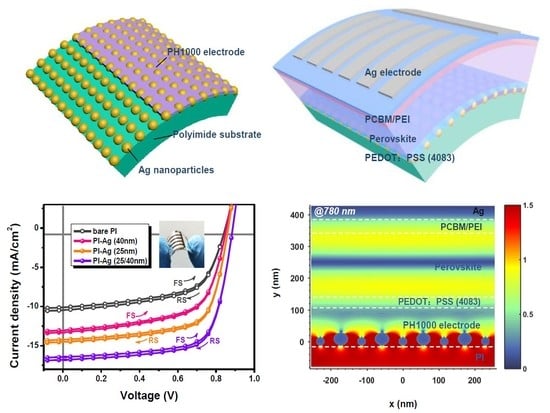
Flexible Perovskite Solar Cells via Surface-Confined Silver Nanoparticles on Transparent Polyimide Substrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Characterization
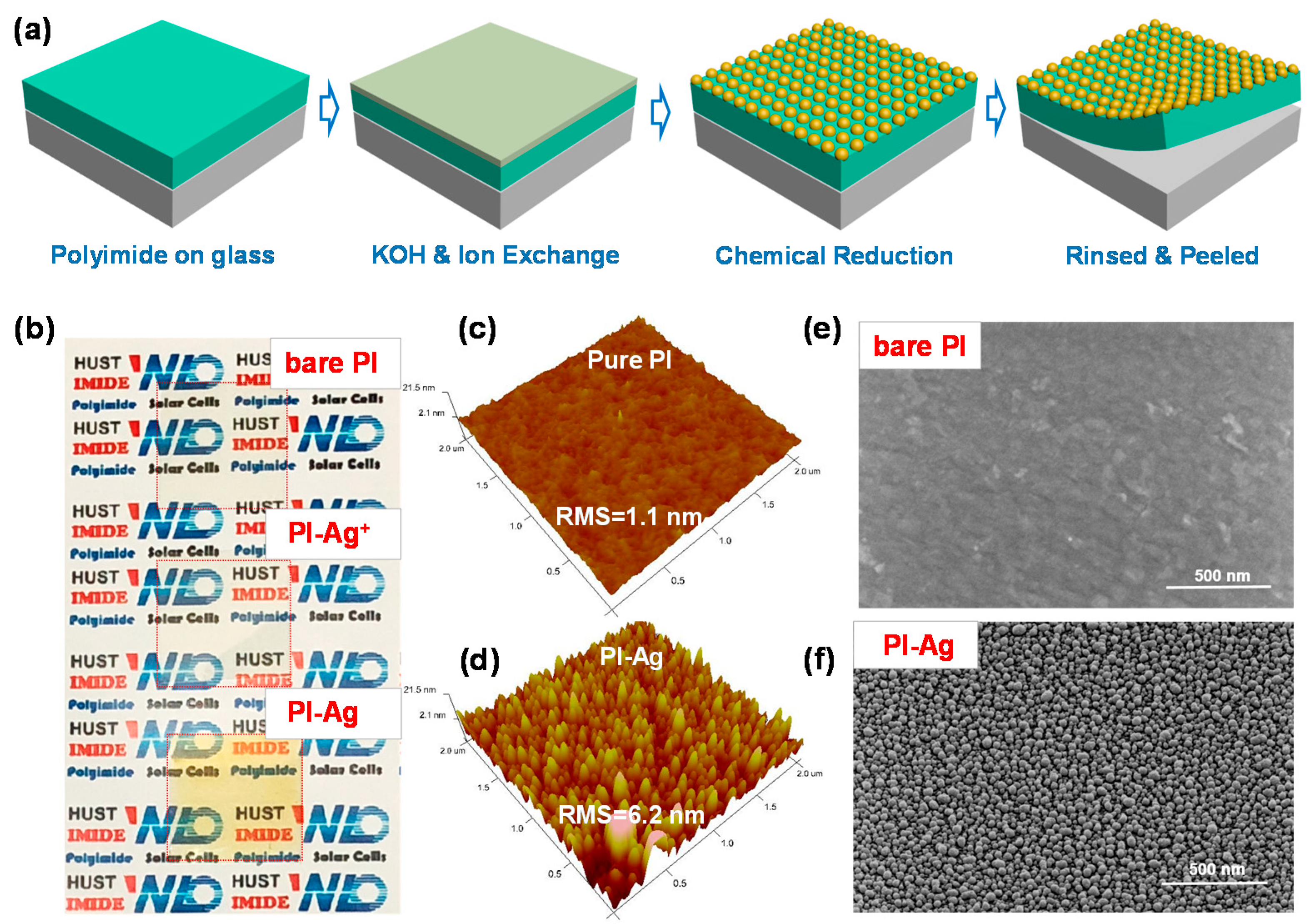
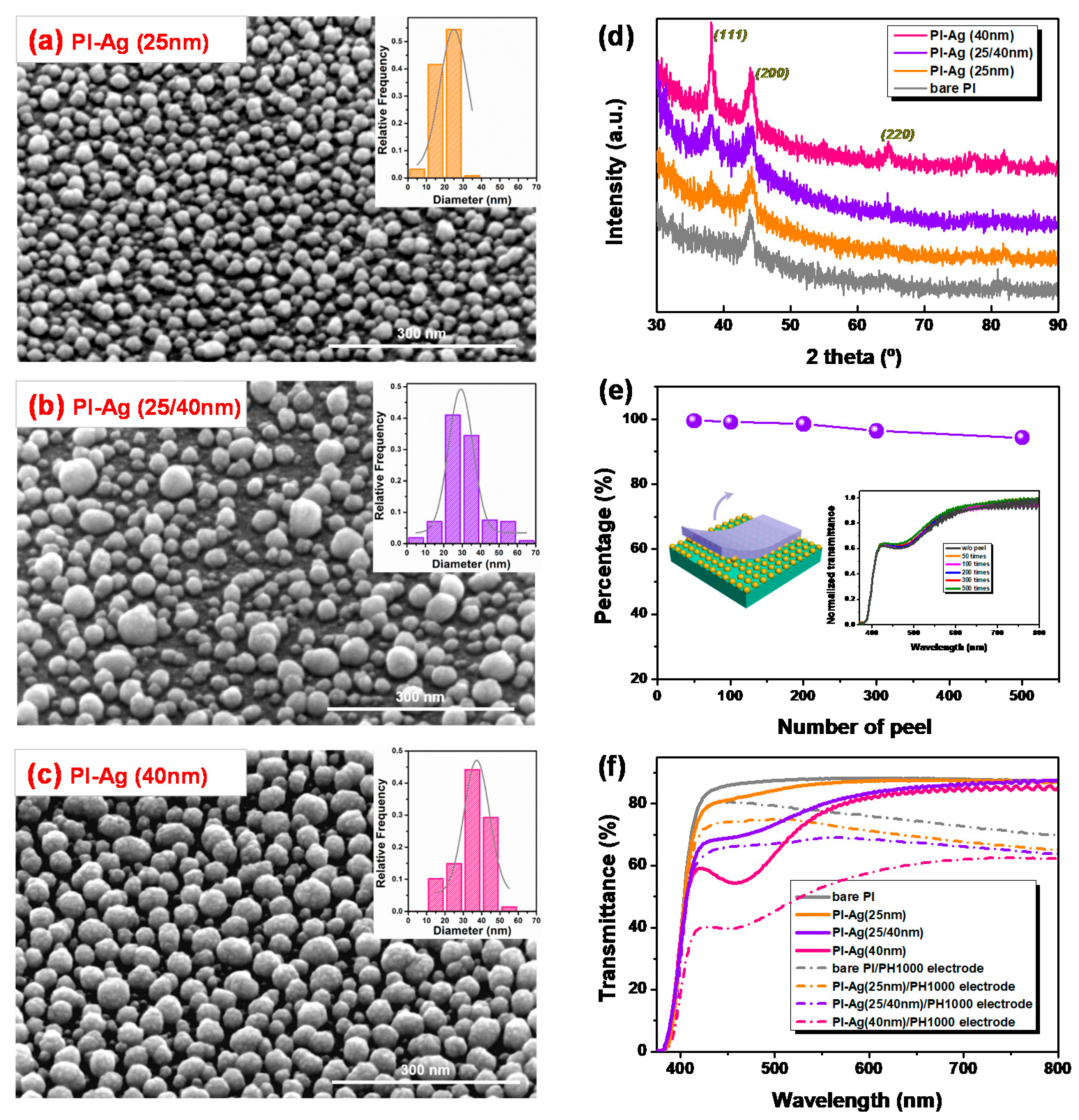
2.2. Synthesis of Surface-Confined Ag Nanoparticles
2.3. Fabrication of a Flexible Device
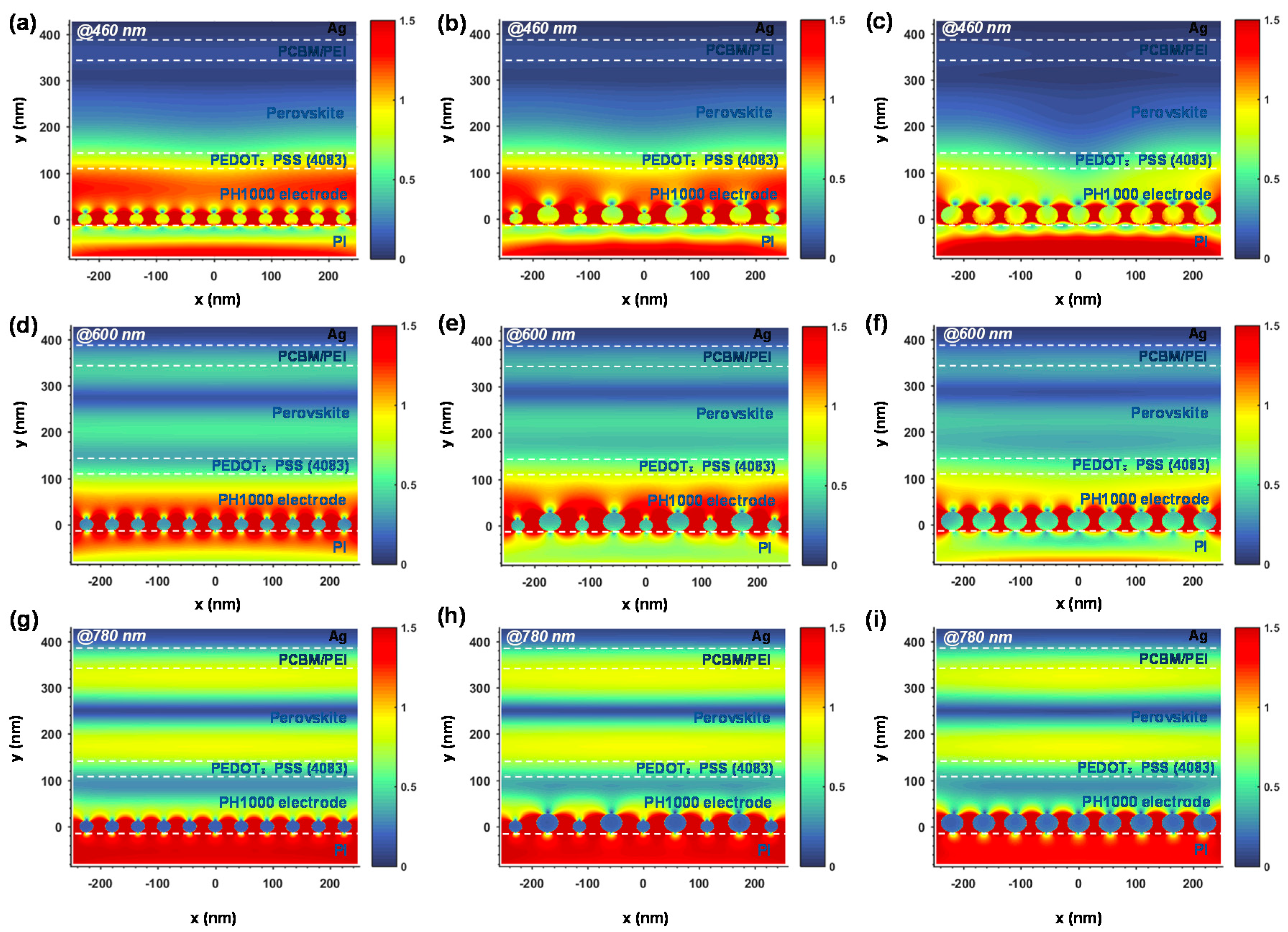
2.4. Theoretical Simulation
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- White, M.S.; Kaltenbrunner, M.; Głowacki, E.D.; Gutnichenko, K.; Kettlgruber, G.; Graz, I.; Aazou, S.; Ulbricht, C.; Egbe, D.A.; Miron, M.C. Ultrathin, highly flexible and stretchable PLEDs. Nat. Photonics 2013, 7, 811. [Google Scholar] [CrossRef]
- Han, T.-H.; Lee, Y.; Choi, M.-R.; Woo, S.-H.; Bae, S.-H.; Hong, B.H.; Ahn, J.-H.; Lee, T.-W. Extremely efficient flexible organic light-emitting diodes with modified graphene anode. Nat. Photonics 2012, 6, 105. [Google Scholar] [CrossRef]
- Luo, D.; Chen, Q.; Liu, B.; Qiu, Y. Emergence of Flexible White Organic Light-Emitting Diodes. Polymers 2019, 11, 384. [Google Scholar] [CrossRef]
- Docampo, P.; Ball, J.M.; Darwich, M.; Eperon, G.E.; Snaith, H.J. Efficient organometal trihalide perovskite planar-heterojunction solar cells on flexible polymer substrates. Nat. Commun. 2013, 4, 2761. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, X.-L.; Tao, H.; Zou, J.; Xu, M.; Wang, L.; Peng, J.; Cao, Y. Manipulation of exciton distribution for high-performance fluorescent/phosphorescent hybrid white organic light-emitting diodes. J. Mater. Chem. C 2017, 5, 7668–7683. [Google Scholar] [CrossRef]
- Yoon, Y.; Park, H.; Lim, Y.; Choi, K.; Lee, K.; Park, G.; Lee, C.J.; Moon, D.; Han, J.; Kim, Y. Effects of parylene buffer layer on flexible substrate in organic light emitting diode. Thin Solid Films 2006, 513, 258–263. [Google Scholar] [CrossRef]
