Polymer/Fullerene Blend Solar Cells with Cadmium Sulfide Thin Film as an Alternative Hole-Blocking Layer
Abstract
1. Introduction
2. Materials and Methods
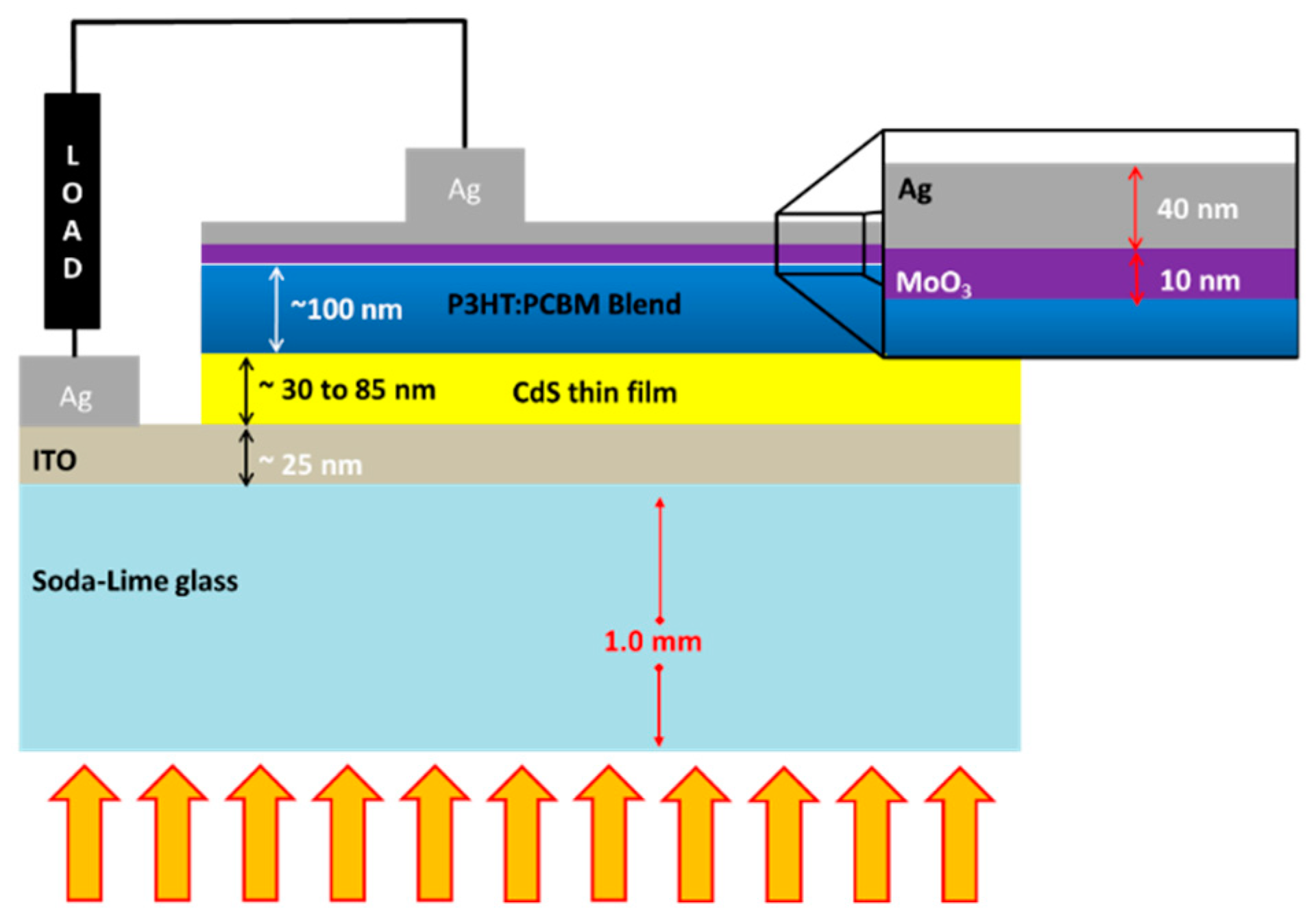
2.1. CdS Thin Film Fabrication
2.2. TiO2 Thin Film Fabrication
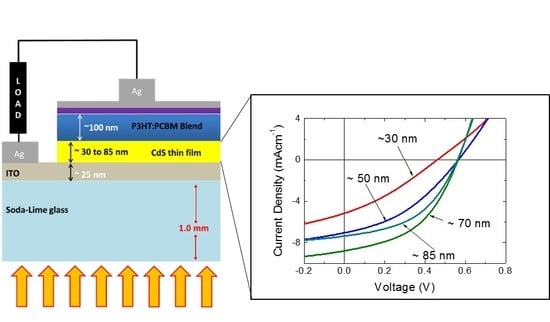
2.3. Solar Cell Fabrication
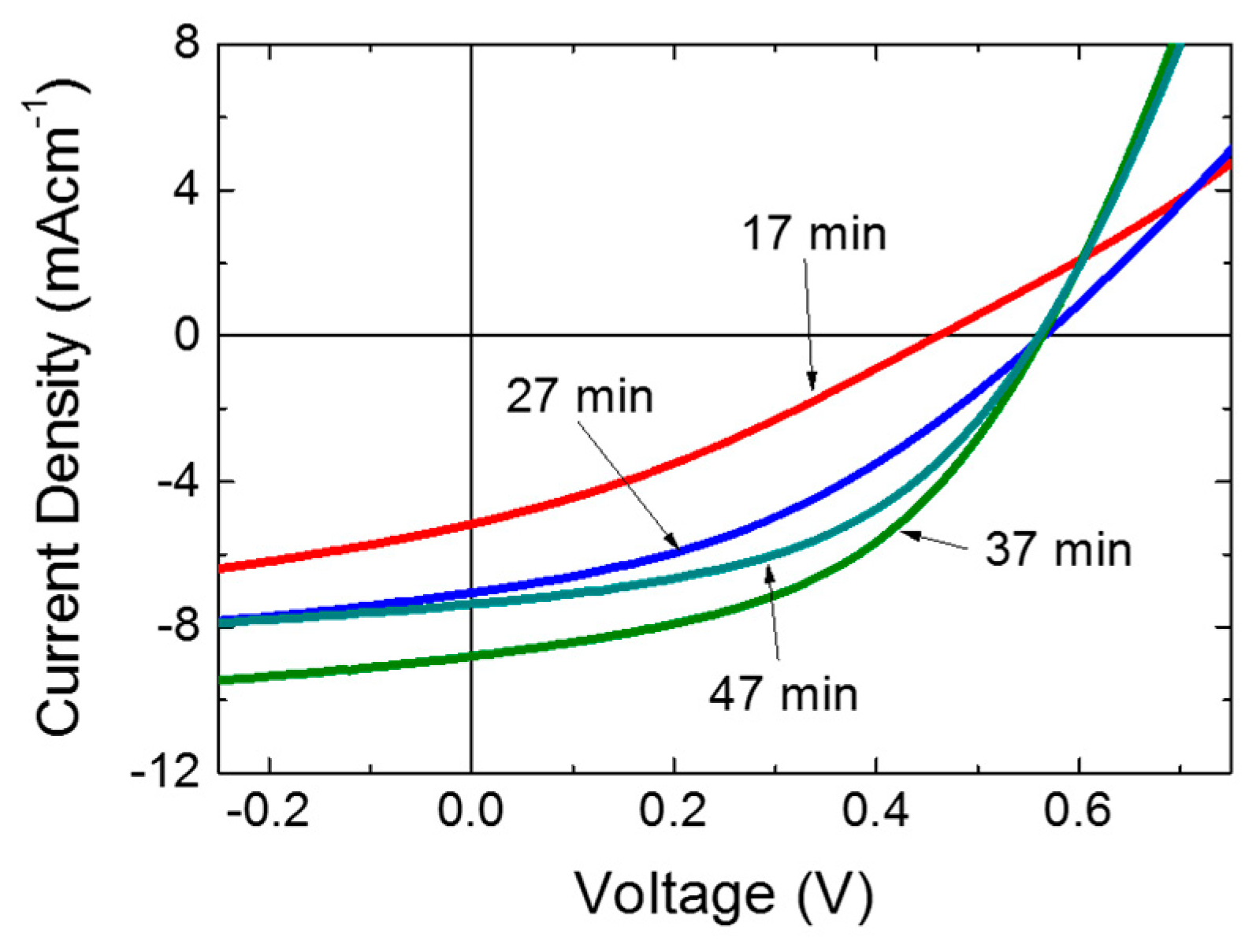
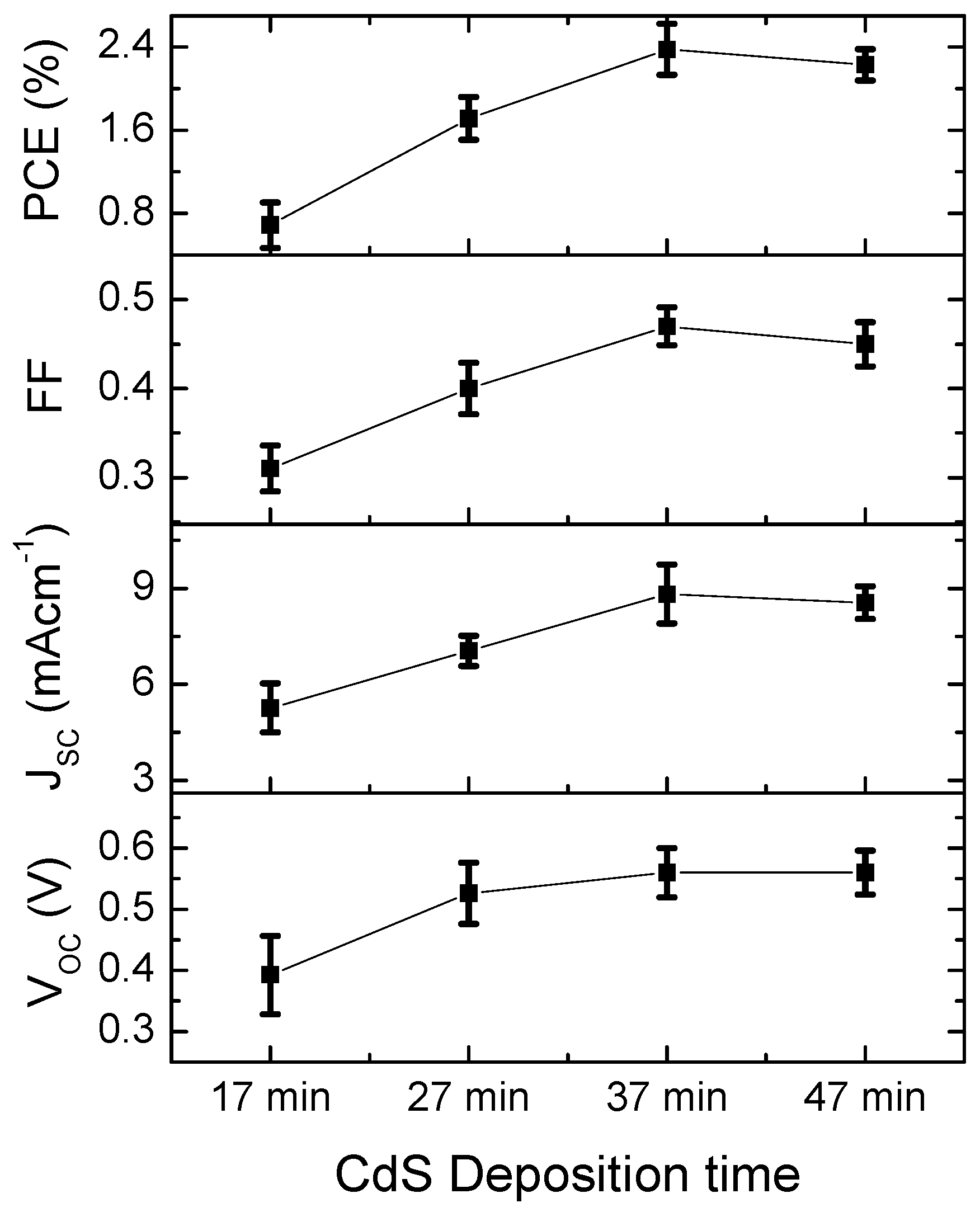
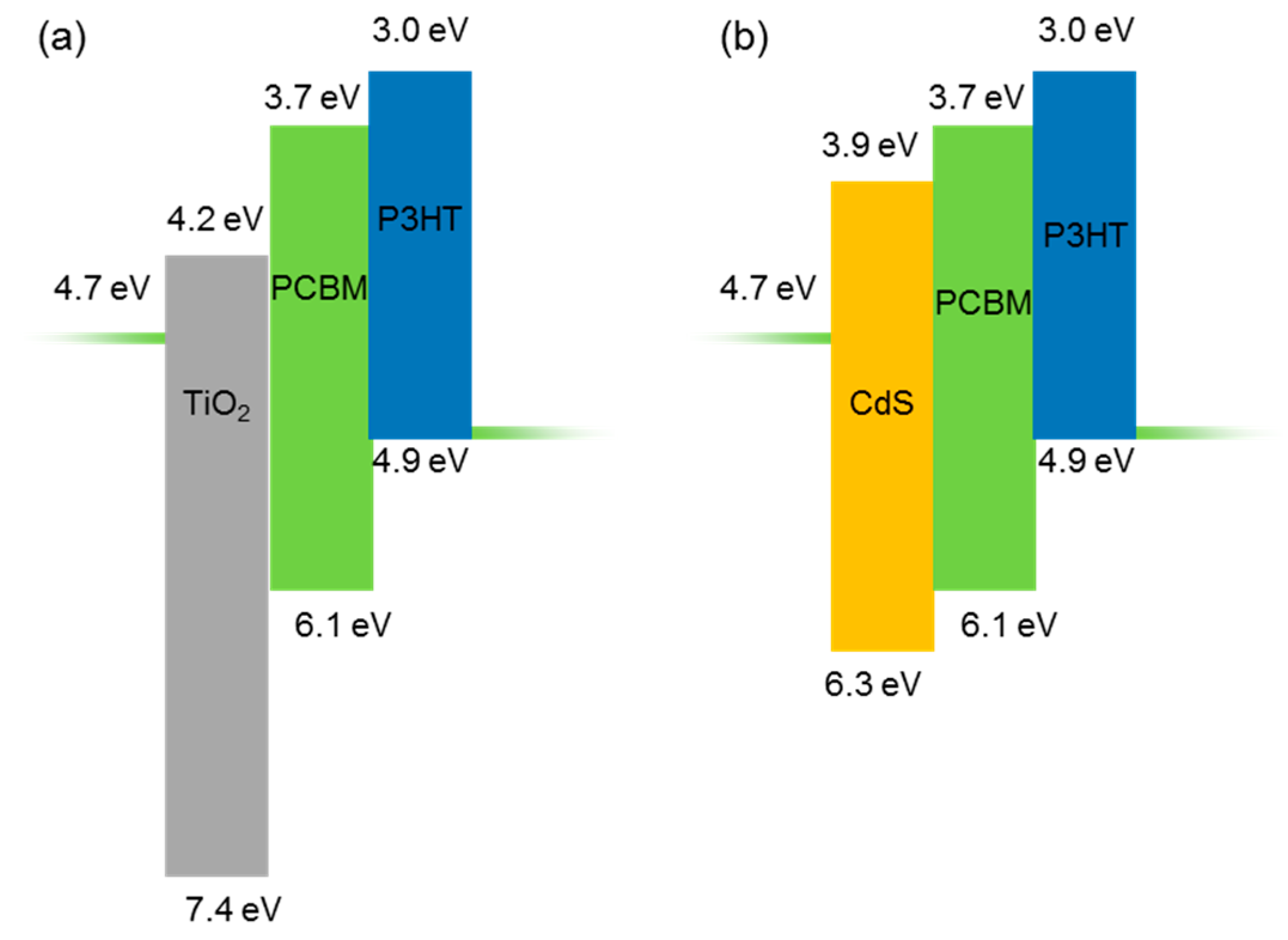
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dennler, G.; Scharber, M.C.; Brabec, C.J. Increasing the surface properties of a vanadium microalloyed steel by current-controlled plasma nitriding. Int. J. Electrochem. Sci. 2015, 10, 1833–1842. [Google Scholar] [CrossRef]
- Mishra, A.; Sahu, S.N. Fullerene-Free Molecular Acceptors for Organic Photovoltaics. In Advances in Solar Energy Research; Springer: Singapore, 2019; pp. 221–279. [Google Scholar]
- Cosentino, M.; Lombardo, G. Correlation between the Modified Mercalli seismic scale and the Medvedev-Sponheuer-Karnik scale for the earthquakes of Sicily and Calabria. Boll. Geofis. Teor. Appl. 1980, 22, 29–37. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, S.; Song, J.; Jin, Y.; Yue, Q.; Qian, Y.; Liu, F.; Zhang, F.; Zhu, X. High-efficiency small-molecule ternary solar cells with a hierarchical morphology enabled by synergizing fullerene and non-fullerene acceptors. Nat. Energy 2018, 3, 952–959. [Google Scholar] [CrossRef]
