Effect of Fructose and Ascorbic Acid on the Performance of Cross-Linked Fish Gelatin Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Fish Gelatin
2.3. Characterization of Fish Gelatin
2.4. Film Preparationc
2.5. Assessment of Cross-linking Extension
2.6. Characterization of Gelatin Films
2.7. Statistical Analysis
3. Results and Discussion
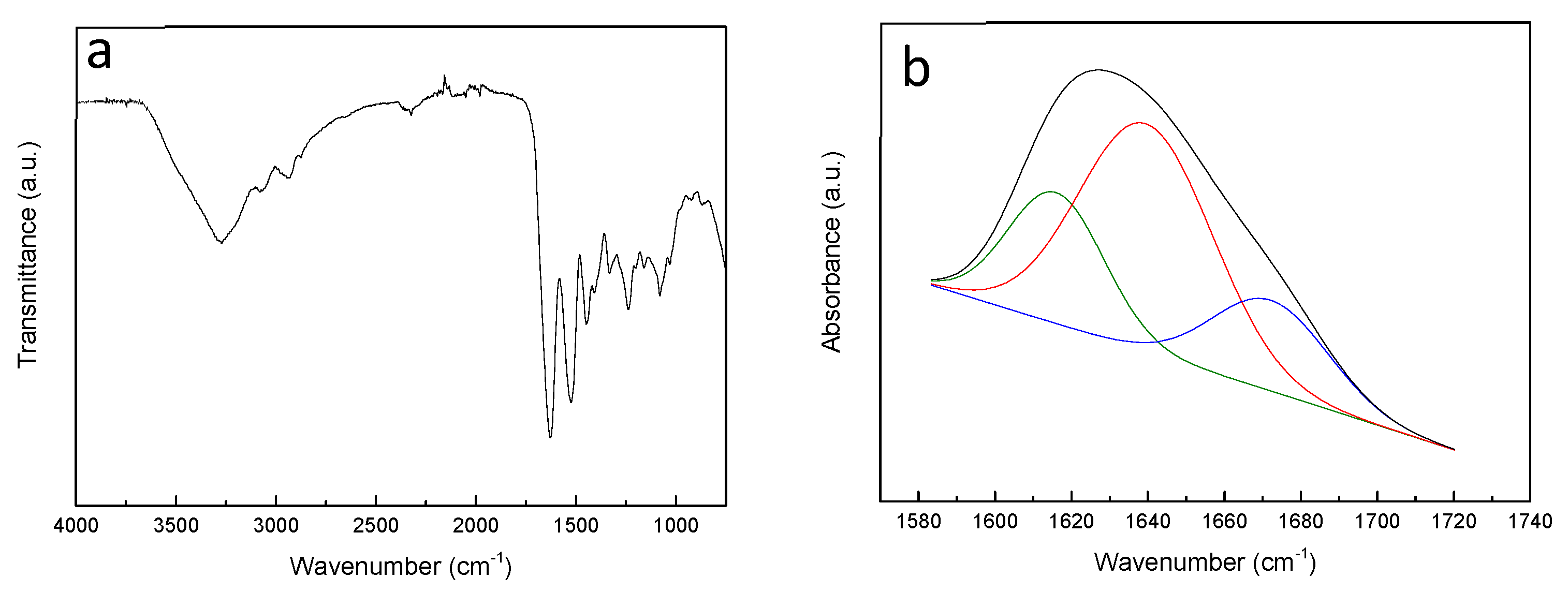
3.1. Characterization of Fish Gelatin
3.2. Assessment of Cross-linking Extension
3.3. Characterization of Gelatin Films
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xiong, X.; Yu, I.K.M.; Tsang, D.C.W.; Bolan, N.S.; Ok, Y.S.; Igalavithana, A.D.; Kirkham, M.B.; Kim, K.; Vikrant, K. Value-added chemicals from food supply chain wastes: State-of-the-art review and future prospects. Chem. Eng. J. 2019, 375, 121983. [Google Scholar] [CrossRef]
- de la Caba, K.; Guerrero, P.; Trung, T.S.; Cruz-Romero, M.; Kerry, J.P.; Fluhr, J.; Maurer, M.; Kruijssen, F.; Albalat, A.; Bunting, S.; et al. From seafood waste to active seafood packaging: An emerging opportunity of the circular economy. J. Clean. Prod. 2019, 208, 86–98. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Ramakrishnan, V.V.; Brooks, M.S.; Budge, S.M.; Dave, D. Fish Processing Wastes as a Potential Source of Proteins, Amino Acids and Oils: A Critical Review. J. Microb. Biochem. Technol. 2013, 5, 107–129. [Google Scholar]
- Available online: http://www.fao.org/news/archive/news-by-date/2018/en/?Page=2&ipp=10&txdynalistpi1%5Bpar%5D=YToxOntzOjE6IkwiO3M6MToiMCI7fQ%3D%3D (accessed on 3 March 2020).
