Inclusion of Cross-Linked Elastin in Gelatin/PEG Hydrogels Favourably Influences Fibroblast Phenotype
Abstract
1. Introduction
2. Materials and Methods
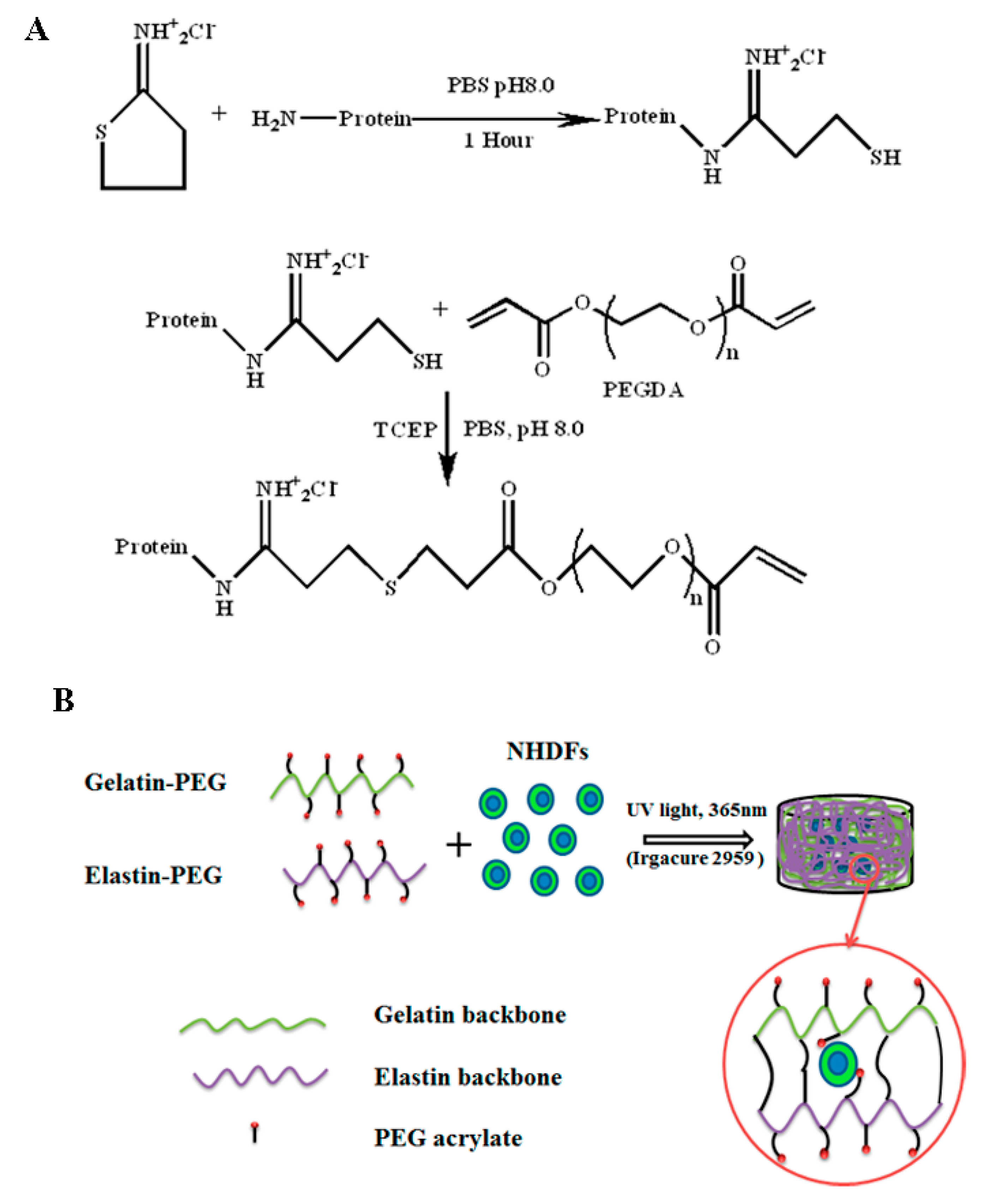
2.1. Gelatin and Elastin PEGylation
2.2. Gelatin and Elastin Hybrid PEG Hydrogel Preparation
2.3. Characterization of Hydrogels
2.4. Cell Encapsulation in Gelatin–PEG Hydrogel
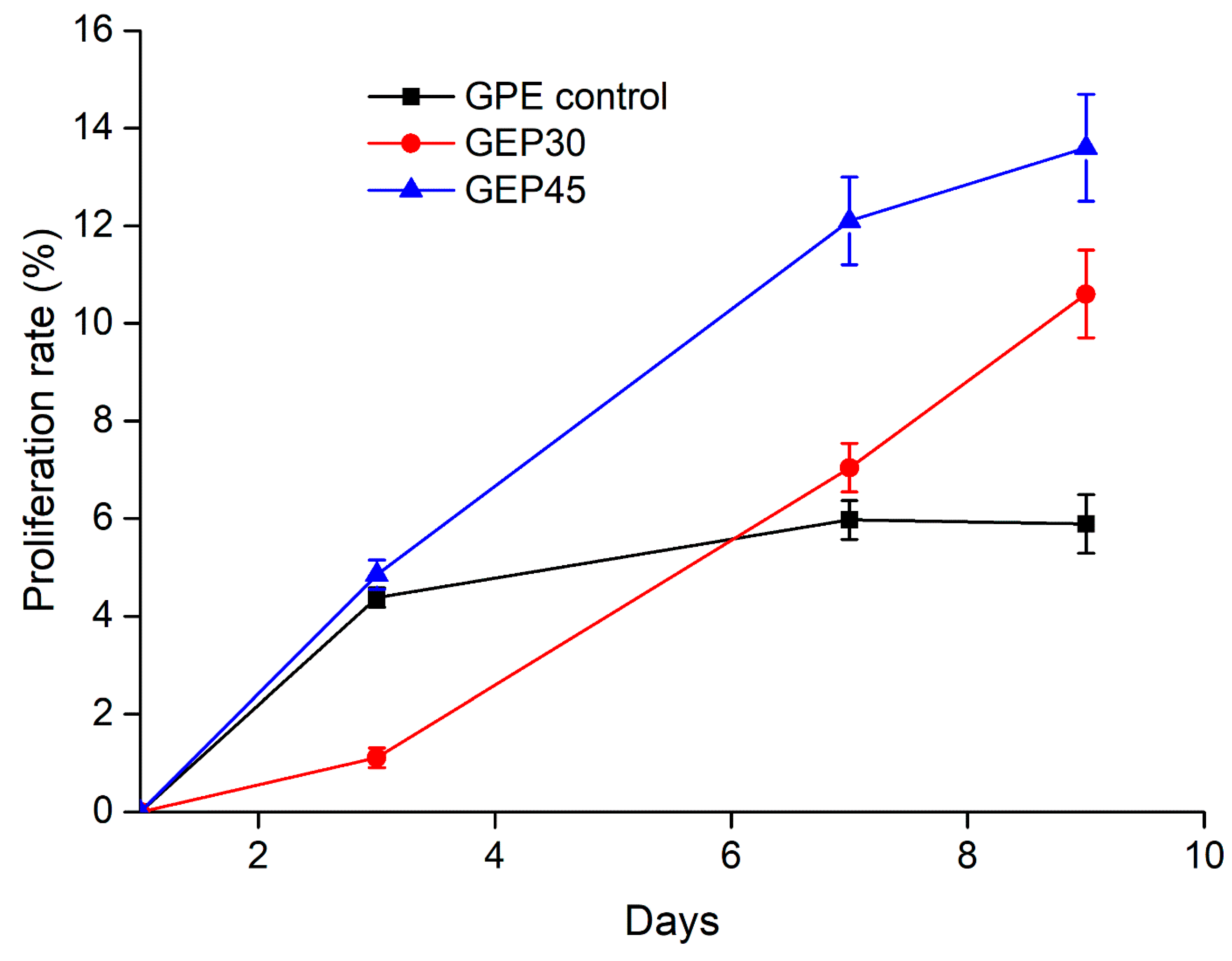
2.5. Cell Proliferation
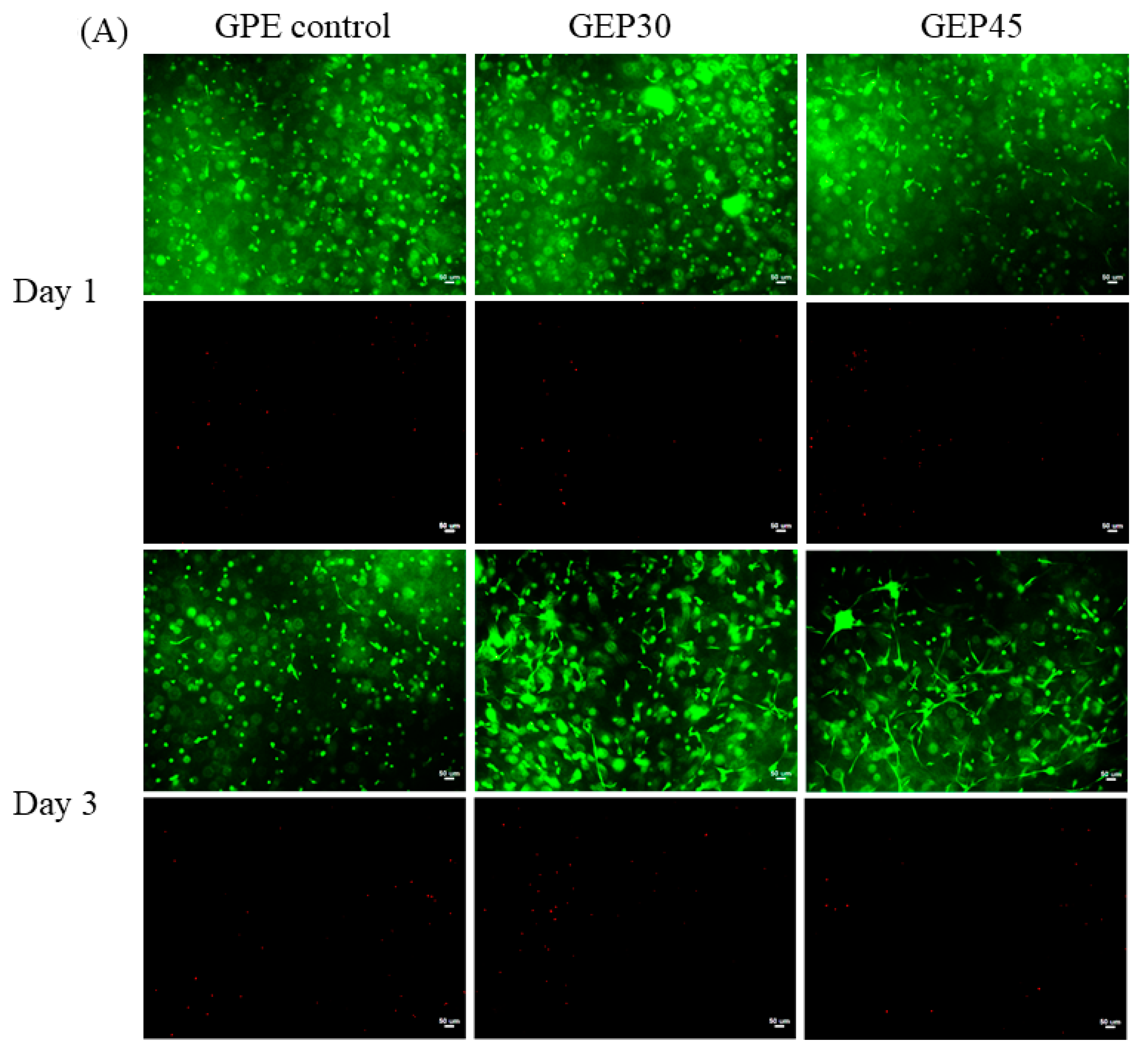
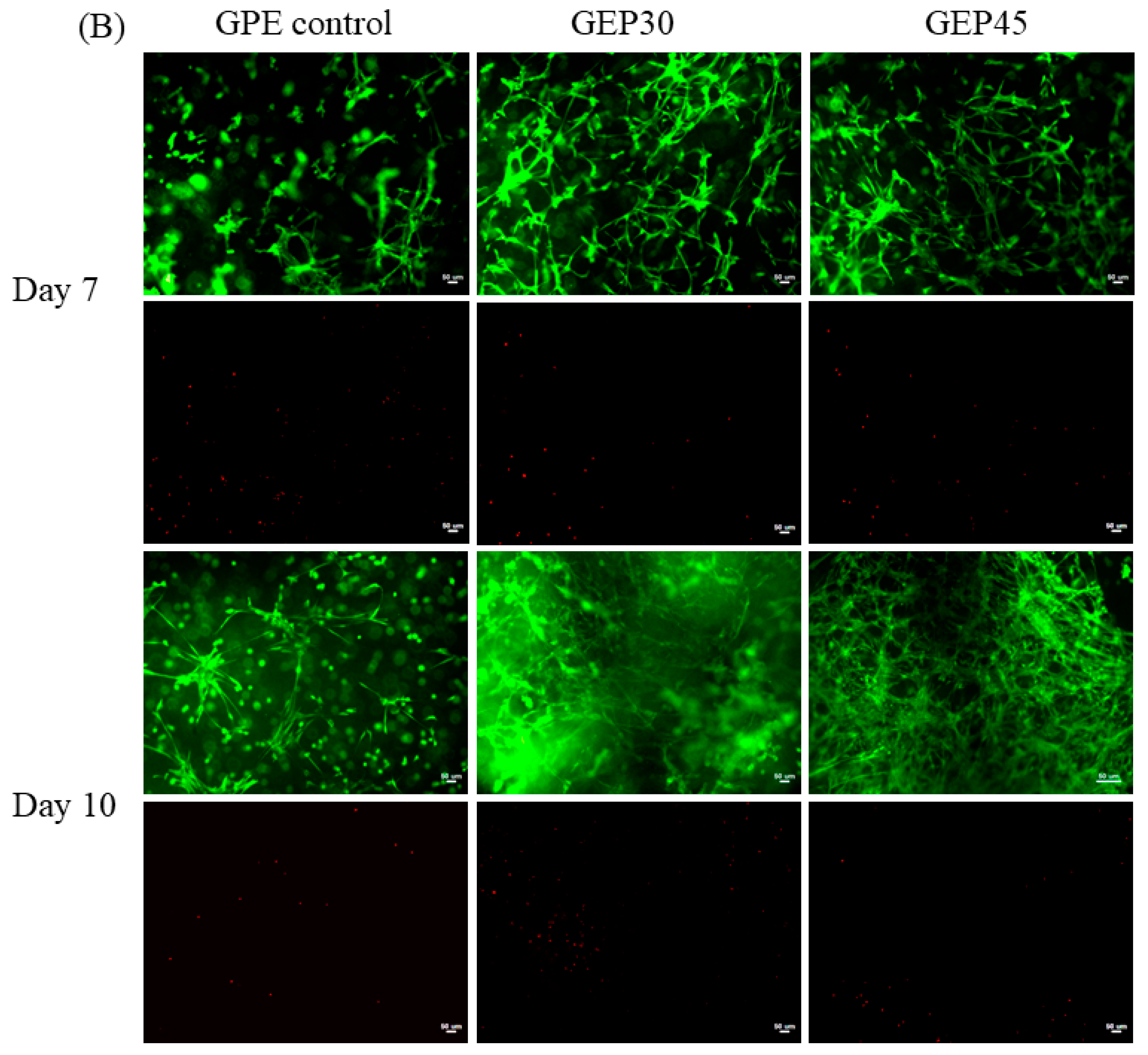
2.6. Cell Live/Dead and Cell Morphology
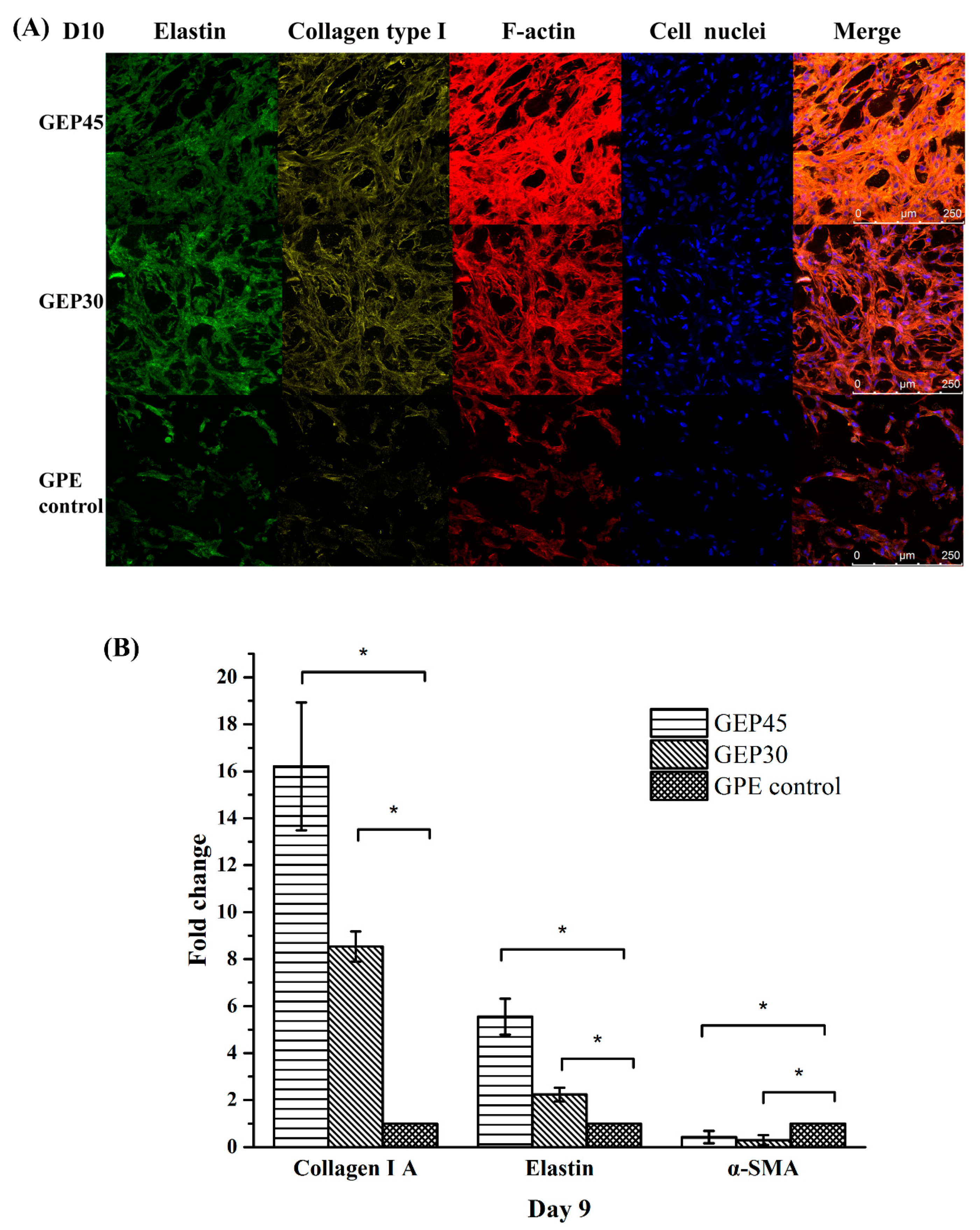
2.7. Immunofluorescence Staining of ECM Protein Deposition
2.8. Gene Expression of Encapsulated Cells
2.9. Statistical Analysis
3. Results and Discussion
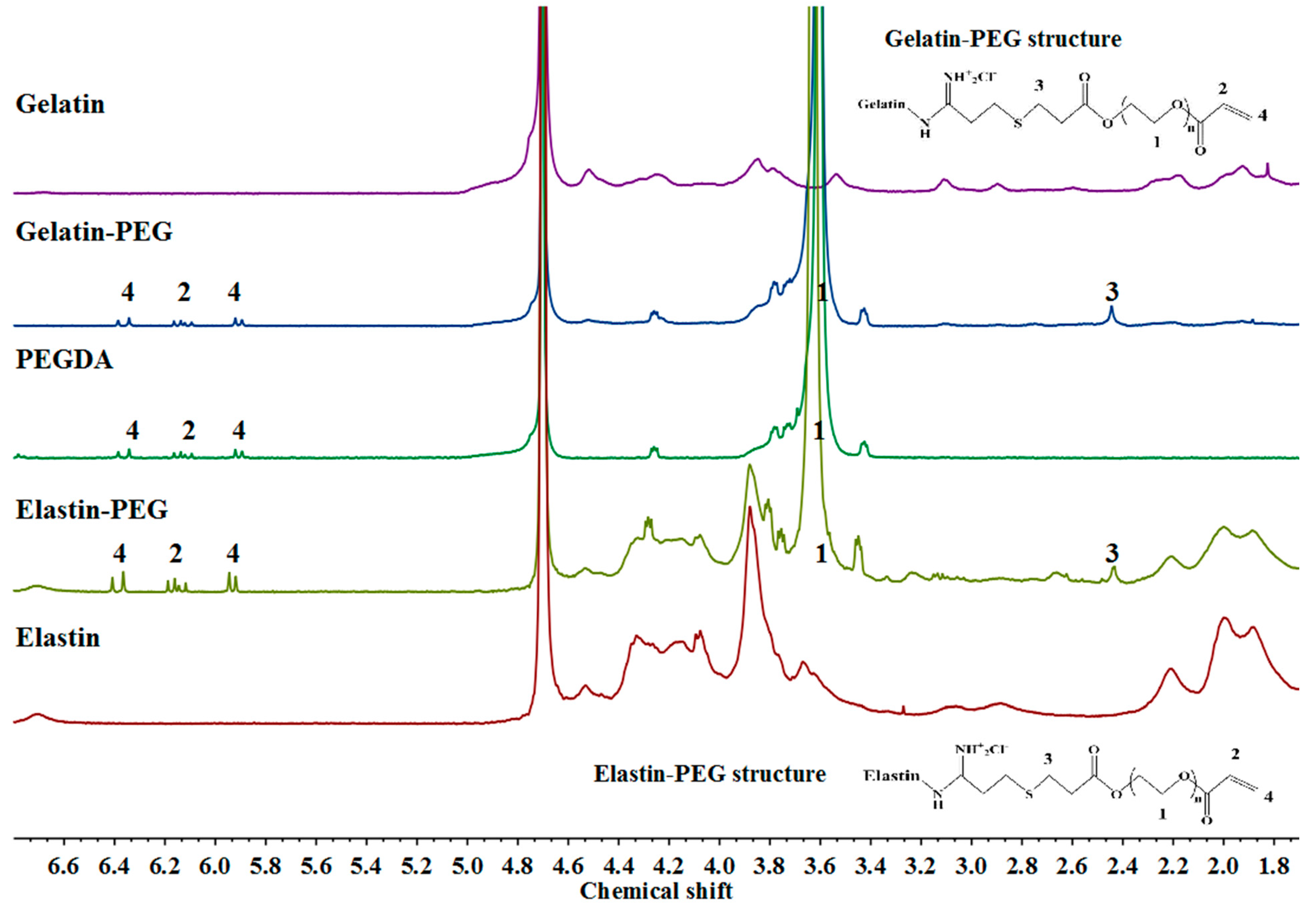
3.1. Characterization of Gelatin–PEG and Elastin–PEG Modification
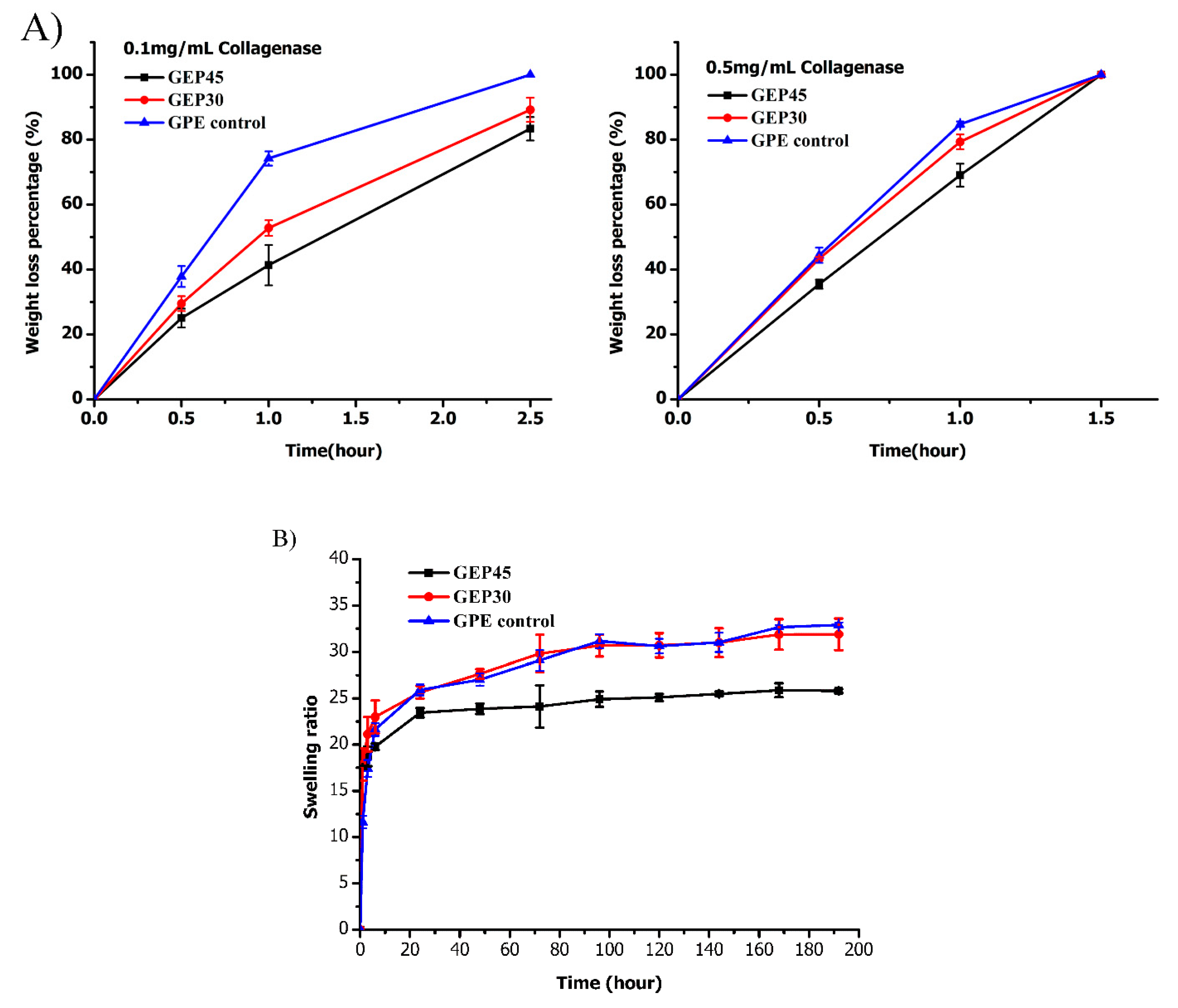
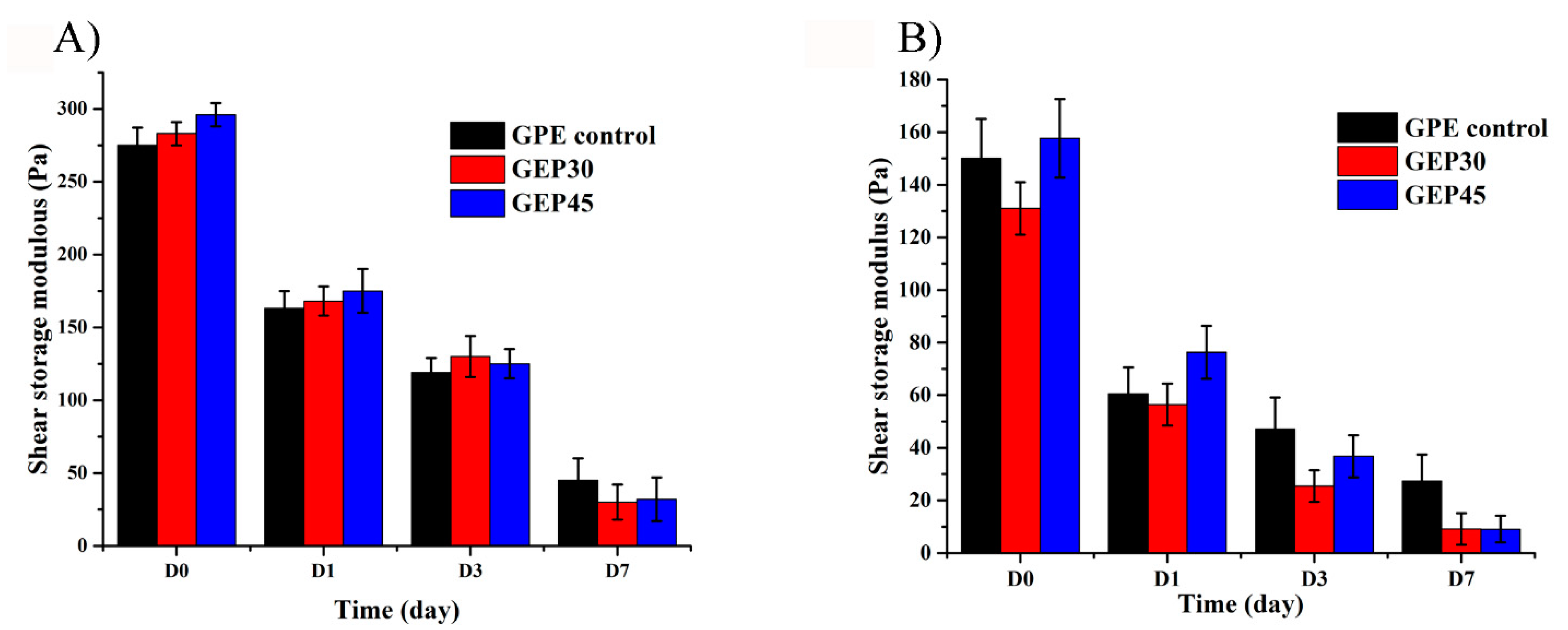
3.2. Hydrogel Swelling, Degradation, and Mechanical Properties
3.3. 3D Cell Encapsulation and Cellular Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ingber, D.E.; Mow, V.C.; Butler, D.; Niklason, L.; Huard, J.; Mao, J.; Yannas, I.; Kaplan, D.; Vunjak-Novakovic, G. Tissue engineering and developmental biology: Going biomimetic. Tissue Eng. 2006, 12, 3265–3283. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.I.; Badwal, P.S.; Gibson, I. Design and fabrication considerations for three dimensional scaffold structures. KnE Eng. 2017, 2, 120–126. [Google Scholar] [CrossRef]
- Higuchi, A.; Ling, Q.-D.; Chang, Y.; Hsu, S.-T.; Umezawa, A. Physical cues of biomaterials guide stem cell differentiation fate. Chem. Rev. 2013, 113, 3297–3328. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.H.; Hou, L.; Huang, N.F. Role of Extracellular Matrix Signaling Cues in Modulating Cell Fate Commitment for Cardiovascular Tissue Engineering. Adv. Healthc. Mater. 2014, 3, 628–641. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R. Extracellular Matrix Mimetic Multi-Functional Scaffolds for Tissue Engineering and Biomedical Applications; Linköping University Electronic Press: Linköping, Sweden, 2018. [Google Scholar]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. Deerfield Beach Weinh. 2006, 18, 1345. [Google Scholar] [CrossRef]
