Tetracarbonatodiruthenium Fragments and Lanthanide(III) Ions as Building Blocks to Construct 2D Coordination Polymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Physical Measurements
2.2. Synthesis
2.2.1. Synthesis of [Pr(DMSO)2(OH2)3][Ru2(CO3)4(DMSO)(OH2)]·5H2O (Prα)
2.2.2. Synthesis of [Ln(OH2)5][Ru2(CO3)4(DMSO)]·3H2O (Ln = Sm (Smβ), Gd (Gdβ))
2.2.3. Synthesis of [Ln(OH2)4][Ru2(CO3)4(OH2)]·xH2O (Ln = Pr (Pr3D), Sm (Sm3D))
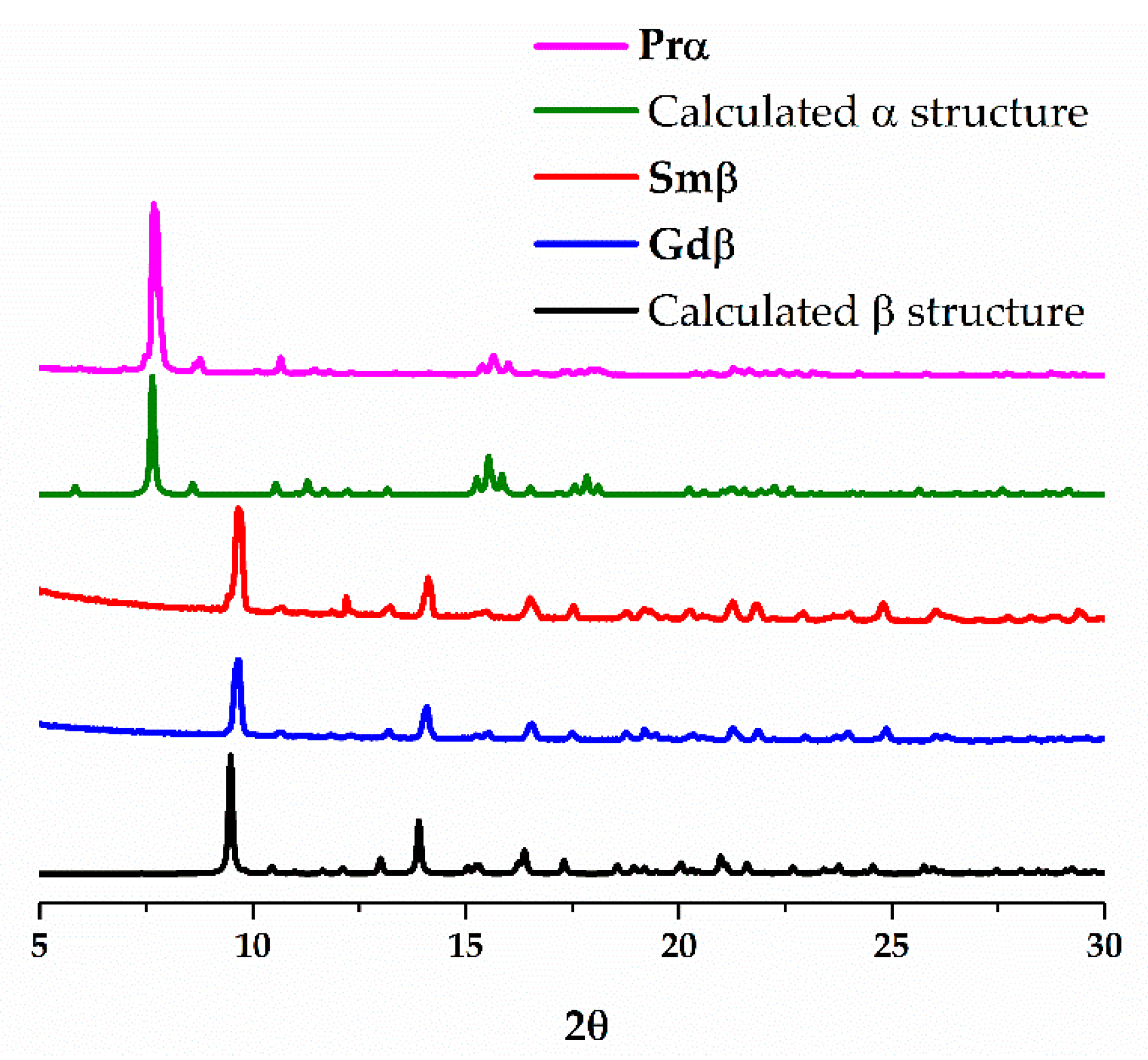
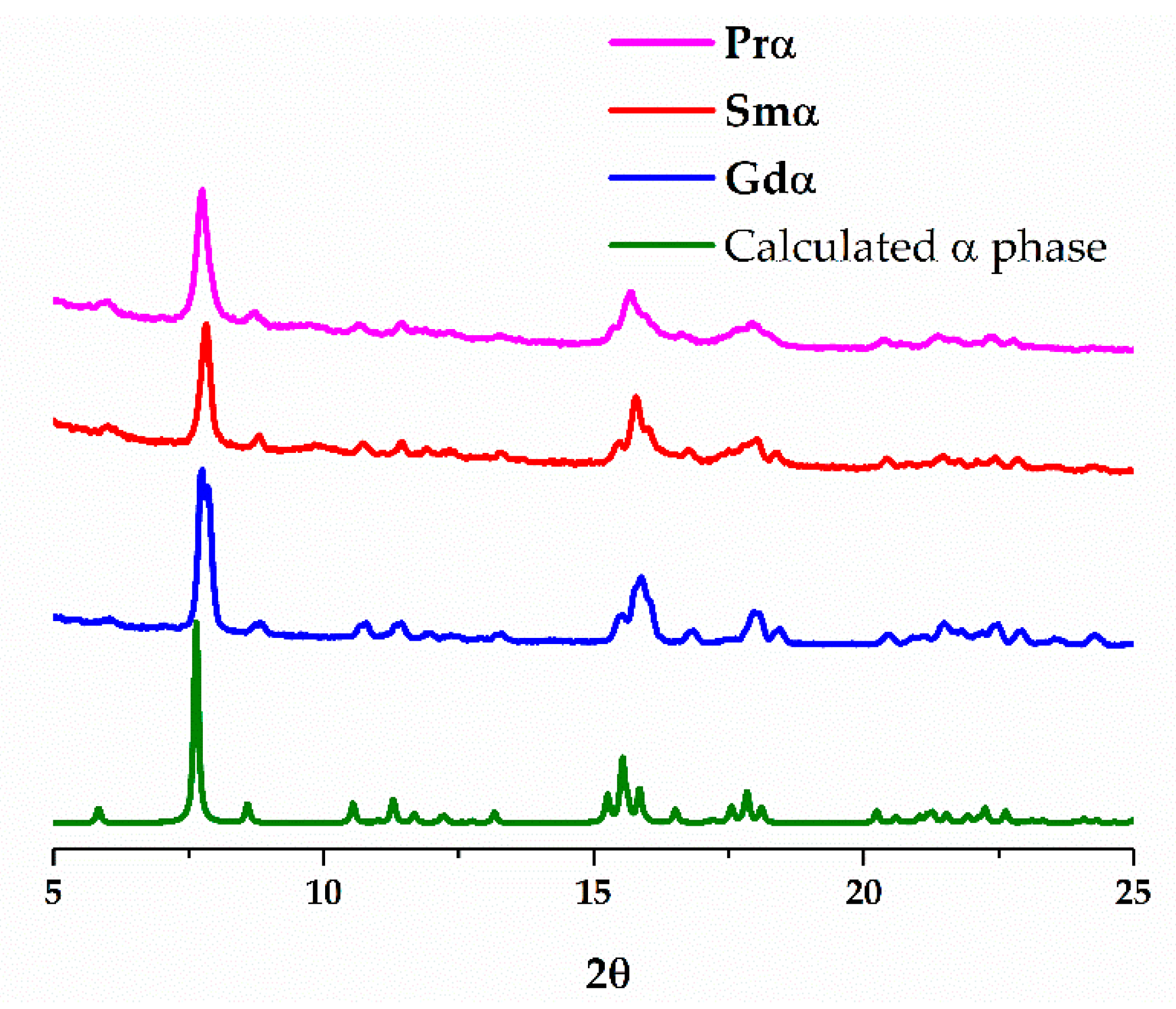
3. Results and Discussion
3.1. Synthesis
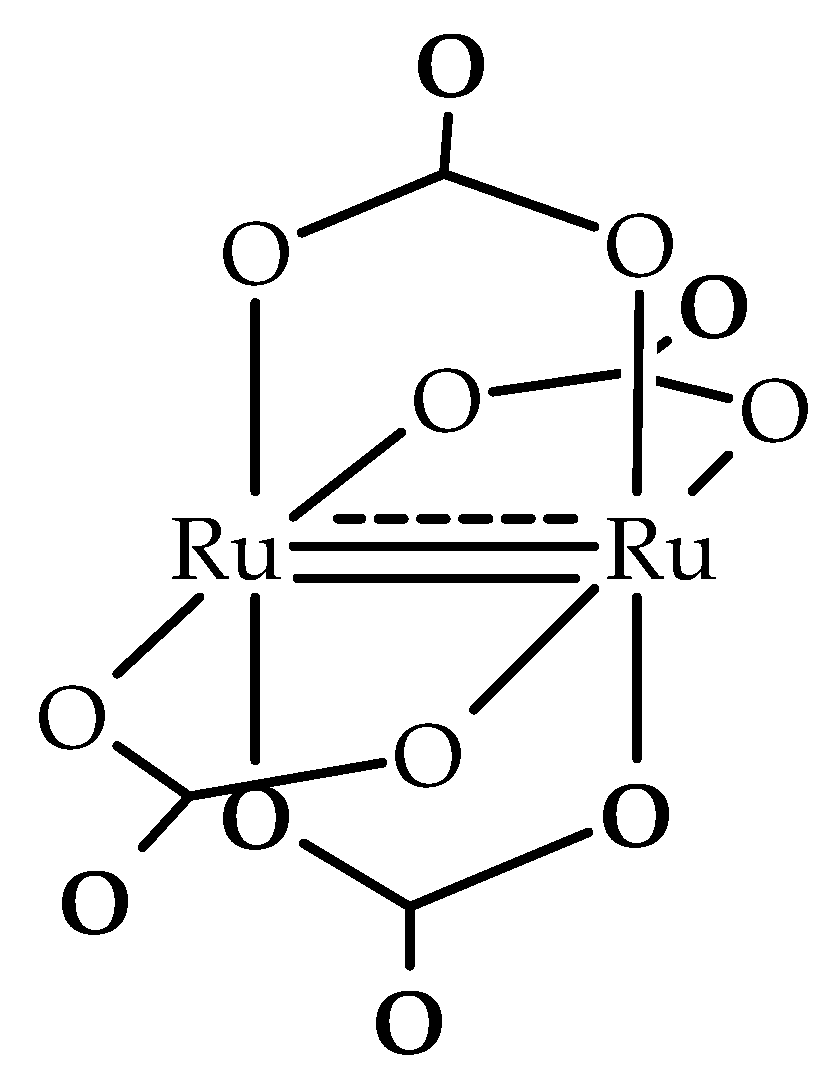
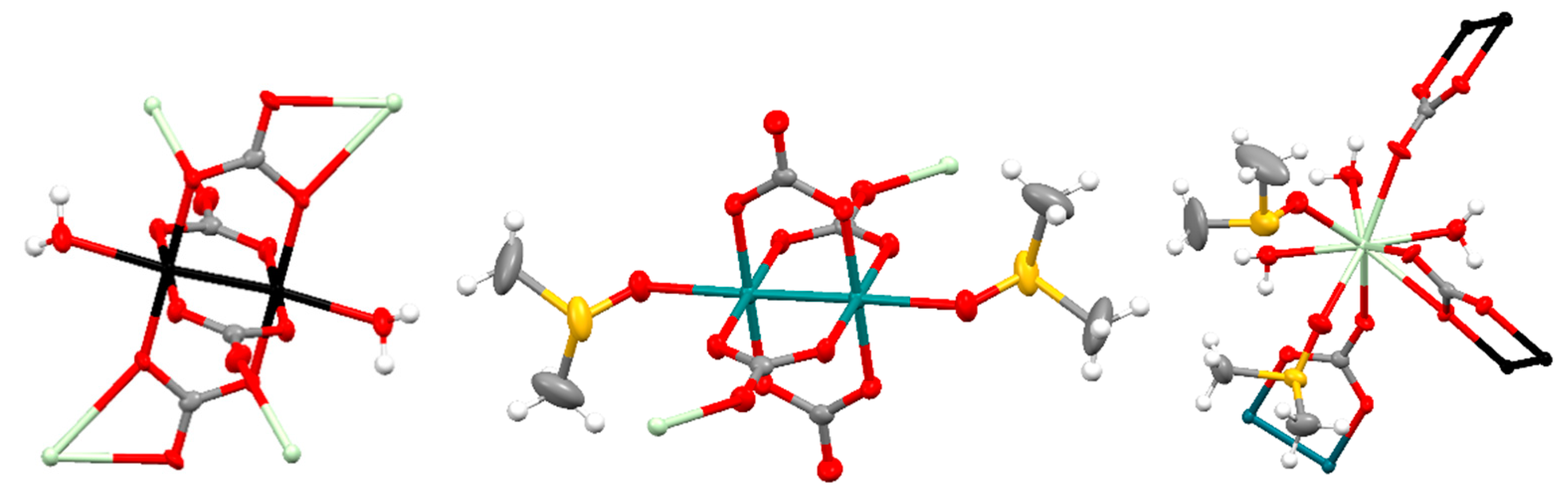
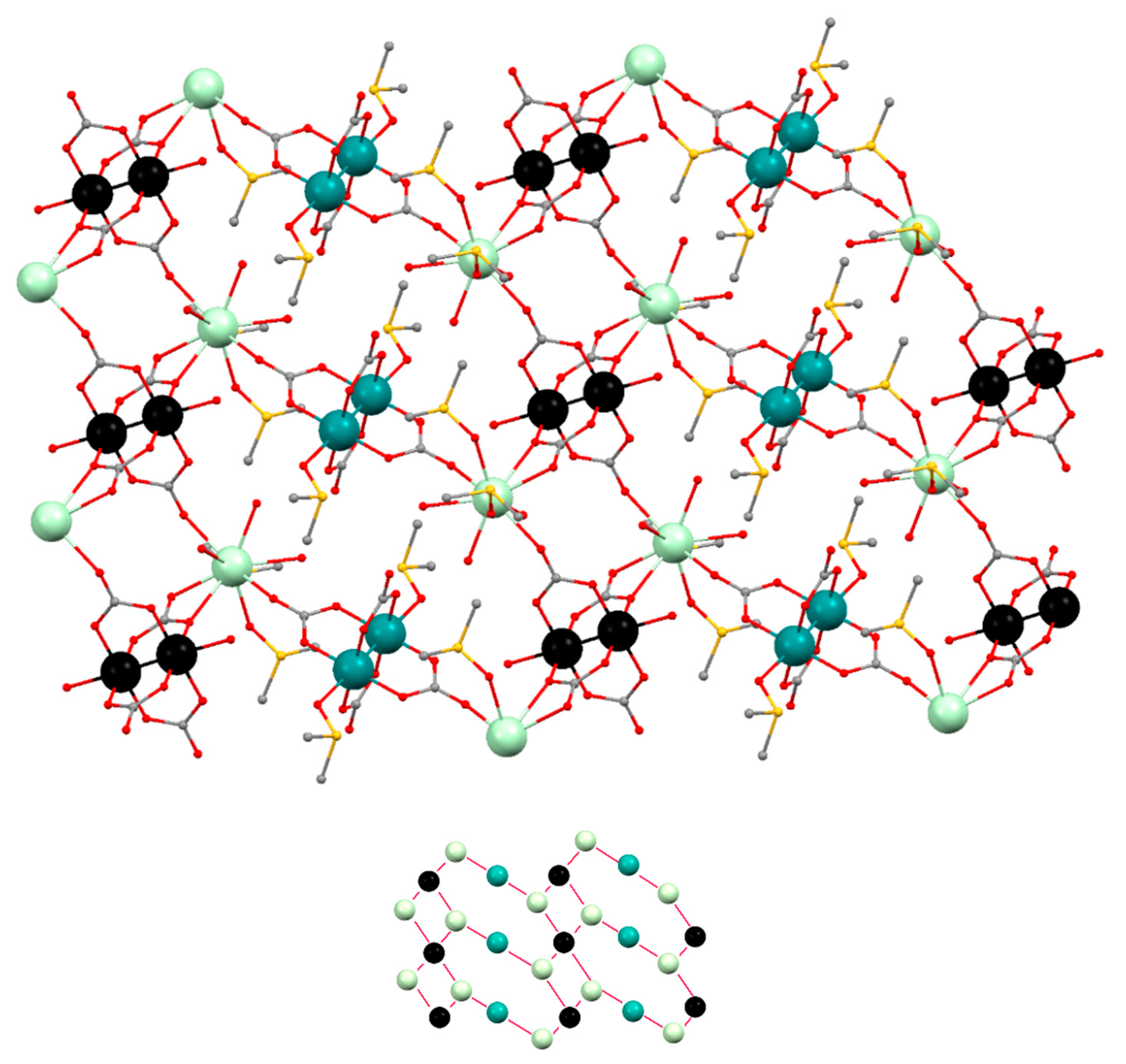
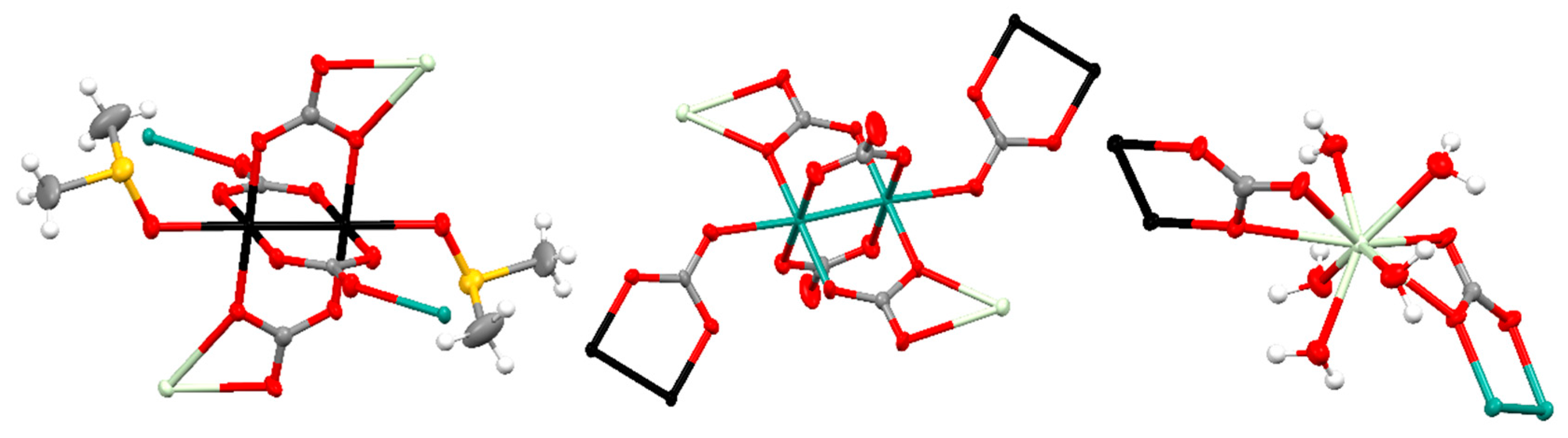
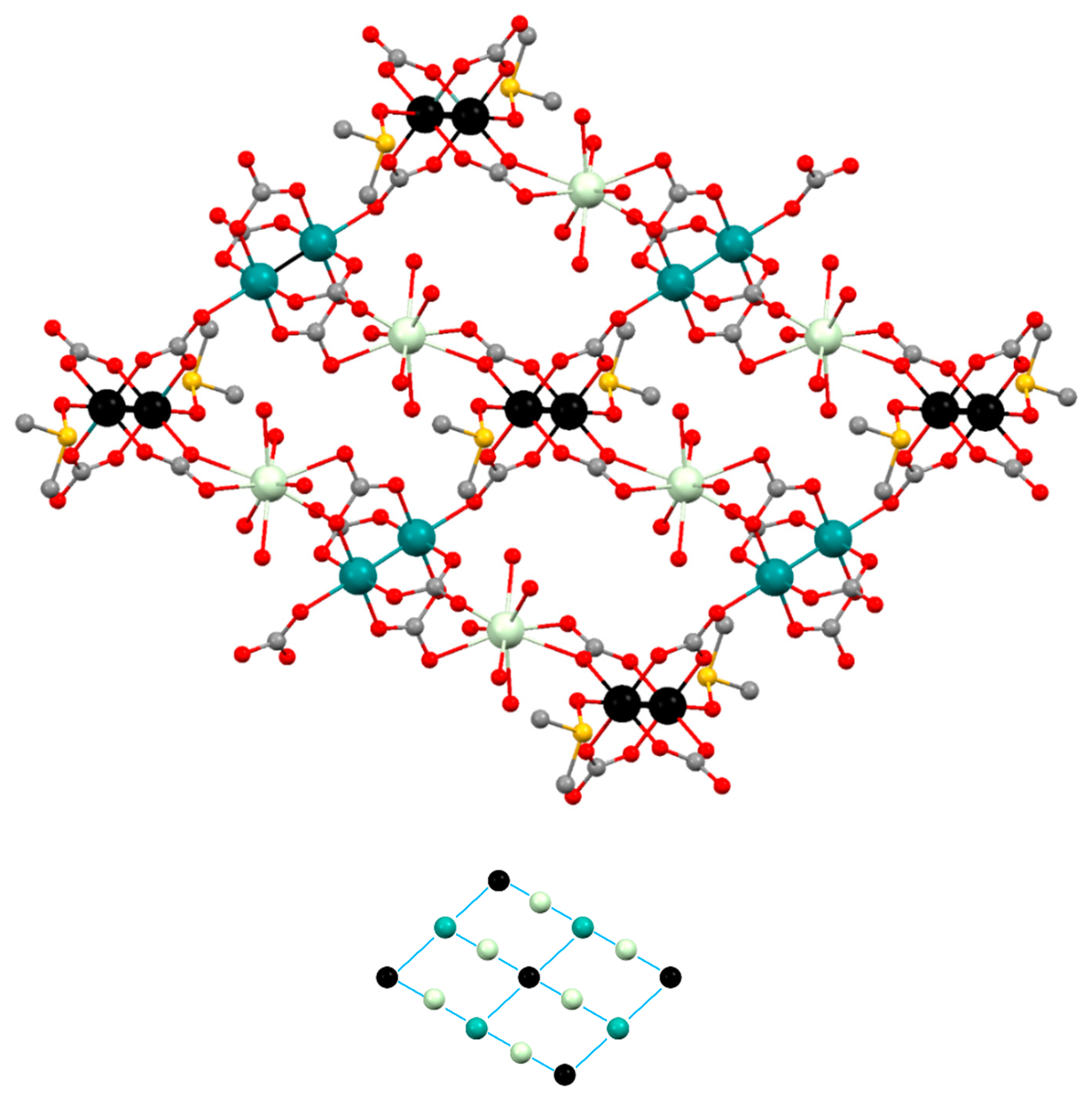
3.2. Structural Description
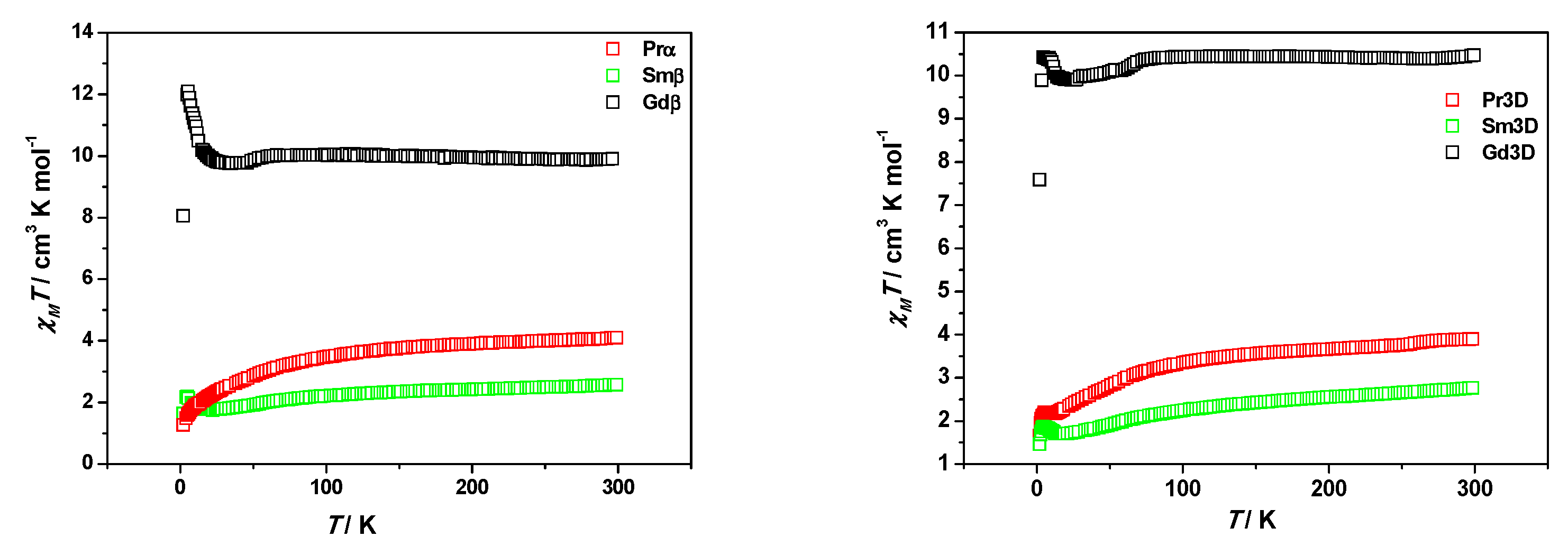
3.3. Magnetic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lindsay, A.J.; Wilkinson, G.; Motevalli, M.; Hursthouse, M.B. Reactions of Tetra-μ-carboxylato-diruthenium(II,II) Compounds. X-Ray Crystal Structures of Ru2(μ-O2CCF3)4(thf)2, Ru2(μ-O2CR)4(NO)2 (R = Et or CF3), and {Na3[Ru2(μ-O2CO)4]·6H2O}n. J. Chem. Soc. Dalton Trans. 1987, 0, 2723–2736. [Google Scholar] [CrossRef]
