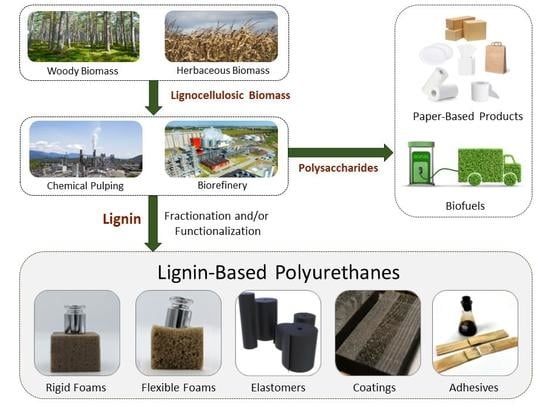
Lignin-Based Polyurethanes: Opportunities for Bio-Based Foams, Elastomers, Coatings and Adhesives
Abstract
1. Introduction
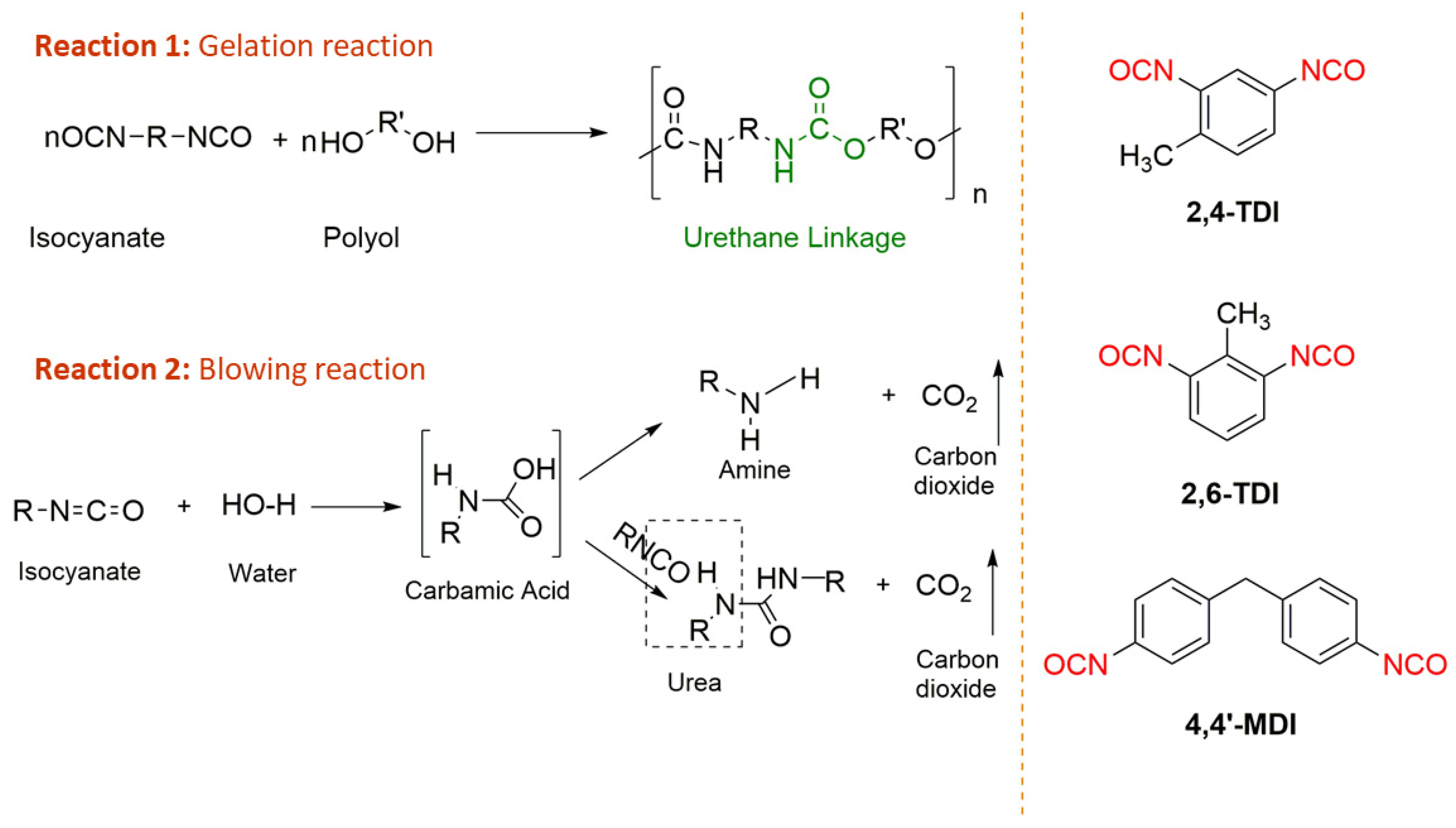
1.1. Polyurethanes
1.2. Opportunities and Challenges for Lignin Incorporation into Polyurethanes
2. Impact of Source, Processing History, and Functionalization on Lignin Structure
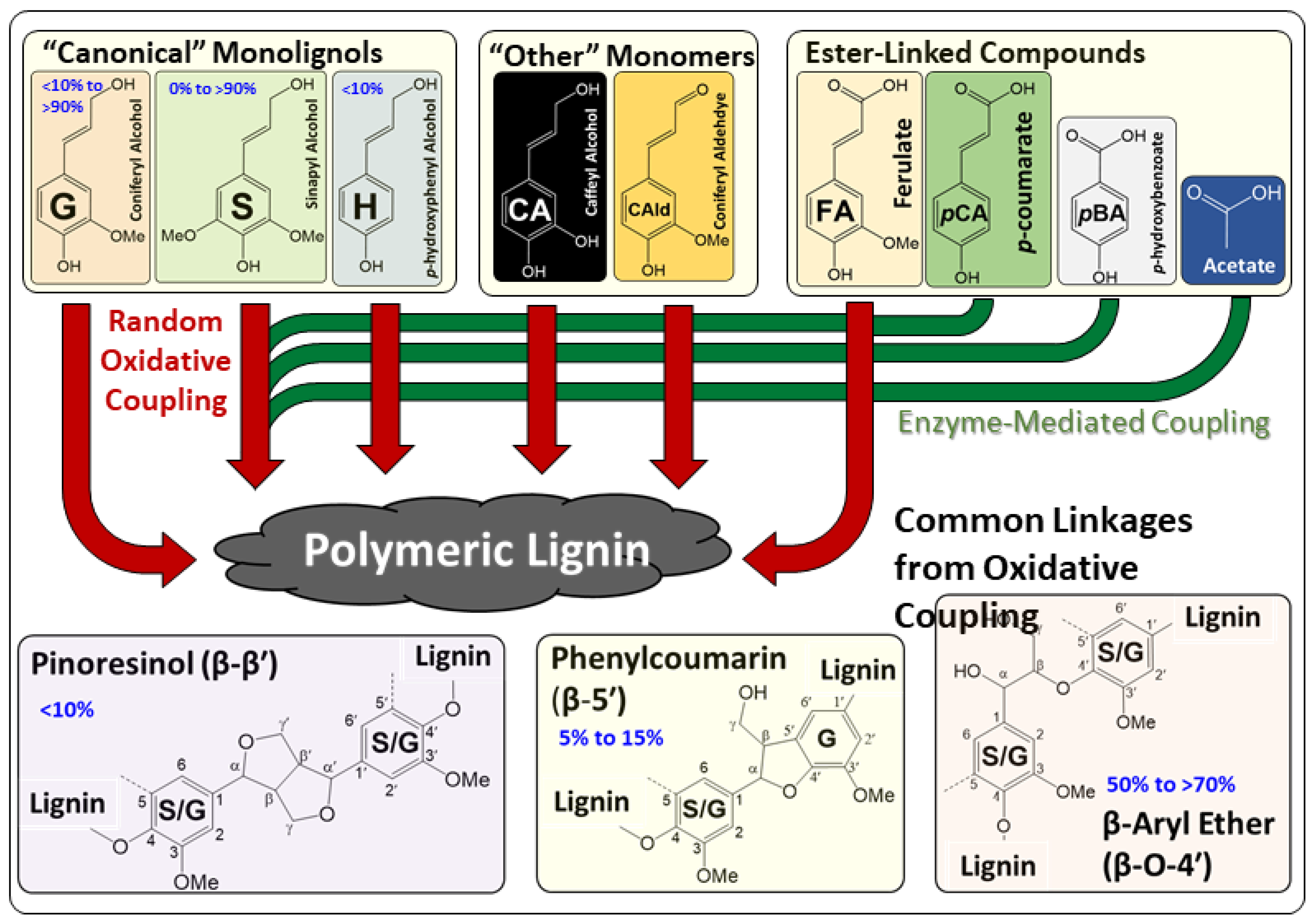
2.1. Diversity of Native Lignins
2.2. Industrial Approaches to Yield Process-Modified Lignins
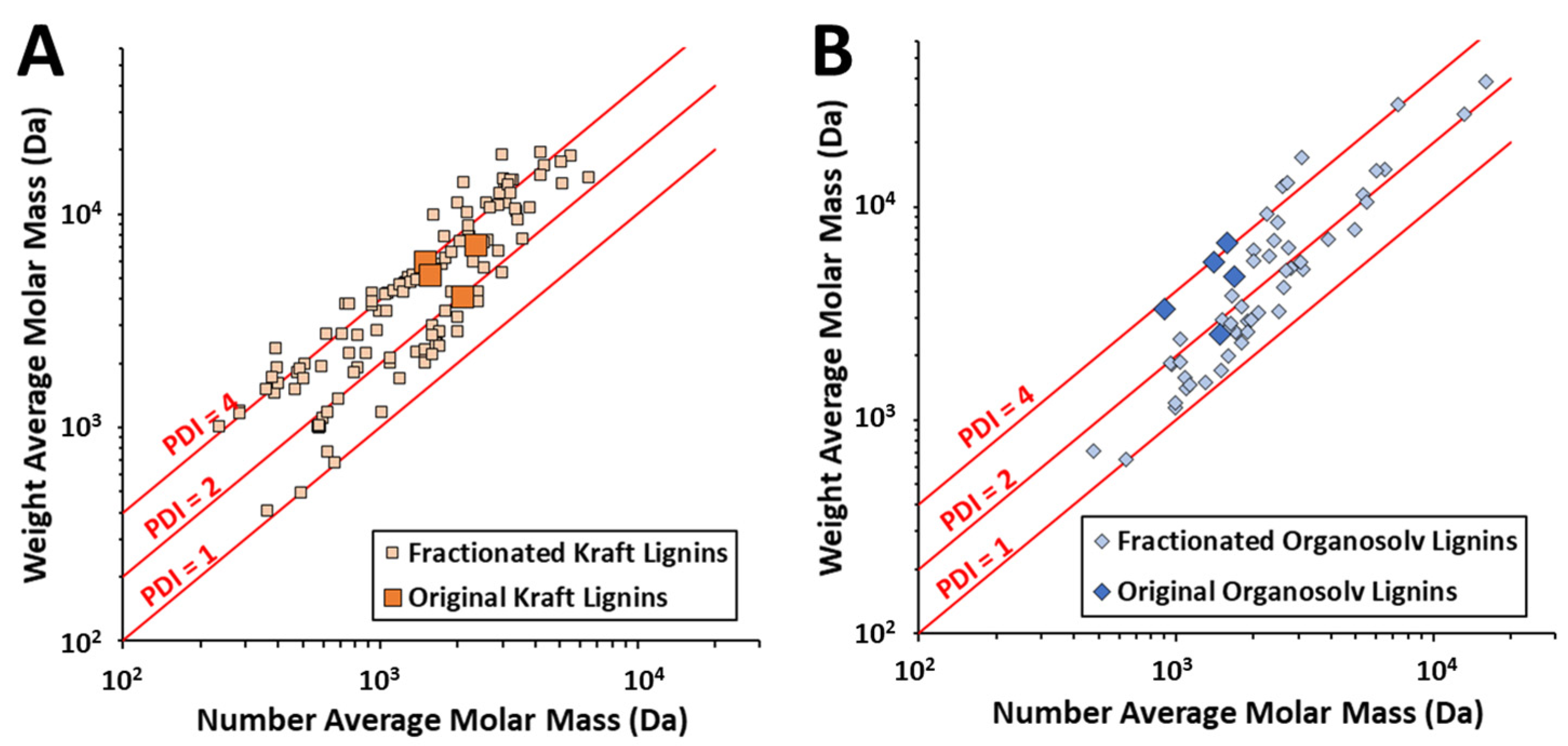
2.3. Lignin Fractionation
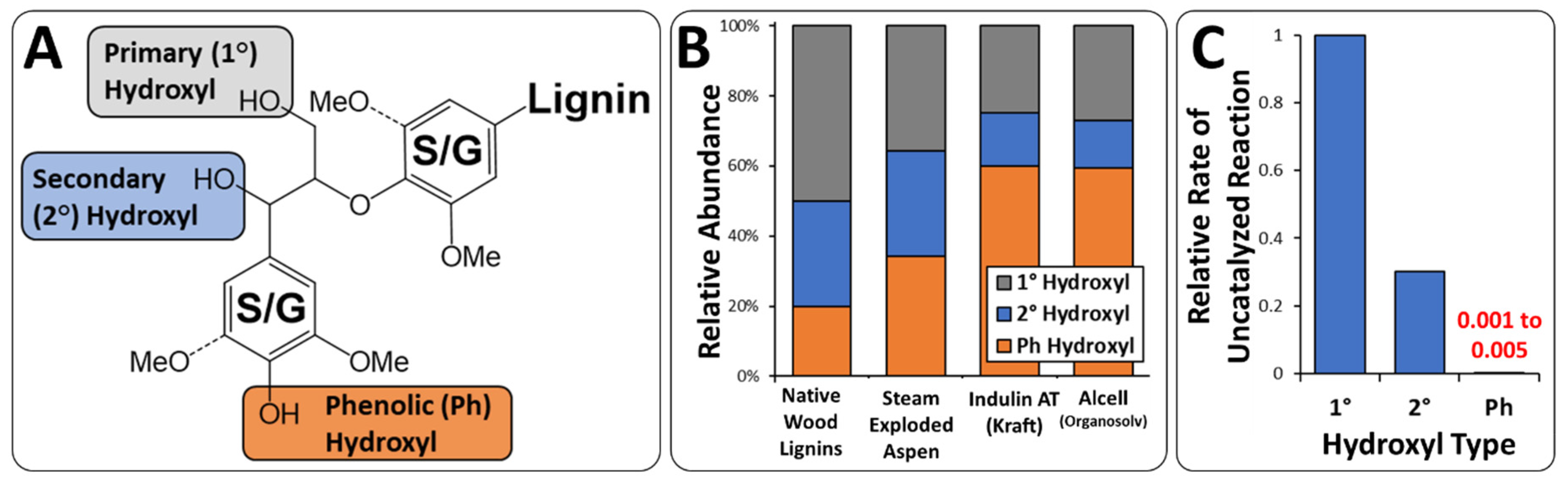
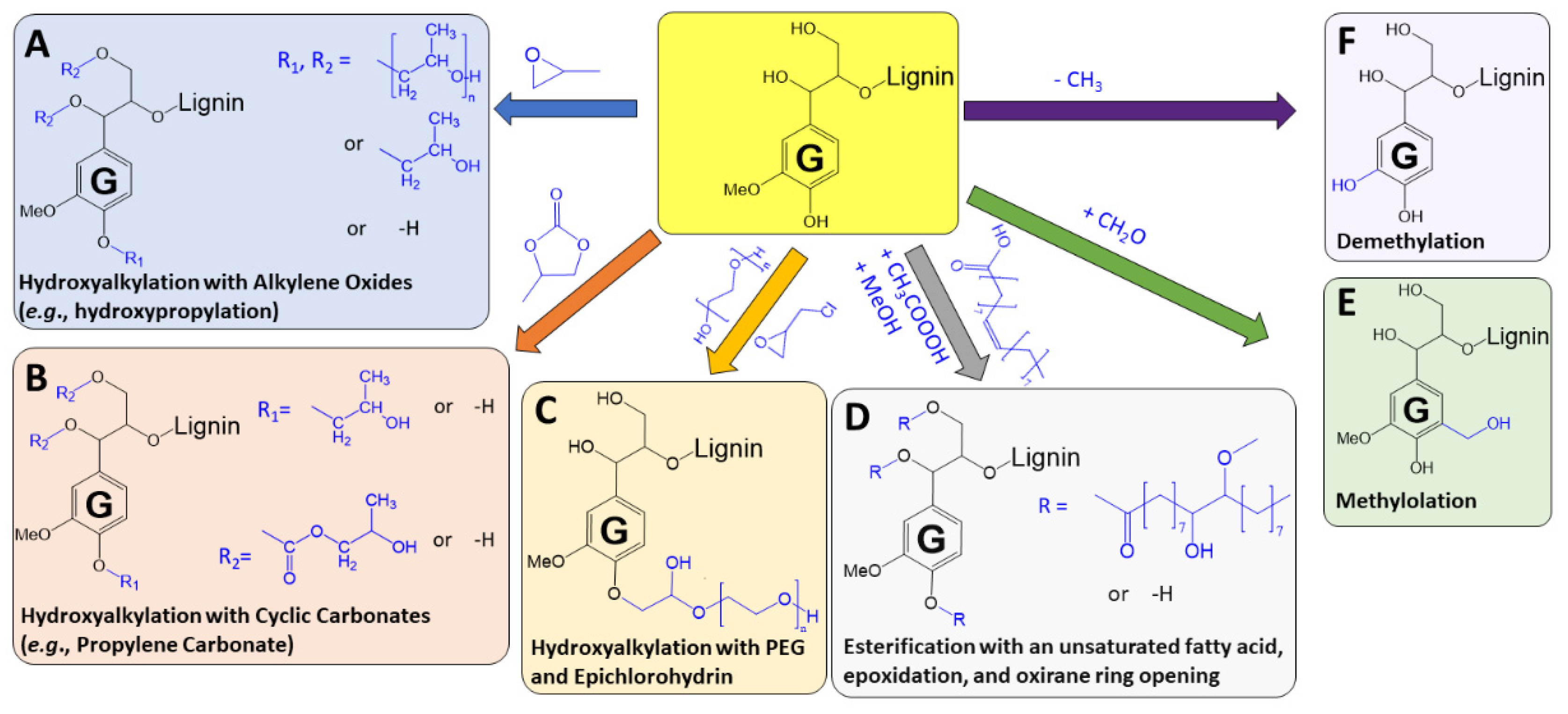
2.4. Lignin Functionalization