- Fernandez-Yague, M.A.; Abbah, S.A.; McNamara, L.; Zeugolis, D.I.; Pandit, A.; Biggs, M.J. Biomimetic approaches in bone tissue engineering: Integrating biological and physicomechanical strategies. Adv. Drug Deliv. Rev. 2015, 84, 1–29. [Google Scholar] [CrossRef]
- Hinderer, S.; Layland, S.L.; Schenke-Layland, K. ECM and ECM-like materials—Biomaterials for applications in regenerative medicine and cancer therapy. Adv. Drug Deliv. Rev. 2016, 97, 260–269. [Google Scholar] [CrossRef]
- Rnjak, J.; Wise, S.G.; Mithieux, S.M.; Weiss, A.S. Severe burn injuries and the role of elastin in the design of dermal substitutes. Tissue Eng. Part B Rev. 2011, 17, 81–91. [Google Scholar] [CrossRef]
- Kamolz, L.P.; Lumenta, D.B. Dermal Replacements in General, Burn, and Plastic Surgery; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Wang, C.; Varshney, R.R.; Wang, D.-A. Therapeutic cell delivery and fate control in hydrogels and hydrogel hybrids. Adv. Drug Deliv. Rev. 2010, 62, 699–710. [Google Scholar] [CrossRef]
- Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular matrix: A dynamic microenvironment for stem cell niche. Biochim. Et Biophys. Acta (Bba)-Gen. Subj. 2014, 1840, 2506–2519. [Google Scholar] [CrossRef]
- Xu, K.; Fu, Y.; Chung, W.; Zheng, X.; Cui, Y.; Hsu, I.C.; Kao, W.J. Thiol–ene-based biological/synthetic hybrid biomatrix for 3-D living cell culture. Acta Biomater. 2012, 8, 2504–2516. [Google Scholar] [CrossRef] [PubMed]
- Kedi, X.; David, A.C.; Yao, F.; Jaehyup, K.; Xiaoxiang, Z.; Periman, H.; Kao, W.J. Thiol-ene Michael-type formation of gelatin/poly(ethylene glycol) biomatrices for three-dimensional mesenchymal stromal/stem cell administration to cutaneous wounds. Acta Biomater. 2013, 11, 8802–8814. [Google Scholar] [CrossRef]
- Gonen-Wadmany, M.; Goldshmid, R.; Seliktar, D. Biological and mechanical implications of PEGylating proteins into hydrogel biomaterials. Biomaterials 2011, 32, 6025–6033. [Google Scholar] [CrossRef]
- Munoz-Pinto, D.J.; Jimenez-Vergara, A.C.; Gharat, T.P.; Hahn, M.S. Characterization of sequential collagen-poly (ethylene glycol) diacrylate interpenetrating networks and initial assessment of their potential for vascular tissue engineering. Biomaterials 2015, 40, 32–42. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef]