- Cotton, F.A.; Labella, L.; Shang, M. Further Study of Tetracarbonato Diruthenium(II,III) Compounds. Inorg. Chem. 1992, 31, 2385–2389. [Google Scholar] [CrossRef]
- Norman, J.G.; Renzoni, G.E.; Case, D.A. Electronic Structure of Ru2(O2CR)4+ and Rh2(O2CR)4+ Complexes. J. Am. Chem. Soc. 1979, 101, 5256–5267. [Google Scholar] [CrossRef]
- Cotton, F.A.; Walton, R.A. Multiple Bonds between Metal Atoms, 2nd ed.; Wiley: New York, NY, USA, 1982. [Google Scholar]
- Cotton, F.A.; Murillo, C.A.; Walton, R.A. Multiple Bonds between Metal Atoms, 3rd ed.; Springer: New York, NY, USA, 2005. [Google Scholar]
- Liddle, S.T. (Ed.) Molecular Metal-Metal Bonds: Compounds, Synthesis, Properties; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Kennon, B.S.; Miller, J.S. Observation of Magnetic Ordering for Layered (2-D) Potassium Diruthenium Tetracarbonate, K3[Ru2II/III(O2CO)4]: A Rare Second Row Transition Metal-based Magnet. Inorg. Chem. 2010, 49, 5542–5545. [Google Scholar] [CrossRef] [PubMed]
