Influence of Commonly Used Endodontic Irrigants on the Setting Time and Metal Composition of Various Base Endodontic Sealers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Characteristics
2.2. Materials
2.3. Sample Preparation
- Sealers not exposed to any endodontic irrigants: final irrigation was conducted with 2 mL of distilled water for a 1 min period;
- For 3% NaOCl Group: final irrigation was conducted with 2 mL of 3% NaOCl for a 1minperiod;
- For 2% CHX Group: final irrigation was conducted with 2 mL of 2% CHX for a 1 min period;
- For 17% EDTA Group: final irrigation was conducted with 2 mL of 17% EDTA for a 1 min period.
2.4. Setting Time Analysis
2.5. Heavy Metal Analysis
2.6. Statistical Analysis
3. Results
4. Discussion
5. Strength, Limitations, and Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Komabayashi, T.; Colmenar, D.; Cvach, N.; Bhat, A.; Primus, C.; Imai, Y. Comprehensive Review of Current Endodontic Sealers. Dent. Mater. J. 2020, 39, 703–720. [Google Scholar] [CrossRef] [Green Version]
- Orstavik, D. Materials Used for Root Canal Obturation: Technical, Biological and Clinical Testing. Endod. Top. 2005, 12, 25–38. [Google Scholar] [CrossRef]
- Marín-Bauza, G.A.; Silva-Sousa, Y.T.C.; da Cunha, S.A.; Rached-Junior, F.J.A.; Bonetti-Filho, I.; Sousa-Neto, M.D.; Miranda, C.E.S. Physicochemical Properties of Endodontic Sealers of Different Bases. J. Appl. Oral Sci. 2012, 20, 455–461. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, W.A.; Leonardo, M.R.; Filho, M.T.; Silva, L.A.B. Evaluation of Apical Sealing of Three Endodontic Sealers. Int. Endod. J. 2000, 33, 25–27. [Google Scholar] [CrossRef]
- Silva, E.J.N.L.; de Carvalho, N.K.; Ronconi, C.T.; De-Deus, G.; Zuolo, M.L.; Zaia, A.A.; Silva, E.J.N.L.; de Carvalho, N.K.; Ronconi, C.T.; De-Deus, G.; et al. Cytotoxicity Profile of Endodontic Sealers Provided by 3D Cell Culture Experimental Model. Braz. Dent. J. 2016, 27, 652–656. [Google Scholar] [CrossRef]
- Brzovic, V.; Miletic, I.; Zeljezic, D.; Mladinic, M.; Kasuba, V.; Ramic, S.; Anic, I. In Vitro Genotoxicity of Root Canal Sealers. Int. Endod. J. 2009, 42, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Vassiliadis, L.P.; Sklavounos, S.A.; Stavrianos, C.K. Depth of Penetration and Appearance of Grossman Sealer in the Dentinal Tubules: An In Vivo Study. J. Endod. 1994, 20, 373–376. [Google Scholar] [CrossRef]
- Szczurko, G.; Pawińska, M.; Łuczaj-Cepowicz, E.; Kierklo, A.; Marczuk-Kolada, G.; Hołownia, A. Effect of Root Canal Sealers on Human Periodontal Ligament Fibroblast Viability: Ex Vivo Study. Odontology 2018, 106, 245–256. [Google Scholar] [CrossRef] [Green Version]
- Ricucci, D.; Rôças, I.N.; Alves, F.R.F.; Loghin, S.; Siqueira, J.F. Apically Extruded Sealers: Fate and Influence on Treatment Outcome. J. Endod. 2016, 42, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Versiani, M.A.; Carvalho-Junior, J.R.; Padilha, M.I.A.F.; Lacey, S.; Pascon, E.A.; Sousa-Neto, M.D. A Comparative Study of Physicochemical Properties of AH PlusTM and EpiphanyTM Root Canal Sealants. Int. Endod. J. 2006, 39, 464–471. [Google Scholar] [CrossRef]
