1. Introduction
Three-dimensional bioprinting (3DP), which has emerged as an innovative additive manufacturing technology [
1,
2,
3], is revolutionizing the field of tissue engineering and thus the future of medicine and medical implants. Similarly, novel biocompatible bio-inks (with or without a drug) is also equally transforming tissue engineering applications and can be used to fabricate complex geometries of personalized medical devices, e.g., scaffolds, providing novel platforms beyond the current state of the art. There is an opportunity for technological innovation in the fabrication of novel scaffolds or biomaterials using 3DP that requires a convergence of expertise in biomaterial, pharmaceutical, and vascular biological fields.
A major challenge for tissue engineering has been to mimic the micro and macro environment of human tissues via a widely used method to generate cell-seeded scaffolds both in anatomically complex geometries and intra-cellular architectures with controlled cell distribution. Studies have revealed that the critical characteristic of a biomaterial, as well as the control of the inner micro- and macro-scale features of the engineered-tissue, is considered a key quality parameter to fabricate complex anatomical, patient-specific structures with high shape fidelity in tissue engineering applications [
4,
5]. In response to this currently unmet need, advances in additive manufacturing and thus 3DP have inspired scientists to employ this innovative technology for biomaterial and tissue engineering strategies [
6,
7]. Bioprinting, in particular, has gained attention for its ability to control and deposit sequential layers of biomaterials, allowing the tailoring of a specific geometry to an object and permitting the placement of cells and biological molecules [
8,
9]. As a result, 3DP offers numerous possibilities for the future of tissue engineering and organ regeneration. Bioprinting can be combined with Computer-Aided Design (CAD) technology using patients’ medical images to allow the biofabrication of biomimetic-shaped 3D structures unique to the target tissue or organ in a personalized manner. Because of the challenges encountered when bioprinting, considerable improvements need to be made in order to bioprint complex constructs or cell-laden 3D tissue constructs by means of developing suitable biomaterials and bioink formulations with optimum properties such as viscosity for successful 3D (bio)printing [
10,
11]. 3D bioprinted scaffolds built for individual patients are favoured over customization of mass-produced products when meeting the specific needs of each patient. The benefits of its clinical application include easy adaptation and fixation, reduced surgical time, and favorable aesthetic results.
Coronary heart disease (CHD) is initiated when the cell lining of arteries (the endothelium) is injured. Endothelial regrowth appears to be an important process limiting CHD. When cells are injured, they activate an inflammatory reaction which induces the expression of the innate repair receptor (IRR), which activates tissue protection and repair [
12]. Despite the early and strong expression of IRR within injured tissues, local production of tissue protective cytokines (TPCs) such as erythropoietin (EPO) is delayed, transient and relatively weak [
13]. This provides an opportunity to intervene with exogenous TPCs that act as innate repair activators, targeting the fundamental processes of tissue injury at a level that controls both the self-damaging and the regenerative components, representing a promising therapeutic approach [
14]. EPO has been shown to be tissue-protective in models of ischaemic, traumatic and inflammatory injury [
15]. Hypoxia enhances the reparative response of ECs to EPO [
16,
17]. This is likely to be mediated by hypoxia inducible factor (HIF)-1. Dimethyloxalylglycine (DMOG) is a HIF-1α inducer and mimics conditions similar to hypoxia [
16,
17,
18,
19,
20].
The use of biomaterials such as biocompatible or biodegradable copolymer (e.g., polylactic acid, pluronic F127 [
21]), is a common strategy for reducing the risk of thrombosis and restenosis. Blood compatibility remains a major issue and several surface modifications have been used to mitigate this problem. We aimed to use a novel bio-therapeutic material, with intrinsic tissue protective activity, to fabricate a novel scaffold by means of an optimized 3D bio-printing technology that can promote EC repair and may offer a promising alternative therapy to existing biomaterials such as polylactic acid (PLA) and pluronic. Among other biomaterials, both polylactic acid (PLA) and pluronic (F127) biopolymers have been used as a suitable polymeric carrier for the development of bioinks because of its superior biocompatibility and printing fidelity [
21]. We also wished to apply this 3DP technology to prevent restenosis, based on triggering the endogenous repair mechanisms of the endothelial cells of the artery wall.
