Synergistic Effects of Boron Nitride (BN) Nanosheets and Silver (Ag) Nanoparticles on Thermal Conductivity and Electrical Properties of Epoxy Nanocomposites
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of AgNPs-BNNSs
2.3. Preparation of EP-AgBN
2.4. Morphology Characterization And Performances Measurement
3. Results and Discussion
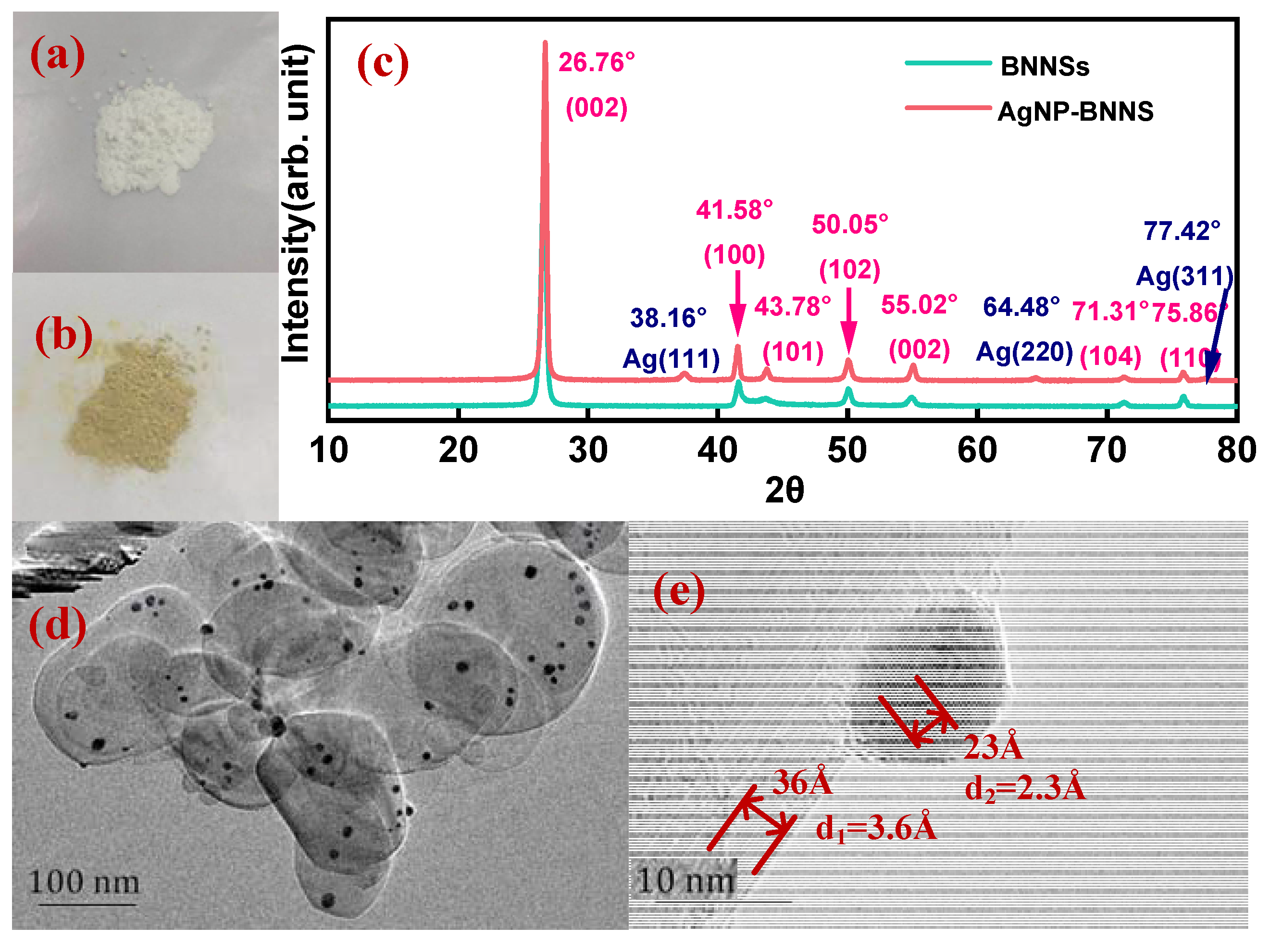
3.1. Morphology Characterization of AgNPs-BNNSs
3.2. Morphology Characterization of EP-AgBN
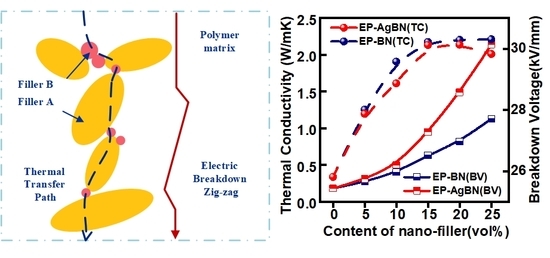
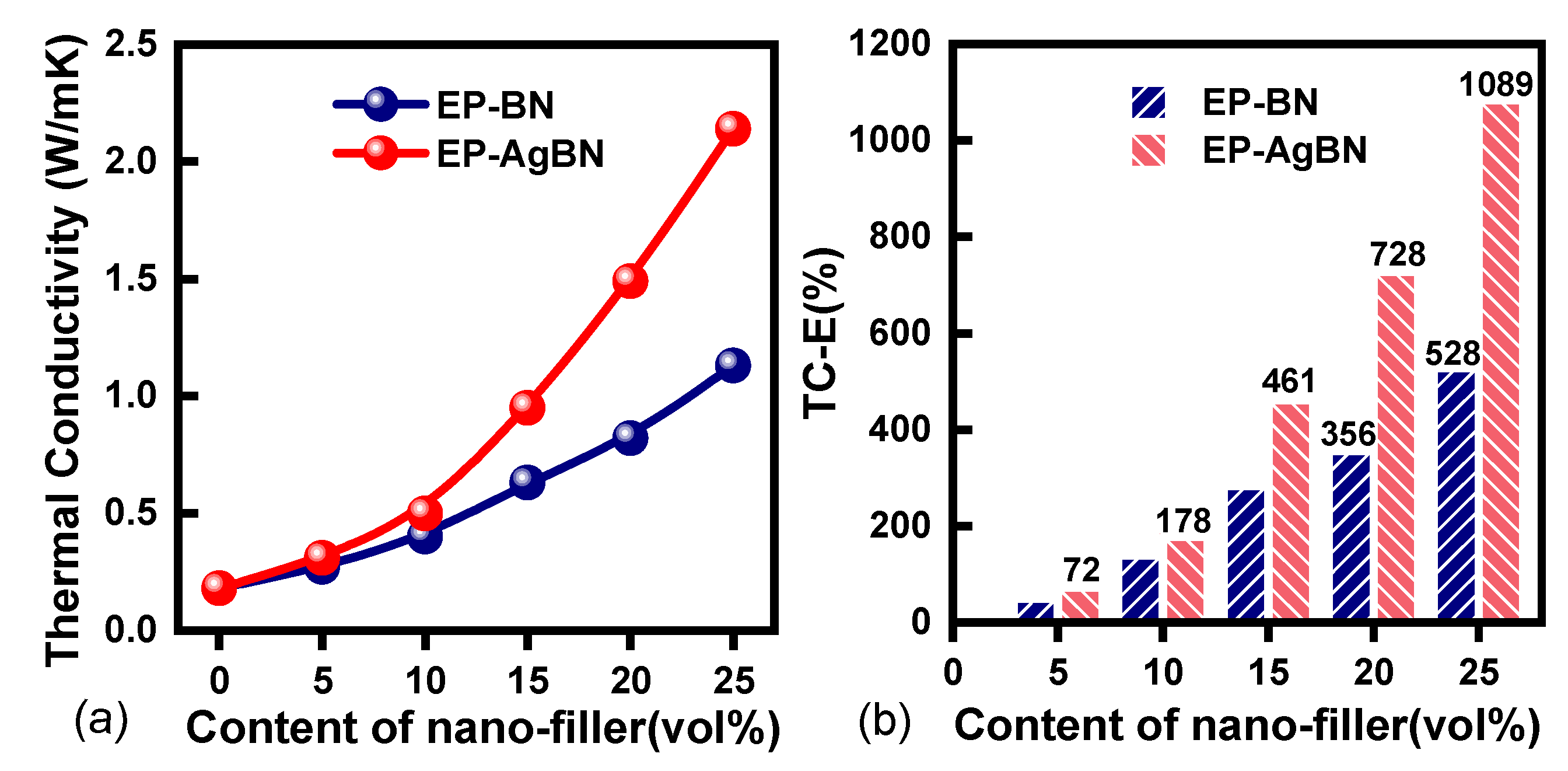
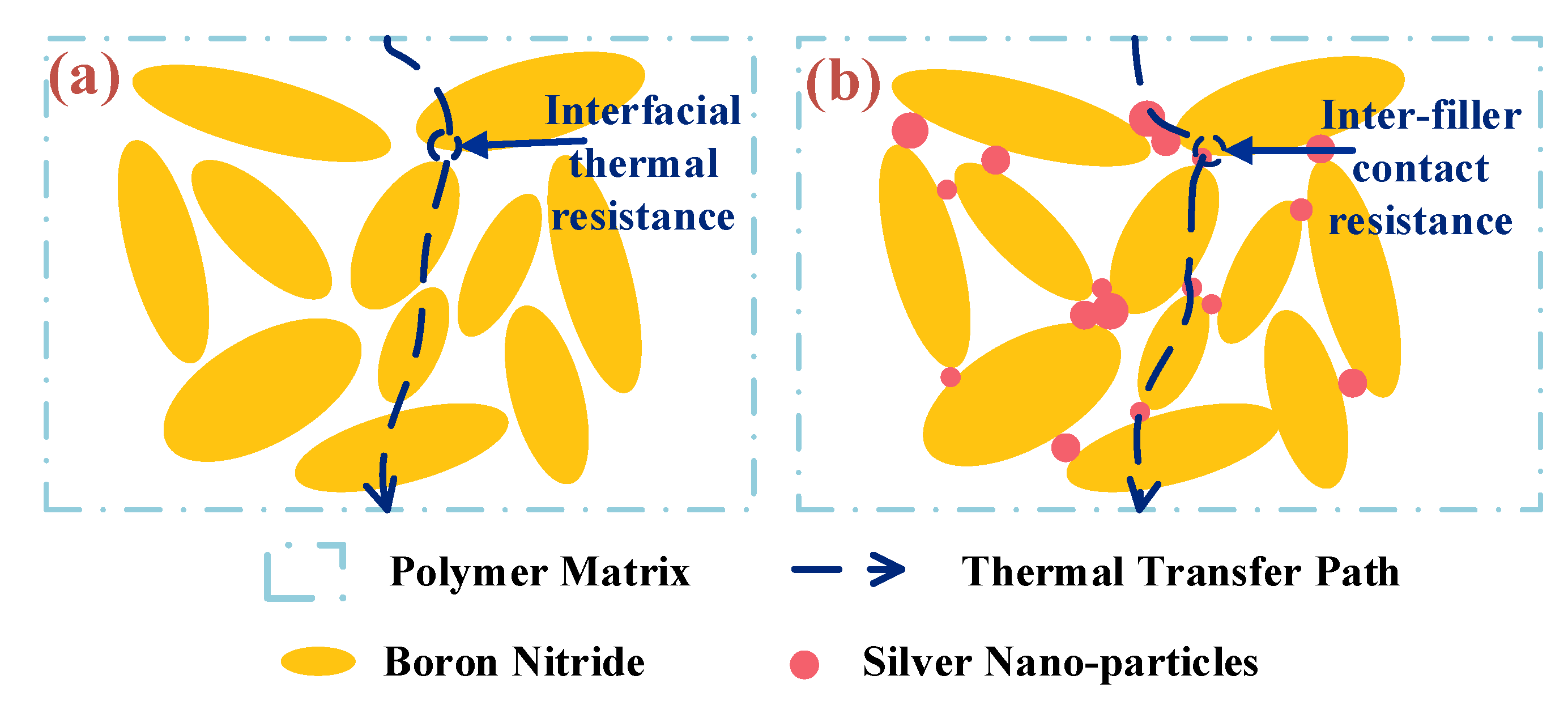
3.3. Thermal Conductivity of EP-AgBN
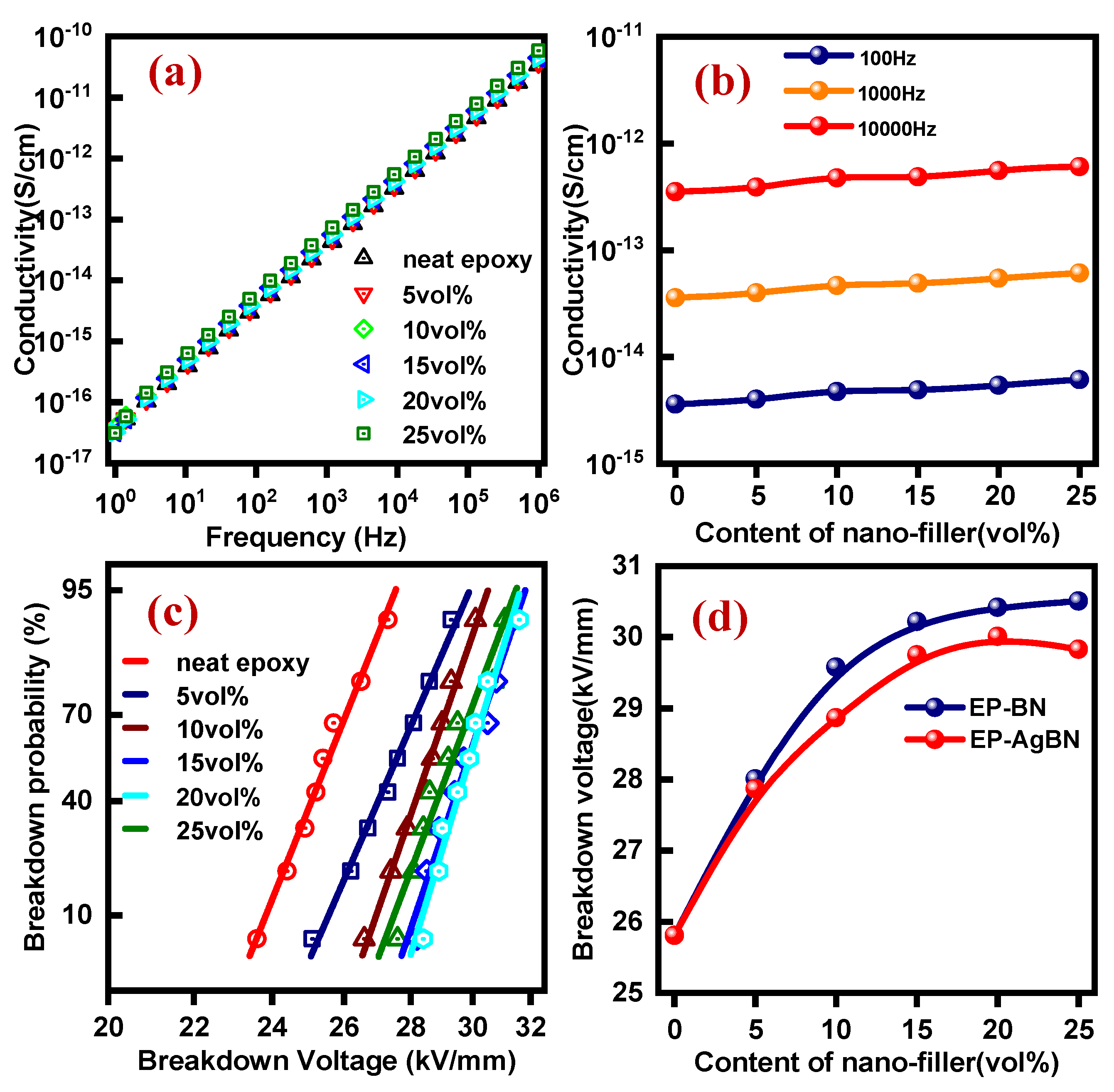
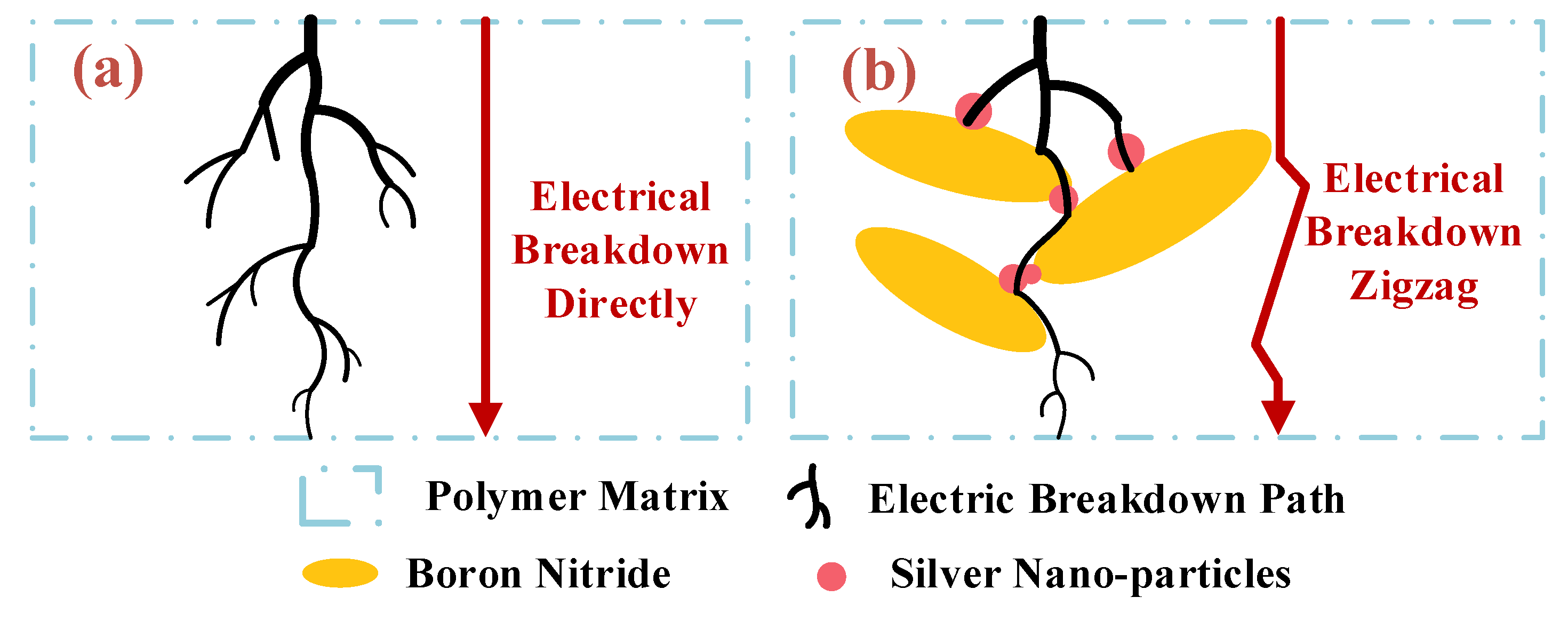
3.4. Electrical Conductivity and Breakdown Voltage of EP-AgBN
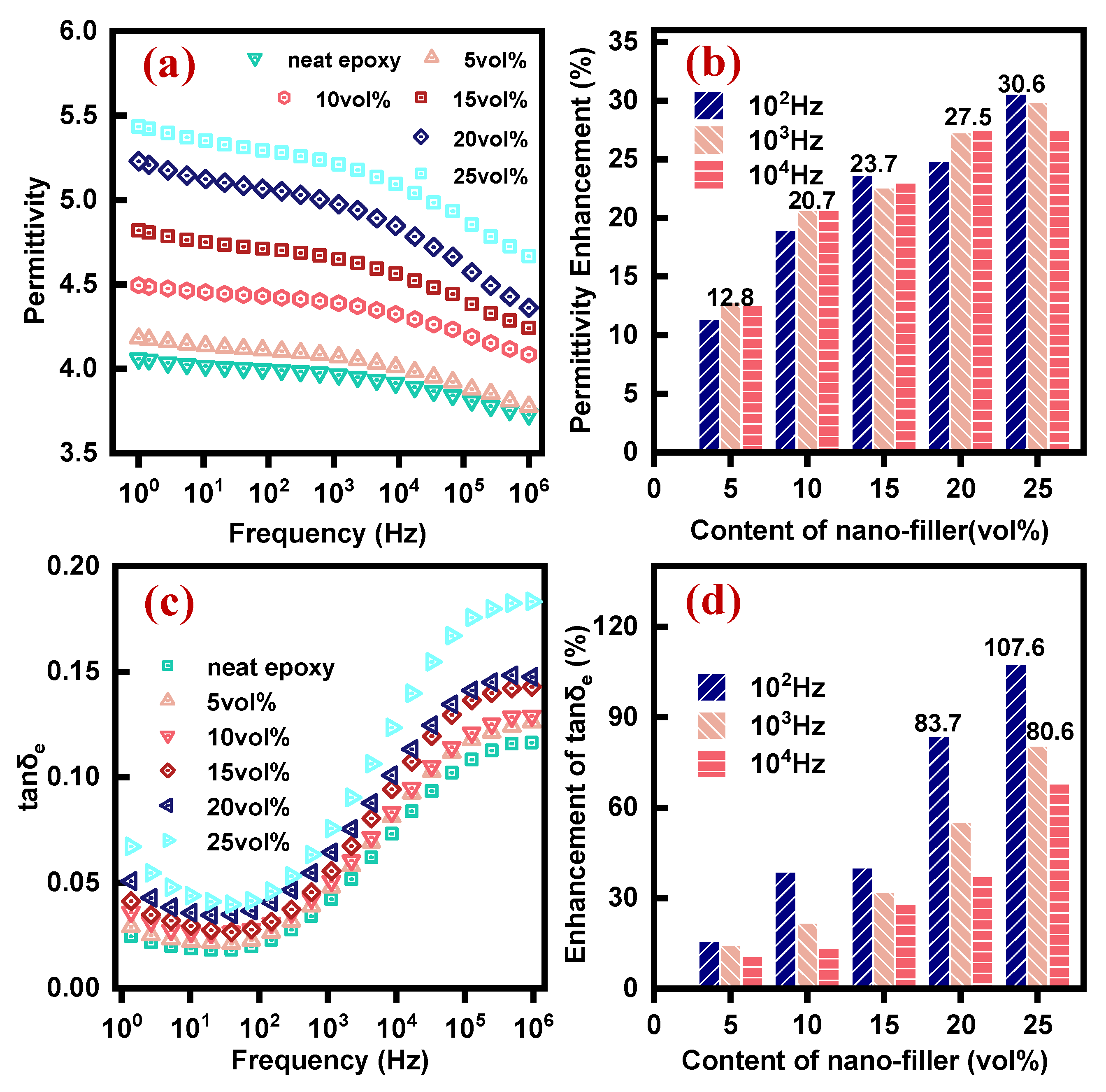
3.5. Permittivity and Dielectric loss of EP-AgBN
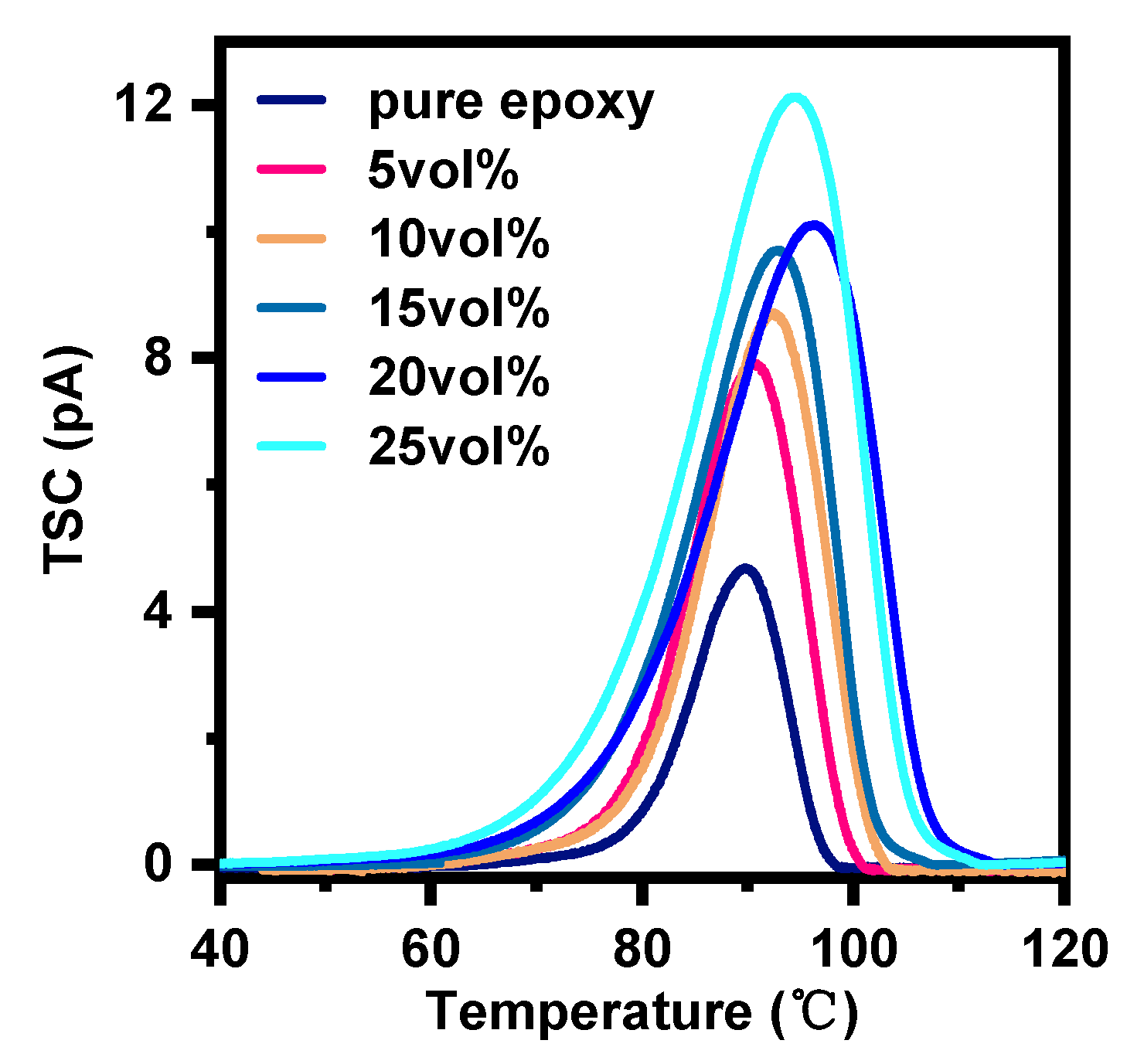
3.6. Thermal Simulated Current of EP-AgBN
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, Y.; Zhang, X.; Zhang, J.; Xie, C.; Shao, X.; Wang, Z.; Xiao, S. Study on the thermal decomposition characteristics of C4F7N-CO2 mixture as eco-friendly gas insulating medium. High Volt. 2019. [Google Scholar] [CrossRef]
- Zhi, C.; Bando, Y.; Terao, T.; Tang, C.; Kuwahara, H.; Golberg, D. Towards Thermoconductive, Electrically Insulating Polymeric Composites with Boron Nitride Nanotubes as Fillers. Adv. Funct. Mater. 2009, 19, 1857–1862. [Google Scholar] [CrossRef]
- Wattanakul, K.; Manuspiya, H.; Yanumet, N. Thermal conductivity and mechanical properties of BN-filled epoxy composite: Effects of filler content, mixing conditions, and BN agglomerate size. J. Compos. Mater. 2011, 45, 1967–1980. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Xiong, H.; Li, Y.; Tang, J.; Xiao, S.; Zhang, D. A first-principles study of the SF6 decomposed products adsorbed over defective WS2 monolayer as promising gas sensing device. IEEE Trans. Device Mater. Reliab. 2019, 19, 473–483. [Google Scholar] [CrossRef]