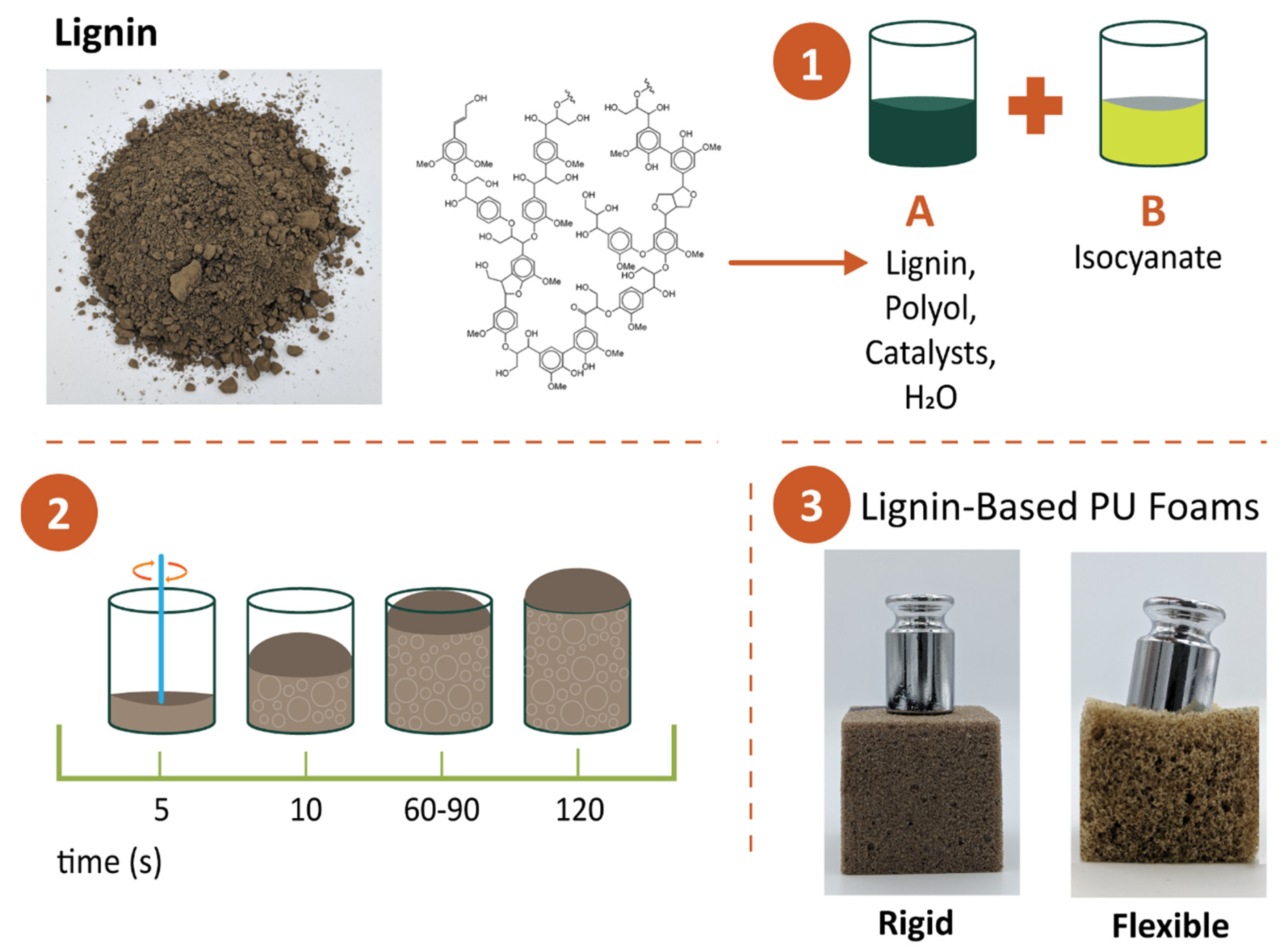
3. Lignin-Based Polyurethanes
3.1. Lignin-Based Rigid Polyurethane Foams
3.2. Lignin Based Flexible Polyurethane Foams
3.3. Lignin Based Polyurethane Elastomers
3.4. Lignin-Based Polyurethane Coatings
3.5. Lignin Based Polyurethane Adhesives
4. Conclusions
Funding
Conflicts of Interest
References
- Sonnenschein, M.F. Polyurethanes: science, technology, markets, and trends; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; ISBN 1118737830. [Google Scholar]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications – a review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Rafiee, Z.; Keshavarz, V. Synthesis and characterization of polyurethane/microcrystalline cellulose bionanocomposites. Prog. Org. Coatings 2015, 86, 190–193. [Google Scholar] [CrossRef]
- Golling, F.E.; Pires, R.; Hecking, A.; Weikard, J.; Richter, F.; Danielmeier, K.; Dijkstra, D. Polyurethanes for coatings and adhesives–chemistry and applications. Polym. Int. 2019, 68, 848–855. [Google Scholar] [CrossRef]
- Ionescu, M. Chemistry and technology of polyols for polyurethanes; Rapra Technology Limited: Shropshire, UK, 2005; ISBN 1859574912. [Google Scholar]
- Plastic Insight. Available online: https://www.plasticsinsight.com/resin-intelligence/resin-prices/polyurethane/%0A%0Ahttps://investor.covestro.com/securedl/14391 (accessed on 1 June 2019).
