Anisotropy of Thin Foils Obtained from Microwave-Irradiated Poly(Vinyl Alcohol) Aqueous Solutions
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of the Samples
2.3. Characterization
2.4. Experimental Device
3. Results and Discussions
3.1. Microwave Oven Efficiency
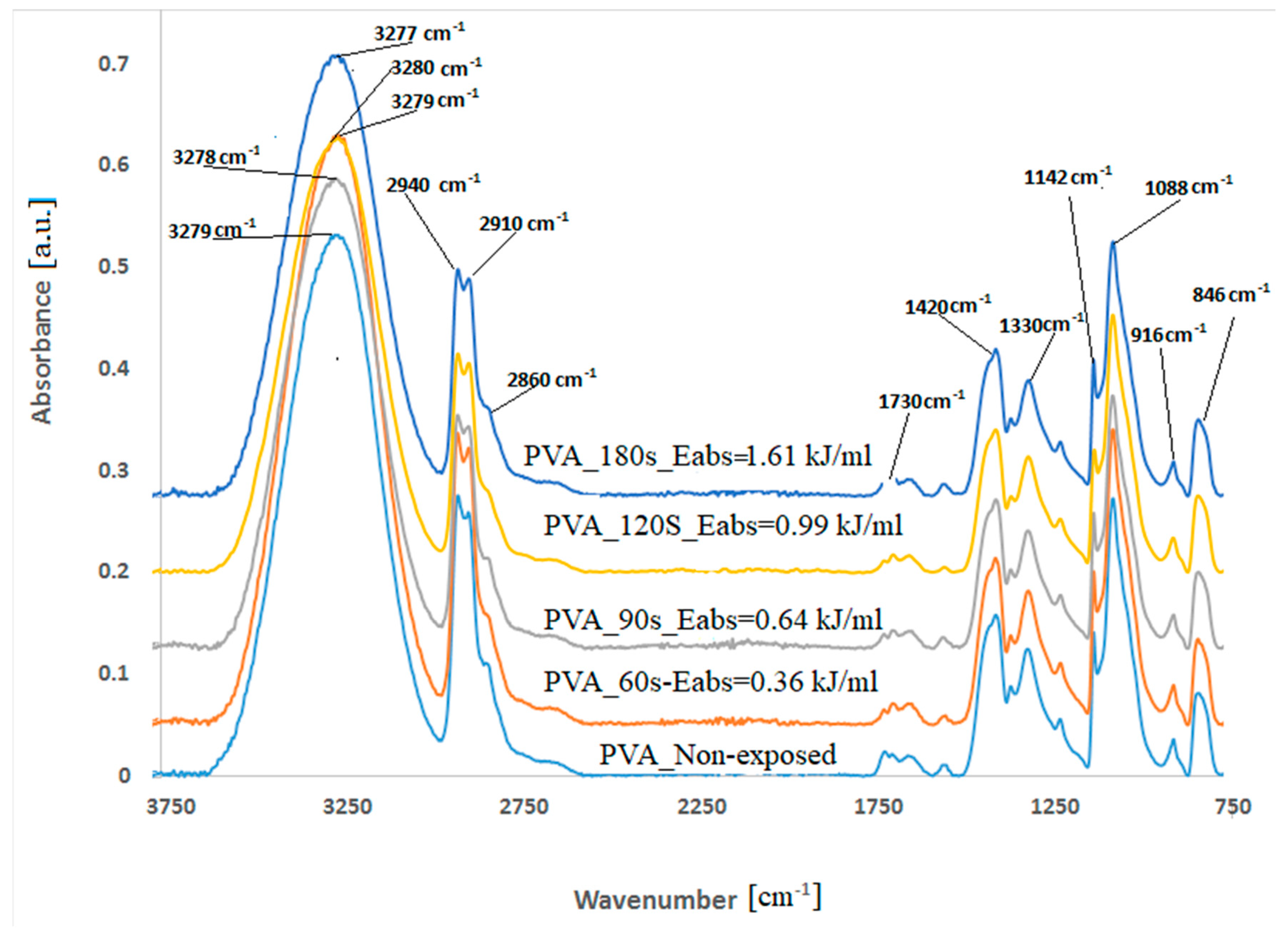
3.2. Spectral Analysis of PVA Foils
3.3. Contact Angle Measurements
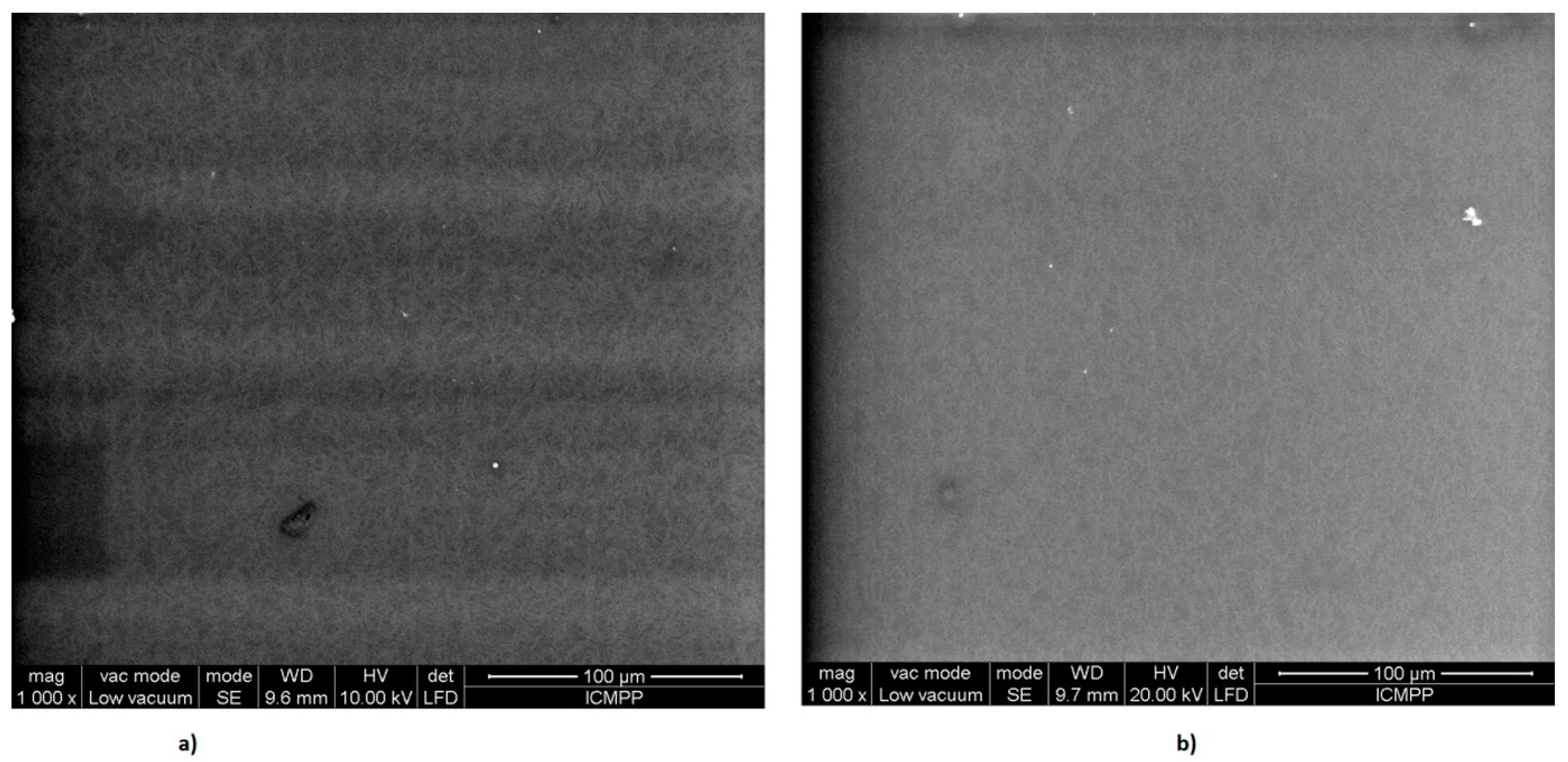
3.4. Scanning Electron Microscopy
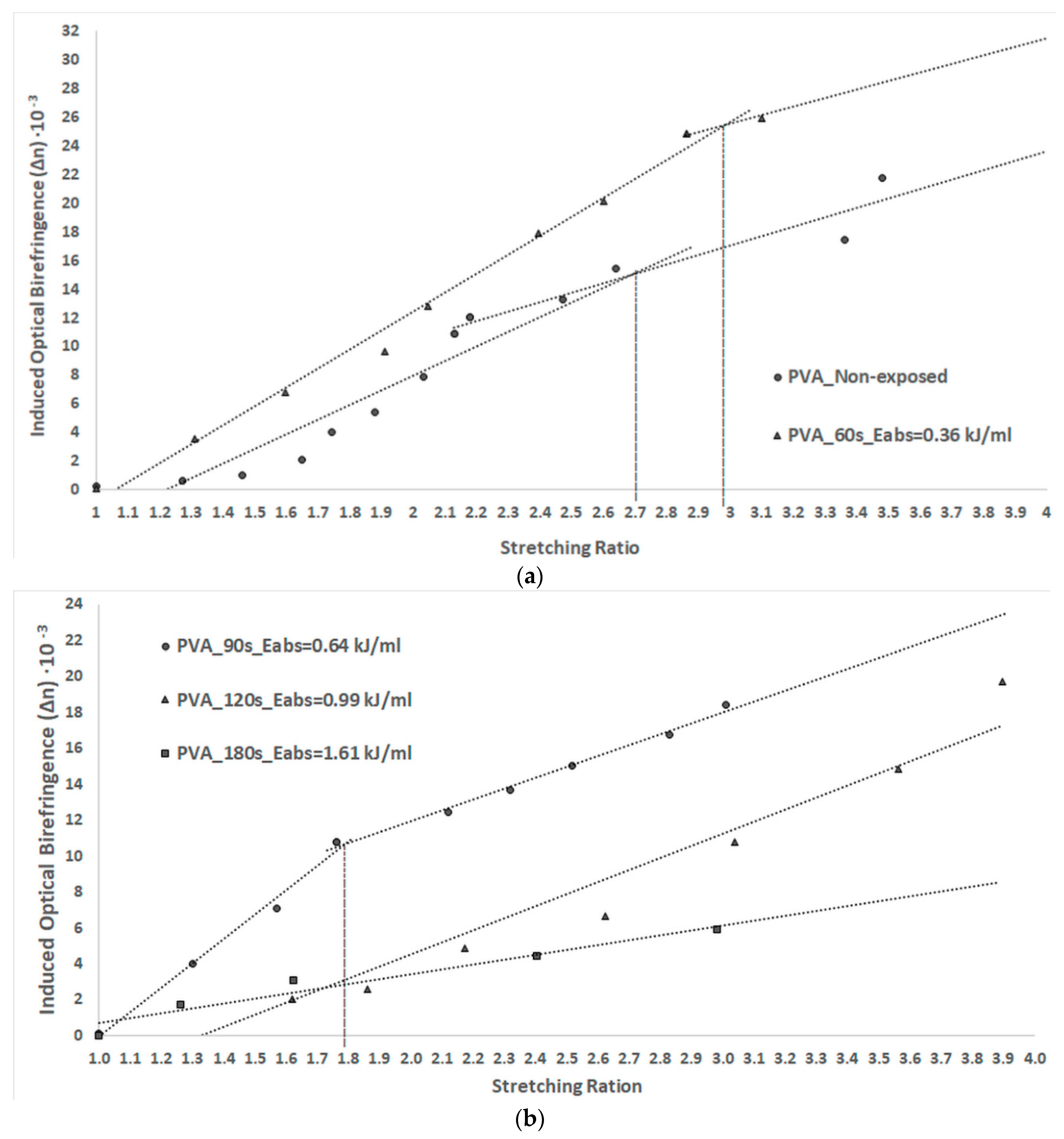
3.5. Anisotropy of PVA Thin Foils
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pop, V.; Dorohoi, D.O.; Cringeanu, E. A New method for determining birefringence dispersion. J. Macromol. Sci.-Phys. B 1994, 33, 373–385. [Google Scholar] [CrossRef]
- Guo, J.; Brady, D.J. Fabrication of high-resolution micropolarizer arrays. Opt. Eng. 1997, 36, 2268–2271. [Google Scholar] [CrossRef]
- Nechifor, C.D.; Angheluta, E.; Dorohoi, D.O. Birefringence of Etired Poly(Vinyl Alcohol) (PVA) Foils. Mat. Plast. 2010, 47, 164–166. [Google Scholar]
- Cui, Y.; Zola, R.S.; Yang, Y.C.; Yang, D.K. Alignment Layers with Variable Anchoring Strengths from Polyvinyl Alcohol. J. Appl. Phys. 2012, 111, 063520. [Google Scholar] [CrossRef]
- Maruyama, K.; Fujiwara, T.; Tanizaki, Y. Orientation-relaxation of polyenes produced in heated PVA film. Polymer 1989, 30, 856–859. [Google Scholar] [CrossRef]
- Rogojanu, A.; Dascalu, C.F.; Zelinschi, B.C.; Caprosu, M.; Dorohoi, D.O. Birefringence and dichroism of poly(vinyl-alcohol) foils containing phthalazinium ylids. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 81, 334–338. [Google Scholar] [CrossRef]
