Electrospun Ultrafine Cationic Cellulose Fibers Produced from Sugarcane Bagasse for Potential Textile Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Purification and Acetylation of Cellulose from Sugarcane Bagasse
2.3. Electrospinning
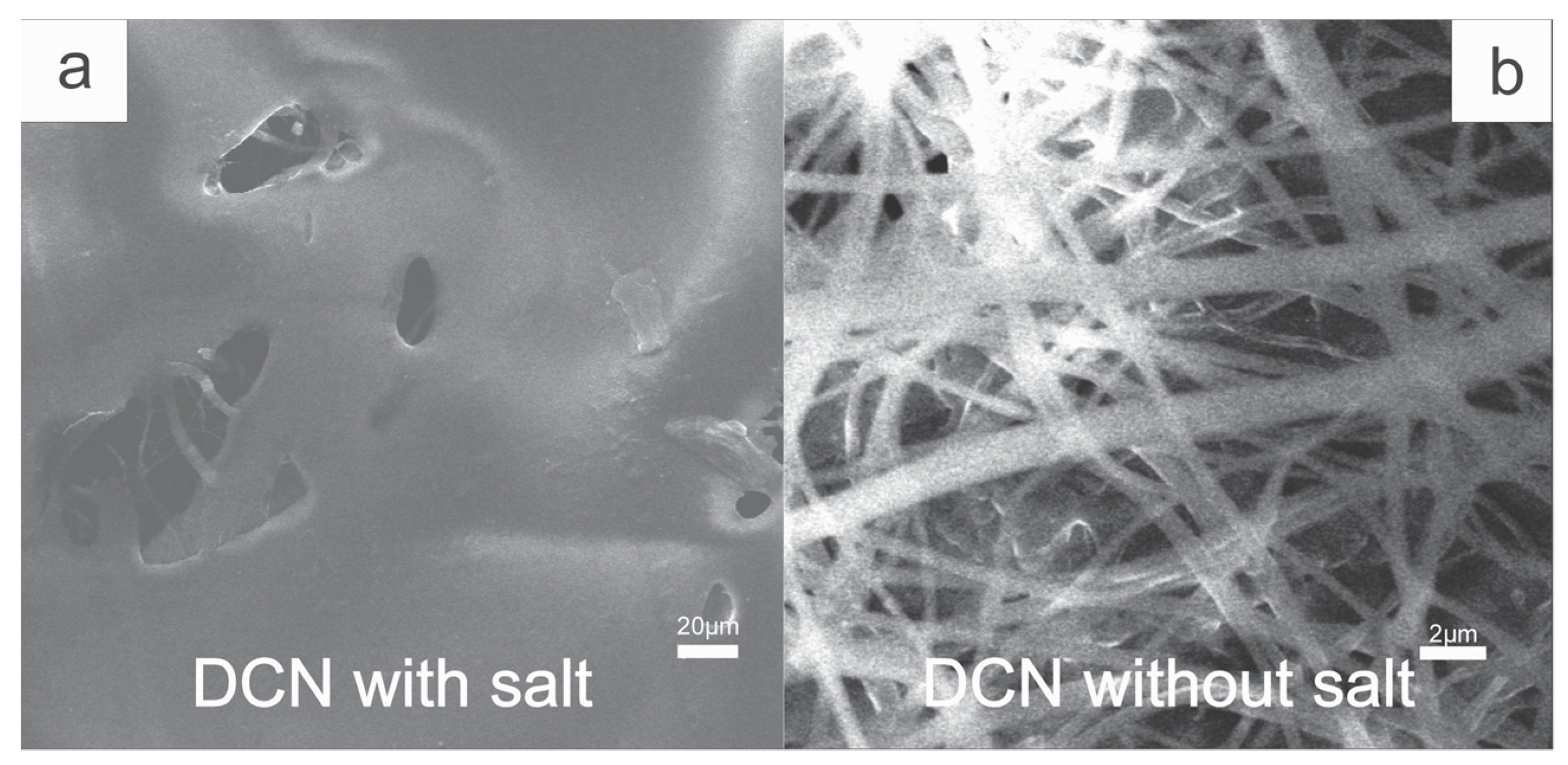
2.4. Fiber Dyeing
2.5. Fiber Characterization
3. Results and Discussion
3.1. Acetyl Groups (%) and Degree of Substitution of Cellulose Acetate
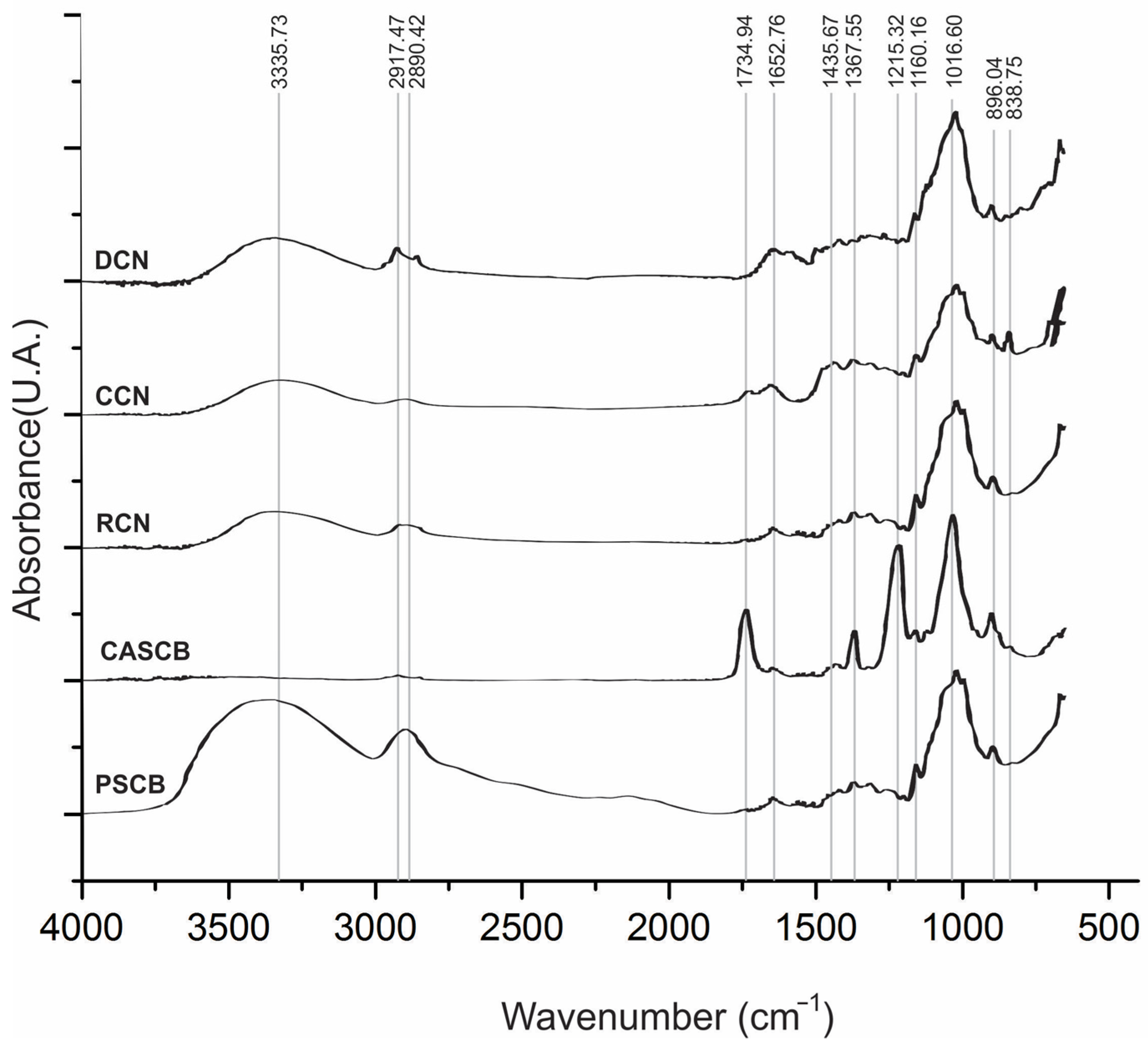
3.2. ATR–FTIR Studies
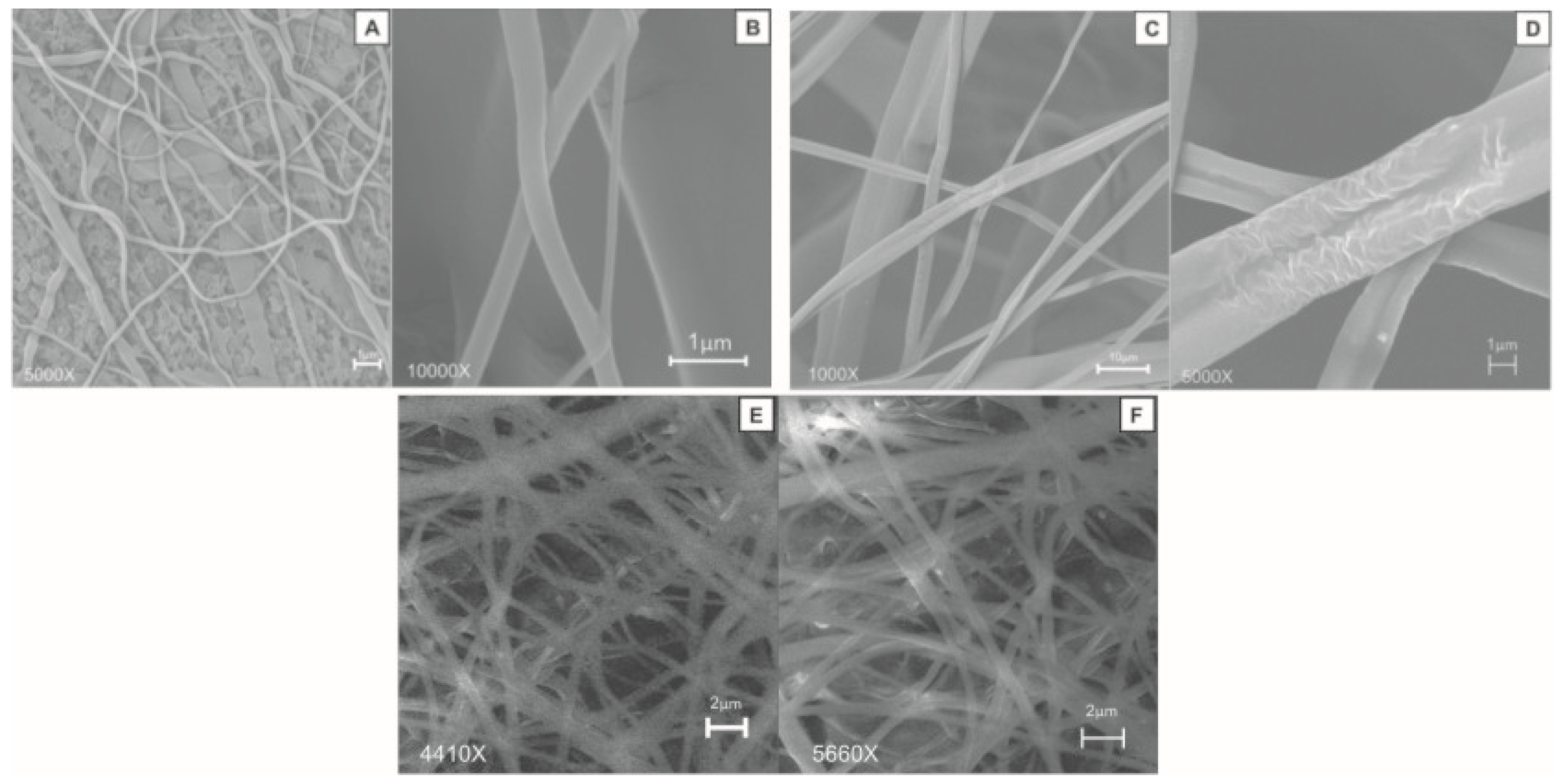
3.3. Fiber Morphology
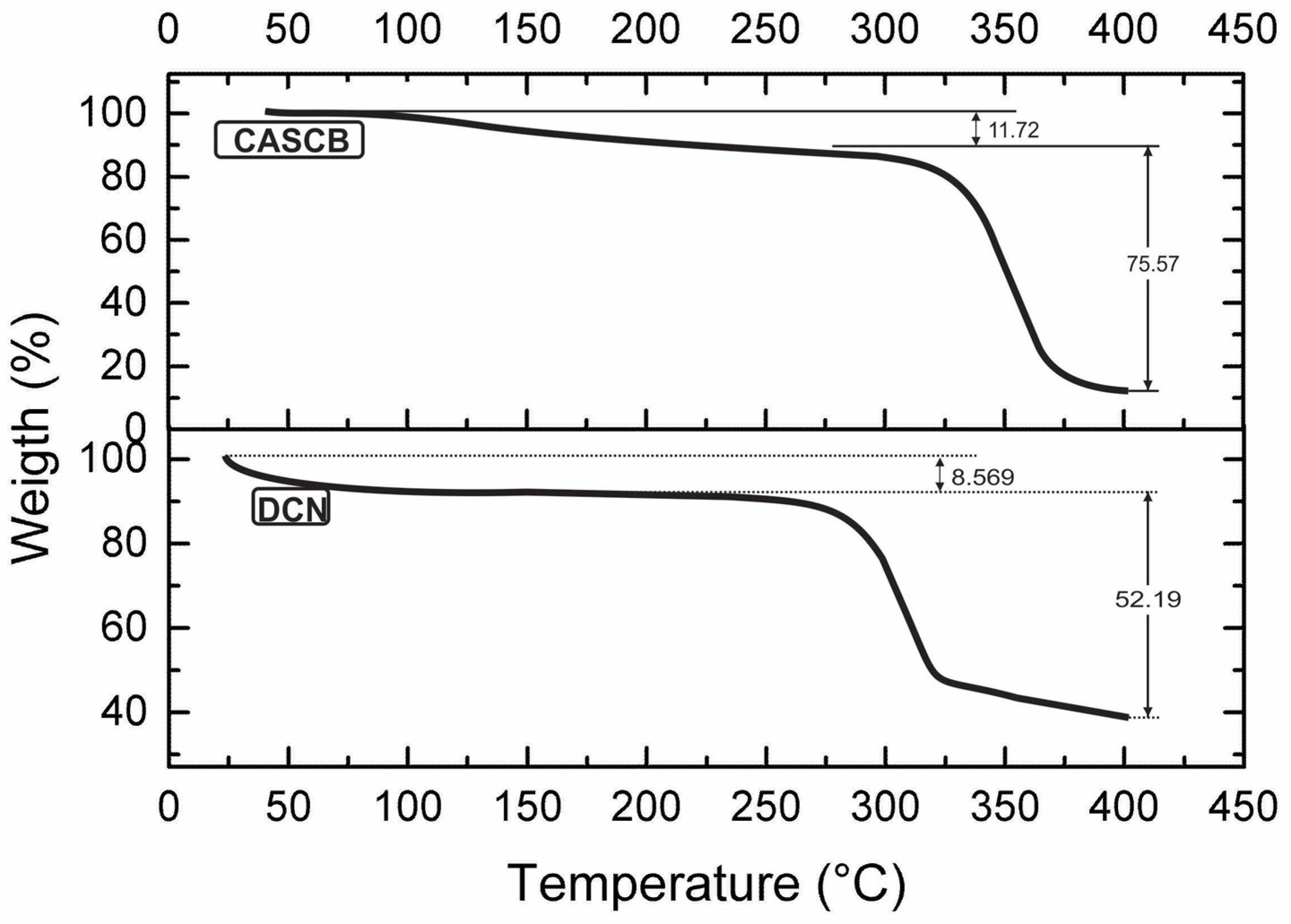
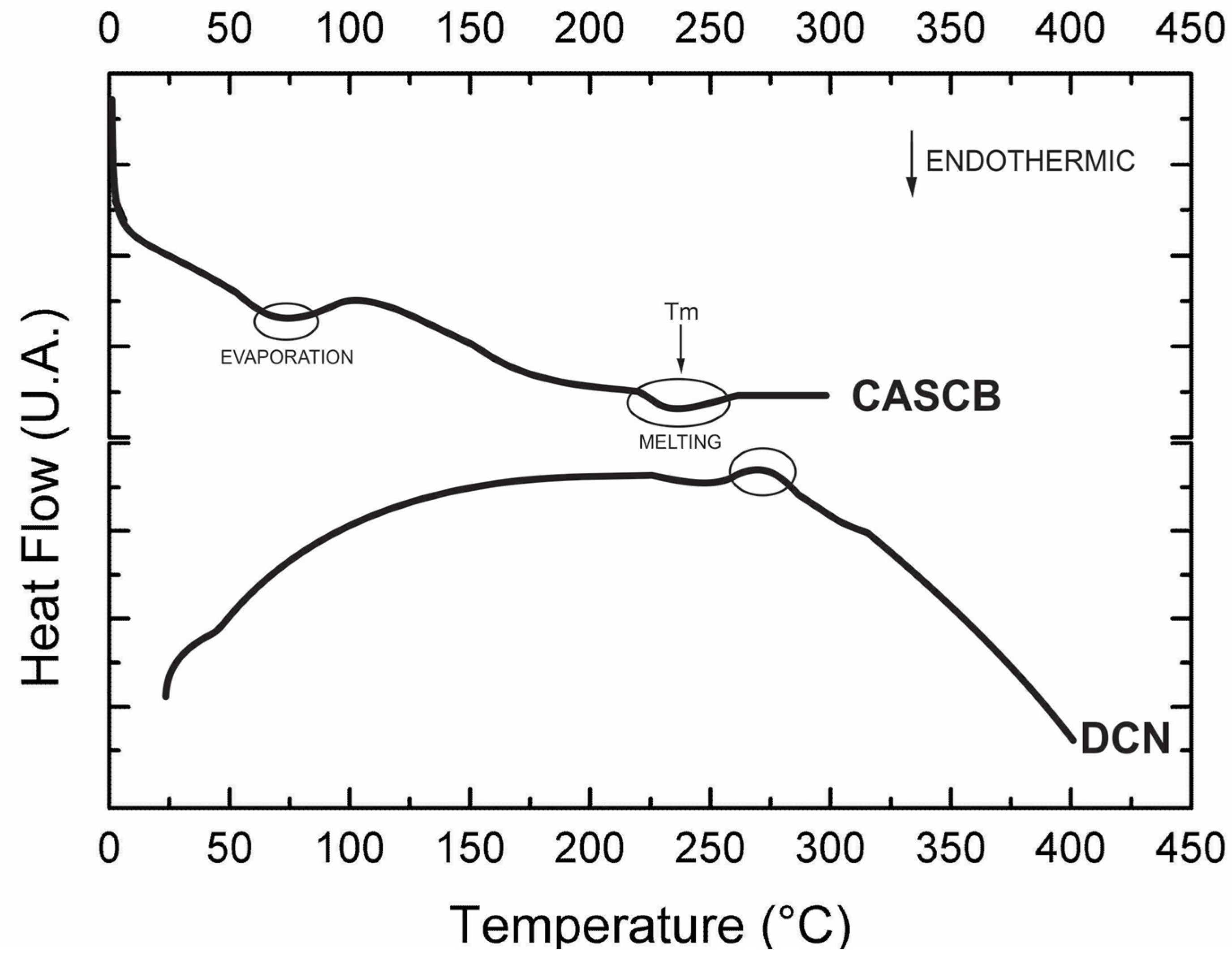
3.4. Thermal Stability
3.5. Color Attributes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Felgueiras, C.; Azoia, N.G.; Gonçalves, C.; Gama, M.; Dourado, F. Trends on the Cellulose-Based Textiles: Raw Materials and Technologies. Front. Bioeng. Biotechnol. 2021, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.M.; Mazzola, P.G.; Silva, J.C.A.R.; Pahl, R.; Pessoa, A.; Costa, S.A. Use of sugar cane straw as a source of cellulose for textile fiber production. Ind. Crop. Prod. 2013, 42, 189–194. [Google Scholar] [CrossRef]