2. Materials and Methods
2.1. Materials
Polylactic acid (PLA) MW 60,000, polyethylene glycol (PEG) MW 400 were purchased from Sigma Aldrich (Gillingham, UK). Pluronic F127 (F127)-based biomaterials were purchased from SE3D (Santa Clara, CA, USA). Dimethyloxalylglycine (DMOG) was purchased from Sigma Aldrich (Dorset, UK) and Erythropoietin (EPO) was purchased from Araim Pharmaceuticals (New York, NY, USA). All materials required for cell culture assessment and analytical studies were purchased from Sigma Aldrich (Dorset, UK) unless otherwise stated. All solvent and chemical were of analytical grade and used as received.
2.2. Preparation of Bioinks Containing EPO and DMOG
The biomaterial matrix was prepared from a mixture of poly (lactic acid) (PLA) and Polyethylene glycol 400 (PEG) and Pluronic F127 (F127) in different concentrations as shown in
Table 1. Briefly, PLA was prepared as a 15% solution by dissolving the PLA pellets in chloroform. PEG was then dissolved in the PLA solution. PLA: PEG matrix was prepared in the following concentrations: 7:0, 6:1 and 5:2
w/
w ratios. A wide range of inks was developed and only the best four (for PLA/PEG only 5:2
w/
w ratio was selected) were used for this part of the study (
Table 1). Model drugs, i.e., EPO and/or DMOG, were loaded on the biomaterial matrix (bioink) to reach a final concentration of 20%–30% (
w/
w).
2.3. Rheology Measurement of the Inks
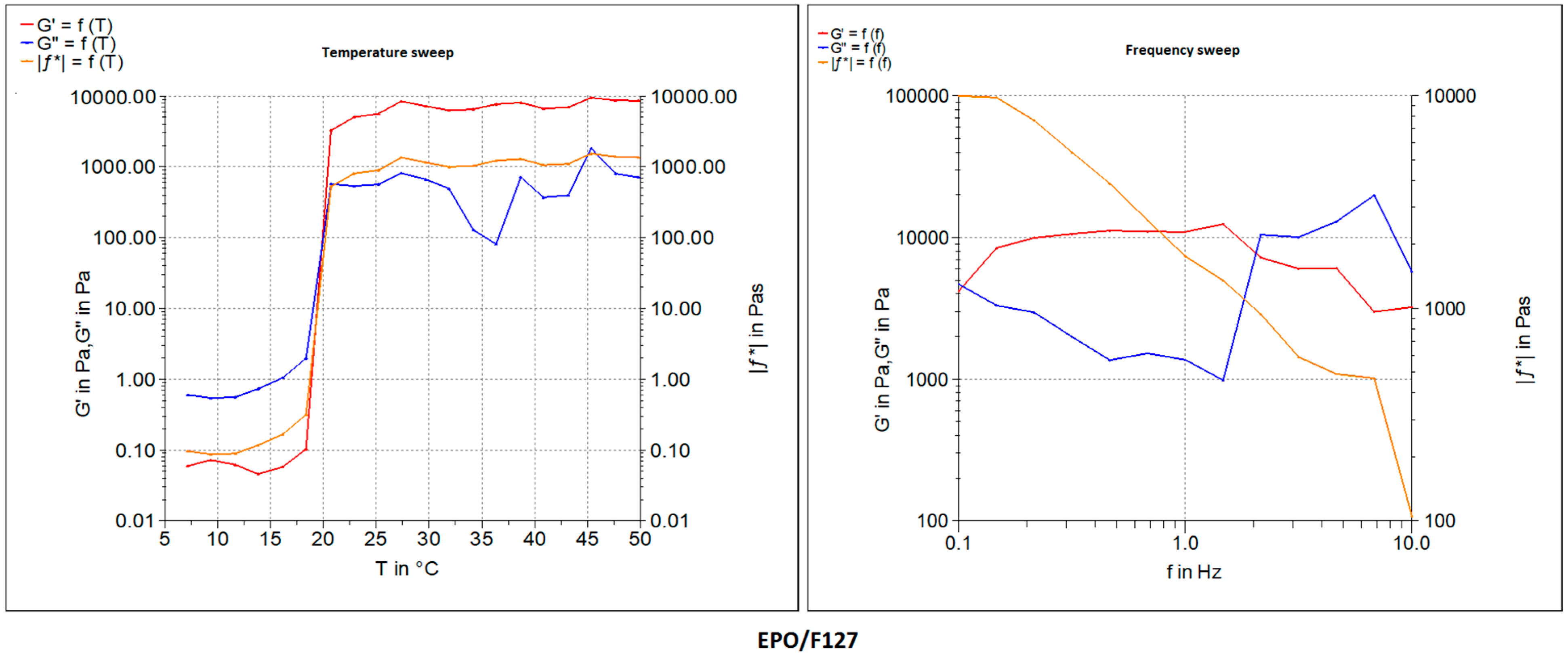
Once optimized, the ink formulations were subject to a rheology measurement study. The viscosity of the inks was measured both at constant and increasing shear rates using a plate rheometer (RheoStress
® RS 1, Karlsruhe, Germany) with a plate–plate distance of 0.052 mm. The viscosity of the pastes was determined by applying a constant shear rate of 10 s
−1 for 500 s. After an initial amplitude sweep test to detect the viscoelastic region, oscillatory frequency sweep tests (
f = 0.01–10 s
−1; 1.0 Hz) were performed at 25 °C on all ink formulations as shown in
Table 1.
2.4. Extrudability/Injectability of the Inks Developed for Bioprinting