- Sariciftci, N.S.; Smilowitz, L.; Heeger, A.J.; Wudl, F. Photoinduced electron transfer from a conducting polymer to buckminsterfullerene. Science 1992, 258, 1474–1476. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudl, F.; Heeger, A.J. Polymer photovoltaic cells: Enhanced efficiencies via a network of internal donor-acceptor heterojunctions. Science 1995, 270, 1789. [Google Scholar] [CrossRef]
- Kleinschmidt, A.T.; Root, S.E.; Lipomi, D.J. Poly(3-hexylthiophene) (P3HT): Fruit fly or outlier in organic solar cell research? J. Mater. Chem. A 2017, 5, 11396–11400. [Google Scholar] [CrossRef]
- Li, G.; Shrotriya, V.; Huang, J.; Yao, Y.; Moriarty, T.; Emery, K.; Yang, Y. High-efficiency solution processable polymer photovoltaic cells by self-organization of polymer blends. Nat. Mater. 2005, 4, 864–868. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, L.M.; Yang, G.; Huang, C.H.; Hou, J.; Wu, Y.; Li, G.; Hsu, C.S.; Yang, Y. Vertical phase separation in poly(3-hexylthiophene): Fullerene derivative blends and its advantage for inverted structure solar cells. Adv. Funct. Mater. 2009, 19, 1227–1234. [Google Scholar] [CrossRef]
- Chen, L.M.; Hong, Z.; Li, G.; Yang, Y. Recent progress in polymer solar cells: Manipulation of polymer: Fullerene morphology and the formation of efficient inverted polymer solar cells. Adv. Mater. 2009, 21, 1434–1449. [Google Scholar] [CrossRef]
