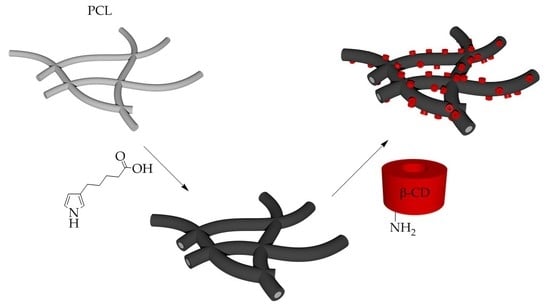
Cyclodextrin-Polypyrrole Coatings of Scaffolds for Tissue Engineering
Abstract
1. Introduction
2. Materials and Methods
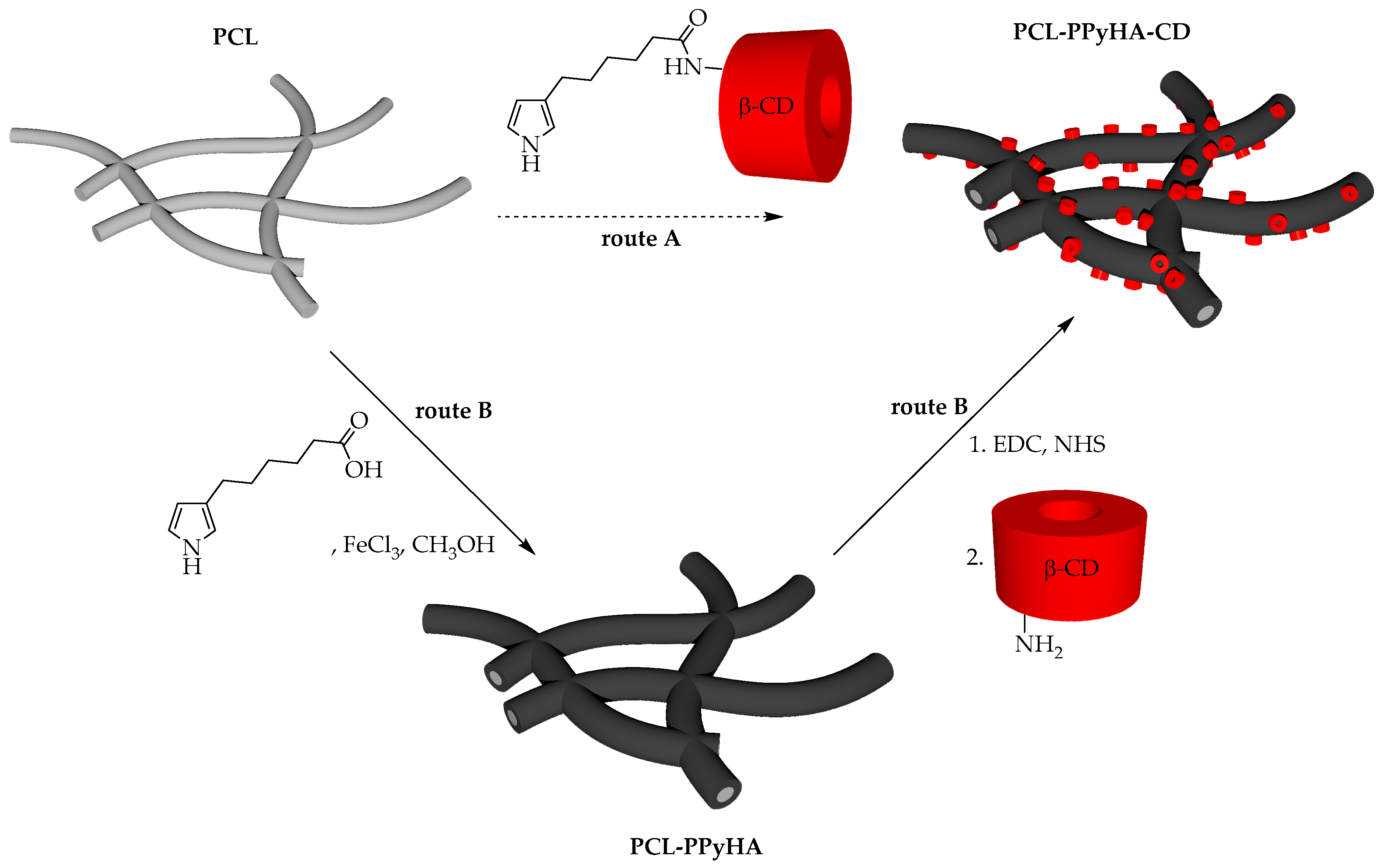
2.1. Synthesis of Pyrrole Monomer
2.2. Preparation of Scaffolds and Their Characterisation
2.2.1. Preparation of Electrospun PCL Fibres
2.2.2. Deposition of PPyHA Layer onto PCL
2.2.3. Immobilisation of CD onto PPyHA Modified PCL
2.3. Cell Culturing
2.4. Analysis of Protein Adsorption to Scaffolds
3. Results and Discussion
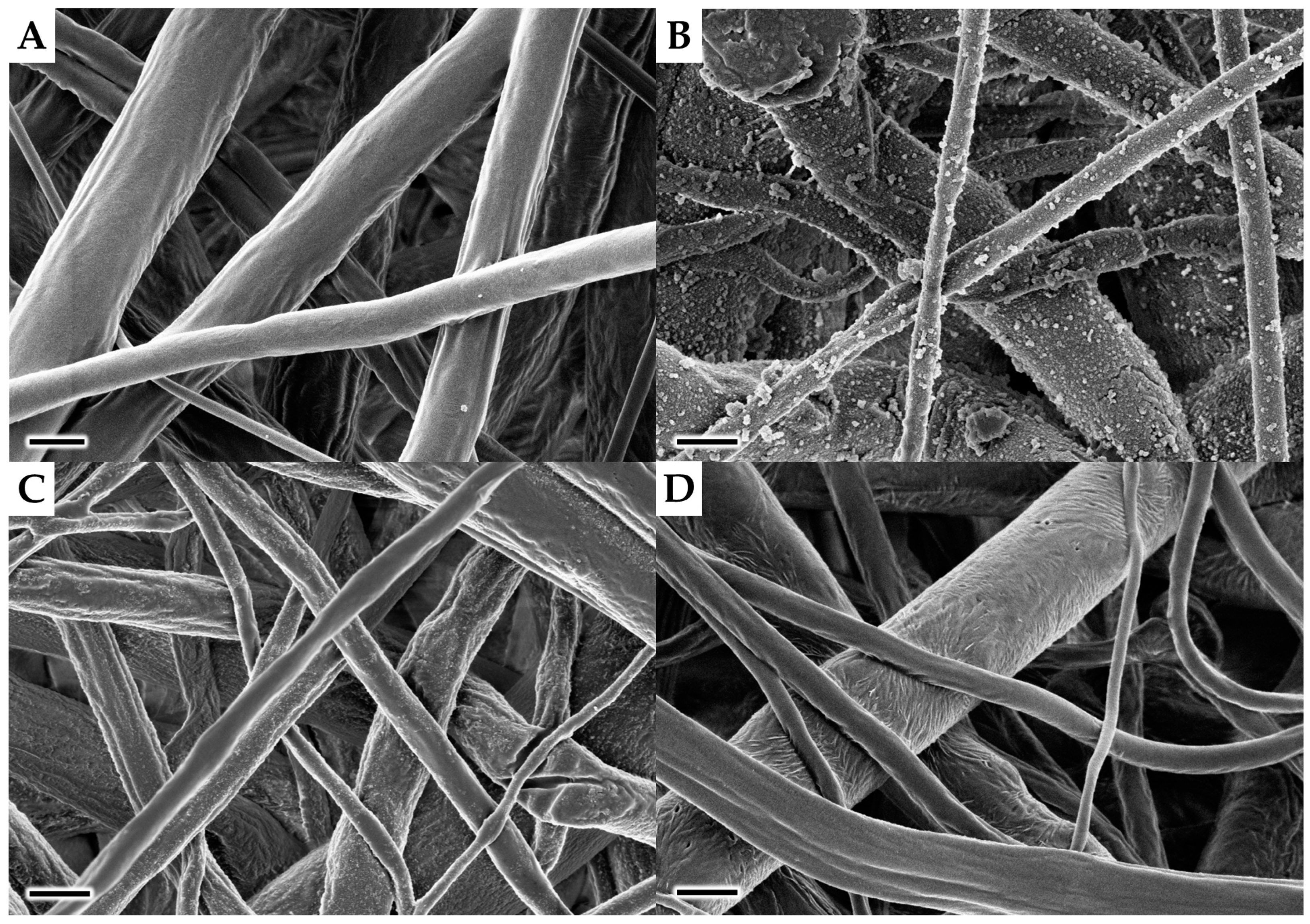
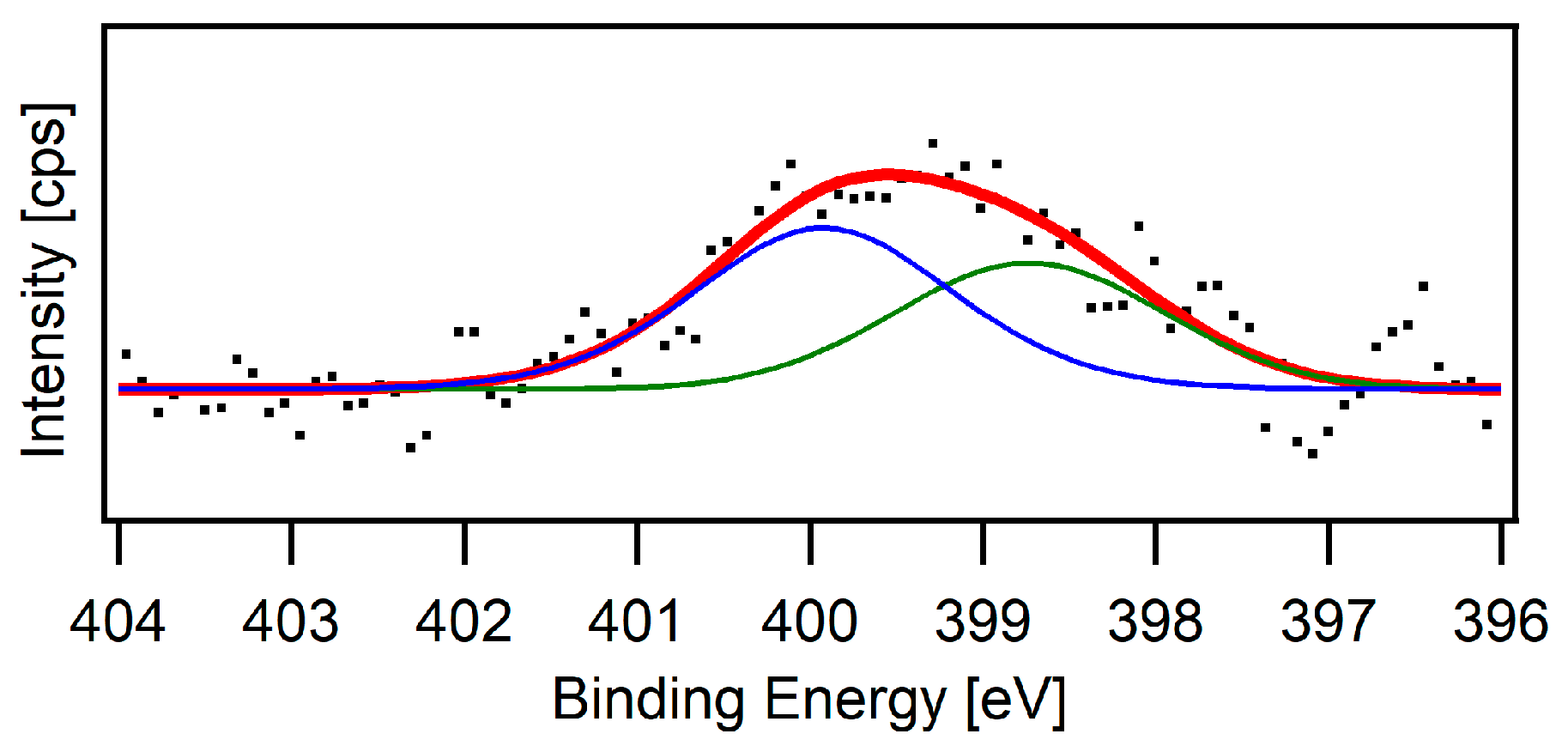
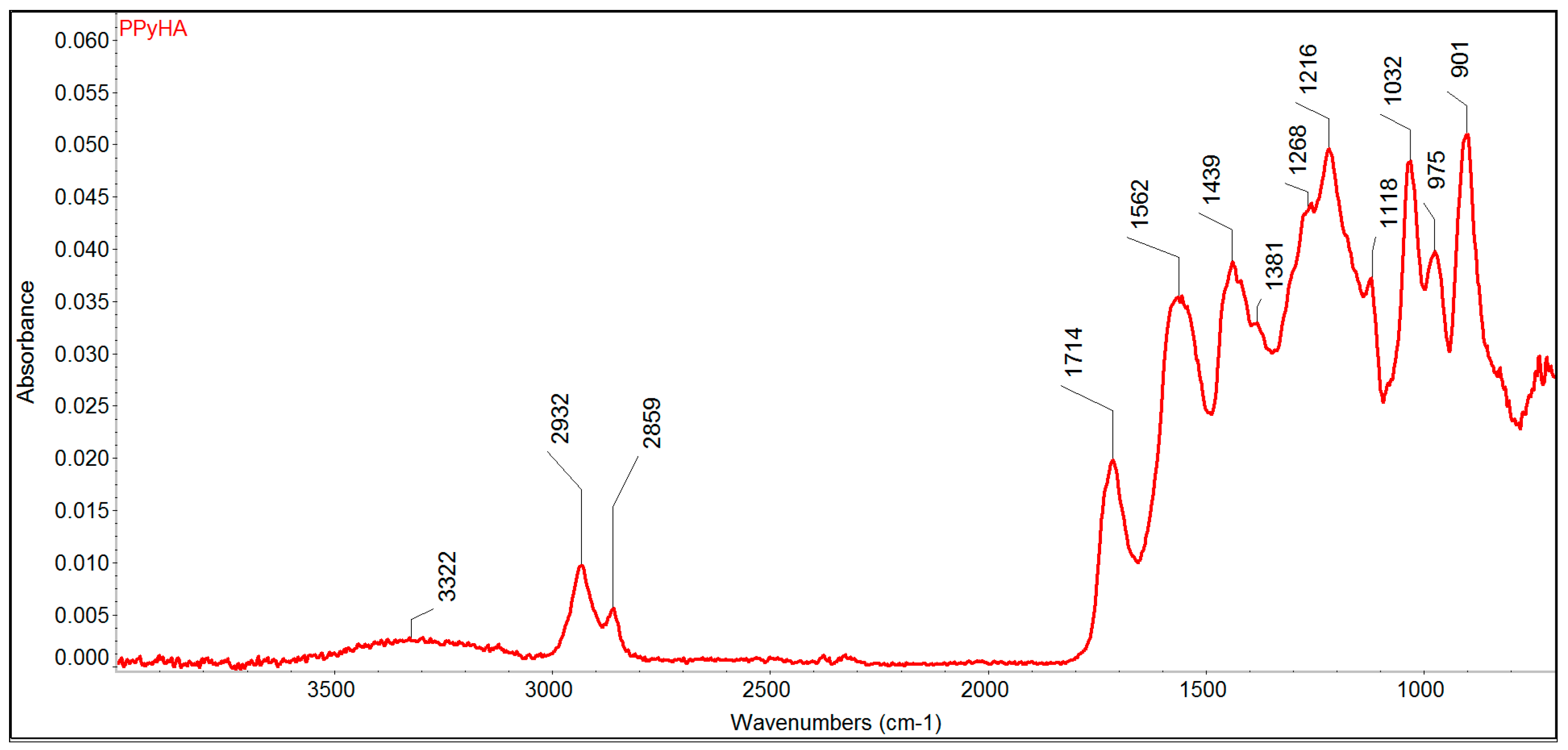
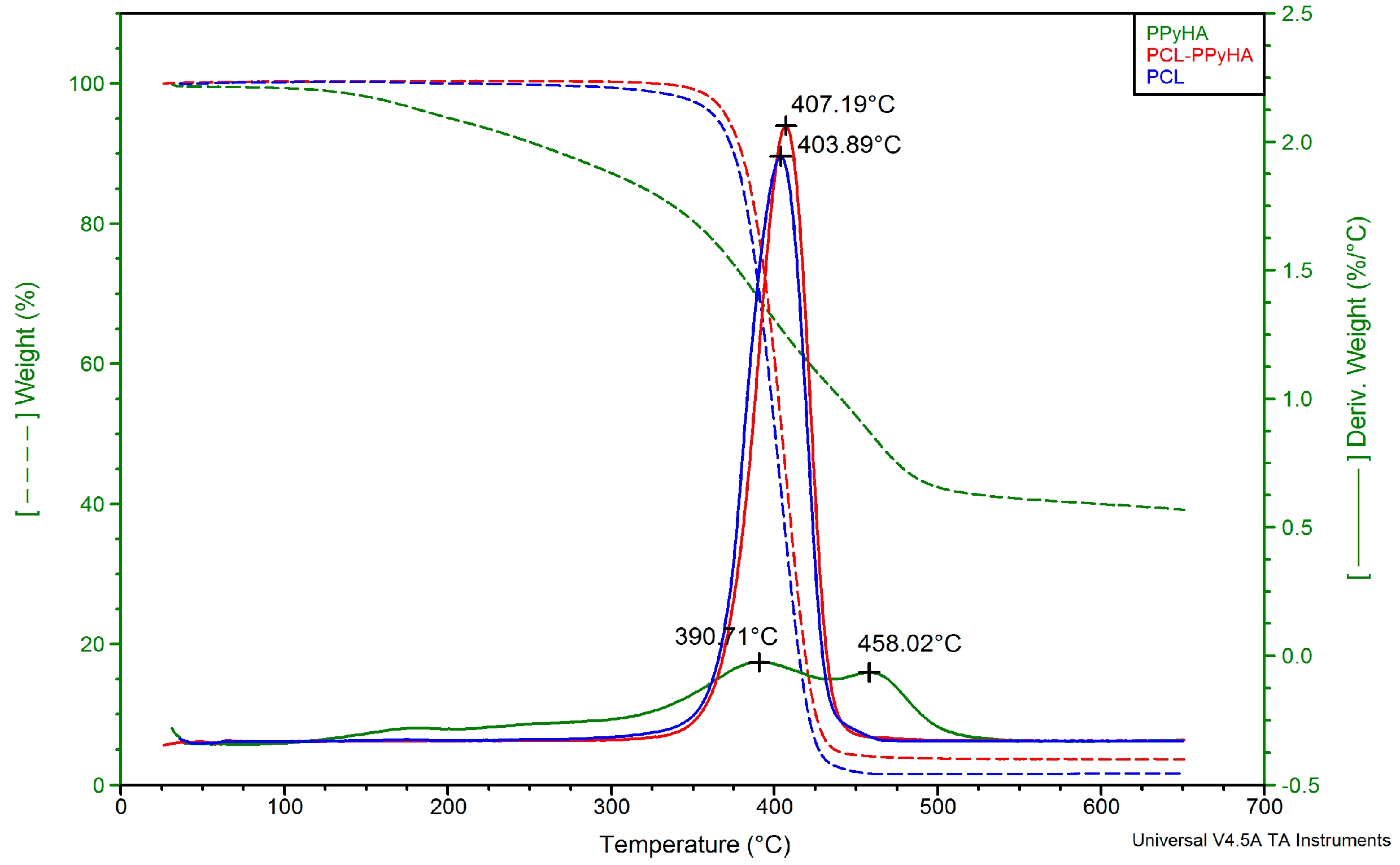
3.1. Preparation of Scaffolds and Their Characterisation
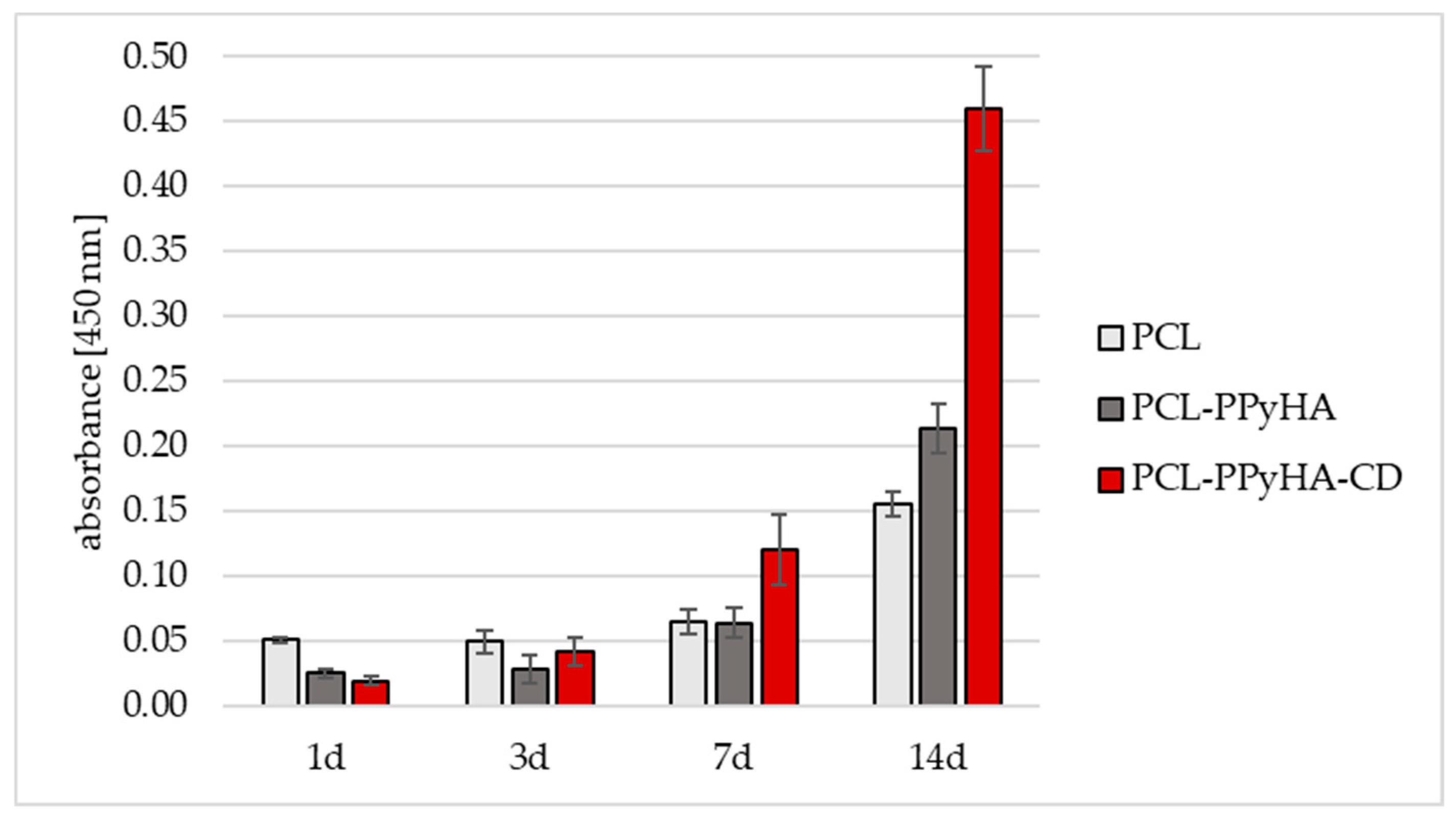
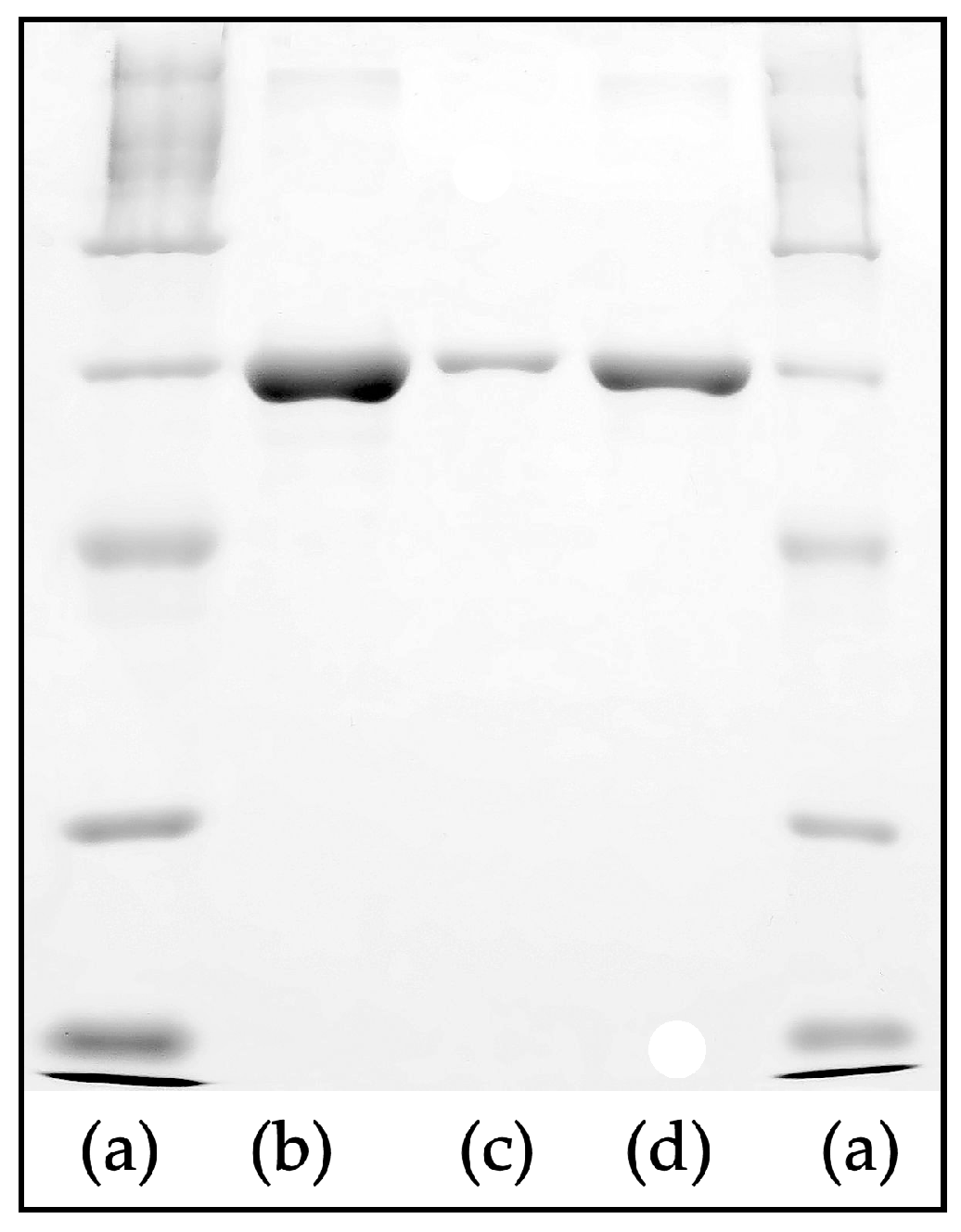
3.2. Cell Culturing and Protein Adsorption to the Scaffolds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef] [PubMed]
- Place, E.S.; Evans, N.D.; Stevens, M.M. Complexity in biomaterials for tissue engineering. Nat. Mater. 2009, 8, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-B.; Yin, G.-F.; Liao, X.-M.; Gu, J.-W. Conducting polypyrrole in tissue engineering applications. Front. Mater. Sci. 2014, 8, 39–45. [Google Scholar] [CrossRef]