- Peinado, P.; Sangiao, S.; De Teresa, J.M. Focused electron and ion beam induced deposition on flexible and transparent polycarbonate substrates. ACS Nano 2015, 9, 6139–6146. [Google Scholar] [CrossRef] [PubMed]
- Ellmer, K. Past achievements and future challenges in the development of optically transparent electrodes. Nat. Photonics 2012, 6, 809–817. [Google Scholar] [CrossRef]
- Forrest, S.R. The path to ubiquitous and low-cost organic electronic appliances on plastic. Nature 2004, 428, 911. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Meng, L.; Yang, Y.M.; Xu, G.; Hong, Z.; Chen, Q.; You, J.; Li, G.; Yang, Y.; Li, Y. High-efficiency robust perovskite solar cells on ultrathin flexible substrates. Nat. Commun. 2016, 7, 10214. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, G.; Cao, Y.; Treacy, G.; Klavetter, F.; Colaneri, N.; Heeger, A. Flexible light-emitting diodes made from soluble conducting polymers. Nature 1992, 357, 477. [Google Scholar] [CrossRef]
- Nomura, K.; Ohta, H.; Takagi, A.; Kamiya, T.; Hirano, M.; Hosono, H. Room-temperature fabrication of transparent flexible thin-film transistors using amorphous oxide semiconductors. Nature 2004, 432, 488. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Cho, H.; Kim, K.; Koh, T.W.; Chung, J.; Lee, J.; Park, Y.; Yoo, S. A Facile Route to Efficient, Low-Cost Flexible Organic Light-Emitting Diodes: Utilizing the High Refractive Index and Built-In Scattering Properties of Industrial-Grade PEN Substrates. Adv. Mater. 2015, 27, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.S.; Yang, W.S.; Noh, J.H.; Suk, J.H.; Jeon, N.J.; Park, J.H.; Kim, J.S.; Seong, W.M.; Seok, S.I. High-performance flexible perovskite solar cells exploiting Zn2SnO4 prepared in solution below 100 C. Nat. Commun. 2015, 6, 7410. [Google Scholar] [CrossRef] [PubMed]