- ASI Adhesive and Sealants. Available online: https://www.adhesivesmag.com/articles/95408-green-and-bio-polyols-market-worth-471-billion-by-2021 (accessed on 1 June 2019).
- Gadhave, R.V.; Mahanwar, P.A.; Gadekar, P.T. Bio-Renewable Sources for Synthesis of Eco-Friendly Polyurethane Adhesives—Review. Open J. Polym. Chem. 2017, 07, 57–75. [Google Scholar] [CrossRef]
- Duval, A.; Lawoko, M. A review on lignin-based polymeric, micro- and nano-structured materials. React. Funct. Polym. 2014, 85, 78–96. [Google Scholar] [CrossRef]
- Xu, C.; Ferdosian, F. Utilization of Lignosulfonate as Dispersants or Surfactants. In Conversion of Lignin into Bio-Based Chemicals and Materials; Springer: Berlin/Heidelberg, Germany, 2017; pp. 81–90. ISBN 9783662549599. [Google Scholar]
- Chung, H.; Washburn, N.R. Improved Lignin Polyurethane Properties with Lewis Acid Treatment. ACS Appl. Mater. Interfaces 2012, 4, 2840–2846. [Google Scholar] [CrossRef]
- Delebecq, E.; Pascault, J.-P.; Boutevin, B.; Ganachaud, F. On the Versatility of Urethane/Urea Bonds: Reversibility, Blocked Isocyanate, and Non-isocyanate Polyurethane. Chem. Rev. 2013, 113, 80–118. [Google Scholar] [CrossRef]
- Sameni, J.; Krigstin, S.; Sain, M. Solubility of Lignin and Acetylated Lignin in Organic Solvents. BioResources 2017, 12, 1548–1565. [Google Scholar] [CrossRef]
- Passoni, V.; Scarica, C.; Levi, M.; Turri, S.; Griffini, G. Fractionation of industrial softwood kraft lignin: solvent selection as a tool for tailored material properties. ACS Sustain. Chem. Eng. 2016, 4, 2232–2242. [Google Scholar] [CrossRef]
- Vishtal, A.; Kraslawski, A. Challenges in industrial applications of technical lignins. BioResources 2011, 6, 3547–3568. [Google Scholar] [CrossRef]
- George, B.; Suttie, E.; Merlin, A.; Deglise, X. Photodegradation and photostabilisation of wood – the state of the art. Polym. Degrad. Stab. 2005, 88, 268–274. [Google Scholar] [CrossRef]
- Jaeger, C.; Nourmamode, A.; Castellan, A. Photodegradation of lignin: A photochemical study of phenolic coniferyl alcohol lignin model molecules. Holzforschung-International J. Biol. Chem. Phys. Technol. Wood 1993, 47, 375–390. [Google Scholar] [CrossRef]
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards lignin-based functional materials in a sustainable world. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- MacLeod, I.T.; Scully, A.D.; Ghiggino, K.P.; Ritchie, P.J.A.; Paravagna, O.M.; Leary, B. Photodegradation at the wood-clearcoat interface. Wood Sci. Technol. 1995, 29, 183–189. [Google Scholar] [CrossRef]
- Vanucci, C.; De Violet, P.F.; Bouas-Laurent, H.; Castellan, A. Photodegradation of lignin: A photophysical and photochemical study of a non-phenolic α-carbonyl β-O-4 lignin model dimer, 3, 4-dimethoxy-α-(2′-methoxypenoxy) acetophenone. J. Photochem. Photobiol. A Chem. 1988, 41, 251–265. [Google Scholar] [CrossRef]
- Yang, W.; Rallini, M.; Wang, D.-Y.; Gao, D.; Dominici, F.; Torre, L.; Kenny, J.M.; Puglia, D. Role of lignin nanoparticles in UV resistance, thermal and mechanical performance of PMMA nanocomposites prepared by a combined free-radical graft polymerization/masterbatch procedure. Compos. Part A Appl. Sci. Manuf. 2018, 107, 61–69. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.H.; Deng, L.; Liao, B.; Guo, Q.Q. Improving aging resistance and mechanical properties of waterborne polyurethanes modified by lignin amines. J. Appl. Polym. Sci. 2013, 130, 1736–1742. [Google Scholar] [CrossRef]
- Kalia, S.; Avérous, L. Biodegradable and Biobased Polymers for Environmental and Biomedical Applications; John Wiley & Sons: Hoboken, NJ, USA, 2016; ISBN 1119117348. [Google Scholar]
- Thring, R.W.; Vanderlaan, M.N.; Griffin, S.L. Polyurethanes from Alcell® lignin. Biomass and Bioenergy 1997, 13, 125–132. [Google Scholar] [CrossRef]
- Crowe, J.D.; Feringa, N.; Pattathil, S.; Merritt, B.; Foster, C.; Dines, D.; Ong, R.G.; Hodge, D.B. Identification of developmental stage and anatomical fraction contributions to cell wall recalcitrance in switchgrass. Biotechnol. Biofuels 2017, 10, 184. [Google Scholar] [CrossRef]
- Glasser, W.G.; Northey, R.A.; Schultz, T.P. Lignin: Historical, Biological, and Materials Perspectives; American Chemical Society: Washington, DC, USA, 1999; pp. 2–531. ISBN 0-8412-3611-9. [Google Scholar]
- Ralph, J.; Lundquist, K.; Brunow, G.; Lu, F.; Kim, H.; Schatz, P.F.; Marita, J.M.; Hatfield, R.D.; Ralph, S.A.; Christensen, J.H. Lignins: natural polymers from oxidative coupling of 4-hydroxyphenyl-propanoids. Phytochem. Rev. 2004, 3, 29–60. [Google Scholar] [CrossRef]
- Ralph, J. Hydroxycinnamates in lignification. Phytochem. Rev. 2010, 9, 65–83. [Google Scholar] [CrossRef]
- Lu, F.; Karlen, S.D.; Regner, M.; Kim, H.; Ralph, S.A.; Sun, R.-C.; Kuroda, K.; Augustin, M.A.; Mawson, R.; Sabarez, H. Naturally p-hydroxybenzoylated lignins in palms. BioEnergy Res. 2015, 8, 934–952. [Google Scholar] [CrossRef]
- Gellerstedt, G.; Tomani, P.; Axegård, P.; Backlund, B. Lignin recovery and lignin-based products. In Integrated Forest Biorefineries–Challenges and Opportunities; Royal Society of Chemistry: Cambridge, UK, 2013; pp. 180–210. ISBN 978-1-84973-321-2. [Google Scholar]
- Chen, J.; Eraghi Kazzaz, A.; AlipoorMazandarani, N.; Hosseinpour Feizi, Z.; Fatehi, P. Production of Flocculants, Adsorbents, and Dispersants from Lignin. Molecules 2018, 23, 868. [Google Scholar] [CrossRef] [PubMed]
- Gierer, J. Chemistry of delignification. Wood Sci. Technol. 1985, 19, 289–312. [Google Scholar] [CrossRef]
- Qureshi, N.; Hodge, D.; Vertes, A. Biorefineries: Integrated biochemical processes for liquid biofuels; Elsevier Science: Amsterdam, The Netherlands, 2014; ISBN 978-0-444-59498-3. [Google Scholar]
- Ong, R.G.; Chundawat, S.P.S.; Hodge, D.B.; Keskar, S.; Dale, B.E. Linking plant biology and pretreatment: understanding the structure and organization of the plant cell wall and interactions with cellulosic biofuel production. In Plants and Bioenergy; Springer: Berlin/Heidelberg, Germany, 2014; pp. 231–253. [Google Scholar]
- Hammett, A.L.; Youngs, R.L.; Sun, X.; Chandra, M. Non-wood fiber as an alternative to wood fiber in Chinas pulp and paper industry. Holzforschung 2001, 55, 219–224. [Google Scholar] [CrossRef]