- Guo, B.; Ma, P.X. Conducting Polymers for Tissue Engineering. Biomacromolecules 2018, 19, 1764–1782. [Google Scholar] [CrossRef] [PubMed]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Gu, X.S.; Yuan, C.W.; Chen, S.J.; Zhang, P.Y.; Zhang, T.Y.; Yao, J.; Chen, F.; Chen, G. Evaluation of biocompatibility of polypyrrole in vitro and in vivo. J. Biomed. Mater. Res. A 2004, 68, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; García-González, C.A.; Concheiro, A. Cyclodextrins as versatile building blocks for regenerative medicine. J. Control. Release 2017, 268, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Venuti, V.; Rossi, B.; Mele, A.; Melone, L.; Punta, C.; Majolino, D.; Masciovecchio, C.; Caldera, F.; Trotta, F. Tuning structural parameters for the optimization of drug delivery performance of cyclodextrin-based nanosponges. Exp. Opin. Drug Deliv. 2017, 14, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rio, L.; Otero-Espinar, F.J.; Luzardo-Alvarez, A.; Blanco-Mendez, J. Cyclodextrin Based Rotaxanes, Polyrotaxanes and Polypseudorotaxanes and their Biomedical Applications. Curr. Top. Med. Chem. 2014, 14, 478–493. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhou, N.; Zhang, T.; Guo, Z.; Hu, W.; Zhu, C.; Ma, D.; Gu, N. In situ formation of multiple stimuli-responsive poly[(methyl vinyl ether)-alt-(maleic acid)]-based supramolecular hydrogels by inclusion complexation between cyclodextrin and azobenzene. RSC Adv. 2016, 6, 13129–13136. [Google Scholar] [CrossRef]
- Yi, W.-J.; Li, L.-J.; He, H.; Hao, Z.; Liu, B.; Chao, Z.-S.; Shen, Y. Synthesis of poly(L-lactide)/beta-cyclodextrin/citrate network modified hydroxyapatite and its biomedical properties. New J. Chem. 2018, 42, 14729–14732. [Google Scholar] [CrossRef]
- Grier, W.K.; Tiffany, A.S.; Ramsey, M.D.; Harley, B.A.C. Incorporating beta-cyclodextrin into collagen scaffolds to sequester growth factors and modulate mesenchymal stem cell activity. Acta Biomater. 2018, 76, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Deluzio, T.G.B.; Penev, K.I.; Mequanint, K. Cyclodextrin Inclusion Complexes as Potential Oxygen Delivery Vehicles in Tissue Engineering. J. Biomater. Tissue Eng. 2014, 4, 957–966. [Google Scholar] [CrossRef]