- López-Córdoba, A.; Castro, G.R.; Goyanes, S. Cellulose-Containing Scaffolds Fabricated by Electrospinning: Applications in Tissue Engineering and Drug Delivery. In Handbook of Composites from Renewable Materials; Thakur, V.K., Thakur, K., Kessler, M.R., Eds.; Scrivener Publishing LLC: Beverly, MA, USA, 2017; Volume 8, pp. 361–388. [Google Scholar]
- Alam, M.N.; Christopher, L.P. A novel, cost-effective and eco-friendly method for preparation of textile fibers from cellulosic pulps. Carbohydr. Polym. 2017, 173, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Fatima, W.; Tarique, M.; Li, M.; Chen, M.; Khatri, M.; Sarwar, M.N.; Kim, I.; Ahmed, F.; Khatri, Z.; Chen, R.; et al. Reactive Dyeing of Electrospun Cellulose Nanofibers by Pad-steam Method. Chem. Res. Chin. Univ. 2021, 37, 535–540. [Google Scholar] [CrossRef]
- Khatri, Z.; Mayakrishnan, G.; Hirata, Y.; Wei, K.; Kim, I.-S. Cationic-cellulose nanofibers: Preparation and dyeability with anionic reactive dyes for apparel application. Carbohydr. Polym. 2013, 91, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Khatri, A.; Ali, S.; Kim, S.H.; Javeed, A. Colouration of polymeric electrospun nanofibrous mats—A mini review. J. Text. Inst. 2020, 111, 765–774. [Google Scholar] [CrossRef]
- Wang, L.; Ma, W.; Zhang, S.; Teng, X.; Yang, J. Preparation of cationic cotton with two-bath pad-bake process and its application in salt-free dyeing. Carbohydr. Polym. 2009, 78, 602–608. [Google Scholar] [CrossRef]
- Khatri, Z.; Ahmed, F.; Jhatial, A.K.; Abro, M.I.; Mayakrishnan, G.; Kim, I.S. Cold pad-batch dyeing of cellulose nanofibers with reactive dyes. Cellulose 2014, 21, 3089–3095. [Google Scholar] [CrossRef]
- Hamawand, I.; da Silva, W.; Seneweera, S.; Bundschuh, J. Value Proposition of Different Methods for Utilisation of Sugarcane Wastes. Energies 2021, 14, 5483. [Google Scholar] [CrossRef]
- Senapitakkul, V.; Vanitjinda, G.; Torgbo, S.; Pinmanee, P.; Nimchua, T.; Rungthaworn, P.; Sukatta, U.; Sukyai, P. Pretreatment of Cellulose from Sugarcane Bagasse with Xylanase for Improving Dyeability with Natural Dyes. ACS Omega 2020, 5, 28168–28177. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.L.; Dall’Antonia, C.B.; Shiga, E.A.; Alvim, L.J.; Pessoni, R.A.B. Sugarcane bagasse as a source of carbon for enzyme production by filamentous fungi1. Hoehnea 2018, 45, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Aristizábal, M.V.; Gómez, P.Á.; Cardona, A.C.A. Biorefineries based on coffee cut-stems and sugarcane bagasse: Furan-based compounds and alkanes as interesting products. Bioresour. Technol. 2015, 196, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Filho, G.R.; da Cruz, S.F.; Pasquini, D.; Cerqueira, D.A.; de Souza Prado, V.; de Assunção, R.M.N. Water flux through cellulose triacetate films produced from heterogeneous acetylation of sugar cane bagasse. J. Memb. Sci. 2000, 177, 225–231. [Google Scholar] [CrossRef]
- Rodrigues Filho, G.; Monteiro, D.S.; da Silva Meireles, C.; de Assunção, R.M.N.; Cerqueira, D.A.; Barud, H.S.; Ribeiro, S.J.L.; Messadeq, Y. Synthesis and characterization of cellulose acetate produced from recycled newspaper. Carbohydr. Polym. 2008, 73, 74–82. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.-P.; Peng, F.; Bian, J.; Yuan, T.-Q.; Xu, F.; Sun, R.-C. Microwave-Assisted Solvent-Free Acetylation of Cellulose with Acetic Anhydride in the Presence of Iodine as a Catalyst. Molecules 2009, 14, 3551–3566. [Google Scholar] [CrossRef] [PubMed]
- Khatri, Z.; Wei, K.; Kim, B.-S.; Kim, I.-S. Effect of deacetylation on wicking behavior of co-electrospun cellulose acetate/polyvinyl alcohol nanofibers blend. Carbohydr. Polym. 2012, 87, 2183–2188. [Google Scholar] [CrossRef]
- Heinze, T.; Liebert, T. Unconventional methods in cellulose functionalization. Prog. Polym. Sci. 2001, 26, 1689–1762. [Google Scholar] [CrossRef]
- Elidrissi, A.; El barkany, S.; Amhamdi, H.; Maaroufi, A.; Hammouti, B. New approach to predict the solubility of polymers application: Cellulose acetate at various DS, prepared from Alfa “Stipa -tenassicima” of Eastern Morocco. J. Mater. Environ. Sci. 2012, 3, 270–285. [Google Scholar]