- Saake, B.; Argyropoulos, D.S.; Beinhoff, O.; Faix, O. A comparison of lignin polymer models (DHPs) and lignins by 31P NMR spectroscopy. Phytochemistry 1996, 43, 499–507. [Google Scholar] [CrossRef]
- Argyropoulos, D. Quantitative Phosphorus-31 NMR Analysis of Six Soluble Lignins. J. Wood Chem. Technol. 1994, 14, 65–82. [Google Scholar] [CrossRef]
- Stoklosa, R.J.; Velez, J.; Kelkar, S.; Saffron, C.M.; Thies, M.C.; Hodge, D.B. Correlating lignin structural features to phase partitioning behavior in a novel aqueous fractionation of softwood Kraft black liquor. Green Chem. 2013, 15, 2904–2912. [Google Scholar] [CrossRef]
- Saito, T.; Perkins, J.H.; Vautard, F.; Meyer, H.M.; Messman, J.M.; Tolnai, B.; Naskar, A.K. Methanol Fractionation of Softwood Kraft Lignin: Impact on the Lignin Properties. ChemSusChem 2014, 7, 221–228. [Google Scholar] [CrossRef]
- Wang, K.; Xu, F.; Sun, R. Molecular Characteristics of Kraft-AQ Pulping Lignin Fractionated by Sequential Organic Solvent Extraction. Int. J. Mol. Sci. 2010, 11, 2988–3001. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Argyropoulos, D.S. Macroscopic Behavior of Kraft Lignin Fractions: Melt Stability Considerations for Lignin–Polyethylene Blends. ACS Sustain. Chem. Eng. 2016, 4, 5160–5166. [Google Scholar] [CrossRef]
- Jiang, X.; Savithri, D.; Du, X.; Pawar, S.; Jameel, H.; Chang, H.; Zhou, X. Fractionation and Characterization of Kraft Lignin by Sequential Precipitation with Various Organic Solvents. ACS Sustain. Chem. Eng. 2017, 5, 835–842. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Tamminen, T.; Liitiä, T.; Peresin, M.S.; Rodríguez, A.; Jääskeläinen, A.S. Aqueous acetone fractionation of kraft, organosolv and soda lignins. Int. J. Biol. Macromol. 2018, 106, 979–987. [Google Scholar] [CrossRef]
- Jääskeläinen, A.-S.; Liitiä, T.; Mikkelson, A.; Tamminen, T. Aqueous organic solvent fractionation as means to improve lignin homogeneity and purity. Ind. Crops Prod. 2017, 103, 51–58. [Google Scholar] [CrossRef]
- Goldmann, W.M.; Ahola, J.; Mikola, M.; Tanskanen, J. Solubility and fractionation of Indulin AT kraft lignin in ethanol-water media. Sep. Purif. Technol. 2019, 209, 826–832. [Google Scholar] [CrossRef]
- Yuan, T.-Q.; He, J.; Xu, F.; Sun, R.-C. Fractionation and physico-chemical analysis of degraded lignins from the black liquor of Eucalyptus pellita KP-AQ pulping. Polym. Degrad. Stab. 2009, 94, 1142–1150. [Google Scholar] [CrossRef]
- Vanderlaan, M.N.; Thring, R.W. Polyurethanes from Alcell® lignin fractions obtained by sequential solvent extraction. Biomass Bioenergy 1998, 14, 525–531. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Wells, T.; Le, R.K.; Sadeghifar, F.; Yuan, J.S.; Jonas Ragauskas, A. Fractionation of Organosolv Lignin Using Acetone:Water and Properties of the Obtained Fractions. ACS Sustain. Chem. Eng. 2017, 5, 580–587. [Google Scholar] [CrossRef]
- Zhang, H.; Bai, Y.; Yu, B.; Liu, X.; Chen, F. A practicable process for lignin color reduction: fractionation of lignin using methanol/water as a solvent. Green Chem. 2017, 19, 5152–5162. [Google Scholar] [CrossRef]
- Cui, C.; Sun, R.; Argyropoulos, D.S. Fractional Precipitation of Softwood Kraft Lignin: Isolation of Narrow Fractions Common to a Variety of Lignins. ACS Sustain. Chem. Eng. 2014, 2, 959–968. [Google Scholar] [CrossRef]
- Kun, D.; Pukánszky, B. Polymer/lignin blends: Interactions, properties, applications. Eur. Polym. J. 2017, 93, 618–641. [Google Scholar] [CrossRef]
- Cateto, C.A.; Barreiro, M.F.; Rodrigues, A.E.; Brochier-Salon, M.C.; Thielemans, W.; Belgacem, M.N. Lignins as macromonomers for polyurethane synthesis: A comparative study on hydroxyl group determination. J. Appl. Polym. Sci. 2008, 109, 3008–3017. [Google Scholar] [CrossRef]
- Ge, Y.; Dababneh, F.; Li, L. Economic Evaluation of Lignocellulosic Biofuel Manufacturing Considering Integrated Lignin Waste Conversion to Hydrocarbon Fuels. Procedia Manuf. 2017, 10, 112–122. [Google Scholar] [CrossRef]
- Gosselink, R.J.A.; De Jong, E.; Guran, B.; Abächerli, A. Co-ordination network for lignin - Standardisation, production and applications adapted to market requirements (EUROLIGNIN). Ind. Crops Prod. 2004, 20, 121–129. [Google Scholar] [CrossRef]
- Quesada-Medina, J.; López-Cremades, F.J.; Olivares-Carrillo, P. Organosolv extraction of lignin from hydrolyzed almond shells and application of the δ-value theory. Bioresour. Technol. 2010, 101, 8252–8260. [Google Scholar] [CrossRef]
- Chauhan, M.; Gupta, M.; Singh, B.; Singh, A.K.; Gupta, V.K. Effect of functionalized lignin on the properties of lignin–isocyanate prepolymer blends and composites. Eur. Polym. J. 2014, 52, 32–43. [Google Scholar] [CrossRef]
- Christian, D.T.; Look, M.; Nobell, A.; Armstrong, T.S. Process for producing polyoxyalkylene ether-polyols from lignin. U.S. Patent No. 3,546,199, 8 December 1970. [Google Scholar]
- Wu, L.C.; Glasser, W.G. Engineering plastics from lignin. I. Synthesis of hydroxypropyl lignin. J. Appl. Polym. Sci. 1984, 29, 1111–1123. [Google Scholar] [CrossRef]
- Hsu, O.H.-H.; Glasser, W.G. Polyurethane foams from carboxylated lignins. J. Appl. Polym. Sci. Appl. Polym. Symp. 1975, 378, 297–307. [Google Scholar]
- Kühnel, I.; Podschun, J.; Saake, B.; Lehnen, R. Synthesis of lignin polyols via oxyalkylation with propylene carbonate. Holzforschung 2015, 69, 531–538. [Google Scholar] [CrossRef]
- Kühnel, I.; Saake, B.; Lehnen, R. A New Environmentally Friendly Approach to Lignin-Based Cyclic Carbonates. Macromol. Chem. Phys. 2018, 219, 1700613. [Google Scholar] [CrossRef]
- Kühnel, I.; Saake, B.; Lehnen, R. Oxyalkylation of lignin with propylene carbonate: Influence of reaction parameters on the ensuing bio-based polyols. Ind. Crops Prod. 2017, 101, 75–83. [Google Scholar] [CrossRef]
- Mimini, V.; Amer, H.; Hettegger, H.; Bacher, M.; Gebauer, I.; Bischof, R.; Fackler, K.; Potthast, A.; Rosenau, T. Lignosulfonate-based polyurethane materials via cyclic carbonates: preparation and characterization. Holzforschung 2019. [Google Scholar] [CrossRef]
- Lin, X.; Zhou, M.; Wang, S.; Lou, H.; Yang, D.; Qiu, X. Synthesis, structure, and dispersion property of a novel lignin-based polyoxyethylene ether from kraft lignin and poly (ethylene glycol). ACS Sustain. Chem. Eng. 2014, 2, 1902–1909. [Google Scholar] [CrossRef]