- Lee, B.H.; Tin, S.P.H.; Chaw, S.Y.; Cao, Y.; Xia, Y.; Steele, T.W.; Seliktar, D.; Bianco-Peled, H.; Venkatraman, S.S. Influence of soluble PEG-OH incorporation in a 3D cell-laden PEG-fibrinogen (PF) hydrogel on smooth muscle cell morphology and growth. J. Biomater. Sci. Polym. Ed. 2014, 25, 394–409. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, H.-W. Emerging properties of hydrogels in tissue engineering. J. Tissue Eng. 2018, 9. [Google Scholar] [CrossRef]
- Amer, L.D.; Holtzinger, A.; Keller, G.; Mahoney, M.J.; Bryant, S.J. Enzymatically degradable poly(ethylene glycol) hydrogels for the 3D culture and release of human embryonic stem cell derived pancreatic precursor cell aggregates. Acta Biomater. 2015, 22, 103–110. [Google Scholar] [CrossRef]
- Mahadevaiah, S.; Robinson, K.G.; Kharkar, P.M.; Kiick, K.L.; Akins, R.E. Decreasing matrix modulus of PEG hydrogels induces a vascular phenotype in human cord blood stem cells. Biomaterials 2015, 62, 24–34. [Google Scholar] [CrossRef]
- Shih, H.; Lin, C.-C. Photo-click hydrogels prepared from functionalized cyclodextrin and poly (ethylene glycol) for drug delivery and in situ cell encapsulation. Biomacromolecules 2015, 16, 1915–1923. [Google Scholar] [CrossRef]
- Cao, Y.; Lee, B.H.; Peled, H.B.; Venkatraman, S.S. Synthesis of stiffness—Tunable and cell—Responsive Gelatin—Poly (ethylene glycol) hydrogel for three—Dimensional cell encapsulation. J. Biomed. Mater. Res. Part A 2016, 104, 2401–2411. [Google Scholar] [CrossRef] [PubMed]
- Yeo, G.C.; Mithieux, S.M.; Weiss, A.S. The elastin matrix in tissue engineering and regeneration. Curr. Opin. Biomed. Eng. 2018, 6, 27–32. [Google Scholar] [CrossRef]
- Vasconcelos, A.; Gomes, A.C.; Cavaco-Paulo, A. Novel silk fibroin/elastin wound dressings. Acta Biomater. 2012, 8, 3049–3060. [Google Scholar] [CrossRef] [PubMed]
- Buttafoco, L.; Engbers—Buijtenhuijs, P.; Poot, A.; Dijkstra, P.; Daamen, W.; Van Kuppevelt, T.; Vermes, I.; Feijen, J. First steps towards tissue engineering of small—Diameter blood vessels: Preparation of flat scaffolds of collagen and elastin by means of freeze drying. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 77, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Grover, C.N.; Cameron, R.E.; Best, S.M. Investigating the morphological, mechanical and degradation properties of scaffolds comprising collagen, gelatin and elastin for use in soft tissue engineering. J. Mech. Behav. Biomed. Mater. 2012, 10, 62–74. [Google Scholar] [CrossRef]