- Aflori, M.; Drobota, M. Combined treatments for the improving of the pet surfaces hydrophilicity. Dig. J. Nanomater. Biostruct. 2015, 10, 587–593. [Google Scholar]
- Aflori, M.; Drobota, M. Modification of Polyethylene Terephthalate. In Poly(Ethylene Terephthalate) Based Blends, Composites and Nanocomposites, 1st ed.; Visakh, P.M., Liang, M., Eds.; Elsevier: Oxford, UK, 2015; pp. 15–39. [Google Scholar]
- Nechifor, C.D.; Zelinschi, C.B.; Dorohoi, D.O. Influence of UV and Gamma radiations on the induced birefringence of stretched poly(vinyl) alcohol foils. J. Mol. Struct. 2014, 1062, 179–184. [Google Scholar] [CrossRef]
- Ku, H.S.; Siores, E.; Ball, J.A.R. Review-Microwave Processing of Materials: Part I. Transactions 2001, 8, 31–37. [Google Scholar] [CrossRef]
- de la Hoz, A.; Díaz-Ortiz, Á.; Moreno, A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 2005, 34, 164–178. [Google Scholar] [CrossRef]
- Guo, J.; Brady, D. Fabrication of thin-film micropolarizer arrays for visible imaging polarimetry. Appl. Opt. 2000, 39, 1486–1492. [Google Scholar] [CrossRef]
- Sólyom, K.; Mato, R.B.; Pérez-Elvira, S.I.; Cocero, M.J. The influence of the energy absorbed from microwave pretreatment on biogas production from secondary wastewater sludge. Bioresour. Technol. 2011, 102, 10849–10854. [Google Scholar] [CrossRef]