- Li, S.; Yu, S.; Feng, Y. Progress in and Prospects for Electrical Insulating Materials. High Volt. 2016, 1, 122–129. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Wen, H. The influence of oxygen on thermal decomposition characteristics of epoxy resins cured by anhydride. Polym. Degrad. Stab. 2018, 156, 125–131. [Google Scholar] [CrossRef]
- Zhang, Y.; Choi, J.R.; Park, S.J. Interlayer polymerization in amine-terminated macromolecular chain-grafted expanded graphite for fabricating highly thermal conductive and physically strong thermoset composites for thermal management applications. Compos. Part A Appl. Sci. 2018, 109, 498–506. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, X.; Xiong, H.; Chen, D.; Cheng, H.; Tang, J.; Tian, Y.; Xiao, S. Dissolved gas analysis in transformer oil using Pt-doped WSe2 monolayer based on first principles method. IEEE Access 2019, 7, 72012–72019. [Google Scholar] [CrossRef]
- Zhang, Y.; Park, S.J. In Situ Shear-induced MercaptoGroup-activated Graphite Nanoplatelets for Fabricating Mechanically Strong and Thermally Conductive Elastomer Composites for Thermal Management Applications. Compos. Part A Appl. Sci. 2018, 112, 40–48. [Google Scholar] [CrossRef]
- Mu, M.; Wan, C.; McNally, T. Thermal Conductivity of 2D Nano-structured Graphitic Materials and Their Composites with Epoxy Resins. 2D Mater. 2017, 4, 042001. [Google Scholar] [CrossRef]
- Hao, W.; Zhang, X.; Rong, X. Thermodynamic simulations of SrTiO3/epoxy nanocomposites with different mass fractions. Sn Appl. Sci. 2019, 1, 354. [Google Scholar]