- Kennon, B.S.; Her, J.-H.; Stephens, P.W.; Shum, W.W.; Miller, J.S. Structure and Magnetic Ordering of KxH1-xNi(OH2)4[Ru2(CO3)4]·zH2O. Inorg. Chem. 2007, 46, 9033–9035. [Google Scholar] [CrossRef] [PubMed]
- Kennon, B.S.; Her, J.-H.; Stephens, P.W.; Miller, J.S. Diruthenium Tetracarbonate Trianion, [Ru2II/III(O2CO)4]3−, Based Molecule-Based Magnets: Three-Dimensional Network Structure and Two-Dimensional Magnetic Ordering. Inorg. Chem. 2009, 48, 6117–6123. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Jia, Y.-Y.; Jin, J.; Liu, X.-M.; Xue, G.-L. Layer structural bimetallic metamagnets obtained from the aggregation of Ru2(CO3)43− and Co2+ in existence of halogen. CrystEngCommun 2013, 15, 4280–4287. [Google Scholar] [CrossRef]
- Jia, Y.-Y.; Liu, B.; Liu, X.-M.; Yang, J.-H. Syntheses, structures and magnetic properties of two heterometallic carbonates: K2Li[Cu(H2O)2Ru2(CO3)4X2]·5H2O (X = Cl, Br). CrystEngCommun 2013, 15, 7936–7942. [Google Scholar] [CrossRef]
- Liu, B.; Wang, D.; Jin, J.; Jia, Y.-Y.; Liu, X.-M.; Xue, G.-L. Heterometallic Co(II)–Ru2(II,III) carbonates: From discrete ionic crystals to three-dimensional network. CrystEngCommun 2013, 15, 5726–5734. [Google Scholar] [CrossRef]
