Dielectric Relaxation in the Hybrid Epoxy/MWCNT/MnFe2O4 Composites
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
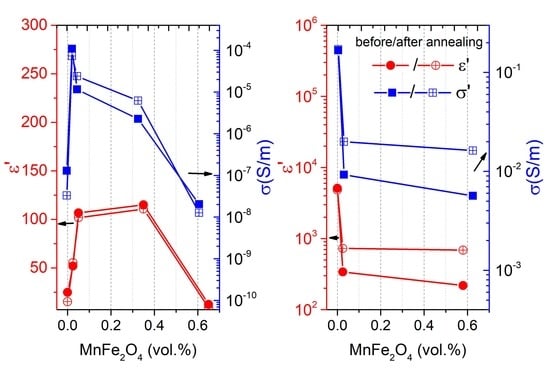
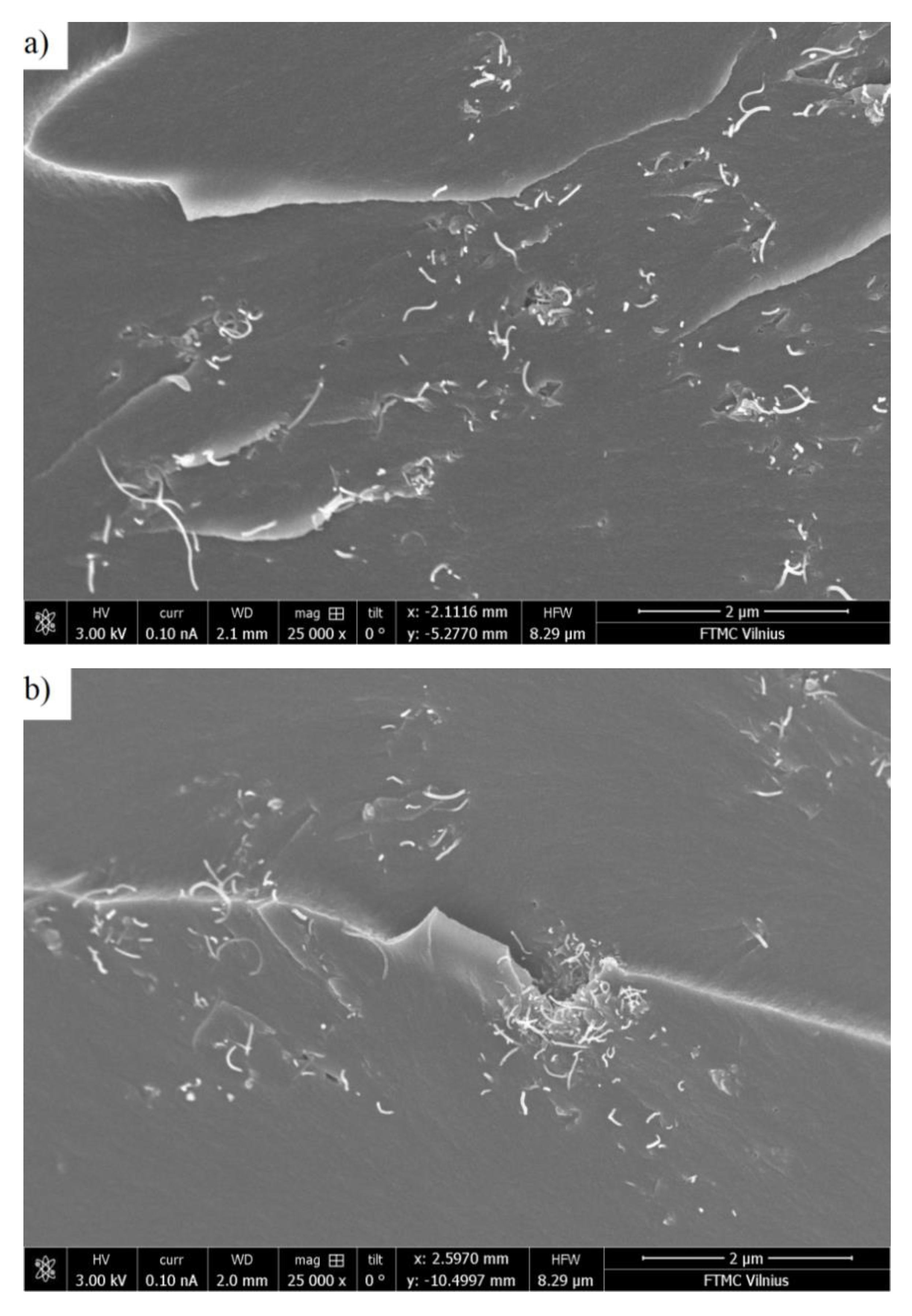
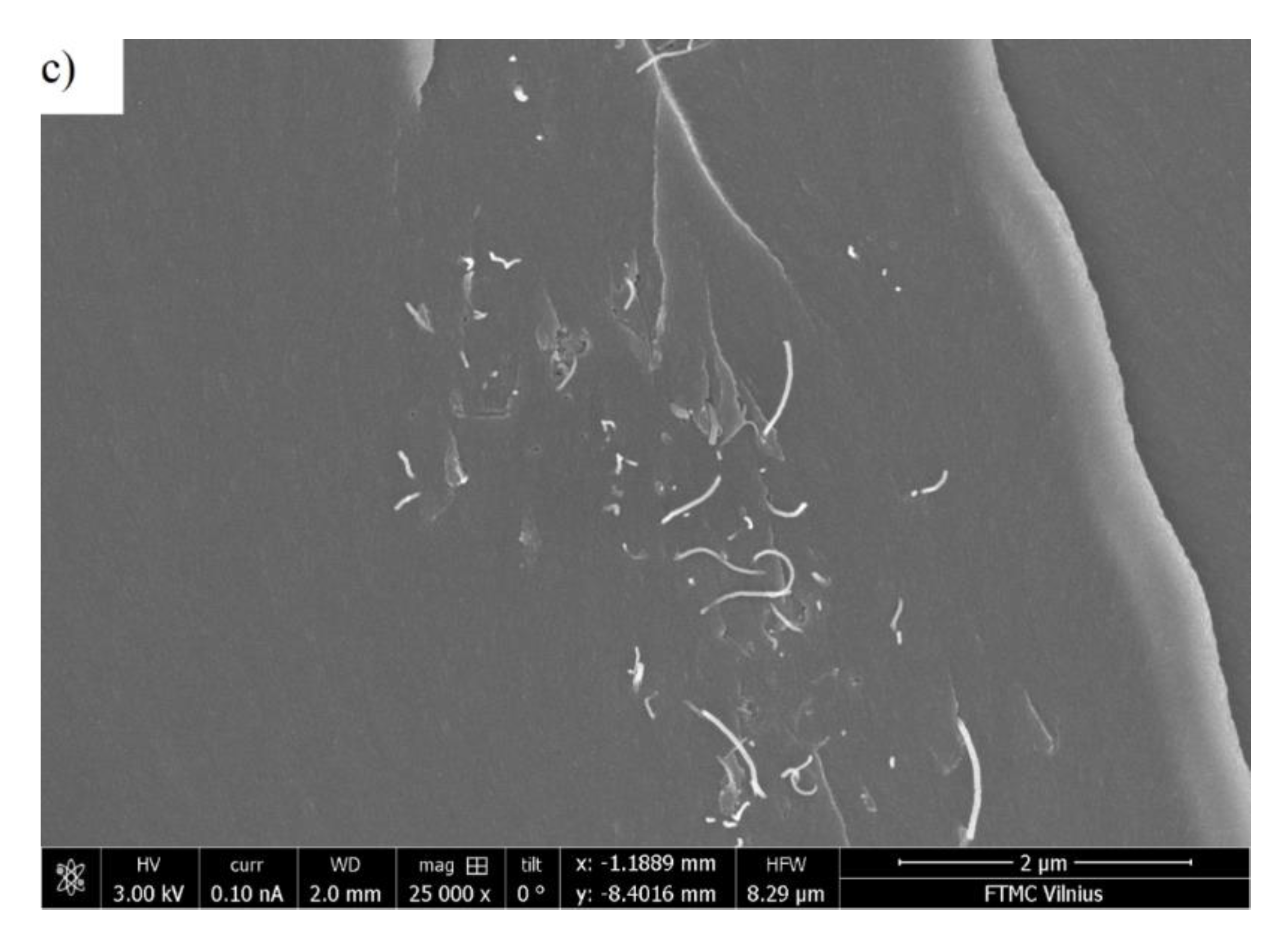
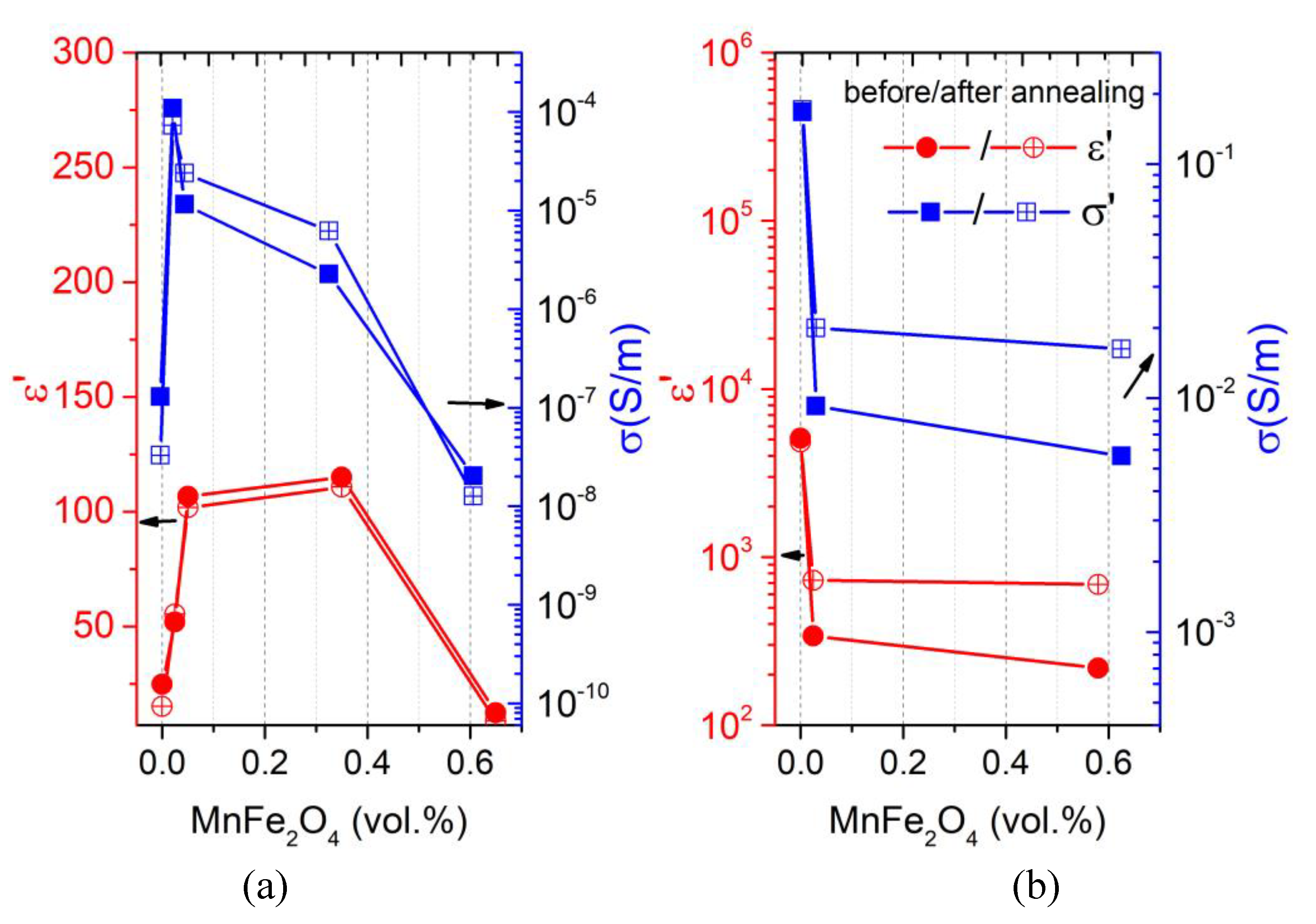
3.1. Room-Temperature Properties
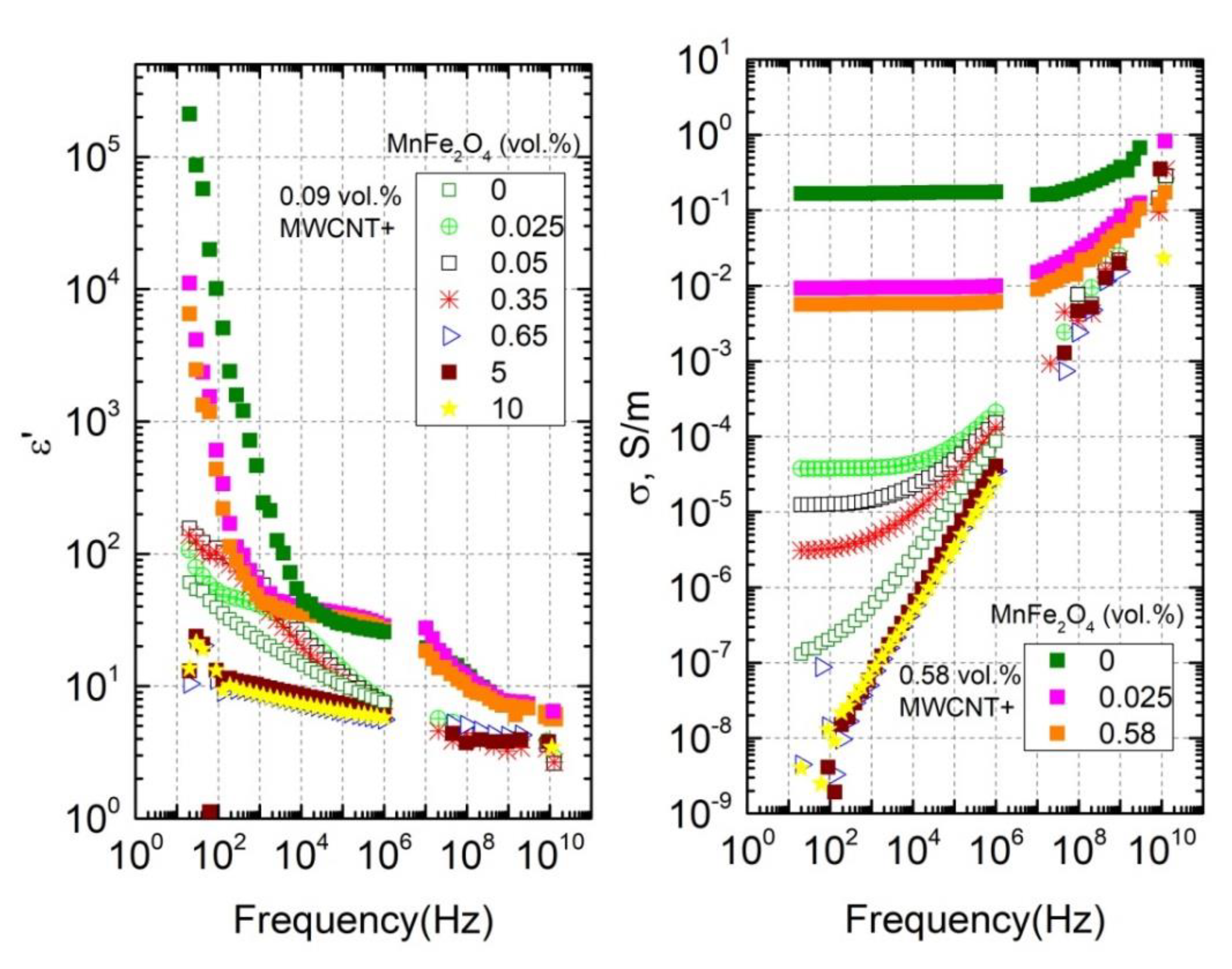
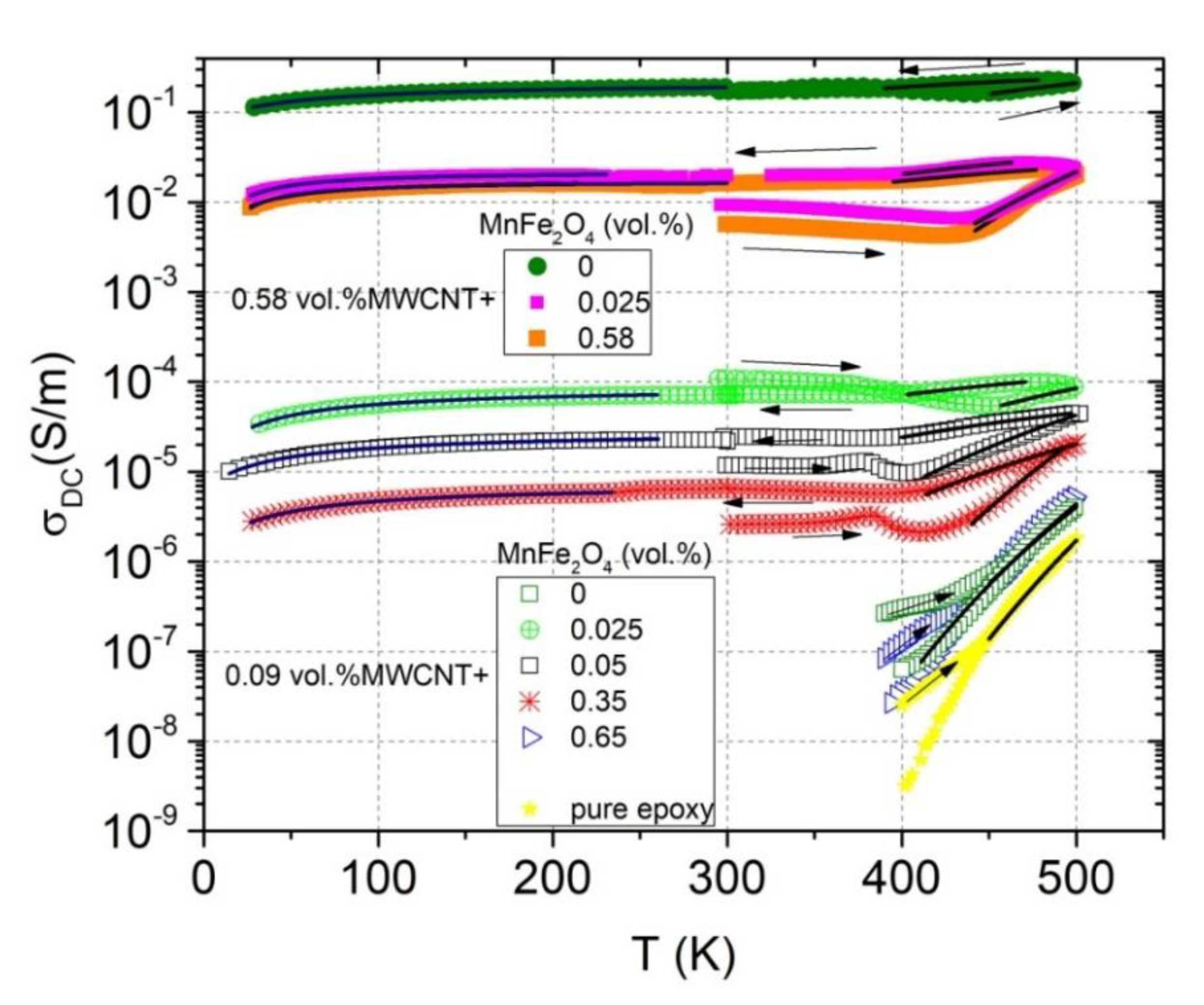
3.2. Temperature-Dependent Region
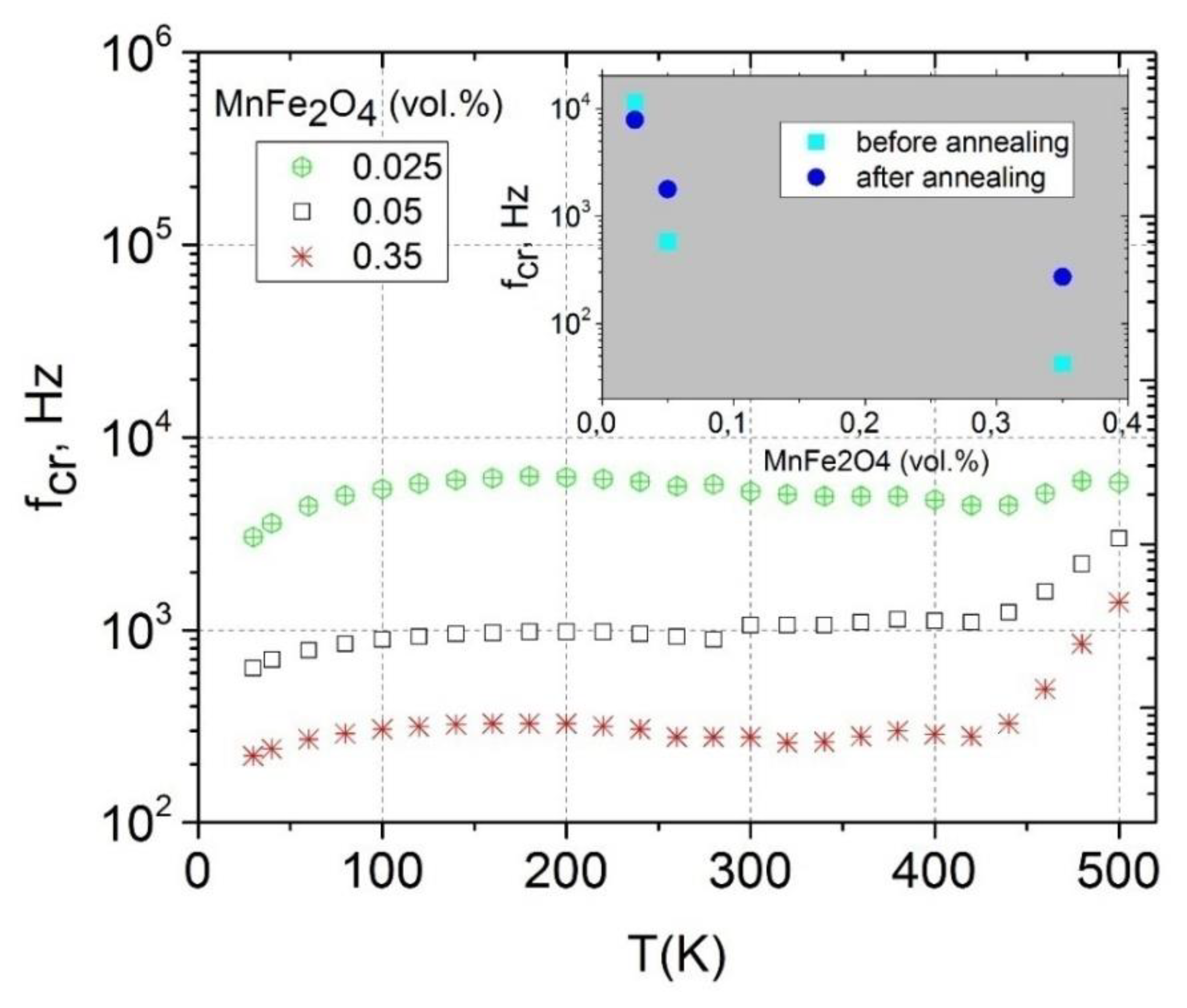
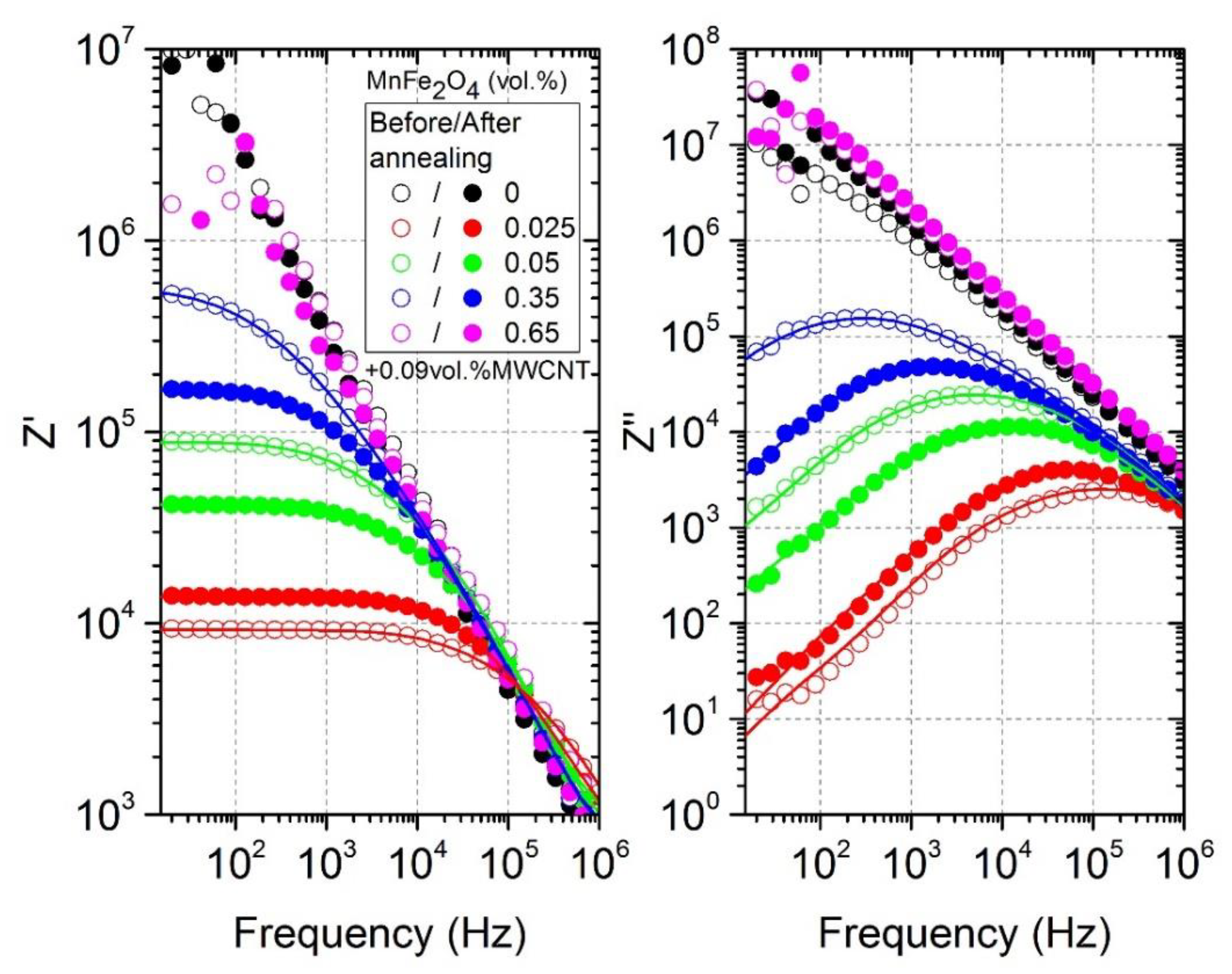
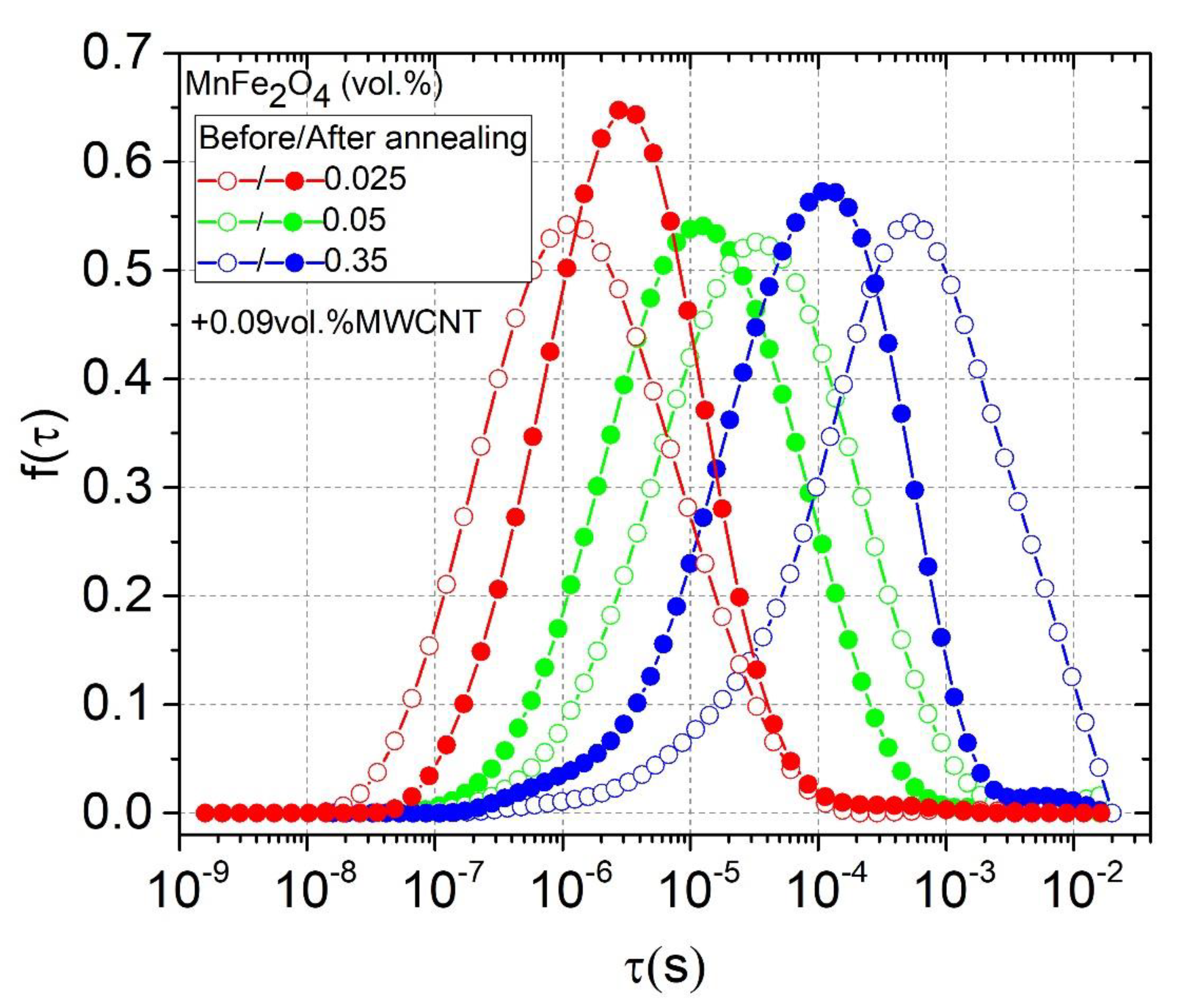
3.3. Relaxation Time Distributions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kango, S.; Kalia, J.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic-inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Cheng, C.; Chen, Z.; Huang, Z.; Zhang, C.; Tusiime, R.; Zhou, J.; Sun, Z.; Liu, Y.; Yu, M.; Zhang, H. Simultaneously improving mode I and