- Fonseca dos Reis, E.; Campos, F.S.; Lage, A.P.; Leite, R.C.; Heneine, L.G.; Vasconcelos, W.L.; Lobato, Z.I.P.; Mansur, H.S. Synthesis and Characterization of Poly(Vinyl Alcohol) Hydrogels and Hybrids for rMPB70 Protein Adsorption. Mater. Res. 2006, 9, 185–191. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra. In A Practical Approach in Encyclopaedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]
- Bernal, A.; Kuritka, I.; Kasparkova, V.; Saha, P. The Effect of Microwave Irradiation on Poly(vinyl alcohol) Dissolved in Ethylene Glycol. J. Appl. Polym. Sci. 2013, 128, 175–180. [Google Scholar] [CrossRef]
- Sone, Y.; Sakurada, I. Studies on the Swelling of Polyvinyl Alcohol, (V) Influence of the Residual Acetyl Group on the Swelling of Polyvinyl Alcohol Films. Chem. High Polym. Jpn. 1957, 14, 139–144. [Google Scholar]
- Araujo, P.H.H.; Sayer, C.; Poco, J.G.R.; Giudici, R. Techniques for reducing residual monomer content in polymers: A review. Polym. Eng. Sci. 2002, 42, 1442–1468. [Google Scholar] [CrossRef]
- Sengwa, R.J.; Kaur, K. Dielectric dispersion studies of poly(vinyl alcohol) in aqueous solutions. Polym. Int. 2000, 49, 1314–1320. [Google Scholar] [CrossRef]
- Jacob, J.; Chia, L.H.L.; Boey, F.Y.C. Thermal and Non-Thermal Interaction of Microwave Radiation with Materials. J. Mater. Sci. 1995, 30, 5321–5327. [Google Scholar] [CrossRef]
- Nagura, M.; Matsuzawa, S.; Jamaura, K.; Ishikawa, H. Tacticity Dependence of Molecular Motion in Crystal of Poly(vinyl alcohol). Polym. J. 1982, 14, 69–72. [Google Scholar] [CrossRef] [Green Version]