- Ryan, A.J.; O’Brien, F.J. Insoluble elastin reduces collagen scaffold stiffness, improves viscoelastic properties, and induces a contractile phenotype in smooth muscle cells. Biomaterials 2015, 73, 296–307. [Google Scholar] [CrossRef]
- Daamen, W.F.; Nillesen, S.T.; Wismans, R.G.; Reinhardt, D.P.; Hafmans, T.; Veerkamp, J.H.; Van Kuppevelt, T.H. A biomaterial composed of collagen and solubilized elastin enhances angiogenesis and elastic fiber formation without calcification. Tissue Eng. Part A 2008, 14, 349–360. [Google Scholar] [CrossRef]
- Daamen, W.; Nillesen, S.; Hafmans, T.; Veerkamp, J.; Van Luyn, M.; Van Kuppevelt, T. Tissue response of defined collagen–elastin scaffolds in young and adult rats with special attention to calcification. Biomaterials 2005, 26, 81–92. [Google Scholar] [CrossRef]
- Annabi, N.; Mithieux, S.M.; Weiss, A.S.; Dehghani, F. The fabrication of elastin-based hydrogels using high pressure CO2. Biomaterials 2009, 30, 1–7. [Google Scholar] [CrossRef]
- Annabi, N.; Mithieux, S.M.; Boughton, E.A.; Ruys, A.J.; Weiss, A.S.; Dehghani, F. Synthesis of highly porous crosslinked elastin hydrogels and their interaction with fibroblasts in vitro. Biomaterials 2009, 30, 4550–4557. [Google Scholar] [CrossRef]
- Bax, D.V.; Rodgers, U.R.; Bilek, M.M.; Weiss, A.S. Cell adhesion to tropoelastin is mediated via the C-terminal GRKRK motif and integrin αVβ3. J. Biol. Chem. 2009, 284, 28616–28623. [Google Scholar] [CrossRef] [PubMed]
- Fathi, A.; Mithieux, S.M.; Wei, H.; Chrzanowski, W.; Valtchev, P.; Weiss, A.S.; Dehghani, F. Elastin based cell-laden injectable hydrogels with tunable gelation, mechanical and biodegradation properties. Biomaterials 2014, 35, 5425–5435. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Nelson, D.M.; Hong, Y.; Wagner, W.R. Thermally responsive injectable hydrogel incorporating methacrylate-polylactide for hydrolytic lability. Biomacromolecules 2010, 11, 1873–1881. [Google Scholar] [CrossRef]
- Browning, M.B.; Cosgriff-Hernandez, E. Development of a biostable replacement for PEGDA hydrogels. Biomacromolecules 2012, 13, 779–786. [Google Scholar] [CrossRef]
- Hermanson, G.T. Bioconjugate Techniques; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Ghobril, C.; Grinstaff, M. The chemistry and engineering of polymeric hydrogel adhesives for wound closure: A tutorial. Chem. Soc. Rev. 2015, 44, 1820–1835. [Google Scholar] [CrossRef]