Injectability of the bioink formulations is an important factor to consider in order to ensure the required dose is delivered effectively and more precisely, and with ease. In general terms, the force which is applied to a syringe plunger during the injection of a formulation via a needle is classified in three ways: stiction, overcoming the resistance force of the syringe plunger; plateau force, energy that accumulates as the formulation glides through the needle under a constant force and finally end constraint force. These three types of forces were recorded manually and were classified as ‘pass’ or ‘fail’, based on whether they did or didn′t expel the formulation steadily out of the syringe, respectively. Based on the preliminary observation and the analysis, only those 4 formulations (
Table 1) were used for 3D bioprinting.
2.5. 3D Bioprinting of Scaffolds
An optimized 3D bioprinting platform (
Figure 1) was used to fabricate all macro-porous scaffolds with a diameter of 10 mm. The thickness of all developed scaffolds was set at ~1–3 mm as a default in order to avoid any possible effect of the varying thickness of the scaffolds on the actual release of the drug. The adopted printing process was later applied for the printing of different scaffolds with various geometries and intricacies of the constructs. The print resolution was set at 100 µm and the construct was directly printed (via r3bEL mini bioprinter, SE3D, Santa Clara, CA, USA) in a petri dish placed on the print bed in ambient temperature (23 ± 1 °C). The various developed viscous printing inks were drawn into a 22-gauge printing syringe with an internal diameter of ~640 µm from which the inks were extruded at a speed of ~100 mm/min to print the constructs. Computer-Aided Design (CAD) was utilized to develop the design of the scaffolds with the required geometry (rectangular pores). Once the fabrication process was optimized, all printed scaffolds were immediately removed from the print bed and the petri dish was stored at 37 °C in an incubator for 24 h to cure the 3D printed scaffolds prior to utilizing it for further analysis.