- Majumdar, S.; Wang, X.; Sommerfeld, S.D.; Chae, J.J.; Athanasopoulou, E.-N.; Shores, L.S.; Duan, X.; Amzel, L.M.; Stellacci, F.; Schein, O.; et al. Cyclodextrin Modulated Type I Collagen Self-Assembly to Engineer Biomimetic Cornea Implants. Adv. Funct. Mater. 2018, 28, 1804076. [Google Scholar] [CrossRef]
- Lee, J.B.; Kim, J.E.; Balikov, D.A.; Bae, M.S.; Heo, D.N.; Lee, D.; Rim, H.J.; Lee, D.-W.; Sung, H.-J.; Kwon, I.K. Poly(l-Lactic Acid)/Gelatin Fibrous Scaffold Loaded with Simvastatin/Beta-Cyclodextrin-Modified Hydroxyapatite Inclusion Complex for Bone Tissue Regeneration. Macromol. Biosci. 2016, 16, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Kim, J.E.; Bae, M.S.; Park, S.A.; Balikov, D.A.; Sung, H.; Jeon, H.B.; Park, H.K.; Um, S.H.; Lee, K.S.; et al. Development of Poly(epsilon-Caprolactone) Scaffold Loaded with Simvastatin and Beta-Cyclodextrin Modified Hydroxyapatite Inclusion Complex for Bone Tissue Engineering. Polymers 2016, 8, 49. [Google Scholar] [CrossRef]