- Abdelaziz, M.; Abdelrazek, E.M. Effect of dopant mixture on structural, optical and electron spin resonance properties of polyvinyl alcohol. Phys. B 2007, 390, 1–9. [Google Scholar] [CrossRef]
- Petrova, N.V.; Evtushenko, A.M.; Chikhacheva, I.P.; Zubov, V.P.; Kubrakova, I.V. Effect of Microwave Irradiation on the Cross-Linking of Polyvinyl Alcohol. Russ. J. Appl. Chem. 2005, 78, 1158–1161. [Google Scholar] [CrossRef]
- Van Oss, C.J. Interfacial Forces in Aqueous Media; Marcel Dekker Inc.: New York, NY, USA, 1994. [Google Scholar]
- Kaelble, D.H. Dispersion-Polar Surface Tension Properties of Organic Solids. J. Adhes. 1970, 2, 66–81. [Google Scholar] [CrossRef]
- Rankl, M.; Laib, S.; Seeger, S. Surface Tension Properties of Surface-Coatings for Application in Biodiagnostics Determined by Contact Angle Measurements. Coll. Surf. B. Biointerf. 2003, 30, 177–186. [Google Scholar] [CrossRef]
- Faibish, R.S.; Yoshida, W.; Cohen, Y. Contact Angle Study on Polymer-Grafted Silicon Wafers. J. Coll. Interf. Sci. 2002, 256, 341–350. [Google Scholar] [CrossRef]


| MW Time (s) | (kJ/mL) | (kJ) | (kJ/mL) | (kJ) | (kJ/mL) | (%) | |
|---|---|---|---|---|---|---|---|
| 1 | 60 | 0.72 | 5.59 | 4.54 | 0.43 | 0.36 | 49 |
| 2 | 90 | 1.08 | 6.79 | 11.80 | 0.72 | 0.64 | 59 |
| 3 | 120 | 1.44 | 7.28 | 21.34 | 1.01 | 0.99 | 69 |
| 4 | 180 | 2.16 | 7.14 | 39.73 | 1.57 | 1.61 | 75 |
| Exposure Time (s) | (kJ/mL) | ||
|---|---|---|---|
| 0 | 0 | 22.39 | 2.27 |
| 60 | 0.36 | 22.27 | 2.31 |
| 90 | 0.64 | 22.31 | 2.19 |
| 120 | 0.99 | 22.44 | 2.18 |
| 180 | 1.61 | 23.14 | 2.24 |
