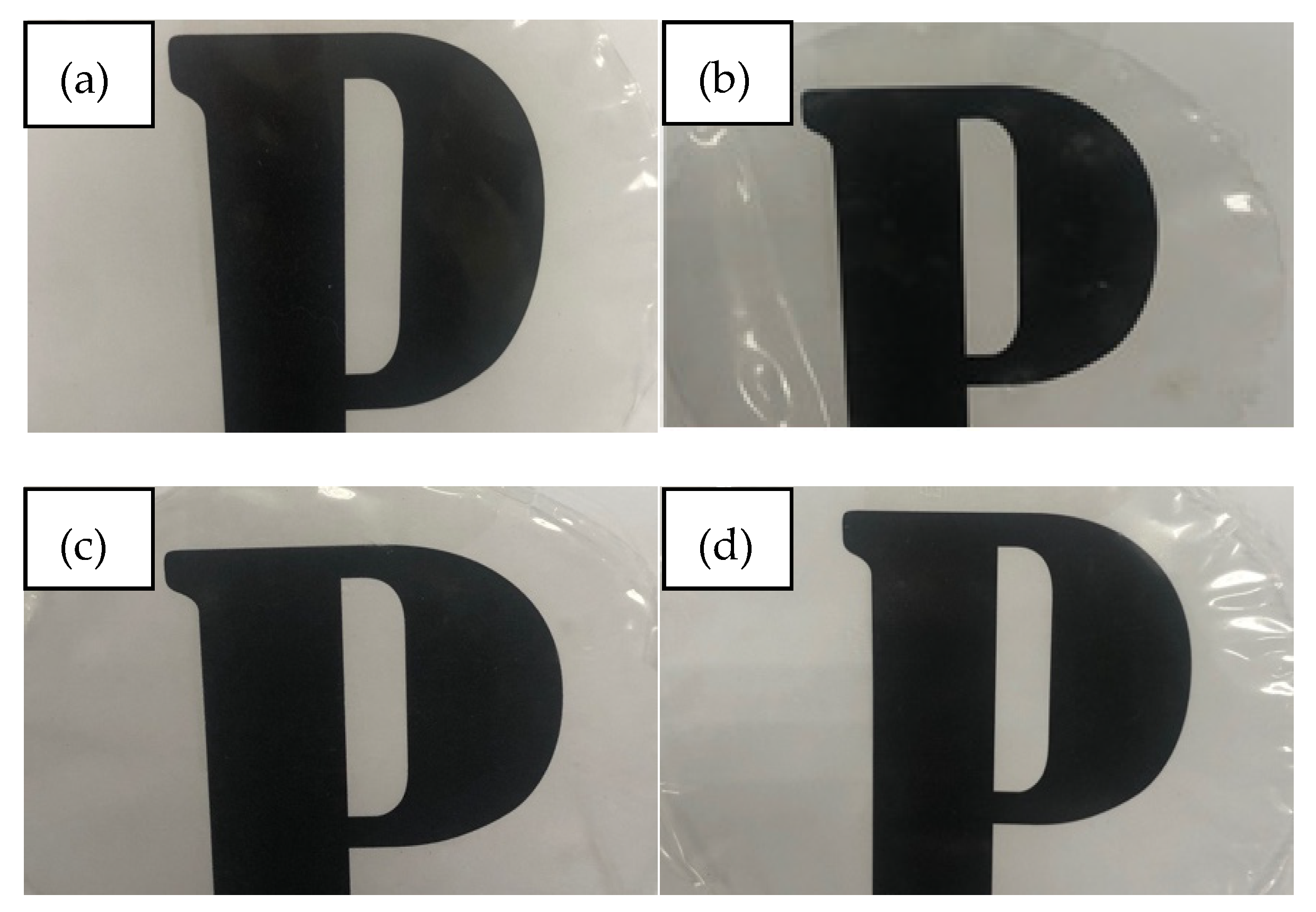


| Concentration of Thymol (wt.%) | Thickness (mm) | L* | a* | b* | ΔE | Opacity (A.mm−1) |
|---|---|---|---|---|---|---|
| 0 | 0.0859 ± 0.0004 a | 92.11 ± 0.26 a | −0.93 ± 0.01 a | 5.46 ± 0.19 a | - | 8.824 ± 0.63 a |
| 3 | 0.0882 ± 0.0010 b | 93.02 ± 0.34 bc | −0.89 ± 0.01 b | 4.25 ± 0.64 b | 1.514 ± 0.06 a | 8.891 ± 0.64 a |
| 5 | 0.0907 ± 0.0010 c | 93.53 ± 0.27 c | −0.88 ± 0.01 b | 4.15 ± 0.75 b | 1.933 ± 0.10 b | 9.209 ± 0.40 ab |
| 7 | 0.0925 ± 0.0006 d | 93.61 ± 0.51 c | −0.95 ± 0.02 ac | 4.07 ± 0.87 b | 2.045 ± 0.11 bc | 9.410 ± 0.39 ab |
| 10 | 0.0945 ± 0.0008 e | 93.68 ± 0.76 c | −0.95 ± 0.01 c | 4.12 ± 0.25 b | 2.064 ± 0.04 c | 9.678 ± 0.65 b |
| E. coli Counts (log CFU/cm2) | |||
|---|---|---|---|
| Films | Day 3 | Day 5 | Day 7 |
| Control (no film) | 9.52 Aa | 9.51 Aa | 8.34 Ab |
| Starch/CNF | 9.31 ABa | 9.29 ABa | 8.32 Ab |
| Starch/CNF/thymol-10 wt.% | 8.13 Ca | 6.12 Cb | 2.58 Bc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Othman, S.H.; Wane, B.M.; Nordin, N.; Noor Hasnan, N.Z.; A. Talib, R.; Karyadi, J.N.W. Physical, Mechanical, and Water Vapor Barrier Properties of Starch/Cellulose Nanofiber/Thymol Bionanocomposite Films. Polymers 2021, 13, 4060. https://doi.org/10.3390/polym13234060
Othman SH, Wane BM, Nordin N, Noor Hasnan NZ, A. Talib R, Karyadi JNW. Physical, Mechanical, and Water Vapor Barrier Properties of Starch/Cellulose Nanofiber/Thymol Bionanocomposite Films. Polymers. 2021; 13(23):4060. https://doi.org/10.3390/polym13234060
Chicago/Turabian StyleOthman, Siti Hajar, Bilguisse Mamadou Wane, Norhazirah Nordin, Noor Zafira Noor Hasnan, Rosnita A. Talib, and Joko Nugroho Wahyu Karyadi. 2021. "Physical, Mechanical, and Water Vapor Barrier Properties of Starch/Cellulose Nanofiber/Thymol Bionanocomposite Films" Polymers 13, no. 23: 4060. https://doi.org/10.3390/polym13234060
APA StyleOthman, S. H., Wane, B. M., Nordin, N., Noor Hasnan, N. Z., A. Talib, R., & Karyadi, J. N. W. (2021). Physical, Mechanical, and Water Vapor Barrier Properties of Starch/Cellulose Nanofiber/Thymol Bionanocomposite Films. Polymers, 13(23), 4060. https://doi.org/10.3390/polym13234060