mode II fracture toughness of the carbon fiber/epoxy composite laminates via interleaved with uniformly aligned PES fiber webs. Compos. Part A 2020, 129, 105696. [Google Scholar] [CrossRef]
- Zhang, R.; Shi, X.; Tang, Li.; Liu, Z.; Zhang, J.; Fuo, Y.; Gu, J. Thermally conductive and insulating composites by synchronously incorporating Si-sol functionalized glass fibers and BN fillers. Chin. J. Polym. Sci. 2019. [Google Scholar] [CrossRef]
- Sandler, J.; Schaffer, M.S.P.; Prasse, T.; Bauhofer, W.; Schulte, K.; Windle, A.H. Development of a dispersion process for carbon nanotubes in an epoxy matrix and the resulting electrical properties. Polymer 1999, 40, 5967–5971. [Google Scholar] [CrossRef]
- Costa, P.; Goncalves, S.; Mora, H.; Carabineiro, S.A.C.; Viana, S.C.; Lanceros-Mendez, J. Highly sensitive piezoresistive graphene-based stretchable composites for sensing applications. Acs Appl. Mater. Interfaces 2019, 11, 46286–46295. [Google Scholar] [CrossRef]
- Daneshjhan, A.; Vij, S.; Siegel, A.P.; Agarival, M. Polyethermide/carbon black composites sensors demonstrate selective detection of medium-chain aldehydes including nonal. Chem. Eng. J. 2020, 383, 123104. [Google Scholar] [CrossRef]
- Mtz Enriques, A.I.; Padmasree, K.P.; Oliva, A.I.; Gomez-Solis, C.; Coutino-Gonzalez, E.; Garcia, C.R.; Esparza, D.; Oliva, J. Tailoring the detection sensitivity of graphene based flexible smoking sensors by decorating with ceramic microparticles. Sens. Actuators B 2020, 305, 127466. [Google Scholar] [CrossRef]
- Wang, X.; Chen, D.L.; Zhang, W.S.; Zhang, L.; Fan, X.; Coi, Z.B.; Zhu, M.H. Experimental and theoretical evaluations of the interfacial interaction between carbon nanotubes and carboxylated butadiene nitrile rubber: Mechanical and damping properties. Mater. Des. 2020, 186, 108318. [Google Scholar]