- Kuwabara, T.; Sugiyama, H.; Yamaguchi, T.; Takahashi, K. Inverted type bulk-heterojunction organic solar cell using electrodeposited titanium oxide thin films as electron collector electrode. Thin Solid Films 2009, 517, 3766–3769. [Google Scholar] [CrossRef]
- Cho, H.S.; Shin, N.; Kim, K.; Kim, B.S.; Kim, D.H. High performance inverted polymer solar cells using ultrathin atomic layer deposited TiO2 films. Synth. Met. 2015, 207, 31–34. [Google Scholar] [CrossRef]
- Rana Bekci, D.; Karsli, A.; Cagatay Cakir, A.; Sarica, H.; Guloglu, A.; Gunes, S.; Erten-Ela, S. Comparison of ZnO interlayers in inverted bulk heterojunction solar cells. Appl. Energy 2012, 96, 417–421. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, Q.; Jiang, L.; Cao, G. ZnO cathode buffer layers for inverted polymer solar cells. Energy Environ. Sci. 2015, 8, 3442–3476. [Google Scholar] [CrossRef]
- Balashangar, K.; Thanihaichelvan, M.; Ravirajan, P.; Mahanama, G.D.K.; Dissanayake, M.A.K.L.; Colegrove, E.; Dhere, R.G.; Sivananthan, S. The Effect of Surface Roughness of Substrates on the Performance of Polycrystalline Cadmium Sulfide/Cadmium Telluride Solar Cells. J. Nanoelectron. Optoelectron. 2015, 10, 435–439. [Google Scholar] [CrossRef]
- Zhu, J.J.; Xu, Z.Q.; Fan, G.Q.; Lee, S.T.; Li, Y.Q.; Tang, J.X. Inverted polymer solar cells with atomic layer deposited CdS film as an electron collection layer. Org. Electron. Phys. Mater. Appl. 2011, 12, 2151–2158. [Google Scholar] [CrossRef]
- Yavuz, N.; Yuksel, S.A.; Karsli, A.; Gunes, S. Inverted structure hybrid solar cells using CdS thin films. Sol. Energy Mater. Sol. Cells 2013, 116, 224–230. [Google Scholar] [CrossRef]