- Kraft, U.; Sejfić, M.; Kang, M.J.; Takimiya, K.; Zaki, T.; Letzkus, F.; Burghartz, J.N.; Weber, E.; Klauk, H. Flexible Low-Voltage Organic Complementary Circuits: Finding the Optimum Combination of Semiconductors and Monolayer Gate Dielectrics. Adv. Mater. 2015, 27, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.Y.; Choi, D.; Jin, M.J.; Kim, I.; Kim, S.H.; Choi, J.Y.; Lee, S.Y.; Kim, J.M.; Kim, S.W. Mechanically powered transparent flexible charge-generating nanodevices with piezoelectric ZnO nanorods. Adv. Mater. 2009, 21, 2185–2189. [Google Scholar] [CrossRef]
- Jeong, H.Y.; Kim, Y.I.; Lee, J.Y.; Choi, S.-Y. A low-temperature-grown TiO2-based device for the flexible stacked RRAM application. Nanotechnology 2010, 21, 115203. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Cho, W.J.; Ha, C.S.; Ando, S.; Kim, Y.K.; Park, C.H.; Lee, K. Flexible Organic Electroluminescent Devices Based on Fluorine-Containing Colorless Polyimide Substrates. Adv. Mater. 2002, 14, 1275–1279. [Google Scholar] [CrossRef]
- Spechler, J.A.; Koh, T.W.; Herb, J.T.; Rand, B.P.; Arnold, C.B. A transparent, smooth, thermally robust, conductive polyimide for flexible electronics. Adv. Funct. Mater. 2015, 25, 7428–7434. [Google Scholar] [CrossRef]
- Zhang, L.; Xiao, W.; Wu, W.; Liu, B. Research Progress on Flexible Oxide-Based Thin Film Transistors. Appl. Sci. 2019, 9, 773. [Google Scholar] [CrossRef]
- Choi, M.-C.; Kim, Y.; Ha, C.-S. Polymers for flexible displays: From material selection to device applications. Prog. Polym. Sci. 2008, 33, 581–630. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Tsai, H.; Asadpour, R.; Blancon, J.-C.; Neukirch, A.J.; Gupta, G.; Crochet, J.J.; Chhowalla, M.; Tretiak, S.; Alam, M.A. High-efficiency solution-processed perovskite solar cells with millimeter-scale grains. Science 2015, 347, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Ahn, N.; Son, D.-Y.; Jang, I.-H.; Kang, S.M.; Choi, M.; Park, N.-G. Highly reproducible perovskite solar cells with average efficiency of 18.3% and best efficiency of 19.7% fabricated via Lewis base adduct of lead (II) iodide. J. Am. Chem. Soc. 2015, 137, 8696–8699. [Google Scholar] [CrossRef] [PubMed]