- Liu, B.; Jia, Y.-Y.; Jin, J.; Wang, X.-M.; Liu, D.; Xue, G.-L. Cadmium diruthenium(II,III) carbonates showing diverse magnetism behavior arising from variety configuration of [Ru2(CO3)4]n3n− layer. Dalton Trans. 2013, 42, 10208–10213. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Jia, Y.-Y.; Yang, H.-Q.; Yang, J.-H.; Xue, G.-L. Incorporation of M(H2O)62+ between layers {M(H2O)2Ru2(CO3)4Cl2}n2n− (M = Zn, Mn): Syntheses, structures and magnetic properties. Dalton Trans. 2013, 42, 16742–16748. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.-F.; Jia, Y.-Y.; Yang, J.-H.; Cao, Z.; Liu, B. Isomeric chain structures of {[Mn(H2O)4]2Ru2(CO3)4Br2}nn−: Syntheses, structural diversity and magnetic properties. Dalton Trans. 2014, 43, 13316–13324. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, B.; Jin, J.; Liu, X.-M.; Jia, Y.-Y.; Xue, G.-L. X-ray single-crystal structure and magnetic properties of KMn(H2O)5Ru2(CO3)4·5H2O: A layered soft magnet. Inorg. Chem. Commun. 2013, 33, 138–141. [Google Scholar] [CrossRef]
- Jia, Y.-Y.; Chang, Z.-G.; Yang, J.-H.; Liu, B. Spin glass ordering in heterometallic carbonates with pillared layer structure: K4[Mn(H2O)4{Ru2(CO3)4}2]·4H2O. Inorg. Chim. Acta 2015, 424, 162–166. [Google Scholar] [CrossRef]