- Zhu, J.-J.; Fan, G.-Q.; Wei, H.-X.; Li, Y.-Q.; Lee, S.-T.; Tang, J.-X. Solution-processed inverted polymer solar cells using chemical bath deposited CdS films as electron collecting layer. CrystEngComm 2012, 14, 8090. [Google Scholar] [CrossRef]
- Li, C.; Yang, C.; Tong, J.; Li, J.; Zhang, P.; Xia, Y. Inverted polymer solar cells using CdS fabricated by thermal decomposition of cadmium xanthate precursor as electron transporting layer. Sol. Energy 2016, 139, 770–775. [Google Scholar] [CrossRef]
- Ravichandran, K.; Porkodi, S. Addressing the issue of under-utilization of precursor material in SILAR process: Simultaneous preparation of CdS in two different forms—Thin film and powder. Mater. Sci. Semicond. Process. 2018, 81, 30–37. [Google Scholar] [CrossRef]
- Mlowe, S.; Nyamen, L.D.; Ndifon, P.T.; Malik, M.A.; Raftery, J.; O’Brien, P.; Revaprasadu, N. Aerosol assisted chemical vapor deposition (AACVD) of CdS thin films from heterocyclic cadmium(II) complexes. Inorg. Chim. Acta 2015, 434, 181–187. [Google Scholar] [CrossRef]
- Paudel, N.R.; Xiao, C.; Yan, Y. Close-space sublimation grown CdS window layers for CdS/CdTe thin-film solar cells. J. Mater. Sci. Mater. Electron. 2014, 25, 1991–1998. [Google Scholar] [CrossRef]
- Esmaeili-Zare, M.; Behpour, M. Fabrication and study of optical properties on CdS semiconductor as buffer layer for Cu(In,Ga)Se2 thin film solar cells. J. Mater. Sci. Mater. Electron. 2017, 28, 10173–10183. [Google Scholar] [CrossRef]