- Ou, Q.D.; Li, Y.Q.; Tang, J.X. Light Manipulation in Organic Photovoltaics. Adv. Sci. 2016, 3, 1600123. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Bartoli, F.J.; Kafafi, Z.H. Plasmonic-enhanced organic photovoltaics: Breaking the 10% efficiency barrier. Adv. Mater. 2013, 25, 2385–2396. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-P.; Lien, D.-H.; Tsai, M.-L.; Lin, C.-A.; Chang, H.-C.; Lai, K.-Y.; He, J.-H. Photon management in nanostructured solar cells. J. Mater. Chem. C 2014, 2, 3144–3171. [Google Scholar] [CrossRef]
- Gómez, D.E.; Davis, T.J.; Funston, A.M. Plasmonics by design: Design principles to structure–function relationships with assemblies of metal nanoparticles. J. Mater. Chem. C 2014, 2, 3077–3087. [Google Scholar] [CrossRef]
- Iftiquar, S.M.; Yi, J. Numerical simulation and light trapping in perovskite solar cell. J. Photon. Energy 2016, 6, 025507. [Google Scholar] [CrossRef]
- Peer, A.; Biswas, R.; Park, J.-M.; Shinar, R.; Shinar, J. Light management in perovskite solar cells and organic LEDs with microlens arrays. Opt. Express 2017, 25, 10704–10709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Saliba, M.; Stranks, S.D.; Sun, Y.; Shi, X.; Wiesner, U.; Snaith, H.J. Enhancement of perovskite-based solar cells employing core–shell metal nanoparticles. Nano Lett. 2013, 13, 4505–4510. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.G.; Shen, G.; John, S. Light-trapping in perovskite solar cells. AIP Adv. 2016, 6, 065002. [Google Scholar] [CrossRef]
- Li, X.; Choy, W.C.H.; Lu, H.; Sha, W.E.; Ho, A.H.P. Efficiency Enhancement of Organic Solar Cells by Using Shape-Dependent Broadband Plasmonic Absorption in Metallic Nanoparticles. Adv. Funct. Mater. 2013, 23, 2728–2735. [Google Scholar] [CrossRef]