- Cheewawuttipong, W.; Fuoka, D.; Tanoue, S. Thermal and mechanical properties of polypropylene/boron nitride composites. Energy Procedia 2013, 34, 808–817. [Google Scholar] [CrossRef]
- Pan, C.; Kou, K.; Zhang, Y.; Li, Z.; Ji, T.; Wu, G. Investigation of the dielectric and thermal conductive properties of core–shell structured HGM/hBN/PTFE composites. Mater. Sci. Eng. B-Adv. 2018, 238, 61–70. [Google Scholar] [CrossRef]
- Spitalsky, Z.; Tasis, D.; Papagelis, K.; Galiotis, C. Carbon Nanotube–polymer Composites: Chemistry Processing Mechanical and Electrical Properties. Prog. Polym. Sci. 2010, 35, 357–401. [Google Scholar] [CrossRef]
- Zhang, X.; Fang, R.; Chen, D.; Zhang, G. Using Pd-Doped γ-Graphyne to Detect Dissolved Gases in Transformer Oil: A Density Functional Theory Investigation. Nanomaterials 2019, 9, 1490. [Google Scholar] [CrossRef]
- Barani, Z.; Mohammadzadeh, A.; Geremew, A.; Huang, C.Y.; Coleman, D.; Mangolini, L.; Balandin, A.A. Thermal Properties of the Binary-Filler Hybrid Composites with Graphene and Copper Nanoparticles. Adv. Funct. Mater. 2019, 1904008. [Google Scholar] [CrossRef]
- Wang, J.; Qiao, J.; Wang, J.; Zhu, Y.; Jiang, L. Bioinspired Hierarchical Alumina–Graphene Oxide–poly (vinyl alcohol) Artificial Nacre with Optimized Strength and Toughness. Acs Appl. Mater. Inter. 2015, 7, 9281–9286. [Google Scholar] [CrossRef]
- Xu, Y.; Chung, D. Increasing the Thermal Conductivity of Boron Nitride and Aluminum Nitride Particle Epoxy-matrix Composites by Particle Surface Treatments. Compos. Interface 2000, 7, 243–256. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, X.; Gu, M.; Xiong, D. Study on Mechanical Thermal and Electrical Characterizations of Nano-SiC/Epoxy Composites. Polym. J. 2009, 41, 51. [Google Scholar] [CrossRef]