- Glavan, G.; Kettl, W.; Brunhuber, A.; Shamonin, M.; Drevensek-Olenik, I. Effect of material composition on tunable surface roughness of magnetoactive elastomers. Polymers 2019, 11, 594. [Google Scholar] [CrossRef]
- Chen, C.T.; Wang, L.; Liu, X.M.; Yang, W.L.; Lin, J.Q.; Chen, G.R.; Yang, X.R. K0.5Na0.5NbO3-SrTiO3/PVDF polymer composite film with low remnant polarization and high discharge energy storage density. Polymers 2019, 11, 310. [Google Scholar] [CrossRef]
- Gao, Z.; Xu, B.; Ma, M.; Feng, A.; Zhang, Y.; Liu, X.; Jia, Z.; Wu, G. Electrostatic self-assembly synthesis of ZnFe2O4 quantum dots (ZnFe2O4@C) and electromagnetic microwave absorption. Compos. Part B 2019, 179, 107417. [Google Scholar] [CrossRef]
- Chen, J.; Du, X.C.; Zhang, W.B.; Yang, J.H.; Zhang, N.; Huang, T.; Wang, Y. Synergistic effect of carbon nanotubes and carbon black on electrical conductivity of PA6/ABS blend. Compos. Sci. Technol. 2013, 81, 1–8. [Google Scholar] [CrossRef]