- Prabaharan, M.; Jayakumar, R. Chitosan-graft-beta-cyclodextrin scaffolds with controlled drug release capability for tissue engineering applications. Int. J. Biol. Macromol. 2009, 44, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Lukášek, J.; Řezanková, M.; Stibor, I.; Řezanka, M. Synthesis of cyclodextrin–pyrrole conjugates possessing tuneable carbon linkers. J. Incl. Phenom. Macrocycl. Chem. 2018, 92, 339–346. [Google Scholar] [CrossRef]
- Siegel, J.; Lyutakov, O.; Rybka, V.; Kolska, Z.; Svorcik, V. Properties of gold nanostructures sputtered on glass. Nanoscale Res. Lett. 2011, 6, 96. [Google Scholar] [CrossRef] [PubMed]
- Martinek, M.; Swar, S.; Zajicova, V.; Volesky, L.; Blazkova, L.; Mullerova, J.; Stuchlik, M.; Rezanka, M.; Stibor, I. Pre-treatment of polyethylene terephthalate by Grignard reagents for high quality polypyrrole coatings and for altering the hydrophobicity. Chem. Pap. 2017, 71, 2403–2415. [Google Scholar] [CrossRef]
- Rapi, S.; Bocchi, V.; Gardini, G.P. Conducting polypyrrole by chemical synthesis in water. Synth. Met. 1988, 24, 217–221. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, D.Y.; Kim, C.Y. Synthesis of soluble polypyrrole of the doped state in organic solvents. Synth. Met. 1995, 74, 103–106. [Google Scholar] [CrossRef]