- Liu, B.; Jin, J.; Liu, X.-M.; Hu, H.-M.; Ding, T.; Zhang, N.; Jia, Y.-Y.; Xue, G.-L. A diruthenium soft ferromagnet showing Tc = 3.0 K: Mn4(H2O)16H[Ru2(CO3)4]2[Ru2(CO3)4(H2O)2]·11H2O. Dalton Trans. 2012, 41, 4748–4750. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-H.; Cheng, R.-M.; Jia, Y.-Y.; Jin, J.; Yang, B.-B.; Cao, Z.; Liu, B. Chlorine and Temperature Directed Self-assembly of Mg-Ru2(II,III) Carbonates and Particle Size Dependent Magnetic Properties. Dalton Trans. 2016, 45, 2945–2954. [Google Scholar] [CrossRef] [PubMed]
- Her, J.-H.; Kennon, B.S.; Shum, W.W.; Stephens, P.W.; Miller, J.S. Structure and magnetic properties of LnIII[Ru2(CO3)4]·8H2O. Inorg. Chim. Acta 2008, 361, 3462–3464. [Google Scholar] [CrossRef]
- Delgado-Martínez, P.; González-Prieto, R.; Herrero, S.; Jiménez-Aparicio, R.; Perles, J.; Priego, J.L.; Torres, M.R.; Sufrate, B. Preparation of Crystalline Phases of 3D Coordination Polymers Based on Tetracarbonatodiruthenium Units and Lanthanide(III) Ions—Magnetic Characterization. Eur. J. Inorg. Chem. 2017, 3161–3168. [Google Scholar] [CrossRef]