- Yuan, W. Effect of Fiber Diameter and Web Porosity on Breathability of Nanofiber Mats at Various Test Conditions. Master’s Thesis, University of Texas at Austin, Austin, TX, USA, 2014. [Google Scholar]
- Wu, S.; Qin, X.; Li, M. The structure and properties of cellulose acetate materials: A comparative study on electrospun membranes and casted films. J. Ind. Text. 2013, 44, 85–98. [Google Scholar] [CrossRef]
| Condition | Parameters | ||
|---|---|---|---|
| Tip-to-Collector Distance (cm) | Electrical Voltage (kV) | Feed Rate (μL/h) | |
| 1 | 12 | 12 | 3 |
| 2 | 14 | 15 | 8 |
| 3 | 15 | 15 | 1000 |
| Condition | Average Diameter (nm) | Average Porosity (%) |
|---|---|---|
| 1 | 261 ± 123 nm | 35.5 |
| 2 | 4452 ± 2999 nm | 30.0 |
| 3 | 678 ± 219 nm | 20.8 |
| CI Reactive | Fabric | L* | a* | b* | C* | h* |
|---|---|---|---|---|---|---|
| Black | DCN without salt | 23.3 ± 0.4 | 3.8 ± 0.1 | −1.5 ± 0.02 | 4.1 ± 0.2 | 338.5 ± 2.1 |
| DCN with salt | 22.8 ± 0.5 | 4.2 ± 0.5 | −0.22 ± 0.02 | 4.2 ± 0.2 | 356.9 ± 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochica Larrota, A.F.; Vera-Graziano, R.; López-Córdoba, A.; Gómez-Pachón, E.Y. Electrospun Ultrafine Cationic Cellulose Fibers Produced from Sugarcane Bagasse for Potential Textile Applications. Polymers 2021, 13, 3927. https://doi.org/10.3390/polym13223927
Ochica Larrota AF, Vera-Graziano R, López-Córdoba A, Gómez-Pachón EY. Electrospun Ultrafine Cationic Cellulose Fibers Produced from Sugarcane Bagasse for Potential Textile Applications. Polymers. 2021; 13(22):3927. https://doi.org/10.3390/polym13223927
Chicago/Turabian StyleOchica Larrota, Andrés Felipe, Ricardo Vera-Graziano, Alex López-Córdoba, and Edwin Yesid Gómez-Pachón. 2021. "Electrospun Ultrafine Cationic Cellulose Fibers Produced from Sugarcane Bagasse for Potential Textile Applications" Polymers 13, no. 22: 3927. https://doi.org/10.3390/polym13223927
APA StyleOchica Larrota, A. F., Vera-Graziano, R., López-Córdoba, A., & Gómez-Pachón, E. Y. (2021). Electrospun Ultrafine Cationic Cellulose Fibers Produced from Sugarcane Bagasse for Potential Textile Applications. Polymers, 13(22), 3927. https://doi.org/10.3390/polym13223927