- Tada, K.; Satake, K.; Onoda, M. In Situ Polymerization of Polypyrrole in Alcohols: Controlling Deposition Rate and Electrical Conductivity. Jpn. J. Appl. Phys. 2002, 41, 6586. [Google Scholar] [CrossRef]
- Papp, C.; Steinrueck, H.-P. In situ high-resolution X-ray photoelectron spectroscopy—Fundamental insights in surface reactions. Surf. Sci. Rep. 2013, 68, 446–487. [Google Scholar] [CrossRef]
- Tabaciarova, J.; Micusik, M.; Fedorko, P.; Omastova, M. Study of polypyrrole aging by XPS, FTIR and conductivity measurements. Polym. Degrad. Stab. 2015, 120, 392–401. [Google Scholar] [CrossRef]
- Hamouma, O.; Oukil, D.; Omastova, M.; Chehimi, M.M. Flexible paper@carbon nanotube@polypyrrole composites: The combined pivotal roles of diazonium chemistry and sonochemical polymerization. Colloids Surf. Physicochem. Eng. Asp. 2018, 538, 350–360. [Google Scholar] [CrossRef]
- Strnadová, K.; Stanislav, L.; Krabicová, I.; Sabol, F.; Lukášek, J.; Řezanka, M.; Lukáš, D.; Jenčová, V. Drawn aligned polymer microfibres for tissue engineering. J. Ind. Text. 2019, in press. [Google Scholar] [CrossRef]