- Thanihaichelvan, M.; Sri Kodikara, M.M.P.; Ravirajan, P.; Velauthapillai, D.; Kodikara, M.; Ravirajan, P.; Velauthapillai, D. Enhanced performance of nanoporous titanium dioxide solar cells using cadmium sulfide and poly(3-hexylthiophene) co-sensitizers. Polymers (Basel) 2017, 9, 467. [Google Scholar] [CrossRef]
- Thanihaichelvan, M.; Sockiah, K.; Balashangar, K.; Ravirajan, P. Cadmium sulfide interface layer for improving the performance of titanium dioxide/poly (3-hexylthiophene) solar cells by extending the spectral response. J. Mater. Sci. Mater. Electron. 2015, 26, 3558–3563. [Google Scholar] [CrossRef]
- Balashangar, K.; Paranthaman, S.; Thanihaichelvan, M.; Amalraj, P.A.; Velauthapillai, D.; Ravirajan, P. Multi-walled carbon nanotube incorporated nanoporous titanium dioxide electrodes for hybrid polymer solar cells. Mater. Lett. 2018, 219, 265–268. [Google Scholar] [CrossRef]
- Prashanthan, K.; Thivakarasarma, T.; Ravirajan, P.; Planells, M.; Robertson, N.; Nelson, J. Enhancement of hole mobility in hybrid titanium dioxide/poly(3-hexylthiophene) nanocomposites by employing an oligothiophene dye as an interface modifier. J. Mater. Chem. C 2017, 5, 11758–11762. [Google Scholar] [CrossRef]
- Enríquez, J.P.; Mathew, X. Influence of the thickness on structural, optical and electrical properties of chemical bath deposited CdS thin films. Sol. Energy Mater. Sol. Cells 2003, 76, 313–322. [Google Scholar] [CrossRef]