- Zhang, X.; Wen, H.; Chen, X.; Wu, Y.; Xiao, S. Study on the Thermal and Dielectric Properties of SrTiO3/Epoxy Nanocomposites. Energies 2017, 10, 692. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, P.; Tanaka, T. A Review of Dielectric Polymer Composites with High Thermal Conductivity. IEEE Electr. Insul. Mag. 2011, 27, 8–16. [Google Scholar] [CrossRef]
- Meng, W.; Huang, Y.; Fu, Y.; Wang, Z.; Zhi, C. Polymer Composites of Boron Nitride Nanotubes and Nanosheets. J. Mater. Chem. C 2014, 2, 10049–10061. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Chen, X.; Wen, H.; Xiao, S. Theoretical Study on Decomposition Mechanism of Insulating Epoxy Resin Cured by Anhydride. Polymers 2017, 9, 341. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Qian, X.; Yang, R. Thermal Conductivity of Polymers and Polymer Nanocomposites. Mat. Sci. Eng. R 2018, 132, 1–22. [Google Scholar] [CrossRef]
- Pan, C.; Kou, K.; Zhang, Y. Enhanced through-plane thermal conductivity of PTFE composites with hybrid fillers of hexagonal boron nitride platelets and aluminum nitride particles. Compos. Part B 2018, 153, 1–8. [Google Scholar] [CrossRef]
- Wang, X.; Yu, Z.; Jiao, L.; Bian, H.; Yang, W.; Wu, W.; Dai, H. Aerogel Perfusion-Prepared h-BN/CNF Composite Film with Multiple Thermally Conductive Pathways and High Thermal Conductivity. Nanomaterials 2019, 9, 1051. [Google Scholar] [CrossRef]
- Kandare, E.; Khatibi, A.A.; Yoo, S.; Wang, R.; Ma, J.; Olivier, P.; Wang, H. Improving the Through-thickness Thermal and Electrical Conductivity of Carbon fibre/Epoxy Laminates by Exploiting Synergy between Graphene and Silver Nano-inclusions. Compos. Part A Appl. Sci. 2015, 69, 72–82. [Google Scholar] [CrossRef]