- Available online: http://mseafood.vasep.com.vn/673/onecontent/sector-profiles.htm (accessed on 3 March 2020).
- Gómez-Guillén, M.C.; Pérez-Mateos, M.; Gómez-Estaca, J.; López-Caballero, E.; Giménez, B.; Montero, P. Fish gelatin: A renewable material for developing active biodegradable films. Trends Food Sci. Technol. 2009, 20, 3–16. [Google Scholar] [CrossRef]
- Etxabide, A.; Guerrero, P.; de la Caba, K. A novel approach to manufacture porous biocomposites using extrusion and injection moulding. Eur. Polym. J. 2016, 82, 324–333. [Google Scholar] [CrossRef]
- Etxabide, A.; Urdanpilleta, M.; Gómez-Arriaran, I.; de la Caba, K.; Guerrero, P. Effect of pH and lactose on cross-linking extension and structure of fish gelatin fish. React. Funct. Polym. 2017, 117, 140–146. [Google Scholar] [CrossRef]
- Kchaou, H.; Benbettaieb, N.; Jridi, M.; Abdelhedi, O.; Karbowiak, T.; Brachais, C.H.; Léonard, M.L.; Debeaufort, F.; Nasri, M. Enhancement of structural, functional and antioxidant properties of fish gelatin films using Maillard reactions. Food Hydrocoll. 2018, 83, 326–339. [Google Scholar] [CrossRef]
- Dills, W.L. Protein fructosylation: Fructose the Maillard reaction. Am. J. Clin. Nutr. 1993, 58, 779S–787S. [Google Scholar] [CrossRef]
- Pischetsrieder, M. Reaction of L-ascorbic acid with L-arginine derivatives. J. Agric. Food Chem. 1996, 44, 2081–2085. [Google Scholar] [CrossRef]
- Schalkwijk, C.G.; Stehouwer, C.D.A.; van Hinsbergh, V.W.M. Fructose-mediated non-enzymatic glycation: Sweet coupling or bad modification. Diabetes Metab. Res. Rev. 2004, 20, 369–382. [Google Scholar] [CrossRef]
- Larisch, B.; Gross, U.; Pischetsrieder, M. On the reaction of L-ascorbic acid with propylamine under various conditions: Quantification of the main products by HPLC/DAD. Z Lebensm. Unters. Forsch. A-Food Res. Technol. 1998, 206, 333–337. [Google Scholar] [CrossRef]
- Tiller, J.; Berlin, P.; Klemm, D. A novel efficient enzyme-immobilization reaction on NH2 polymers by means of L-ascorbic acid. Biotechnol. Appl. Biochem. 1999, 30, 155–162. [Google Scholar] [PubMed]
- Koubaa, M.; Roohinehad, S.; Mungure, T.E.; El-Din, B.A.; Greiner, R.; Mallikarjunan, K. Effect of emerging processing technologies on Maillard reactions. Encycl. Food Chem. 2019, 2, 76–82. [Google Scholar]
- Dewhirst, R.A.; Clarkson, G.J.J.; Rothwell, S.D.; Fry, S.C. Novel insights into ascorbate retention and degradation during the washing and post-harvest storage of spinach and other salad leaves. Food Chem. 2017, 233, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Nooshkam, M.; Varidi, M.; Bashash, M. The Maillard reaction products as food-born antioxidant and antibrowning agents in model and real food systems. Food Chem. 2019, 275, 644–660. [Google Scholar] [CrossRef] [PubMed]
- STANDARD TESTING METHODS FOR EDIBLE GELATIN. Available online: http://www.gelatin-gmia.com/uploads/1/1/8/4/118450438/gmia_official_methods_2019.pdf (accessed on 3 March 2020).