- Wen, Z.; Tian-mo, L. Gas-sensing properties of SnO2-TiO2-based sensor for volatile organic compound gas and its sensing mechanism. Phys. B Condens. Matter 2010, 405, 1345–1348. [Google Scholar] [CrossRef]
- Khallaf, H.; Oladeji, I.O.; Chai, G.; Chow, L. Characterization of CdS thin films grown by chemical bath deposition using four different cadmium sources. Thin Solid Films 2008, 516, 7306–7312. [Google Scholar] [CrossRef]
- Rodríguez-Rosales, K.; Quiñones-Galván, J.G.; Guillén-Cervantes, A.; Campos-González, E.; Santos-Cruz, J.; Mayén-Hernández, S.A.; Arias-Cerón, J.S.; De La Olvera, M.L.; Zelaya-Angel, O.; Hernández-Hernández, L.A.; et al. Nanocrystalline-CdS thin films grown on flexible PET-substrates by chemical bath deposition. Mater. Res. Express 2017, 4, 75904. [Google Scholar] [CrossRef]
- Liu, F.; Lai, Y.; Liu, J.; Wang, B.; Kuang, S.; Zhang, Z.; Li, J.; Liu, Y. Characterization of chemical bath deposited CdS thin films at different deposition temperature. J. Alloys Compd. 2010, 493, 305–308. [Google Scholar] [CrossRef]
- Lisco, F.; Kaminski, P.M.; Abbas, A.; Bass, K.; Bowers, J.W.; Claudio, G.; Losurdo, M.; Walls, J.M. The structural properties of CdS deposited by chemical bath deposition and pulsed direct current magnetron sputtering. Thin Solid Films 2015, 582, 323–327. [Google Scholar] [CrossRef]
- Islam, M.A.; Hossain, M.S.; Aliyu, M.M.; Chelvanathan, P.; Huda, Q.; Karim, M.R.; Sopian, K.; Amin, N. Comparison of structural and optical properties of CdS thin films grown by CSVT, CBD and sputtering techniques. Energy Procedia 2013, 33, 203–213. [Google Scholar] [CrossRef]