- Münchow, E.A.; Vitti, R.P.; Sinhoreti, M.A.C.; Piva, E.; Ogliari, F.A.; Zanchi, C.H. Pentaerythritol Tetrasalicylate in the Chemical Composition of Root Canal Sealers. Braz. Dent. J. 2018, 29, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Desai, S.; Chandler, N. Calcium Hydroxide—Based Root Canal Sealers: A Review. J. Endod. 2009, 35, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Filho, J.E.; Watanabe, S.; Lodi, C.S.; Cintra, L.T.A.; Nery, M.J.; Filho, J.A.O.; Dezan, E.; Bernabé, P.F.E. Rat Tissue Reaction to MTA FILLAPEX®. Dent. Traumatol. 2012, 28, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Bioceramic Materials in Endodontics. Endod. Top. 2015, 32, 3–30. [Google Scholar] [CrossRef]
- Vitti, R.P.; Prati, C.; Sinhoreti, M.A.C.; Zanchi, C.H.; e Silva, M.G.; Ogliari, F.A.; Piva, E.; Gandolfi, M.G. Chemical—Physical Properties of Experimental Root Canal Sealers Based on Butyl Ethylene Glycol Disalicylate and MTA. Dent. Mater. 2013, 29, 1287–1294. [Google Scholar] [CrossRef]
- Chang, S.-W.; Baek, S.-H.; Yang, H.-C.; Seo, D.-G.; Hong, S.-T.; Han, S.-H.; Lee, Y.; Gu, Y.; Kwon, H.-B.; Lee, W.; et al. Heavy Metal Analysis of Ortho MTA and ProRoot MTA. J. Endod. 2011, 37, 1673–1676. [Google Scholar] [CrossRef]
- ISO 9917-1:2007 (En), Dentistry—Water-Based Cements—Part 1: Powder/Liquid Acid-Base Cements. Available online: https://www.iso.org/obp/ui/#iso:std:iso:9917:-1:ed-2:v1:en (accessed on 29 April 2021).
- Zehnder, M. Root Canal Irrigants. J. Endod. 2006, 32, 389–398. [Google Scholar] [CrossRef]
- Lee, O.Y.S.; Khan, K.; Li, K.Y.; Shetty, H.; Abiad, R.S.; Cheung, G.S.P.; Neelakantan, P. Influence of Apical Preparation Size and Irrigation Technique on Root Canal Debridement: A Histological Analysis of Round and Oval Root Canals. Int. Endod. J. 2019, 52, 1366–1376. [Google Scholar] [CrossRef] [PubMed]
- ISO 6876:2012(En), Dentistry—Root Canal Sealing Materials. Available online: https://www.iso.org/obp/ui/#iso:std:iso:6876:ed-3:v1:en (accessed on 29 April 2021).
- Siddique, R.; Sureshbabu, N.M.; Somasundaram, J.; Jacob, B.; Selvam, D. Qualitative and Quantitative Analysis of Precipitate Formation Following Interaction of Chlorhexidine with Sodium Hypochlorite, Neem, and Tulsi. J. Conserv. Dent. 2019, 22, 40. [Google Scholar] [CrossRef]
- Camargo, C.H.R.; Oliveira, T.R.; Silva, G.O.; Rabelo, S.B.; Valera, M.C.; Cavalcanti, B.N. Setting Time Affects In Vitro Biological Properties of Root Canal Sealers. J. Endod. 2014, 40, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Nunes, V.H.; Silva, R.G.; Alfredo, E.; Sousa-Neto, M.D.; Silva-Sousa, Y.T.C. Adhesion of Epiphany and AH Plus Sealers to Human Root Dentin Treated with Different Solutions. Braz. Dent. J. 2008, 19, 46–50. [Google Scholar] [CrossRef] [Green Version]
- Barros, J.; Silva, M.G.; Rodrigues, M.A.; Alves, F.R.F.; Lopes, M.A.; Pina-Vaz, I.; Siqueira, J.F. Antibacterial, Physicochemical and Mechanical Properties of Endodontic Sealers Containing Quaternary Ammonium Polyethylenimine Nanoparticles. Int. Endod. J. 2014, 47, 725–734. [Google Scholar] [CrossRef]
- Rocha, A.W.; de Andrade, C.D.; Leitune, V.C.B.; Collares, F.M.; Samuel, S.M.W.; Grecca, F.S.; de Figueiredo, J.A.P.; dos Santos, R.B. Influence of Endodontic Irrigants on Resin Sealer Bond Strength to Radicular Dentin. Bull. Tokyo Dent. Coll. 2012, 53, 1–7. [Google Scholar] [CrossRef] [Green Version]