- Laurichesse, S.; Huillet, C.; Avérous, L. Original polyols based on organosolv lignin and fatty acids: New bio-based building blocks for segmented polyurethane synthesis. Green Chem. 2014, 16, 3958–3970. [Google Scholar] [CrossRef]
- Meister, J.J. Modification of lignin. J. Macromol. Sci. Part C Polym. Rev. 2002, 42, 235–289. [Google Scholar] [CrossRef]
- Sawamura, K.; Tobimatsu, Y.; Kamitakahara, H.; Takano, T. Lignin functionalization through chemical demethylation: preparation and tannin-like properties of demethylated guaiacyl-type synthetic lignins. ACS Sustain. Chem. Eng. 2017, 5, 5424–5431. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, G.; Xu, J.; Gao, C.; Wu, Y. Recent advances on ligin-derived polyurethane polymers. Rev. Adv. Mater. Sci. 2015, 40, 146–154. [Google Scholar]
- Randall, D.; Lee, S. The Polyurethanes Book; Wiley: Hoboken, NJ, USA, 2002; ISBN 0470850418. [Google Scholar]
- Xu, C.; Ferdosian, F. Lignin-Based Polyurethane (PU) Resins and Foam. In Conversion of Lignin into Bio-Based Chemicals and Materials; Green Chemistry and Sustainable Technology; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2017; pp. 133–156. ISBN 9783662549599. [Google Scholar]
- Al-Homoud, M.S. Performance characteristics and practical applications of common building thermal insulation materials. Build. Environ. 2005, 40, 353–366. [Google Scholar] [CrossRef]
- Landrock, A.H. Handbook of Plastic Foams: Types, Properties, Manufacture and Applications; Elsevier: Amsterdam, The Netherlands, 1995; pp. 88–139. ISBN 9780815513575. [Google Scholar]
- Tawade, B.V.; Shingte, R.D.; Kuhire, S.S.; Sadavarte, N.V.; Garg, K.; Maher, D.M.; Ichake, A.B.; More, A.S.; Wadgaonkar, P.P. Bio-Based Di-/Poly-isocyanates for Polyurethanes: An Overview. Polyurethanes Today 2017. Technical Article. [Google Scholar] [CrossRef]
- Sivertsen, K. POLYMER FOAMS 3.063 Polymer Physics Spring 2007; Massachusetts Institue of Technology (MIT): Cambridge, MA, USA, 2007. [Google Scholar]
- Szycher, M. Szycher’s Handbook of Polyurethanes; CRC Press: Boca Raton, FL, USA, 2013; Volume 37, ISBN 1439839581. [Google Scholar]
- Seo, W.J.; Park, J.H.; Sung, Y.T.; Hwang, D.H.; Kim, W.N.; Lee, H.S. Properties of water-blown rigid polyurethane foams with reactivity of raw materials. J. Appl. Polym. Sci. 2004, 93, 2334–2342. [Google Scholar] [CrossRef]
- Li, Y.; Ragauskas, A.J. Kraft lignin-based rigid polyurethane foam. J. Wood Chem. Technol. 2012, 32, 210–224. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in Green Polymer Composites from Lignin for Multifunctional Applications: A Review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rabbani, M.M.; Saha, J.K. Polyurethane and Its Derivatives. In Functional Polymers; Jafar Mazumder, M.A., Sheardown, H., Al-Ahmed, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–16. ISBN 9783319920672. [Google Scholar]
- Helling, R.K.; Russell, D.A. Use of life cycle assessment to characterize the environmental impacts of polyol production options. Green Chem. 2009, 11, 380–389. [Google Scholar] [CrossRef]
- Pawlik, H.; Prociak, A. Influence of Palm Oil-Based Polyol on the Properties of Flexible Polyurethane Foams. J. Polym. Environ. 2012, 20, 438–445. [Google Scholar] [CrossRef]
- Shogren, R.L.; Petrovic, Z.; Liu, Z.; Erhan, S.Z. Biodegradation Behavior of Some Vegetable Oil-Based Polymers. J. Polym. Environ. 2004, 12, 173–178. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Lee, H.W.; Lee, S.M.; Jae, J.; Park, Y.-K. Overview of the recent advances in lignocellulose liquefaction for producing biofuels, bio-based materials and chemicals. Bioresour. Technol. 2019. [Google Scholar] [CrossRef]
- Huang, X.; De Hoop, C.F.; Xie, J.; Hse, C.-Y.; Qi, J.; Hu, T. Characterization of Biobased Polyurethane Foams Employing Lignin Fractionated from Microwave Liquefied Switchgrass. Int. J. Polym. Sci. 2017, 1–8. [Google Scholar] [CrossRef]
- Faruk, O.; Sain, M.; Farnood, R.; Pan, Y.; Xiao, H. Development of Lignin and Nanocellulose Enhanced Bio PU Foams for Automotive Parts. J. Polym. Environ. 2014, 22, 279–288. [Google Scholar] [CrossRef]
- Evtuguin, D.; Andreolety, J.; Gandini, A. Polyurethanes based on oxygen-organosolv lignin. Eur. Polym. J. 1998, 34, 1163–1169. [Google Scholar] [CrossRef]
- Mahmood, N.; Yuan, Z.; Schmidt, J.; Xu, C. (Charles) Depolymerization of lignins and their applications for the preparation of polyols and rigid polyurethane foams: A review. Renew. Sustain. Energy Rev. 2016, 60, 317–329. [Google Scholar] [CrossRef]
- Xue, B.-L.; Wen, J.-L.; Sun, R.-C. Lignin-Based Rigid Polyurethane Foam Reinforced with Pulp Fiber: Synthesis and Characterization. ACS Sustain. Chem. Eng. 2014, 2, 1474–1480. [Google Scholar] [CrossRef]
- Xie, J.; Qi, J.; Hse, C.-Y.; Shupe, T.F. Effect of Lignin Derivatives in the Bio-Polyols from Microwave Liquefied Bamboo on the Properties of Polyurethane Foams. BioResources 2013, 9, 578–588. [Google Scholar] [CrossRef]
- Obaid, N.; Kortschot, M.T.; Sain, M. Lignin-Based Foaming Materials. In Lignin in Polymer Composites; Elsevier: Amsterdam, The Netherlands, 2016; pp. 217–232. ISBN 9780323355667. [Google Scholar]
- Yoshida, H.; Mörck, R.; Kringstad, K.P.; Hatakeyama, H. Kraft lignin in polyurethanes. II. Effects of the molecular weight of kraft lignin on the properties of polyurethanes from a kraft lignin–polyether triol–polymeric MDI system. J. Appl. Polym. Sci. 1990, 40, 1819–1832. [Google Scholar] [CrossRef]
- Cinelli, P.; Anguillesi, I.; Lazzeri, A. Green synthesis of flexible polyurethane foams from liquefied lignin. Eur. Polym. J. 2013, 49, 1174–1184. [Google Scholar] [CrossRef]
- Pan, X.; Saddler, J.N. Effect of replacing polyol by organosolv and kraft lignin on the property and structure of rigid polyurethane foam. Biotechnol. Biofuels 2013, 6, 12. [Google Scholar] [CrossRef]
- Mahmood, N.; Yuan, Z.; Schmidt, J.; Tymchyshyn, M.; Xu, C. Hydrolytic liquefaction of hydrolysis lignin for the preparation of bio-based rigid polyurethane foam. Green Chem. 2016, 18, 2385–2398. [Google Scholar] [CrossRef]
- Carriço, C.S.; Fraga, T.; Pasa, V.M.D. Production and characterization of polyurethane foams from a simple mixture of castor oil, crude glycerol and untreated lignin as bio-based polyols. Eur. Polym. J. 2016, 85, 53–61. [Google Scholar] [CrossRef]