- Lim, E.L.; Yap, C.C.; Mat Teridi, M.A.; Teh, C.H.; Mohd Yusoff, A.R.B.; Hj Jumali, M.H. A review of recent plasmonic nanoparticles incorporated P3HT: PCBM organic thin film solar cells. Org. Electron. 2016, 36, 12–28. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Y.; Wang, L.-L.; Ringe, E. Small morphology variations effects on plasmonic nanoparticle dimer hotspots. J. Mater. Chem. C 2018, 6, 9607–9614. [Google Scholar] [CrossRef]
- Mai, R.; Wu, X.; Jiang, Y.; Meng, Y.; Liu, B.; Hu, X.; Roncali, J.; Zhou, G.; Liu, J.-M.; Kempa, K. An efficient multi-functional material based on polyether-substituted indolocarbazole for perovskite solar cells and solution-processed non-doped OLEDs. J. Mater. Chem. A 2019, 7, 1539–1547. [Google Scholar] [CrossRef]
- Carretero-Palacios, S.; Calvo, M.E.; Míguez, H. Absorption enhancement in organic–inorganic halide perovskite films with embedded plasmonic gold nanoparticles. J. Phys. Chem. C 2015, 119, 18635–18640. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Pan, X.; Ma, Y.; Li, Y.; Zheng, L.; Zhang, D.; Xu, Q.; Chen, Z.; Wang, S.; Qu, B. Plasmonic-enhanced perovskite solar cells using alloy popcorn nanoparticles. RSC Adv. 2015, 5, 11175–11179. [Google Scholar] [CrossRef]
- Saliba, M.; Zhang, W.; Burlakov, V.M.; Stranks, S.D.; Sun, Y.; Ball, J.M.; Johnston, M.B.; Goriely, A.; Wiesner, U.; Snaith, H.J. Plasmonic-Induced Photon Recycling in Metal Halide Perovskite Solar Cells. Adv. Funct. Mater. 2015, 25, 5038–5046. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, R.; You, P.; Zhu, X.; Wang, J.; Yan, F. Au/Ag core–shell nanocuboids for high-efficiency organic solar cells with broadband plasmonic enhancement. Energy Environ. Sci. 2016, 9, 898–905. [Google Scholar] [CrossRef]
- Kakavelakis, G.; Vangelidis, I.; Heuer-Jungemann, A.; Kanaras, A.G.; Lidorikis, E.; Stratakis, E.; Kymakis, E. Plasmonic Backscattering Effect in High-Efficient Organic Photovoltaic Devices. Adv. Energy Mater. 2016, 6, 1501640. [Google Scholar] [CrossRef]