- de Assis, D.F.; do Prado, M.; Simão, R.A. Evaluation of the Interaction between Endodontic Sealers and Dentin Treated with Different Irrigant Solutions. J. Endod. 2011, 37, 1550–1552. [Google Scholar] [CrossRef]
- Vertucci, F.J. Root Canal Morphology of Mandibular Premolars. J. Am. Dent. Assoc. 1978, 97, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Uygun, A.D.; Gündoğdu, E.C.; Arslan, H.; Ersoy, İ. Efficacy of XP-Endo Finisher and TRUShape 3D Conforming File Compared to Conventional and Ultrasonic Irrigation in Removing Calcium Hydroxide. Aust. Endod. J. 2017, 43, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Cerroni, L.; Iorio, L.; Dall’Asta, L.; Cianconi, L. FESEM Evaluation of Smear Layer Removal Using Different Irrigant Activation Methods (EndoActivator, EndoVac, PUI and LAI). An in Vitro Study. Clin. Oral Investig. 2018, 22, 993–999. [Google Scholar] [CrossRef]
- Khademi, A.; Mehdizadeh, M.; Sanei, M.; Sadeqnejad, H.; Khazaei, S. Comparative Evaluation of Root Canal Morphology of Mandibular Premolars Using Clearing and Cone Beam Computed Tomography. Dent. Res. J. 2017, 14, 321–325. [Google Scholar] [CrossRef]
- Alghamdi, F.T.; Khalil, W.A. Root Canal Morphology and Symmetry of Mandibular Second Premolars Using Cone-Beam Computed Tomography. Oral Radiol. 2021, 1–13. [Google Scholar] [CrossRef]
- Bürklein, S.; Heck, R.; Schäfer, E. Evaluation of the Root Canal Anatomy of Maxillary and Mandibular Premolars in a Selected German Population Using Cone-Beam Computed Tomographic Data. J. Endod. 2017, 43, 1448–1452. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, J.; Versiani, M.A.; de Sousa-Neto, M.D.; Plazas-Garzon, A.; Basrani, B. Evaluation of the Shaping Characteristics of ProTaper Gold, ProTaper Next, and ProTaper Universal in Curved Canals. J. Endod. 2015, 41, 1718–1724. [Google Scholar] [CrossRef] [PubMed]
- Shashirekha, G.; Jena, A.; Pattanaik, S.; Rath, J. Assessment of Pain and Dissolution of Apically Extruded Sealers and Their Effect on the Periradicular Tissues. J. Conserv. Dent. JCD 2018, 21, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Nawal, R.R.; Parande, M.; Sehgal, R.; Naik, A.; Rao, N.R. A Comparative Evaluation of Antimicrobial Efficacy and Flow Properties for Epiphany, Guttaflow and AH-Plus Sealer: Evaluation of Three Obturation Systems. Int. Endod. J. 2011, 44, 307–313. [Google Scholar] [CrossRef]
- da Silva, E.J.N.L.; Santos, C.C.; Zaia, A.A. Long-Term Cytotoxic Effects of Contemporary Root Canal Sealers. J. Appl. Oral Sci. 2013, 21, 43–47. [Google Scholar] [CrossRef] [Green Version]
- Scelza, M.Z.; Linhares, A.B.; da Silva, L.E.; Granjeiro, J.M.; Alves, G.G. A Multiparametric Assay to Compare the Cytotoxicity of Endodontic Sealers with Primary Human Osteoblasts: Multiparametric Cytotoxicity Test of Endodontic Sealers. Int. Endod. J. 2012, 45, 12–18. [Google Scholar] [CrossRef]
- List of Classifications—IARC Monographs on the Identification of Carcinogenic Hazards to Humans. Available online: https://monographs.iarc.who.int/list-of-classifications (accessed on 2 May 2021).