- Oyarzabal, I.; Artetxe, B.; Rodríguez-Diéguez, A.; García, J.A.; Seco, J.M.; Colacio, E. A family of acetato-diphenoxo triply bridged dimetallic ZnIILnIII complexes: SMM behavior and luminescent properties. Dalton Trans. 2016, 45, 9712–9726. [Google Scholar] [CrossRef] [PubMed]
- Kahn, O. Molecular Magnetism; VCH: New York, NY, USA, 1991. [Google Scholar]
- Woodruff, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide Single-Molecule Magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef] [PubMed]
| Crystallographic Parameters | Prα | Smβ | Gdβ | Sm3D |
|---|---|---|---|---|
| Formula | PrRu2C10H26O19 S3·5H2O | SmRu2C6H16O18 S·3H2O | GdRu2C6H16O18 S·2H2O | SmRu2C4H12O18 2H2O |
| fw | 979.62 | 814.80 | 803.67 | 736.67 |
| Space group | P-1 | P-1 | P-1 | C2/c |
| a/Å | 8.4508(4) | 9.7951(5) | 9.7687(6) | 25.063(2) |
| b/Å | 12.4403(6) | 9.8502(5) | 9.8237(6) | 9.8420(8) |
| c/Å | 15.5262(8) | 12.8759(6) | 12.8403(7) | 14.0568(12) |
| α/° | 78.243(1) | 75.608(1) | 75.657(1) | 90 |
| β/° | 89.339(1) | 70.296(1) | 70.560(1) | 95.092(2) |
| γ/° | 72.147(1) | 73.885(1) | 73.951(1) | 90 |