- Baek, S.W.; Hun Kim, J.; Kang, J.; Lee, H.; Young Park, J.; Lee, J.Y. Enhancing the Internal Quantum Efficiency and Stability of Organic Solar Cells via Metallic Nanofunnels. Adv. Energy Mater. 2015, 5, 1501393. [Google Scholar] [CrossRef]
- Angmo, D.; Larsen-Olsen, T.T.; Jørgensen, M.; Søndergaard, R.R.; Krebs, F.C. Roll-to-Roll Inkjet Printing and Photonic Sintering of Electrodes for ITO Free Polymer Solar Cell Modules and Facile Product Integration. Adv. Energy Mater. 2013, 3, 172–175. [Google Scholar] [CrossRef]
- Jung, E.; Kim, C.; Kim, M.; Chae, H.; Cho, J.H.; Cho, S.M. Roll-to-roll preparation of silver-nanowire transparent electrode and its application to large-area organic light-emitting diodes. Org. Electron. 2017, 41, 190–197. [Google Scholar] [CrossRef]
- Shen, J.; Li, F.; Cao, Z.; Barat, D.; Tu, G. Light Scattering in Nanoparticle Doped Transparent Polyimide Substrates. ACS Appl. Mater. Interfaces 2017, 9, 14990–14997. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Meng, W.; Tong, J.; Zhao, C.; Qin, F.; Jiang, F.; Xiong, S.; Zeng, S.; Xu, L.; Hu, B. A nonionic surfactant simultaneously enhancing wetting property and electrical conductivity of PEDOT: PSS for vacuum-free organic solar cells. Sol. Energy Mater. Sol. C 2015, 137, 311–318. [Google Scholar] [CrossRef]
- Thomas, R.R. Quantification of ionizable functional groups on a hydrolyzed polyimide surface. Langmuir 1996, 12, 5247–5249. [Google Scholar] [CrossRef]
- Akamatsu, K.; Ikeda, S.; Nawafune, H.; Deki, S. Surface modification-based synthesis and microstructural tuning of nanocomposite layers: Monodispersed copper nanoparticles in polyimide resins. Chem. Mater. 2003, 15, 2488–2491. [Google Scholar] [CrossRef]
- Ikeda, S.; Akamatsu, K.; Nawafune, H.; Nishino, T.; Deki, S. Formation and growth of copper nanoparticles from ion-doped precursor polyimide layers. J. Phys. Chem. B 2004, 108, 15599–15607. [Google Scholar] [CrossRef]