- Han, J.; Jian, Y.; He, Y.; Liu, Y.; Xiong, X.; Cha, L.; Krishnakumar, V.; Schimper, H.J. Nanostructures of CdS thin films prepared by various technologies for thin film solar cells. Mater. Lett. 2016, 177, 5–8. [Google Scholar] [CrossRef]
- Loheeswaran, S.; Thanihaichelvan, M.; Ravirajan, P.; Nelson, J. Controlling recombination kinetics of hybrid poly-3-hexylthiophene (P3HT)/titanium dioxide solar cells by self-assembled monolayers. J. Mater. Sci. Mater. Electron. 2017, 28, 4732–4737. [Google Scholar] [CrossRef]
- Ishwara, T.; Bradley, D.D.C.; Nelson, J.; Ravirajan, P.; Vanseveren, I.; Cleij, T.; Vanderzande, D.; Lutsen, L.; Tierney, S.; Heeney, M.; et al. Influence of polymer ionization potential on the open-circuit voltage of hybrid polymer/TiO2 solar cells. Appl. Phys. Lett. 2008, 92, 53308. [Google Scholar] [CrossRef]
- Brabec, C.J.; Cravino, A.; Meissner, D.; Serdar Sariciftci, N.; Fromherz, T.; Rispens, M.T.; Sanchez, L.; Hummelen, J.C. Origin of the open circuit voltage of plastic solar cells. Adv. Funct. Mater. 2001, 11, 374–380. [Google Scholar] [CrossRef]


| CdS Thin Film Fabrication Method | Optimum CdS Layer Thickness | Optimum Device Performance | Ref. | |||
|---|---|---|---|---|---|---|
| JSC * | VOC # | FF | PCE (%) | |||
| Thermal decomposition of cadmium xanthate precursor | 10 nm | 8.28 | 0.60 | 0.63 | 3.22 | [19] |