| V/Å3 | 1518.81(13) | 1107.0(10) | 1100.07(11) | 3453.7(5) |
| Z | 2 | 2 | 2 | 8 |
| d calc/g·cm−3 | 2.142 | 2.447 | 2.426 | 2.833 |
| μ/mm−1 | 2.857 | 4.151 | 4.517 | 5.185 |
| R indices (all data) | R1 = 0.0743 wR2 = 0.0974 | R1 = 0.0504 wR2 = 0.1100 | R1 = 0.0553 wR2 = 0.1241 | R1 = 0.0585 wR2 = 0.0983 |
| GooF on F2 | 1.082 | 1.039 | 1.073 | 1.052 |
| Compound | gRu | gLn1 | D [cm−1] | λ [cm−1] | θ [K] | TIP [emu/mol] | σ 2 |
|---|---|---|---|---|---|---|---|
| Gdβ | 2.28 | 2.00 | 39 | 0.92 | 2.17 × 10−4 | 1.12 × 10−2 | |
| Gdβ 2 | 2.10 | 2.00 | 70 | 1.98 | 4.14 × 10−12 | 7.11 × 10−3 | |
| Smβ | 2.18 | 0.29 | 73 | 256 | 1.73 | 5.27 × 10−11 | 5.12 × 10−5 |
| Sm3D | 2.17 | 0.29 | 77 | 270 | 1.11 | 7.76 × 10−4 | 4.88 × 10−5 |
| Prα | 2.07 | 0.80 | 78 | −5.73 | 2.20 × 10−3 | 9.14 × 10−3 | |
| Pr3D | 2.08 | 0.80 | 73 | −7.09 | 3.27 × 10−3 | 7.95 × 10−4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Martín, D.; Cortijo, M.; Martín-Humanes, Á.; González-Prieto, R.; Delgado-Martínez, P.; Herrero, S.; Priego, J.L.; Jiménez-Aparicio, R. Tetracarbonatodiruthenium Fragments and Lanthanide(III) Ions as Building Blocks to Construct 2D Coordination Polymers. Polymers 2019, 11, 426. https://doi.org/10.3390/polym11030426