- Pashayi, K.; Fard, H.R.; Lai, F.; Iruvanti, S.; Plawsky, J.; Borca-Tasciuc, T. Self-constructed Tree-shape High Thermal Conductivity Nanosilver Networks in Epoxy. Nanoscale 2014, 6, 4292–4296. [Google Scholar] [CrossRef]
- Wang, F.; Zeng, X.; Yao, Y.; Sun, R.; Xu, J.; Wong, C.P. Silver Nanoparticle-Deposited Boron Nitride Nanosheets as Fillers for Polymeric Composites with High Thermal Conductivity. Sci. Rep. 2016, 6, 19394. [Google Scholar] [CrossRef]
- Pullanchiyodan, A.; Nair, K.; Surendran, K.P. Silver-decorated Boron Nitride Nanosheets as an Effective Hybrid Filler in PMMA for High-thermal-conductivity Electronic Substrates. Acs Omega 2017, 2, 8825–8835. [Google Scholar] [CrossRef]
- Zhi, C.; Bando, Y.; Tan, C.; Golberg, D. Effective Precursor for High Yield Synthesis of Pure BN Nanotubes. Solid State Commun. 2005, 135, 67–70. [Google Scholar] [CrossRef]
- Shen, J.; Shi, M.; Li, N.; Yan, B.; Ma, H.; Hu, Y.; Ye, M. Facile Synthesis and Application of Ag-chemically Converted Graphene Nanocomposite. Nano Res. 2010, 3, 339–349. [Google Scholar] [CrossRef]
- Liu, K.; Chen, S.; Luo, Y.; Jia, D.; Gao, H.; Hu, G.; Liu, L. Noncovalently Functionalized Pristine Graphene/metal Nanoparticle Hybrid for Conductive Composites. Compos. Sci. Technol. 2014, 94, 1–7. [Google Scholar] [CrossRef]
- Zhan, Y.; Ren, Y.; Wan, X.; Zhang, J.; Zhang, S. Dielectric Thermally Conductive and Stable Poly (arylene ether nitrile) Composites Filled with Silver Nanoparticles Decorated Hexagonal Boron Nitride. Ceram Int. 2018, 44, 2021–2029. [Google Scholar] [CrossRef]
- Jang, I.; Shin, K.H.; Yang, I.; Kim, H.; Kim, J.; Kim, W.H.; Kim, J.P. Enhancement of Thermal Conductivity of BN/Epoxy Composite through Surface Modification with Cilane Coupling Agents. Colloids Surf. A 2017, 518, 64–72. [Google Scholar] [CrossRef]