- Szeluga, U.; Kumanek, B.; Trebicka, B. Synergy in hybrid polymer/nanocarbon composites: A review. Compos. Part A 2015, 73, 204–231. [Google Scholar] [CrossRef]
- Dang, Z.M.; Zheng, M.S.; Zha, J.W. 1D/2D carbon nanomaterial-polymer dielectric composites with high permittivity for power energy storage applications. Small 2016, 12, 1688–1701. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.; Mendez, N.; Gonzalez, M.; Baselga, S.; Pozuelo, J. Synergistic effect of magnetite nanoparticles and carbon nanofibres in electromagnetic absorbing composites. Carbon 2014, 74, 63–72. [Google Scholar] [CrossRef]
- Sun, S.H.; Zeng, H.; Robinson, D.B.; Raoux, J.; Rice, P.M.; Wang, S.X.; Li, G.X. Monodisperse MnFe2O4 (M=Fe, Co, Mn) nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef]
- Gloag, L.; Mehdipour, M.; Chen, D.; Tilley, R.D.; Gooding, J.J. Advances in the application of magnet nanoparticles for sensing. Adv. Mater. 2019, 31, 1904385. [Google Scholar]
- Sriplai, N.; Mangayil, R.; Pammo, A.; Santala, V.; Tuukannen, S.; Pinitsoontorn, S. Enhancing piezoelectric properties of bacterial cellulose films by incorporation of MnFe2O4 nanoparticles. Carbohydr. Polym. 2020, 231, 115730. [Google Scholar] [CrossRef]
- Yin, P.; Zhang, L.; Wang, J.; Feng, X.; Zhao, L.; Rao, H.; Wang, Y.; Dai, J. Preparation of SiO2-MnFe2O4 composites via one-pot hydrothermal synthesis method and microwave absorption investigation in S-Band. Molecules 2019, 24, 2605. [Google Scholar] [CrossRef]
- Meisak, D.; Macutkevic, J.; Selskis, A.; Banys, J.; Kuzhir, P. Dielectric properties and electrical percolation in MnFe2O4/epoxy resin composites. Phys. Status Solidi (A) 2019. [Google Scholar] [CrossRef]