- Davis, M.; Walton, R.; Rivera, E. Sealer Distribution in Coronal Dentin. J. Endod. 2002, 28, 464–466. [Google Scholar] [CrossRef]
- Kum, K.-Y.; Zhu, Q.; Safavi, K.; Gu, Y.; Bae, K.-S.; Chang, S.W. Analysis of Six Heavy Metals in Ortho Mineral Trioxide Aggregate and ProRoot Mineral Trioxide Aggregate by Inductively Coupled Plasma-Optical Emission Spectrometry: Six Heavy Metal Analysis of Ortho MTA. Aust. Endod. J. 2013, 39, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A. Asymptomatic Aspergillosis of the Maxillary Sinus Associated with Foreign Body of Endodontic Origin. Int. J. Oral Maxillofac. Surg. 1995, 24, 243–244. [Google Scholar] [CrossRef]
- Nicolai, P.; Mensi, M.; Marsili, F.; Piccioni, M.; Salgarello, S.; Gilberti, E.; Apostoli, P. Maxillary Fungus Ball: Zinc-Oxide Endodontic Materials as a Risk Factor. Acta Otorhinolaryngol. Ital. 2015, 35, 93–96. [Google Scholar]
- Sanz, J.L.; López-García, S.; Lozano, A.; Pecci-Lloret, M.P.; Llena, C.; Guerrero-Gironés, J.; Rodríguez-Lozano, F.J.; Forner, L. Microstructural Composition, Ion Release, and Bioactive Potential of New Premixed Calcium Silicate–Based Endodontic Sealers Indicated for Warm Vertical Compaction Technique. Clin. Oral Investig. 2021, 25, 1451–1462. [Google Scholar] [CrossRef]
- Hsieh, S.-C.; Teng, N.-C.; Lin, Y.-C.; Lee, P.-Y.; Ji, D.-Y.; Chen, C.-C.; Ke, E.-S.; Lee, S.-Y.; Yang, J.-C. A Novel Accelerator for Improving the Handling Properties of Dental Filling Materials. J. Endod. 2009, 35, 1292–1295. [Google Scholar] [CrossRef]
- Wongsorachai, R.N.; Thanatvarakorn, O.; Prasansuttiporn, T.; Jittidecharaks, S.; Hosaka, K.; Foxton, R.M.; Nakajima, M. Effect of Polymerization Accelerator on Bond Strength to Eugenol-Contaminated Dentin. J. Adhes. Dent. 2018, 20, 541–547. [Google Scholar] [PubMed]
- Patini, R.; Spagnuolo, G.; Guglielmi, F.; Staderini, E.; Simeone, M.; Camodeca, A.; Gallenzi, P. Clinical Effects of Mercury in Conservative Dentistry: A Systematic Review, Meta-Analysis, and Trial Sequential Analysis of Randomized Controlled Trials. Int. J. Dent. 2020, 2020, e8857238. [Google Scholar] [CrossRef] [PubMed]
- Dumlu, A.; Yalcinkaya, S.; Olgac, V.; Güvercin, M. Osteomyelitis Due to Arsenic Trioxide Use for Tooth Devitalization. Int. Endod. J. 2007, 40, 317–322. [Google Scholar] [CrossRef]