- Xiong, S.; Tong, J.; Mao, L.; Li, Z.; Qin, F.; Jiang, F.; Meng, W.; Liu, T.; Li, W.; Zhou, Y. Double-side responsive polymer near-infrared photodetectors with transfer-printed electrode. J. Mater. Chem. C 2016, 4, 1414–1419. [Google Scholar] [CrossRef]
- Anger, P.; Bharadwaj, P.; Novotny, L. Enhancement and quenching of single-molecule fluorescence. Phys. Rev. Lett. 2006, 96, 113002. [Google Scholar] [CrossRef] [PubMed]
- Konda, R.B.; Mundle, R.; Mustafa, H.; Bamiduro, O.; Pradhan, A.K.; Roy, U.N.; Cui, Y.; Burger, A. Surface plasmon excitation via Au nanoparticles in n-CdSe/p-Si heterojunction diodes. Appl. Phys. Lett. 2007, 91, 191111. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.H.; Kim, J.S.; Lee, D.Y.; Cho, K. High efficiency polymer solar cells with wet deposited plasmonic gold nanodots. Org. Electron. 2009, 10, 416–420. [Google Scholar] [CrossRef]
- Wu, J.-L.; Chen, F.-C.; Hsiao, Y.-S.; Chien, F.-C.; Chen, P.; Kuo, C.-H.; Huang, M.H.; Hsu, C.-S. Surface plasmonic effects of metallic nanoparticles on the performance of polymer bulk heterojunction solar cells. ACS Nano 2011, 5, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Hu, Q.; Liu, T.; Zhao, L.; Luo, D.; Wu, J.; Zhang, Y.; Zhang, W.; Liu, F.; Russell, T.P.; et al. Charge-Carrier Balance for Highly Efficient Inverted Planar Heterojunction Perovskite Solar Cells. Adv. Mater. 2016, 28, 10718–10724. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Song, H.-J.; Lee, G.; Ko, Y.; Ahn, K.; Choi, H.; Kim, J.Y.; Ha, K.; Song, J.; Lee, J.-K. Plasmonic organic solar cells employing nanobump assembly via aerosol-derived nanoparticles. ACS Nano 2014, 8, 2590–2601. [Google Scholar] [CrossRef] [PubMed]

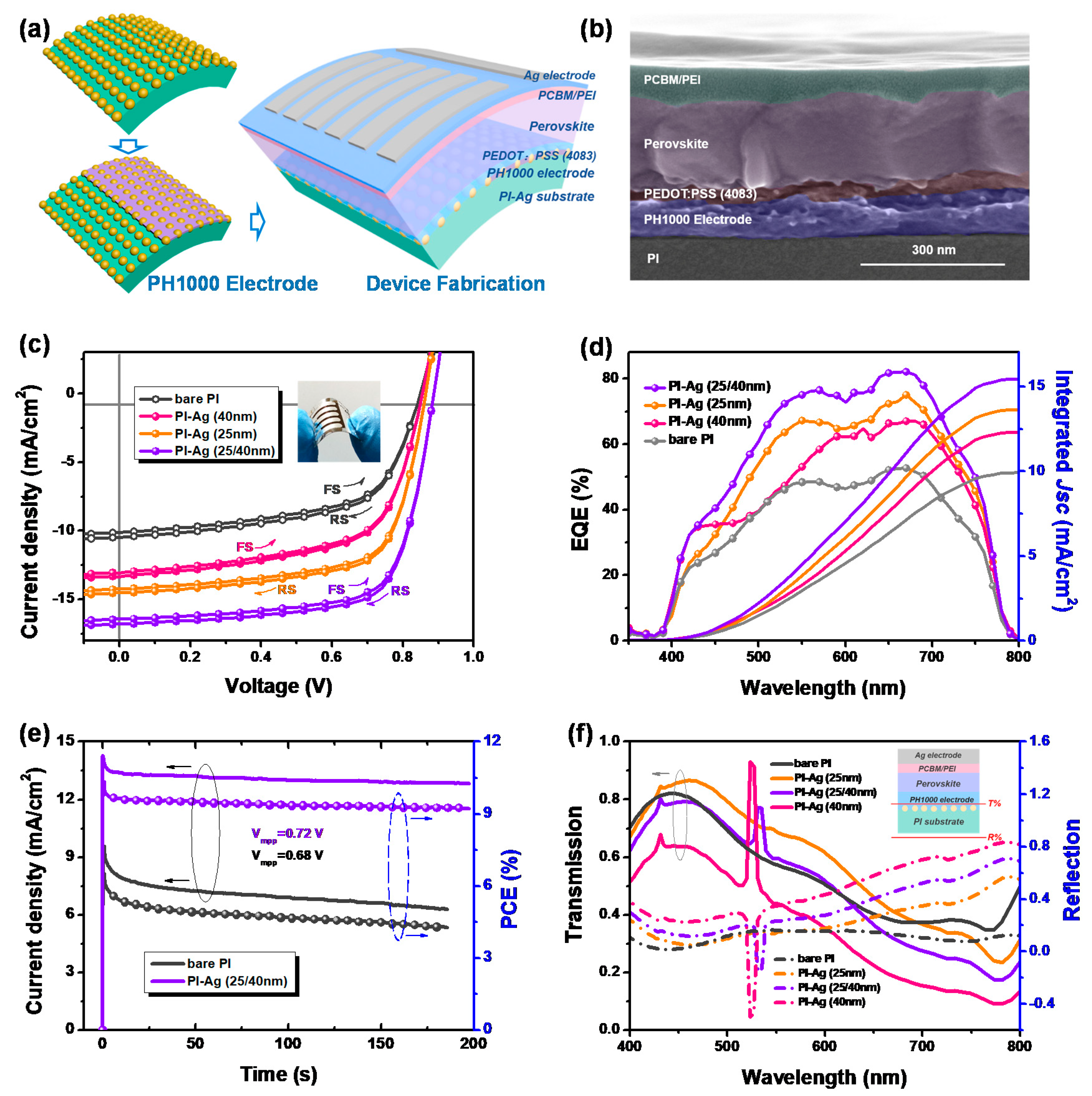
| Substrate | Scan direction | Jsc (mA/cm2) | Voc (V) | FF | PCE (%) |
|---|---|---|---|---|---|