- Sridhar, B.V.; Dailing, E.A.; Brock, J.L.; Stansbury, J.W.; Randolph, M.A.; Anseth, K.S. A biosynthetic scaffold that facilitates chondrocyte-mediated degradation and promotes articular cartilage extracellular matrix deposition. Regen. Eng. Transl. Med. 2015, 1, 11–21. [Google Scholar] [CrossRef]
- Fu, Y.; Xu, K.; Zheng, X.; Giacomin, A.J.; Mix, A.W.; Kao, W.J. 3D cell entrapment in crosslinked thiolated gelatin-poly (ethylene glycol) diacrylate hydrogels. Biomaterials 2012, 33, 48–58. [Google Scholar] [CrossRef]
- Abreu, E. Review of “Scaffolding in Tissue Engineering”, by Peter X. Ma and Jennifer Elisseeff (Editors). Biomed. Eng. Online 2006, 5, 52. [Google Scholar] [CrossRef]
- Canal, T.; Peppas, N.A. Correlation between mesh size and equilibrium degree of swelling of polymeric networks. J. Biomed. Mater. Res. 1989, 23, 1183–1193. [Google Scholar] [CrossRef]
- Bryant, S.J.; Anseth, K.S. Photopolymerization of hydrogel scaffolds. In Scaffolding in Tissue Engineering; CRC Press: Boca Raton, FL, USA, 2005; pp. 80–99. [Google Scholar]
- Temenoff, J.S.; Athanasiou, K.A.; Lebaron, R.G.; Mikos, A.G. Effect of poly (ethylene glycol) molecular weight on tensile and swelling properties of oligo (poly (ethylene glycol) fumarate) hydrogels for cartilage tissue engineering. J. Biomed. Mater. Res. 2002, 59, 429–437. [Google Scholar] [CrossRef]
- Deslee, G.; Woods, J.C.; Moore, C.M.; Liu, L.; Conradi, S.H.; Milne, M.; Gierada, D.S.; Pierce, J.; Patterson, A.; Lewit, R.A. Elastin expression in very severe human COPD. Eur. Respir. J. 2009, 34, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kats, L.; Blättler, W.A.; Lambert, J.M. Formation of N-substituted 2-iminothiolanes when amino groups in proteins and peptides are modified by 2-iminothiolane. Anal. Biochem. 1996, 236, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.X.; Tsang, K.M.; Simon, G.P.; Boyd, R.L.; Evans, R.A.; Thissen, H.; Forsythe, J.S. Photodegradable Gelatin-Based Hydrogels Prepared by Bioorthogonal Click Chemistry for Cell Encapsulation and Release. Biomacromolecules 2015, 16, 2246–2253. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.H.; Gilbert, M.; Virdi, A.S.; Sena, K.; Sumner, D.R.; Healy, K.E. Biomimetic artificial ECMs stimulate bone regeneration. J. Biomed. Mater. Res. Part A 2006, 79, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Ariyoshi, Y. Angiotensin-converting enzyme inhibitors derived from food proteins. Trends Food Sci. Technol. 1993, 4, 139–144. [Google Scholar] [CrossRef]