- Poggio, C.; Riva, P.; Chiesa, M.; Colombo, M.; Pietrocola, G. Comparative Cytotoxicity Evaluation of Eight Root Canal Sealers. J. Clin. Exp. Dent. 2017, 9, e574–e578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinna, L.; Brackett, M.G.; Lockwood, P.E.; Huffman, B.P.; Mai, S.; Cotti, E.; Dettori, C.; Pashley, D.H.; Tay, F.R. In Vitro Cytotoxicity Evaluation of a Self-Adhesive, Methacrylate Resin-Based Root Canal Sealer. J. Endod. 2008, 34, 1085–1088. [Google Scholar] [CrossRef]
- Shrivastava, R.; Upreti, R.K.; Seth, P.K.; Chaturvedi, U.C. Effects of Chromium on the Immune System. FEMS Immunol. Med. Microbiol. 2002, 34, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Janani, K.; Teja, K.V.; Alam, M.K.; Shrivastava, D.; Iqbal, A.; Khattak, O.; Al-Johani, K.; Hamza, M.O.; Jose, J.; Karobari, M.I.; et al. Efficacy of Oregano Essential Oil Extract in the Inhibition of Bacterial Lipopolysaccharide (LPS)-Induced Osteoclastogenesis Using RAW 264.7 Murine Macrophage Cell Line & mdash; An In-Vitro Study. Separations 2021, 8, 240. [Google Scholar] [CrossRef]
- Teja, K.V.; Janani, K.; Srivastava, K.C.; Shrivastava, D.; Jose, J.; Marya, A.; Karobari, M.I. Comparison of Herbal Agents with Sodium Hypochlorite as Root Canal Irrigant: A Systematic Review of In Vitro Studies. Evid. Based Complement. Alternat. Med. 2021, 2021, e8967219. [Google Scholar] [CrossRef]
- Janani, K.; Teja, K.V.; Sandhya, R.; Alam, M.K.; Al-Qaisi, R.K.; Shrivastava, D.; Alnusayri, M.O.; Alkhalaf, Z.A.; Sghaireen, M.G.; Srivastava, K.C. Monomer Elution from Three Resin Composites at Two Different Time Interval Using High Performance Liquid Chromatography—An In-Vitro Study. Polymers 2021, 13, 4395. [Google Scholar] [CrossRef]

| Materials | Composition | Manufacturer |
|---|---|---|
| Sealapex | calcium hydroxide, barium sulfate, zinc oxide, titanium dioxide, and zinc stearate. | SybronEndo, Orange, CA, USA |
| AH Plus | Paste A:bisphenol A epoxy resin, bisphenol F epoxy resin, calcium tungstate, zirconium oxide, aerosol, and iron oxide Paste B: dibenzyldiamine, adamantane amine, tricyclodecane-diamine, calcium tungstate, zirconium oxide, aerosol, and silicon oil | Dentsply DeTrey GmbH, Konstanz, Germany |