- Pang, H.; Sahu, R.P.; Duan, Y.; Puri, I.L. MnFe2O4-coated carbon nanotubes with enhanced microwave absorption: Effect of CNT content and hydrothermal reaction time. Diam. Relat. Mater. 2019, 96, 13–43. [Google Scholar] [CrossRef]
- Available online: https://www.us-nano.com/inc/sdetail/7019 (accessed on 20 March 2020).
- Okotrub, A.V.; Bulusheva, A.G.; Kudashov, A.G.; Belavin, V.V. Komogortsev, S.V.; Arrays of carbon nanotubes aligned perpendicular to the substrate surface: Anisotropy of structure and properties. Nanotechnologies Russ. 2008, 3, 191–200. [Google Scholar] [CrossRef]
- Bychanok, D.; Gorokhov, G.; Meisak, D.; Kuzhir, P.; Maksimenko, S.; Wang, Y.; Han, Z.; Gao, X.; Yue, H. Desing of carbon nanotube-based broadband radar absorber for Ka-band frequency range. Prog. Electromagn. Res. 2017, 53, 9–16. [Google Scholar] [CrossRef][Green Version]
- Available online: https://www.sigmaaldrich.com/catalog/product/aldrich/90460?lang=en®ion=LT (accessed on 20 March 2020).