- Izadifar, Z.; Chen, X.; Kulyk, W. Strategic Design and Fabrication of Engineered Scaffolds for Articular Cartilage Repair. J. Funct. Biomater. 2012, 3, 799–838. [Google Scholar] [CrossRef]
- Oda, H.; Konno, T.; Ishihara, K. The use of the mechanical microenvironment of phospholipid polymer hydrogels to control cell behavior. Biomaterials 2013, 34, 5891–5896. [Google Scholar] [CrossRef]
- Rydholm, A.E.; Anseth, K.S.; Bowman, C.N. Effects of neighboring sulfides and pH on ester hydrolysis in thiol–acrylate photopolymers. Acta Biomater. 2007, 3, 449–455. [Google Scholar] [CrossRef]
- Mauck, R.L.; Seyhan, S.L.; Ateshian, G.A.; Hung, C.T. Influence of Seeding Density and Dynamic Deformational Loading on the Developing Structure/Function Relationships of Chondrocyte-Seeded Agarose Hydrogels. Ann. Biomed. Eng. 2002, 30, 1046–1056. [Google Scholar] [CrossRef]
- Blaeser, A.; Duarte Campos, D.F.; Puster, U.; Richtering, W.; Stevens, M.M.; Fischer, H. Controlling shear stress in 3D bioprinting is a key factor to balance printing resolution and stem cell integrity. Adv. Healthc. Mater. 2016, 5, 326–333. [Google Scholar] [CrossRef]
- Madaghiele, M.; Demitri, C.; Sannino, A.; Ambrosio, L. Polymeric hydrogels for burn wound care: Advanced skin wound dressings and regenerative templates. Burn. Trauma 2014, 2, 153. [Google Scholar] [CrossRef] [PubMed]
- Berthod, F.; Germain, L.; Li, H.; Xu, W.; Damour, O.; Auger, F.A. Collagen fibril network and elastic system remodeling in a reconstructed skin transplanted on nude mice. Matrix Biol. 2001, 20, 463–473. [Google Scholar] [CrossRef]
- Lamme, E.N.; De Vries, H.; van Veen, H.; Gabbiani, G.; Westerhof, W.; Middelkoop, E. Extracellular matrix characterization during healing of full-thickness wounds treated with a collagen/elastin dermal substitute shows improved skin regeneration in pigs. J. Histochem. Cytochem. 1996, 44, 1311–1322. [Google Scholar] [CrossRef] [PubMed]
- Middelkoop, E.; de Vries, H.J.; Ruuls, L.; Everts, V.; Wildevuur, C.H.; Westerhof, W. Adherence, proliferation and collagen turnover by human fibroblasts seeded into different types of collagen sponges. Cell Tissue Res. 1995, 280, 447–453. [Google Scholar] [CrossRef]