| MTA Fillapex | After mixing: salicylate resin, natural resin, diluting resin, bismuth trioxide, nanoparticulated silica, MTA, and pigments. | Angelus Soluções Odontológicas, Londrina, Brazil |
| Tubli-Seal | Base:zinc oxide, oleo resin, bismuth trioxide, thymol iodide, oils, and waxes. Catalyst:eugenol, polymerized resin, and annidalin | Kerr Dental, Orange, CA, USA |
| Elements (ppm)(Mean ± SD) | AH Plus | AH Plus | AH Plus | AH Plus | MTA Fillapex | MTA Fillapex | MTA Fillapex | MTA Fillapex | Tubli-Seal | Tubli-Seal | Tubli-Seal | Tubli-Seal | Sealapex | Sealapex | Sealapex | Sealapex |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| - | CHX | NaOCl | EDTA | - | CHX | NaOCl | EDTA | - | CHX | NaOCl | EDTA | - | CHX | NaOCl | EDTA | |
| Cr | 5.24 ± 1.24 | 0.84 ± 0.005 | 0.38 ± 0.006 | 0.50 ± 0.002 | 1.35 ± 0.08 | 0.31 ± 0.0002 | 0.71 ± 0.0002 | 0.71 ± 0.0002 | 1.42 ± 0.05 | 0.79 ± 0.08 | 0.96 ± 0.006 | 1.42 ± 0.85 | 0.22 ± 0.007 | 0.44 ± 0.04 | 0.93 ± 0.0006 | 0.78 ± 0.008 |
| Ni | 0.51 ± 0.01 | 0.30 ± 0.005 | 0.91 ± 0.009 | 0.38 ± 0.069 | 2.84 ± 1.08 | 0.23 ± 0.45 | 1.45 ± 0.81 | 0.36 ± 0.08 | 0.56 ± 0.08 | 0.10 ± 0.0002 | 1.54 ± 0.08 | 1.47 ± 0.65 | 0.17 ± 0.005 | 0.17 ± 0.02 | 0.66 ± 0.001 | 1.35 ±0.18 |
| Co | 0.08 ± 0.005 | 0.09 ± 0.002 | 0.18 ± 0.001 | 0.07 ± 0.0002 | 0.58 ± 0.006 | 0.05 ± 0.00004 | 0.26 ± 0.10 | 0.13 ± 0.002 | 0.13 ± 0.001 | 0.04 ± 0.0002 | 0.31 ± 0.001 | 0.28 ± 0.002 | 0.04 ± 0.0001 | 0.04 ± 0.0001 | 0.09 ±0.0005 | 0.24 ± 0.10 |
| Cd | 0.08 ± 0.005 | 0.06 ± 0.002 | 0.05 ± 0.0004 | 0.17 ± 0.0012 | 0.29 ± 0.08 | 0.08 ± 0.0002 | 0.08 ± 0.0002 | 0.07 ± 0.002 | 0.13 ± 0.045 | 0.09 ± 0.001 | 0.13 ± 0.0221 | 0.09 ± 0.0002 | 0.09 ± 0.0002 | 0.04 ± 0.0001 | 0.09 ± 0.0005 | 0.10 ± 0.002 |
| As | 2.15 ± 0.55 | 0.33 ± 0.15 | 0.29 ± 0.0085 | 0.98 ± 0.034 | 2.26 ± 1.00002 | 0.45 ± 0.0002 | 1.01 ± 0.45 | 0.51 ± 0.006 | 2.02 ± 0.18 | 0.81 ± 0.002 | 1.20 ± 0.08 | 1.62 ± 0.15 | 0.00 | 0.17 ± 0.0023 | 0.45 ± 0.002 | 0.00 |
| Hg | 0.00 | 0.93 ± 0.45 | 1.54 ± 0.65 | 1.96 ± 0.04 | 2.79 ± 0.02 | 1.19 ± 0.006 | 1.98 ± 0.91 | 1.07 ± 0.058 | 1.55 ± 0.045 | 1.07 ± 0.004 | 1.75 ± 0.78 | 1.14 ± 0.28 | 3.14 ± 1.02 | 3.36 ± 0.006 | 0.27 ± 0.002 | 1.49 ± 0.59 |