- Řezanka, M. Monosubstituted Cyclodextrins as Precursors for Further Use. Eur. J. Org. Chem. 2016, 2016, 5322–5334. [Google Scholar] [CrossRef]
- Řezanka, M. Synthesis of substituted cyclodextrins. Environ. Chem. Lett. 2019, in press. [Google Scholar] [CrossRef]
- Walkey, C.D.; Chan, W.C.W. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem. Soc. Rev. 2012, 41, 2780–2799. [Google Scholar] [CrossRef] [PubMed]
- Louie, S.M.; Tilton, R.D.; Lowry, G.V. Critical review: Impacts of macromolecular coatings on critical physicochemical processes controlling environmental fate of nanomaterials. Environ. Sci.-Nano 2016, 3, 283–310. [Google Scholar] [CrossRef]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef] [PubMed]
- Anand, G.; Sharma, S.; Dutta, A.K.; Kumar, S.K.; Belfort, G. Conformational Transitions of Adsorbed Proteins on Surfaces of Varying Polarity. Langmuir 2010, 26, 10803–10811. [Google Scholar] [CrossRef] [PubMed]
- Benesch, J.; Hungerford, G.; Suhling, K.; Tregidgo, C.; Mano, J.F.; Reis, R.L. Fluorescence probe techniques to monitor protein adsorption-induced conformation changes on biodegradable polymers. J. Colloid Interface Sci. 2007, 312, 193–200. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukášek, J.; Hauzerová, Š.; Havlíčková, K.; Strnadová, K.; Mašek, K.; Stuchlík, M.; Stibor, I.; Jenčová, V.; Řezanka, M. Cyclodextrin-Polypyrrole Coatings of Scaffolds for Tissue Engineering. Polymers 2019, 11, 459. https://doi.org/10.3390/polym11030459
Lukášek J, Hauzerová Š, Havlíčková K, Strnadová K, Mašek K, Stuchlík M, Stibor I, Jenčová V, Řezanka M. Cyclodextrin-Polypyrrole Coatings of Scaffolds for Tissue Engineering. Polymers. 2019; 11(3):459. https://doi.org/10.3390/polym11030459
Chicago/Turabian StyleLukášek, Jan, Šárka Hauzerová, Kristýna Havlíčková, Kateřina Strnadová, Karel Mašek, Martin Stuchlík, Ivan Stibor, Věra Jenčová, and Michal Řezanka. 2019. "Cyclodextrin-Polypyrrole Coatings of Scaffolds for Tissue Engineering" Polymers 11, no. 3: 459. https://doi.org/10.3390/polym11030459
APA StyleLukášek, J., Hauzerová, Š., Havlíčková, K., Strnadová, K., Mašek, K., Stuchlík, M., Stibor, I., Jenčová, V., & Řezanka, M. (2019). Cyclodextrin-Polypyrrole Coatings of Scaffolds for Tissue Engineering. Polymers, 11(3), 459. https://doi.org/10.3390/polym11030459