- De Vries, H.J.; Middelkoop, E.; Mekkes, J.R.; Dutrieux, R.P.; Wildevuur, C.H.; Westerhof, W. Dermal regeneration in native non—Cross—Linked collagen sponges with different extracellular matrix molecules. Wound Repair Regen. 1994, 2, 37–47. [Google Scholar] [CrossRef]
- Giancotti, F.G.; Ruoslahti, E. Integrin signaling. Science 1999, 285, 1028–1033. [Google Scholar] [CrossRef]
- Almine, J.F.; Bax, D.V.; Mithieux, S.M.; Nivison-Smith, L.; Rnjak, J.; Waterhouse, A.; Wise, S.G.; Weiss, A.S. Elastin-based materials. Chem. Soc. Rev. 2010, 39, 3371–3379. [Google Scholar] [CrossRef]
- Kamoun, A.; Landeau, J.-M.; Godeau, G.; Wallach, J.; Duchesnay, A.; Pellat, B.; Hornebeck, W. Growth stimulation of human skin fibroblasts by elastin-derived peptides. Cell Commun. Adhes. 1995, 3, 273–281. [Google Scholar] [CrossRef]
- Senior, R.M.; Griffin, G.L.; Mecham, R.P. Chemotactic responses of fibroblasts to tropoelastin and elastin-derived peptides. J. Clin. Investig. 1982, 70, 614. [Google Scholar] [CrossRef]
- Nivison-Smith, L.; Weiss, A. Elastin Based Constructs; INTECH Open Access Publisher: London, UK, 2011. [Google Scholar]
- Bradford, J.A.; Clarke, S.T. Dual-Pulse Labeling Using 5-Ethynyl-2′-Deoxyuridine (EdU) and 5-Bromo-2′-Deoxyuridine (BrdU) in Flow Cytometry. In Current Protocols in Cytometry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Mahoney, M.J.; Anseth, K.S. Three-dimensional growth and function of neural tissue in degradable polyethylene glycol hydrogels. Biomaterials 2006, 27, 2265–2274. [Google Scholar] [CrossRef]
- Truong, A.-T.N.; Kowal-Vern, A.; Latenser, B.A.; Wiley, D.E.; Walter, R.J. Comparison of dermal substitutes in wound healing utilizing a nude mouse model. J. Burn. Wounds 2005, 4, e4. [Google Scholar]
- Hinek, A.; Wang, Y.; Liu, K.; Mitts, T.F.; Jimenez, F. Proteolytic digest derived from bovine Ligamentum Nuchae stimulates deposition of new elastin-enriched matrix in cultures and transplants of human dermal fibroblasts. J. Dermatol. Sci. 2005, 39, 155–166. [Google Scholar] [CrossRef]
- Darby, I.A.; Laverdet, B.; Bonté, F.; Desmoulière, A. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 2014, 7, 301. [Google Scholar] [PubMed]
- Lamme, E.N.; van Leeuwen, R.T.J.; Jonker, A.; van Marle, J.; Middelkoop, E. Living Skin Substitutes: Survival and Function of Fibroblasts Seeded in a Dermal Substitute in Experimental Wounds. J. Investig. Dermatol. 1998, 111, 989–995. [Google Scholar] [CrossRef] [PubMed]