| Pb | 0.34 ± 0.05 | 0.18 ± 0.08 | 0.22 ± 0.04 | 0.19 ± 0.002 | 1.01 ± 0.02 | 0.09 ± 0.05 | 0.4 ± 0.002 | 0.23 ± 0. 005 | 0.43 ± 0.004 | 0.16 ± 0.002 | 0.70 ± 0.25 | 0.50 ± 0.20 | 8.66 ± 3.02 | 5.17 ± 2.05 | 151.78 ± 22.45 | 346.83 ± 28.65 |
| Be | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
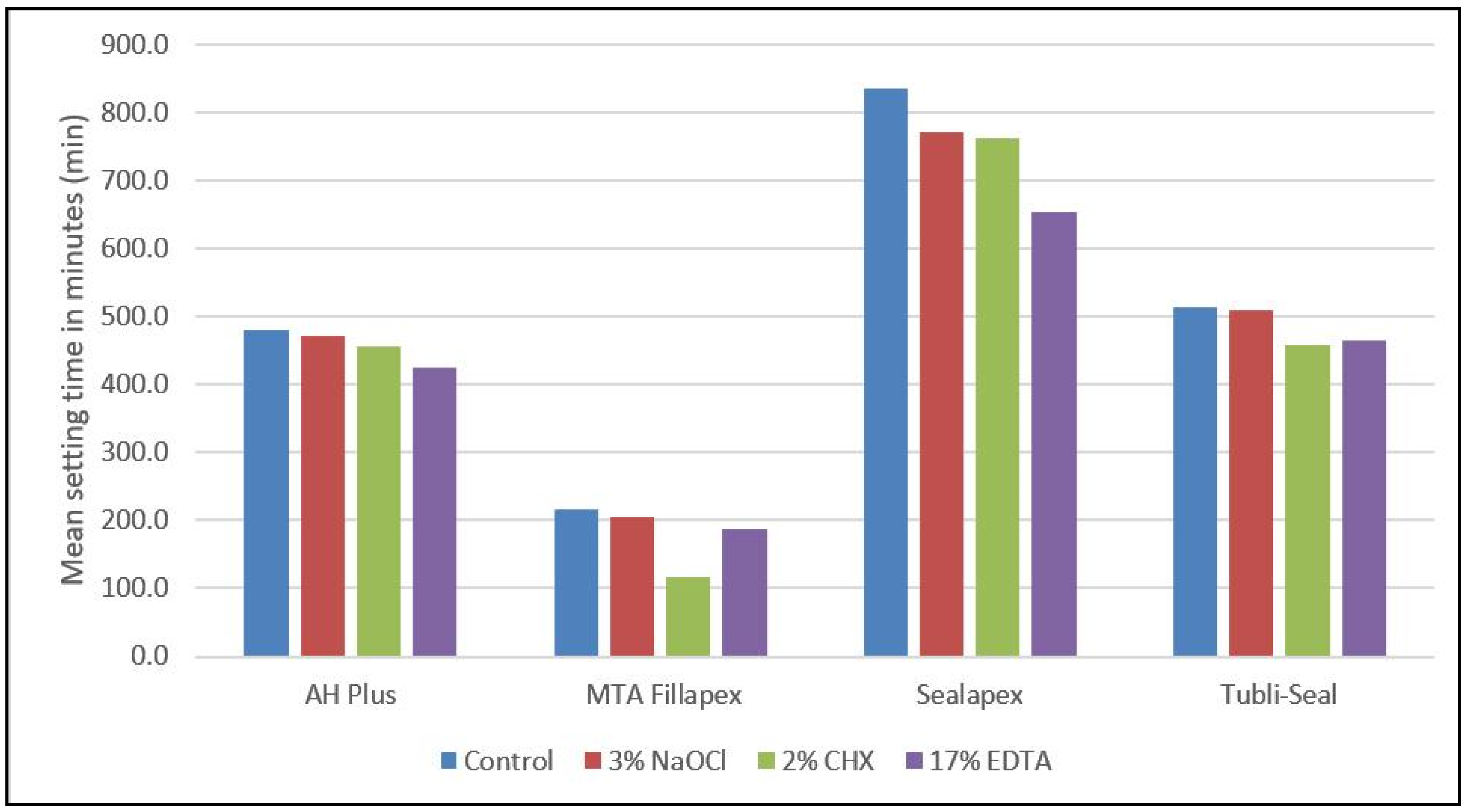
| Endodontic Irrigants | Endodontic Sealers | Mean | Standard Deviation | F-Value | p-Value |
|---|---|---|---|---|---|
| No interaction with any endodontic irrigants | AH Plus | 479.6 | 10.2 | 834.50 | 0.0005 ** |
| MTA Fillapex | 215.7 | 6.3 | |||
| Sealapex | 834.6 | 26.8 | |||
| Tubli-Seal | 514.7 | 7.9 | |||
| On interaction with 3% NaOCl | AH Plus | 471.5 | 17.4 | 239.65 | 0.0005 ** |
| MTA Fillapex | 204.9 | 4.2 | |||
| Sealapex | 772.0 | 48.6 | |||
| Tubli-Seal | 509.9 | 3.7 | |||
| On interaction with 2% CHX | AH Plus | 456.4 | 11.7 | 311.06 | 0.0005 ** |
| MTA Fillapex | 115.7 | 6.2 | |||
| Sealapex | 762.1 | 49.9 | |||
| Tubli-Seal | 457.8 | 4.6 | |||
| On interaction with 17% EDTA | AH Plus | 423.9 | 11.3 | 816.45 | 0.0005 ** |
| MTA Fillapex | 187.3 | 4.5 | |||
| Sealapex | 652.6 | 19.4 | |||
| Tubli-Seal | 465.2 | 4.0 |
| Dependent Variable | MD (I-J) | Std. Error | p-Value | 95% C.I | |||
|---|---|---|---|---|---|---|---|
| LB | UB | ||||||
| Control | AH Plus | MTA Fillapex | 263.9000 | 12.414 | 0.0005 ** | 224.1 | 303.7 |
| Sealapex | −355.0000 | 12.414 | 0.0005 ** | −394.8 | −315.2 | ||
| Tubli-Seal | −35.1000 | 12.414 | 0.085 # | −74.9 | 4.7 | ||
| MTA Fillapex | Sealapex | −618.9000 | 12.414 | 0.0005 ** | −658.7 | −579.1 | |
| Tubli-Seal | −299.0000 | 12.414 | 0.0005 ** | −338.8 | −259.2 | ||
| Sealapex | Tubli-Seal | 319.9000 | 12.414 | 0.0005 ** | 280.1 | 359.7 | |
| 3% NaOCl | AH Plus | MTA Fillapex | 266.6000 | 21.20 | 0.0005 ** | 198.7 | 334.5 |
| Sealapex | −300.5000 | 21.20 | 0.0005 ** | −368.4 | −232.6 | ||
| Tubli-Seal | −38.4000 | 21.20 | 0.335 # | −106.3 | 29.5 | ||
| MTA Fillapex | Sealapex | −567.1000 | 21.20 | 0.0005 ** | −635.0 | −499.2 | |
| Tubli-Seal | −305.0000 | 21.20 | 0.0005 ** | −372.9 | −237.1 | ||
| Sealapex | Tubli-Seal | 262.1000 | 21.20 | 0.0005 ** | 194.2 | 330.0 | |
| 2% CHX | AH Plus | MTA Fillapex | 340.7000 | 21.18 | 0.0005 ** | 272.9 | 408.5 |