- Xue, Y.; Jin, X.; Fan, Y.; Tian, R.; Xu, X.; Li, J.; Lin, J.; Zhang, J.; Hu, L.; Tang, C. Large-scale synthesis of hexagonal boron nitride nanosheets and their improvement in thermal properties of epoxy composites. Polym. Compos. 2014, 35, 1707–1715. [Google Scholar] [CrossRef]
- Hou, J.; Li, G.; Yang, N.; Qin, L.; Grami, M.E.; Zhang, Q.; Wang, N.; Qu, X. Preparation and characterization of surface modified boron nitride epoxy composites with enhanced thermal conductivity. Rsc. Adv. 2014, 4, 44282–44290. [Google Scholar] [CrossRef]
- Zhi, C.Y.; Bando, Y.; Wang, W.L. Mechanical and thermal properties of polymethyl methacrylate-BN nanotube composites. J. Nanomater. 2008, 2008, 642036. [Google Scholar] [CrossRef]
- Zhu, B.L.; Ma, J.; Wu, J. Study on the properties of the epoxy-matrix composites filled with thermally conductive AlN and BN ceramic particles. J. Appl. Polym. Sci. 2010, 118, 2754–2764. [Google Scholar] [CrossRef]
- Bian, W.; Yao, T.; Chen, M.; Zhang, C.; Shao, T.; Yang, Y. The Synergistic Effects of the Micro-BN and Nano-Al2O3 in Micro-nano Composites on Enhancing the Thermal Conductivity for Insulating Epoxy Resin. Compos. Sci. Technol. 2018, 168, 420–428. [Google Scholar] [CrossRef]
- Huang, X.; Zhi, C.; Jiang, P.; Golberg, D.; Bando, Y.; Tanaka, T. Polyhedral Oligosilsesquioxane-Modified Boron Nitride Nanotube Based Epoxy Nanocomposites: An Ideal Dielectric Material with High Thermal Conductivity. Adv. Funct Mater. 2013, 23, 1824–1831. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, Y.; Yang, M.; Huang, J.; Cao, D.; Chen, S.; Wu, H. Dielectric Properties and Thermal Conductivity of Epoxy Composites Using Core/Shell Structured Si/SiO2/Polydopamine. Compos. Part B 2018, 140, 83–90. [Google Scholar] [CrossRef]