| Spraying equimolar solutions of CdCl2 and CS(NH2)2 | 330 nm | 1.91 | 0.502 | 0.30 | 0.29 | [17] |
| Chemical bath deposition | 21.3 nm | 5.33 | 0.62 | 0.43 | 1.42 | [18] |
| Atomic layer deposition | 17.5 nm | 8.94 | 0.61 | 0.61 | 3.33 | [16] |
| Chemical bath deposition | ~70 nm | 9.04 | 0.59 | 0.48 | 2.81 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thanihaichelvan, M.; Loheeswaran, S.; Balashangar, K.; Velauthapillai, D.; Ravirajan, P. Polymer/Fullerene Blend Solar Cells with Cadmium Sulfide Thin Film as an Alternative Hole-Blocking Layer. Polymers 2019, 11, 460. https://doi.org/10.3390/polym11030460
Thanihaichelvan M, Loheeswaran S, Balashangar K, Velauthapillai D, Ravirajan P. Polymer/Fullerene Blend Solar Cells with Cadmium Sulfide Thin Film as an Alternative Hole-Blocking Layer. Polymers. 2019; 11(3):460. https://doi.org/10.3390/polym11030460
Chicago/Turabian StyleThanihaichelvan, Murugathas, Selvadurai Loheeswaran, Kailasapathy Balashangar, Dhayalan Velauthapillai, and Punniamoorthy Ravirajan. 2019. "Polymer/Fullerene Blend Solar Cells with Cadmium Sulfide Thin Film as an Alternative Hole-Blocking Layer" Polymers 11, no. 3: 460. https://doi.org/10.3390/polym11030460
APA StyleThanihaichelvan, M., Loheeswaran, S., Balashangar, K., Velauthapillai, D., & Ravirajan, P. (2019). Polymer/Fullerene Blend Solar Cells with Cadmium Sulfide Thin Film as an Alternative Hole-Blocking Layer. Polymers, 11(3), 460. https://doi.org/10.3390/polym11030460