| Sealapex | −305.7333 | 21.18 | 0.0005 ** | −373.6 | −237.9 | ||
| Tubli-Seal | −1.4000 | 21.18 | 1.00 # | −69.2 | 66.4 | ||
| MTA Fillapex | Sealapex | −646.4333 | 21.18 | 0.0005 ** | −714.3 | −578.6 | |
| Tubli-Seal | −342.1000 | 21.18 | 0.0005 ** | −409.9 | −274.3 | ||
| Sealapex | Tubli-Seal | 304.3333 | 21.18 | 0.0005 ** | 236.5 | 372.2 | |
| 17% EDTA | AH Plus | MTA Fillapex | 236.6000 | 9.47 | 0.0005 ** | 206.29 | 266.91 |
| Sealapex | −228.7333 | 9.47 | 0.0005 ** | −259.05 | −198.42 | ||
| Tubli-Seal | −41.3000 | 9.47 | 0.010 ** | −71.61 | −10.99 | ||
| MTA Fillapex | Sealapex | −465.3333 | 9.47 | 0.0005 ** | −495.65 | −435.02 | |
| Tubli-Seal | −277.9000 | 9.47 | 0.0005 ** | −308.21 | −247.59 | ||
| Sealapex | Tubli-Seal | 187.4333 | 9.47 | 0.0005 ** | 157.12 | 217.75 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jose, J.; Teja, K.V.; Ranjan, M.; Mohamed, R.N.; Alam, M.K.; Shrivastava, D.; Natoli, V.; Nagarajappa, A.K.; Janani, K.; Srivastava, K.C. Influence of Commonly Used Endodontic Irrigants on the Setting Time and Metal Composition of Various Base Endodontic Sealers. Polymers 2022, 14, 27. https://doi.org/10.3390/polym14010027
Jose J, Teja KV, Ranjan M, Mohamed RN, Alam MK, Shrivastava D, Natoli V, Nagarajappa AK, Janani K, Srivastava KC. Influence of Commonly Used Endodontic Irrigants on the Setting Time and Metal Composition of Various Base Endodontic Sealers. Polymers. 2022; 14(1):27. https://doi.org/10.3390/polym14010027
Chicago/Turabian StyleJose, Jerry, Kavalipurapu Venkata Teja, Manish Ranjan, Roshan Noor Mohamed, Mohammad Khursheed Alam, Deepti Shrivastava, Valentino Natoli, Anil Kumar Nagarajappa, Krishnamachari Janani, and Kumar Chandan Srivastava. 2022. "Influence of Commonly Used Endodontic Irrigants on the Setting Time and Metal Composition of Various Base Endodontic Sealers" Polymers 14, no. 1: 27. https://doi.org/10.3390/polym14010027
APA StyleJose, J., Teja, K. V., Ranjan, M., Mohamed, R. N., Alam, M. K., Shrivastava, D., Natoli, V., Nagarajappa, A. K., Janani, K., & Srivastava, K. C. (2022). Influence of Commonly Used Endodontic Irrigants on the Setting Time and Metal Composition of Various Base Endodontic Sealers. Polymers, 14(1), 27. https://doi.org/10.3390/polym14010027