- Kouisni, L.; Gagné, A.; Maki, K.; Holt-Hindle, P.; Paleologou, M. LignoForce System for the Recovery of Lignin from Black Liquor: Feedstock Options, Odor Profile, and Product Characterization. ACS Sustain. Chem. Eng. 2016, 4, 5152–5159. [Google Scholar] [CrossRef]
- Lee, A.; Deng, Y. Green polyurethane from lignin and soybean oil through non-isocyanate reactions. Eur. Polym. J. 2015, 63, 67–73. [Google Scholar] [CrossRef]
- Naseem, A.; Tabasum, S.; Zia, K.M.; Zuber, M.; Ali, M.; Noreen, A. Lignin-derivatives based polymers, blends and composites: A review. Int. J. Biol. Macromol. 2016, 93, 296–313. [Google Scholar] [CrossRef]
- Hon, D.N.-S. Chemical Modification of Lignocellulosic Materials; Marcel Dekker. Inc.: New York, NY, USA, 1995; ISBN 0-8247-9472-9. [Google Scholar]
- Nadji, H.; Bruzzèse, C.; Belgacem, M.N.; Benaboura, A.; Gandini, A. Oxypropylation of Lignins and Preparation of Rigid Polyurethane Foams from the Ensuing Polyols. Macromol. Mater. Eng. 2005, 290, 1009–1016. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Zhang, X.; Jeremic, D.; Kim, Y.; Street, J.; Shmulsky, R. Effects of surface functionalization of lignin on synthesis and properties of rigid bio-based polyurethanes foams. Polymers 2018, 10. [Google Scholar] [CrossRef]
- Cateto, C.A.; Barreiro, M.F.; Ottati, C.; Lopretti, M.; Rodrigues, A.E.; Belgacem, M.N. Lignin-based rigid polyurethane foams with improved biodegradation. J. Cell. Plast. 2014, 50, 81–95. [Google Scholar] [CrossRef]
- Gao, L.; Zheng, G.; Zhou, Y.; Hu, L.; Feng, G. Improved mechanical property, thermal performance, flame retardancy and fire behavior of lignin-based rigid polyurethane foam nanocomposite. J. Therm. Anal. Calorim. 2015, 120, 1311–1325. [Google Scholar] [CrossRef]
- Chang, L.; Sain, M.; Kortschot, M. Improvement in Compressive Behavior of Alkali-treated Wood Polyurethane Foams. Cell. Polym. 2014, 33, 139–158. [Google Scholar] [CrossRef]
- Jeong, H.; Park, J.; Kim, S.; Lee, J.; Ahn, N. Compressive viscoelastic properties of softwood kraft lignin-based flexible polyurethane foams. Fibers Polym. 2013, 14, 1301–1310. [Google Scholar] [CrossRef]
- Li, H.; Sun, J.-T.; Wang, C.; Liu, S.; Yuan, D.; Zhou, X.; Tan, J.; Stubbs, L.; He, C. High Modulus, Strength, and Toughness Polyurethane Elastomer Based on Unmodified Lignin. ACS Sustain. Chem. Eng. 2017, 5, 7942–7949. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Kosugi, R.; Hatakeyama, T. Thermal properties of lignin-and molasses-based polyurethane foams. J. Therm. Anal. Calorim. 2008, 92, 419–424. [Google Scholar] [CrossRef]
- Li, Y.; Ragauskas, A.J. Ethanol organosolv lignin-based rigid polyurethane foam reinforced with cellulose nanowhiskers. RSC Adv. 2012, 2, 3347. [Google Scholar] [CrossRef]
- Lu, W.; Li, Q.; Zhang, Y.; Yu, H.; Hirose, S.; Hatakeyama, H.; Matsumoto, Y.; Jin, Z. Lignosulfonate/APP IFR and its flame retardancy in lignosulfonate-based rigid polyurethane foams. J. Wood Sci. 2018, 64, 287–293. [Google Scholar] [CrossRef]
- Gama, N.; Ferreira, A.; Barros-Timmons, A. Polyurethane Foams: Past, Present, and Future. Materials (Basel). 2018, 11, 1841. [Google Scholar] [CrossRef]
- Silvestre, A.J.D.; Neto, C.P.; Gandini, A. Cork and Suberins: Major Sources, Properties and Applications. In Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2008; pp. 305–320. ISBN 9780080453163. [Google Scholar]
- Xue, B.-L.; Huang, P.-L.; Sun, Y.-C.; Li, X.-P.; Sun, R.-C. Hydrolytic depolymerization of corncob lignin in the view of a bio-based rigid polyurethane foam synthesis. RSC Adv. 2017, 7, 6123–6130. [Google Scholar] [CrossRef]
- Mahmood, N.; Yuan, Z.; Schmidt, J.; (Charles) Xu, C. Production of polyols via direct hydrolysis of kraft lignin: Effect of process parameters. Bioresour. Technol. 2013, 139, 13–20. [Google Scholar] [CrossRef]
- Bernardini, J.; Anguillesi, I.; Coltelli, M.-B.B.; Cinelli, P.; Lazzeri, A. Optimizing the lignin based synthesis of flexible polyurethane foams employing reactive liquefying agents. Polym. Int. 2015, 64, 1235–1244. [Google Scholar] [CrossRef]
- White, E.F.T. Polyurethane handbook Edited by G. Oertel, Hanser Publishers, Munich, 1985. pp. 629, price E104.70. ISBN 3-446-13671-1. Br. Polym. J. 1986, 18, 403–404. [Google Scholar] [CrossRef]
- Wypych, G. Handbook of Foaming and Blowing Agents; ChemTec publishing: Toronto, ON, Canada, 2017; Volume 1, ISBN 9781895198997. [Google Scholar]
- Woods, G. Flexible Polyurethane Foams, Chemistry and Technology; Springer: Dordrecht, The Netherlands, 1982; ISBN 9780853349815. [Google Scholar]
- Santos, O.S.H.; Coelho da Silva, M.; Silva, V.R.; Mussel, W.N.; Yoshida, M.I. Polyurethane foam impregnated with lignin as a filler for the removal of crude oil from contaminated water. J. Hazard. Mater. 2017, 324, 406–413. [Google Scholar] [CrossRef]
- Bernardini, J.; Cinelli, P.; Anguillesi, I.; Coltelli, M.-B.; Lazzeri, A. Flexible polyurethane foams green production employing lignin or oxypropylated lignin. Eur. Polym. J. 2015, 64, 147–156. [Google Scholar] [CrossRef]
- Wang, S.; Liu, W.; Yang, D.; Qiu, X. Highly Resilient Lignin-Containing Polyurethane Foam. Ind. Eng. Chem. Res. 2019, 58, 496–504. [Google Scholar] [CrossRef]
- Gómez-Fernández, S.; Ugarte, L.; Calvo-Correas, T.; Peña-Rodríguez, C.; Corcuera, M.A.; Eceiza, A. Properties of flexible polyurethane foams containing isocyanate functionalized kraft lignin. Ind. Crops Prod. 2017, 100, 51–64. [Google Scholar] [CrossRef]
- Wysocka, K.; Szymona, K.; McDonald, A.G.; Mamiński, M.Ł. Characterization of Thermal and Mechanical Properties of Lignosulfonate- and Hydrolyzed Lignosulfonate-based Polyurethane Foams. BioResources 2016, 11, 7355–7364. [Google Scholar] [CrossRef]
- Zia, K.M.; Bhatti, H.N.; Ahmad Bhatti, I. Methods for polyurethane and polyurethane composites, recycling and recovery: A review. React. Funct. Polym. 2007, 67, 675–692. [Google Scholar] [CrossRef]
- Feldman, D.; Lacasse, M.A. Polymer–filler interaction in polyurethane kraft lignin polyblends. J. Appl. Polym. Sci. 1994, 51, 701–709. [Google Scholar] [CrossRef]
- Lang, J.M.; Shrestha, U.M.; Dadmun, M. The Effect of Plant Source on the Properties of Lignin-Based Polyurethanes. Front. Energy Res. 2018, 6, 1–12. [Google Scholar] [CrossRef]
- Gang, H.; Lee, D.; Choi, K.Y.; Kim, H.N.; Ryu, H.; Lee, D.S.; Kim, B.G. Development of High Performance Polyurethane Elastomers Using Vanillin-Based Green Polyol Chain Extender Originating from Lignocellulosic Biomass. ACS Sustain. Chem. Eng. 2017, 5, 4582–4588. [Google Scholar] [CrossRef]