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Extraction and physico-chemical characterisation of Nile perch (Lates niloticus) skin and bone gelatin. Food Hydrocoll. 2004, 581–592. [Google Scholar] [CrossRef]
- Horwitz, W.; Chichilo, P.; Reynolds, H.A. Official methods of analysis of the Association of Official Analytical Chemists. In Official methods of analysis of the Association of Official Analytical Chemists; Horwitz, W., Chichilo, P., Reynolds, H., Eds.; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Panzavolta, S.; Gioffrè, M.; Focarete, M.L.; Gualandi, C.; Foroni, L.; Bigi, A. Electrospun gelatin nanofibers: Optimization of genipin cross-linking to preserve fiber morphology after exposure to wáter. Acta Biomater. 2011, 7, 1702–1709. [Google Scholar] [CrossRef]
- ASTM D1708-13. Standard test method for tensile properties of plastics by use of microtensile specimens. In Annual book of ASTM standards; American Society of Testing and Materials: Philadelphia, PA, USA, 2013. [Google Scholar]
- Giménez, B.; Gómez-Estaca, J.; Alemán, A.; Gómez-Guillén, M.C.; Montero, M.P. Physico-chemical and film forming properties of giant squid (Dosidicus gigas) gelatin. Food Hydrocoll. 2009, 23, 585–592. [Google Scholar] [CrossRef]
- Santos, J.P.; Esquerdo, V.M.; Moura, C.M.; Pinto, L.A.A. Crosslinking agents effect on gelatins from carp and tilapia skins and in their biopolymeric films. Colloid Surf. A-Physicochem. Eng. Asp. 2018, 539, 184–191. [Google Scholar] [CrossRef]
- Li, H.; Liu, B.L.; Gao, L.Z.; Chen, H.L. Studies on bullfrog skin collagen. Food Chem. 2004, 84, 65–69. [Google Scholar] [CrossRef]
- Coppola, M.; Djabourov, M.; Ferrand, M. Unified phase diagram of gelatin films plasticized by hydrogen bonded liquids. Polymer 2012, 53, 1483–1493. [Google Scholar] [CrossRef]
- Duconseille, A.; Wien, F.; Audonnet, F.; Traore, A.; Refregiers, M.; Astruc, T.; Santé-Lhoutellier, V. The effect of origin if the gelatin and ageing on the secondary structure and water dissolution. Food Hydrocoll. 2017, 66, 378–388. [Google Scholar] [CrossRef]
- Liu, H.; Li, D.; Guo, S. Rheological properties of channel catfish (Ictalurus punctaus) gelatine from fish skins preserved by different methods. LWT-Food Sci. Technol. 2008, 41, 414–419. [Google Scholar] [CrossRef]
- Etxabide, A.; Urdanpilleta, M.; Guerrero, P.; de la Caba, K. Effects of cross-linking in nanostructure and physicochemical properties of fish gelatins for bio-applications. React. Funct. Polym. 2015, 94, 55–62. [Google Scholar] [CrossRef]
- Uranga, J.; Puertas, A.I.; Etxabide, A.; Dueñas, M.T.; Guerrero, P.; de la Caba, K. Citric acid-incorporated fish gelatin/chitosan composite films. Food Hydrocoll. 2019, 86, 95–103. [Google Scholar] [CrossRef]
- Devi, A.F.; Buckow, R.; Singh, T.; Hemar, Y.; Kasapis, S. Colour change and proteolysis of skim milk during high pressure thermal-processing. J. Food Eng. 2015, 147, 102–111. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.S. Effect of reaction pH on enolization and racemization reactions of glucose and fructose on heating with amino acid enantiomers and formation of melanoidins as result of the Maillard reaction. Food Chem. 2008, 108, 582–592. [Google Scholar] [CrossRef]