| Exposure Time (s) | (kJ/mL) | Contact Angle (°) | ||
|---|---|---|---|---|
| Water | Ethanol | Glycerol | ||
| 0 | 0 | 62 | 21 | 56 |
| 60 | 0.36 | 59 | 13 | 53 |
| 90 | 0.64 | 68 | 12 | 57 |
| 120 | 0.99 | 69 | 13 | 63 |
| 180 | 1.61 | 70 | 19 | 66 |
| Exposure Time (s) | (kJ/mL) | (mN/m) | (mN/m) | (mN/m) | P (%) |
|---|---|---|---|---|---|
| 0 | 0 | 0.86 | 86.58 | 87.44 | 0.99 |
| 60 | 0.36 | 0.80 | 92.60 | 93.40 | 0.86 |
| 90 | 0.64 | 1.97 | 71.57 | 73.54 | 2.68 |
| 120 | 0.99 | 5.20 | 41.47 | 46.67 | 11.14 |
| 180 | 1.61 | 5.69 | 36.54 | 42.23 | 13.47 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nechifor, C.-D.; Aflori, M.; Dorohoi, D.-O. Anisotropy of Thin Foils Obtained from Microwave-Irradiated Poly(Vinyl Alcohol) Aqueous Solutions. Polymers 2019, 11, 1072. https://doi.org/10.3390/polym11061072
Nechifor C-D, Aflori M, Dorohoi D-O. Anisotropy of Thin Foils Obtained from Microwave-Irradiated Poly(Vinyl Alcohol) Aqueous Solutions. Polymers. 2019; 11(6):1072. https://doi.org/10.3390/polym11061072
Chicago/Turabian StyleNechifor, Cristina-Delia, Magdalena Aflori, and Dana-Ortansa Dorohoi. 2019. "Anisotropy of Thin Foils Obtained from Microwave-Irradiated Poly(Vinyl Alcohol) Aqueous Solutions" Polymers 11, no. 6: 1072. https://doi.org/10.3390/polym11061072
APA StyleNechifor, C.-D., Aflori, M., & Dorohoi, D.-O. (2019). Anisotropy of Thin Foils Obtained from Microwave-Irradiated Poly(Vinyl Alcohol) Aqueous Solutions. Polymers, 11(6), 1072. https://doi.org/10.3390/polym11061072