- Singha, S.; Thomas, M.J. Dielectric Properties of Epoxy Nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 12–23. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Li, Y.; Li, Y.; Chen, Q.; Zhang, G.; Xiao, S.; Tang, J. AC breakdown and decomposition characteristics of environmental friendly gas C5F10O/Air and C5F10O/N2. IEEE Access 2019, 7, 73954. [Google Scholar] [CrossRef]
- Luo, H.; Zhang, D.; Jiang, C.; Yuan, X.; Chen, C.; Zhou, K. Improved Dielectric Properties and Energy Storage Density of Poly (vinylidene fluoride-co-hexafluoropropylene) Nanocomposite with Hydantoin Epoxy Resin Coated BaTiO3. Acs Appl. Mater. Inter. 2015, 7, 8061–8069. [Google Scholar] [CrossRef]
- Dang, Z.M.; Yu, Y.F.; Xu, H.P.; Bai, J. Study on Microstructure and Dielectric Property of the BaTiO3/Epoxy Resin Composites. Compos. Sci. Technol. 2008, 68, 171–177. [Google Scholar] [CrossRef]
- Kuo, D.H.; Chang, C.; Su, T.Y.; Wang, K.; Lin, B.Y. Dielectric Behaviours of Multi-doped BaTiO3/epoxy Composites. J. Eur. Ceram Soc. 2001, 21, 1171–1177. [Google Scholar] [CrossRef]
- Tanaka, T. Dielectric nanocomposites with insulating properties. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 914–928. [Google Scholar] [CrossRef]
- Wang, W.; Min, D.; Li, S. Understanding the Conduction and Breakdown Properties of Polyethylene Nanodielectrics: Effect of deep traps. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 564–572. [Google Scholar] [CrossRef]

| Matrix | Fillers | TC (m-1 K-1) | TC-E | Reference |
|---|---|---|---|---|
| epoxy | 60wt% micro BN | 1.052 | 420% | [3] |
| polypropylene | 30vol% micro BN | ~2 | ~900% | [12] |
| epoxy | 25wt% BNNSs | 0.65 | 180% | [36] |
| epoxy | 25vol% BNNSs | 1.13 | 528% | this paper |
| epoxy | 30wt% h-BN | 1.178 | 514% | [37] |
| polymethylmethacrylate | 10wt% BNNTs | 0.5 | 194 | [38] |
| epoxy | 22.5vol% micro+nano BN | 1.4 | 250% | [39] |