- Garrido, T.; Leceta, I.; Cabezudo, S.; Guerrero, P.; de la Caba, K. Tailoring soy protein film properties by selecting casting or compression as processing methods. Eur. Polym. J. 2016, 85, 499–507. [Google Scholar] [CrossRef]
- Nur Hanani, Z.A.; Cheng, F.Y.; Nor-Khaizura, M.A.R. Effect of pomegranate (Punica granatum L.) peel powder on the antioxidant and antimicrobial properties of fish gelatin films as active packaging. Food Hydrocoll. 2019, 89, 253–259. [Google Scholar] [CrossRef]
- Hazaveh, P.; Nafchi, A.M.; Abbaspour, H. The effects of sugars on moisture sorption isotherm and functional properties of cold water fish gelatin films. Int. J. Biol. Macromol. 2015, 79, 370–376. [Google Scholar] [CrossRef]
- Hoque, M.S.; Benjakul, S.; Prodpran, T. Properties of film from cuttlefish (Sepia pharaonis) skin gelatin incorporated with cinnamon, clove and star anise extracts. Food Hydrocoll. 2011, 25, 1085–1097. [Google Scholar] [CrossRef]



| Amino Acids | Concentration (%) |
|---|---|
| Hydroxyproline | 8.55 |
| Aspartic acid | 4.28 |
| Threonine | 2.19 |
| Serine | 2.85 |
| Glutamic acid | 7.16 |
| Proline | 11.13 |
| Glycine | 33.15 |
| Alanine | 13.58 |
| Valine | 1.77 |
| Methionine | 1.36 |
| Isoleucine | 0.76 |
| Leucine | 1.73 |
| Tyrosine | 0.50 |
| Phenylalanine | 1.52 |
| Histidine | 0.52 |
| Lysine | 2.62 |
| Arginine | 4.96 |
| Film | CE (%) |
|---|---|
| 10F | 37.58 ± 0.48a |
| 20F | 59.72 ± 1.12b |
| 10AA | 37.88 ± 1.32a |
| 20AA | 65.62 ± 1.15c |
| Film | L* | a* | b* | ΔE* | BI (%) |
|---|---|---|---|---|---|
| Control | 95.422 ± 0.285a | −0.001 ± 0.050a | 3.041 ± 0.181a | - | - |
| 10F | 92.742 ± 0.484b | −2.138 ±0.116b | 20.406 ± 1.696b | 17.705 ± 1.735a | 22.261 ± 2.329a |
| 20F | 91.252 ± 0.887c | −1.759 ±0.513c | 31.657 ± 3.208c | 28.986 ± 3.208b | 39.602 ± 5.711b |
| 10AA | 90.642 ± 0.492c | −1.510 ± 0.174c | 31.507 ± 1.719c | 28.910 ± 3.253b | 39.788 ± 3.188b |
| 20AA | 88.835 ± 0.653d | −0.800 ± 0.327d | 39.546 ± 2.248d | 37.105 ± 2.315c | 55.547 ± 5.008c |
| Film | TS (MPa) | EB (%) |
|---|---|---|
| Control | 71.78 ± 3.82a | 5.14 ± 0.17a |
| 10F | 78.62 ± 4.74b | 5.24 ± 0.89a |
| 20F | 59.74 ± 2.88c | 10.23 ± 0.46c |
| 10AA | 77.19 ± 4.10a,b | 7.93 ± 0.54b |
| 20AA | 54.25 ± 3.28c | 19.18 ± 0.88d |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrero, P.; Zugasti, I.; Etxabide, A.; Bao, H.N.D.; Trang Si, T.; Peñalba, M.; de la Caba, K. Effect of Fructose and Ascorbic Acid on the Performance of Cross-Linked Fish Gelatin Films. Polymers 2020, 12, 570. https://doi.org/10.3390/polym12030570
Guerrero P, Zugasti I, Etxabide A, Bao HND, Trang Si T, Peñalba M, de la Caba K. Effect of Fructose and Ascorbic Acid on the Performance of Cross-Linked Fish Gelatin Films. Polymers. 2020; 12(3):570. https://doi.org/10.3390/polym12030570
Chicago/Turabian StyleGuerrero, Pedro, Iraitz Zugasti, Alaitz Etxabide, Huynh Nguyen Duy Bao, Trung Trang Si, Miriam Peñalba, and Koro de la Caba. 2020. "Effect of Fructose and Ascorbic Acid on the Performance of Cross-Linked Fish Gelatin Films" Polymers 12, no. 3: 570. https://doi.org/10.3390/polym12030570
APA StyleGuerrero, P., Zugasti, I., Etxabide, A., Bao, H. N. D., Trang Si, T., Peñalba, M., & de la Caba, K. (2020). Effect of Fructose and Ascorbic Acid on the Performance of Cross-Linked Fish Gelatin Films. Polymers, 12(3), 570. https://doi.org/10.3390/polym12030570