- Bertasius, P.; Meisak, D.; Macutkevic, J.; Kuzhir, P.; Selskis, A.; Volnyanko, E.; Banys, J. Fine tuning of electrical transport and dielectric properties of epoxy/carbon nanotubes composites via magnesium oxide additives. Polymers 2019, 11, 2044. [Google Scholar] [CrossRef] [PubMed]
- Grigas, J. Microwave Dielectric Spectroscopy of Ferroelectrics and Related Materials, 1st ed.; Gordon and Breach Publishers: London, UK, 1996; pp. 1–416. [Google Scholar]
- Macutkevic, J.; Kuzhir, P.; Paddubskaya, A.; Maksimenko, S.; Banys, J.; Celzard, A.; Fierro, V.; Bistarelli, S.; Cataldo, A.; Micciulla, F. Electrical transport in carbon black-epoxy resin composites at different temperatures. J. Appl. Phys. 2013, 114, 033707. [Google Scholar] [CrossRef]
- Sheng, P.; Sichel, E.K.; Gittleman, J.K. Fluctuation-induced tunneling conduction in carbon-polyvinylchloride composites. Phys. Rev. Lett. 1978, 40, 1197. [Google Scholar] [CrossRef]
- Jager, K.M.; McQueen, D.H.; Vilcakova, J. Ac conductance and capacitance of carbon black polymer composites during thermal cycling and isothermal annealing. J. Phys. D: Appl. Phys. 2002, 35, 1068–1075. [Google Scholar] [CrossRef]
- Macutkevic, J.; Banys, J.; Matulis, A. Determination of the distribution of relaxation times from dielectric spectra. Nonlinear Anal. Model. Control 2004, 9, 75–84. [Google Scholar] [CrossRef]
- Bychanok, D.; Angelova, P.; Paddubskaya, A.; Meisak, D.; Shashkova, L.; Demidenko, M.; Plyushch, A.; Ivanov, E.; Krastev, R.; Kotsilkova, R.; et al. Terahertz absorption in graphite nanoplatelets/polylactic acid composites. J. Phys. D: Appl. Phys. 2018, 51, 145307. [Google Scholar] [CrossRef]

| Sample | Before Annealing | After Annealing | ||
|---|---|---|---|---|
| EA/kB, K | EA/kB, K | |||
| Pure epoxy | 1.3 × 104 | 11,363 | 1.6 × 105 | 12,433 |
| 0.09 vol.% MWCNT | 2.2 × 102 | 8892 | 5.6 × 102 | 9338 |
| 0.09 vol.% MWCNT + 0.025 vol.% MnFe2O4 | 9.5 × 10−3 | 2357 | 6.7 × 10−4 | 898 |
| 0.09 vol.% MWCNT + 0.05 vol.% MnFe2O4 | 7.5 × 10−2 | 3736 | 5.7 × 10−4 | 1266 |
| 0.09 vol.% MWCNT + 0.35 vol.% MnFe2O4 | 2.0 × 102 | 7998 | 1.0 × 10−2 | 3103 |
| 0.09 vol.% MWCNT + 0.65 vol.% MnFe2O4 | 4.1 × 104 | 11,380 | 1.7 × 103 | 9755 |
| 0.58 vol.% MWCNT | 3.0 | 1272 | 0.58 × 10−1 | 446 |
| 0.58 vol.% MWCNT + 0.025 vol.% MnFe2O4 | 5.0 × 102 | 5056 | 1.9 × 10−1 | 897 |
| 0.58 vol.% MWCNT +0.58 vol.% MnFe2O4 | 1.6 × 103 | 5623 | 1.1 × 10−1 | 724 |
| Sample | ||||
|---|---|---|---|---|
| 0.09 vol.% MWCNT + 0.025 vol.% MnFe2O4 | 8.7 × 10−5 | 55.3 | 26.2 | 2.1 |
| 0.09 vol.% MWCNT + 0.05 vol.% MnFe2O4 | 2.8 × 10−5 | 59.5 | 40.4 | 1.5 |
| 0.09 vol.% MWCNT + 0.35 vol.% MnFe2O4 | 7.3 × 10−6 | 59.3 | 32.9 | 1.8 |
| 0.58 vol.% MWCNT | 2.1 × 10−1 | 49.2 | 47.4 | 1.0 |
| 0.58 vol.% MWCNT + 0.025 vol.% MnFe2O4 | 2.2 × 10−2 | 20.7 | 5.3 | 3.9 |
| 0.58 vol.% MWCNT + 0.58 vol.% MnFe2O4 | 1.8 × 10−2 | 27.3 | 11.6 | 2.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meisak, D.; Macutkevic, J.; Plyushch, A.; Kuzhir, P.; Selskis, A.; Banys, J. Dielectric Relaxation in the Hybrid Epoxy/MWCNT/MnFe2O4 Composites. Polymers 2020, 12, 697. https://doi.org/10.3390/polym12030697
Meisak D, Macutkevic J, Plyushch A, Kuzhir P, Selskis A, Banys J. Dielectric Relaxation in the Hybrid Epoxy/MWCNT/MnFe2O4 Composites. Polymers. 2020; 12(3):697. https://doi.org/10.3390/polym12030697
Chicago/Turabian StyleMeisak, Darya, Jan Macutkevic, Artyom Plyushch, Polina Kuzhir, Algirdas Selskis, and Juras Banys. 2020. "Dielectric Relaxation in the Hybrid Epoxy/MWCNT/MnFe2O4 Composites" Polymers 12, no. 3: 697. https://doi.org/10.3390/polym12030697
APA StyleMeisak, D., Macutkevic, J., Plyushch, A., Kuzhir, P., Selskis, A., & Banys, J. (2020). Dielectric Relaxation in the Hybrid Epoxy/MWCNT/MnFe2O4 Composites. Polymers, 12(3), 697. https://doi.org/10.3390/polym12030697