| epoxy | 25wt%BN +7.5wt%Al2O3 | 1.182 | 700% | [40] |
| epoxy | 25.1vol%BNNS/AgNPs | 3.05 | 1123% | [29] |
| epoxy | 25vol% AgNPs-BNNSs | 2.14 | 1089% | this paper |
| Sample | Peak Value (pA) | Temperature of Peak Value (°C) | Trap Depth(eV) | Polarization Charges (pC) |
|---|---|---|---|---|
| Pure epoxy | 4.76 | 89.77 | 3.285 | 329.34 |
| 5vol% | 8.04 | 90.61 | 2.770 | 663.24 |
| 10vol% | 8.85 | 92.43 | 2.664 | 766.02 |
| 15vol% | 9.73 | 92.96 | 2.353 | 956.04 |
| 20vol% | 10.13 | 96.34 | 1.980 | 1205.28 |
| 25vol% | 12.16 | 94.62 | 1.966 | 1441.92 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Zhang, X.; Negi, A.; He, J.; Hu, G.; Tian, S.; Liu, J. Synergistic Effects of Boron Nitride (BN) Nanosheets and Silver (Ag) Nanoparticles on Thermal Conductivity and Electrical Properties of Epoxy Nanocomposites. Polymers 2020, 12, 426. https://doi.org/10.3390/polym12020426
Wu Y, Zhang X, Negi A, He J, Hu G, Tian S, Liu J. Synergistic Effects of Boron Nitride (BN) Nanosheets and Silver (Ag) Nanoparticles on Thermal Conductivity and Electrical Properties of Epoxy Nanocomposites. Polymers. 2020; 12(2):426. https://doi.org/10.3390/polym12020426
Chicago/Turabian StyleWu, Yunjian, Xiaoxing Zhang, Ankit Negi, Jixiong He, Guoxiong Hu, Shuangshuang Tian, and Jun Liu. 2020. "Synergistic Effects of Boron Nitride (BN) Nanosheets and Silver (Ag) Nanoparticles on Thermal Conductivity and Electrical Properties of Epoxy Nanocomposites" Polymers 12, no. 2: 426. https://doi.org/10.3390/polym12020426
APA StyleWu, Y., Zhang, X., Negi, A., He, J., Hu, G., Tian, S., & Liu, J. (2020). Synergistic Effects of Boron Nitride (BN) Nanosheets and Silver (Ag) Nanoparticles on Thermal Conductivity and Electrical Properties of Epoxy Nanocomposites. Polymers, 12(2), 426. https://doi.org/10.3390/polym12020426