| Samples | Gelatin–PEG Concentration (wt/v %) | Elastin–PEG Concentration (wt/v %) | Elastin Concentration (wt/v %) | Elastin, as % of Solids | PEGDA Concentration (wt/v %) | Total Solid (wt/v %) | Network Cross-Linking Density (mol∙cm−3) | Initial Mesh Size (nm) |
|---|---|---|---|---|---|---|---|---|
| GEP45 | 4.80 | 4.50 | 0 | 27.1% | 0 | 9.30 | 6.34 × 10−5 ± 0.67 × 10−5 | 22.4 ± 3.5 |
| GEP30 | 4.80 | 3.00 | 0 | 21.5% | 0 | 7.80 | 4.04 ×10−5 ± 0.58 × 10−5 | 30.3 ± 4.1 |
| GPE control | 4.80 | 0 | 1.50 | 23.8%- soluble, leaches out | 0.90 | 7.20 | 2.15 ×10−5 ± 0.17 × 10−5 | 46.4 ± 4.2 |
| Samples | Protein Ratio (%) | Protein Concentration (mg/mL) | PEG Ratio (%) | TNBSA (µg/mL) | Conjugation Efficiency (%) |
|---|---|---|---|---|---|
| Gelatin–PEG–acrylate | 60.2 ± 4.5 | 32.5 ± 1.5 | 39.8 ± 4.5 | 160.3 ± 10.4 | 52.8 ± 6.5 |
| Elastin–PEG–acrylate | 56.4 ± 5.8 | 1.69 ± 1.7 | 43.6 ± 5.8 | 2.5 ± 0.1 | 59.6 ± 2.2 |
| Samples | Viscosity (Pa∙s) | ||
|---|---|---|---|
| Shear Rate (s−1) 0.1 | Shear Rate (s−1) 230 | Shear Rate (s−1) 500 | |
| GPE control | 0.0506 | 0.0133 | 0.0128 |
| GEP30 | 648 | 0.510 | 0.2620 |
| GEP45 | 1080 | 0.675 | 0.3450 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Lee, B.H.; Irvine, S.A.; Wong, Y.S.; Bianco Peled, H.; Venkatraman, S. Inclusion of Cross-Linked Elastin in Gelatin/PEG Hydrogels Favourably Influences Fibroblast Phenotype. Polymers 2020, 12, 670. https://doi.org/10.3390/polym12030670
Cao Y, Lee BH, Irvine SA, Wong YS, Bianco Peled H, Venkatraman S. Inclusion of Cross-Linked Elastin in Gelatin/PEG Hydrogels Favourably Influences Fibroblast Phenotype. Polymers. 2020; 12(3):670. https://doi.org/10.3390/polym12030670
Chicago/Turabian StyleCao, Ye, Bae Hoon Lee, Scott Alexander Irvine, Yee Shan Wong, Havazelet Bianco Peled, and Subramanian Venkatraman. 2020. "Inclusion of Cross-Linked Elastin in Gelatin/PEG Hydrogels Favourably Influences Fibroblast Phenotype" Polymers 12, no. 3: 670. https://doi.org/10.3390/polym12030670
APA StyleCao, Y., Lee, B. H., Irvine, S. A., Wong, Y. S., Bianco Peled, H., & Venkatraman, S. (2020). Inclusion of Cross-Linked Elastin in Gelatin/PEG Hydrogels Favourably Influences Fibroblast Phenotype. Polymers, 12(3), 670. https://doi.org/10.3390/polym12030670