| bare PI/PH1000 | RS | 10.44 ± 0.39 (10.56) | 0.82 ± 0.03 (0.84) | 0.60 ± 0.04 (0.61) | 5.13 ± 0.79 (5.41) |
| FS | 10.09 ± 0.40 (10.21) | 0.82 ± 0.03 (0.84) | 0.60 ± 0.04 (0.61) | 4.96 ± 0.81 (5.23) | |
| PI-Ag (40nm)/PH1000 | RS | 13.09 ± 0.35 (13.42) | 0.83 ± 0.03 (0.85) | 0.61 ± 0.03 (0.62) | 6.63 ± 0.78 (7.07) |
| FS | 12.97 ± 0.37 (13.15) | 0.83 ± 0.03 (0.85) | 0.61 ± 0.03 (0.62) | 6.57 ± 0.80 (6.93) | |
| PI-Ag (25nm)/PH1000 | RS | 14.37 ± 0.35 (14.65) | 0.85 ± 0.03 (0.86) | 0.67 ± 0.03 (0.68) | 8.18 ± 0.76 (8.56) |
| FS | 14.09 ± 0.36 (14.36) | 0.85 ± 0.03 (0.86) | 0.67 ± 0.03 (0.68) | 8.02 ± 0.78 (8.39) | |
| PI-Ag (25/40nm)/PH1000 | RS | 16.80 ± 0.28 (16.90) | 0.87 ± 0.03 (0.88) | 0.69 ± 0.03 (0.70) | 10.08 ± 0.75 (10.41) |
| FS | 16.34 ± 0.33 (16.54) | 0.87 ± 0.03 (0.88) | 0.69 ± 0.03 (0.70) | 9.80 ± 0.74 (10.18) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Hu, L.; Wang, R.; Li, J.; Gu, H.; Liu, S.; Zhou, Y.; Tu, G. Flexible Perovskite Solar Cells via Surface-Confined Silver Nanoparticles on Transparent Polyimide Substrates. Polymers 2019, 11, 427. https://doi.org/10.3390/polym11030427
Liu X, Hu L, Wang R, Li J, Gu H, Liu S, Zhou Y, Tu G. Flexible Perovskite Solar Cells via Surface-Confined Silver Nanoparticles on Transparent Polyimide Substrates. Polymers. 2019; 11(3):427. https://doi.org/10.3390/polym11030427
Chicago/Turabian StyleLiu, Xiangfu, Lin Hu, Rongwen Wang, Junli Li, Honggang Gu, Shiyuan Liu, Yinhua Zhou, and Guoli Tu. 2019. "Flexible Perovskite Solar Cells via Surface-Confined Silver Nanoparticles on Transparent Polyimide Substrates" Polymers 11, no. 3: 427. https://doi.org/10.3390/polym11030427
APA StyleLiu, X., Hu, L., Wang, R., Li, J., Gu, H., Liu, S., Zhou, Y., & Tu, G. (2019). Flexible Perovskite Solar Cells via Surface-Confined Silver Nanoparticles on Transparent Polyimide Substrates. Polymers, 11(3), 427. https://doi.org/10.3390/polym11030427