Gutiérrez-Martín D, Cortijo M, Martín-Humanes Á, González-Prieto R, Delgado-Martínez P, Herrero S, Priego JL, Jiménez-Aparicio R. Tetracarbonatodiruthenium Fragments and Lanthanide(III) Ions as Building Blocks to Construct 2D Coordination Polymers. Polymers. 2019; 11(3):426. https://doi.org/10.3390/polym11030426
Chicago/Turabian StyleGutiérrez-Martín, Daniel, Miguel Cortijo, Álvaro Martín-Humanes, Rodrigo González-Prieto, Patricia Delgado-Martínez, Santiago Herrero, José L. Priego, and Reyes Jiménez-Aparicio. 2019. "Tetracarbonatodiruthenium Fragments and Lanthanide(III) Ions as Building Blocks to Construct 2D Coordination Polymers" Polymers 11, no. 3: 426. https://doi.org/10.3390/polym11030426
APA StyleGutiérrez-Martín, D., Cortijo, M., Martín-Humanes, Á., González-Prieto, R., Delgado-Martínez, P., Herrero, S., Priego, J. L., & Jiménez-Aparicio, R. (2019). Tetracarbonatodiruthenium Fragments and Lanthanide(III) Ions as Building Blocks to Construct 2D Coordination Polymers. Polymers, 11(3), 426. https://doi.org/10.3390/polym11030426