- Meier-Westhues, U. Polyurethanes: Coatings, Adhesives and Sealants; Vincentz Network: Hanover, Germany, 2007; ISBN 0815515642. [Google Scholar]
- Wang, Z.; Yang, X.; Zhou, Y.; Liu, C. Mechanical and thermal properties of polyurethane films from peroxy-acid wheat straw lignin. BioResources 2013, 8, 3833–3843. [Google Scholar] [CrossRef]
- Klein, S.E.; Rumpf, J.; Kusch, P.; Albach, R.; Rehahn, M.; Witzleben, S.; Schulze, M. Unmodified kraft lignin isolated at room temperature from aqueous solution for preparation of highly flexible transparent polyurethane coatings. Rsc Adv. 2018, 8, 40765–40777. [Google Scholar] [CrossRef]
- Griffini, G.; Passoni, V.; Suriano, R.; Levi, M.; Turri, S. Polyurethane Coatings Based on Chemically Unmodified Fractionated Lignin. ACS Sustain. Chem. Eng. 2015, 3, 1145–1154. [Google Scholar] [CrossRef]
- Jia, Z.; Lu, C.; Zhou, P.; Wang, L. Preparation and characterization of high boiling solvent lignin-based polyurethane film with lignin as the only hydroxyl group provider. RSC Adv. 2015, 5, 53949–53955. [Google Scholar] [CrossRef]
- Mozheiko, L.N.; Gromova, M.F.; Bakalo, L.A.; Sergeyeva, V.N. Polyurethanes prepared from oxypropylated lignin. Polym. Sci. USSR 1981, 23, 141–149. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, J. Effects of nitrolignin on mechanical properties of polyurethane–nitrolignin films. J. Appl. Polym. Sci. 2001, 80, 1213–1219. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L. Structure and properties of regenerated cellulose films coated with polyurethane–nitrolignin graft-IPNs coating. J. Appl. Polym. Sci. 2002, 86, 1799–1806. [Google Scholar] [CrossRef]
- Christiansen, A.W.; Vick, C.B.; Okkonen, E.A. Enhanced durability of one-part polyurethane bonds to wood due to the use of HMR primer. In Proceedings of the Forest Produtcs Society Annual Meeting, South Lake Tahoe, NV, USA, 22–23 June 2000; pp. 489–494. [Google Scholar]
- Rowell, R. Handbook of Wood Chemistry and Wood Composites; Taylor & Francis Group: Boca Raton, FL, USA, 2013; ISBN 1439853819. [Google Scholar]
- Lima García, J.; Pans, G.; Phanopoulos, C. Use of lignin in polyurethane-based structural wood adhesives. J. Adhes. 2018, 94, 814–828. [Google Scholar] [CrossRef]
- Hemmilä, V.; Adamopoulos, S.; Karlsson, O.; Kumar, A. Development of sustainable bio-adhesives for engineered wood panels – A Review. RSC Adv. 2017, 7, 38604–38630. [Google Scholar] [CrossRef]
- Sen, S.; Patil, S.; Argyropoulos, D.S. Thermal properties of lignin in copolymers, blends, and composites: A review. Green Chem. 2015, 17, 4862–4887. [Google Scholar] [CrossRef]
- Chahar, S.; Dastidar, M.G.; Choudhary, V.; Sharma, D.K. Synthesis and characterisation of polyurethanes derived from waste black liquor lignin. J. Adhes. Sci. Technol. 2004, 18, 169–179. [Google Scholar] [CrossRef]
- Tavares, L.B.; Boas, C.V.; Schleder, G.R.; Nacas, A.M.; Rosa, D.S.; Santos, D.J. Bio-based polyurethane prepared from Kraft lignin and modified castor oil. Express Polym. Lett. 2016, 10, 927–940. [Google Scholar] [CrossRef]
- Hsu, O.H.H.; Glasser, W.G. Polyurethane adhesives and coatings from modified lignin. Wood Sci. 1976, 9, 97–103. [Google Scholar]
- Bonini, C.; D’Auria, M.; Emanuele, L.; Ferri, R.; Pucciariello, R.; Sabia, A.R. Polyurethanes and polyesters from lignin. J. Appl. Polym. Sci. 2005, 98, 1451–1456. [Google Scholar] [CrossRef]
- Newman, W.H.; Glasser, W.G. Engineering plastics from lignin-XII. Synthesis and performance of lignin adhesives with isocyanate and melamine. Holzforschung-International J. Biol. Chem. Phys. Technol. Wood 1985, 39, 345–353. [Google Scholar] [CrossRef]
- Glasser, W.G.; Saraf, V.P.; Newman, W.H. Hydroxy Propylated Lignin-Isocyanate Combinations as Bonding Agents for Wood and Cellulosic Fibers. J. Adhes. 1982, 14, 233–255. [Google Scholar] [CrossRef]
- Saraf, V.P.; Glasser, W.G.; Wilkes, G.L. Engineering plastics from lignin. VII. Structure property relationships of poly(butadiene glycol)-containing polyurethane networks. J. Appl. Polym. Sci. 1985, 30, 3809–3823. [Google Scholar] [CrossRef]
| Lignin Property | Impact on PU Synthesis or Performance | Potential Solution | ||
|---|---|---|---|---|
| Sulfur content | Odor problems in final product, yellowing in coating applications | Selection of appropriate lignin source | ||
| Low solubility | Poor incorporation into polymer matrix and low interaction with the co-reactant (NCO) | Depolymerization or fractionation of the lignin | Functionalization of the lignin | |
| Low reactivity | Poor incorporation into polymer matrix | |||
| High Tg | Brittleness | |||
| High polydispersity | Inconsistent performance, differing reactivities and solubilities | |||
| Dark color | Limited utility in some coating applications where light color is required | |||
| Ultraviolet (UV) instability | Product degradation in outdoor applications | Ensuring the reaction between isocyanate and lignin phenolic hydroxyl groups; Addition of UV stabilizers | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alinejad, M.; Henry, C.; Nikafshar, S.; Gondaliya, A.; Bagheri, S.; Chen, N.; Singh, S.K.; Hodge, D.B.; Nejad, M. Lignin-Based Polyurethanes: Opportunities for Bio-Based Foams, Elastomers, Coatings and Adhesives. Polymers 2019, 11, 1202. https://doi.org/10.3390/polym11071202
Alinejad M, Henry C, Nikafshar S, Gondaliya A, Bagheri S, Chen N, Singh SK, Hodge DB, Nejad M. Lignin-Based Polyurethanes: Opportunities for Bio-Based Foams, Elastomers, Coatings and Adhesives. Polymers. 2019; 11(7):1202. https://doi.org/10.3390/polym11071202
Chicago/Turabian StyleAlinejad, Mona, Christián Henry, Saeid Nikafshar, Akash Gondaliya, Sajad Bagheri, Nusheng Chen, Sandip K. Singh, David B. Hodge, and Mojgan Nejad. 2019. "Lignin-Based Polyurethanes: Opportunities for Bio-Based Foams, Elastomers, Coatings and Adhesives" Polymers 11, no. 7: 1202. https://doi.org/10.3390/polym11071202
APA StyleAlinejad, M., Henry, C., Nikafshar, S., Gondaliya, A., Bagheri, S., Chen, N., Singh, S. K., Hodge, D. B., & Nejad, M. (2019). Lignin-Based Polyurethanes: Opportunities for Bio-Based Foams, Elastomers, Coatings and Adhesives. Polymers, 11(7), 1202. https://doi.org/10.3390/polym11071202