2.6. Surface Morphology of the Inks and Scaffolds
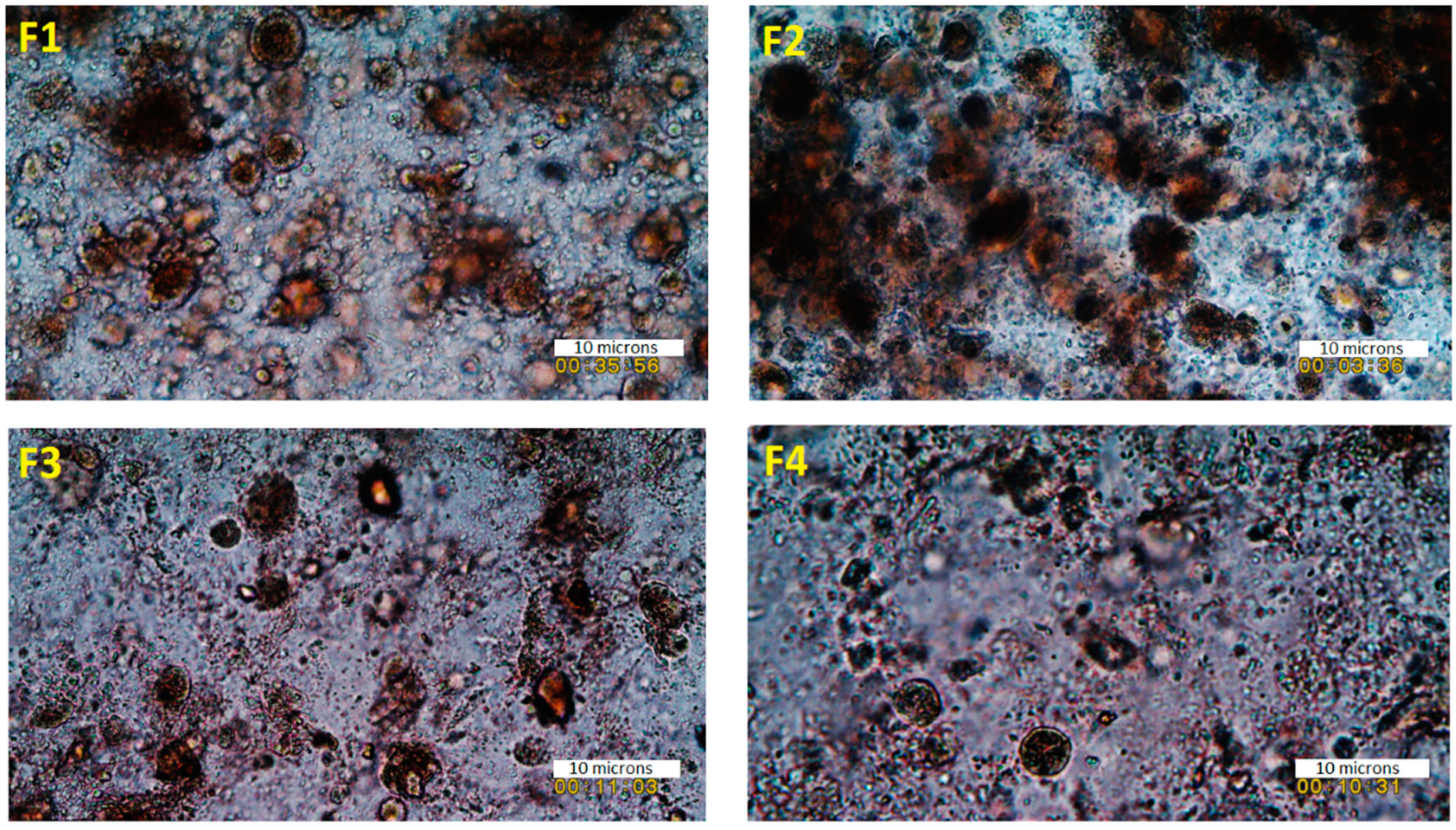
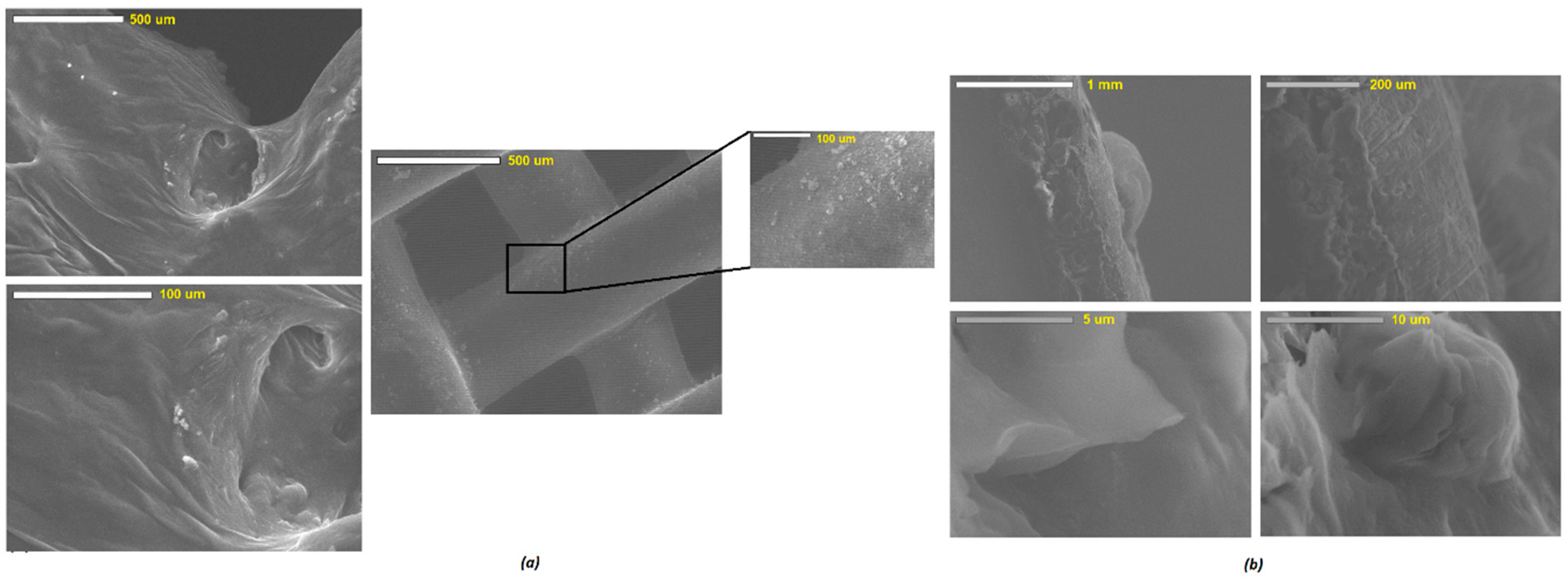
The surface morphology of the fabricated scaffolds was studied using a scanning electron microscope (Jeol JMS 820, Freising, Germany). The samples were placed on a double-sided carbon tape and sputter-coated with gold using a sputter coater (Edwards S-150 sputter coater, Edwards High Vacuum Co. International, Sanborn, NY, USA). After the samples were sputter-coated, they were placed into the SEM where the surface structure was then observed and recorded at various magnifications using the SEM operating at 3 kV. An optical microscope (JEOL JEM1400-Plus, 120 kV, LaB6, Peabody, MA, USA) was also utilized to investigate the surface of the viscous inks as well as scaffolds to visualize the texture of the ink formulations prior to the 3D printing and the surface of the developed scaffolds to determine the distribution of the deposited substances on the surface of the scaffolds.
2.7. Mechanical Properties of the Scaffolds Developed
Uniaxial compressive tests were applied to scaffolds and Young′s modulus and compressive strength were obtained from the data. For the purpose of this study, the scaffold strips (3–5 mm in length) were attached to a 75-mm-diameter adhesive rig probe with a double-sided adhesive tape on a TA.HD.plus Texture Analyser (Stable Micro Systems, Surrey, UK) fitted with a 5-kg load cell in compression mode. The probe, lined with the scaffolds, was lowered towards the surface at a pretest speed of 0.5 mm/s, test speed of 0.5 mm/s and post speed of 1.00 mm/s. The maximum force required to penetrate the scaffolds was determined. The mechanical analysis was conducted at room temperature (23 ± 1 °C) and run in triplicate (n = 3).
2.8. Thermal Analysis
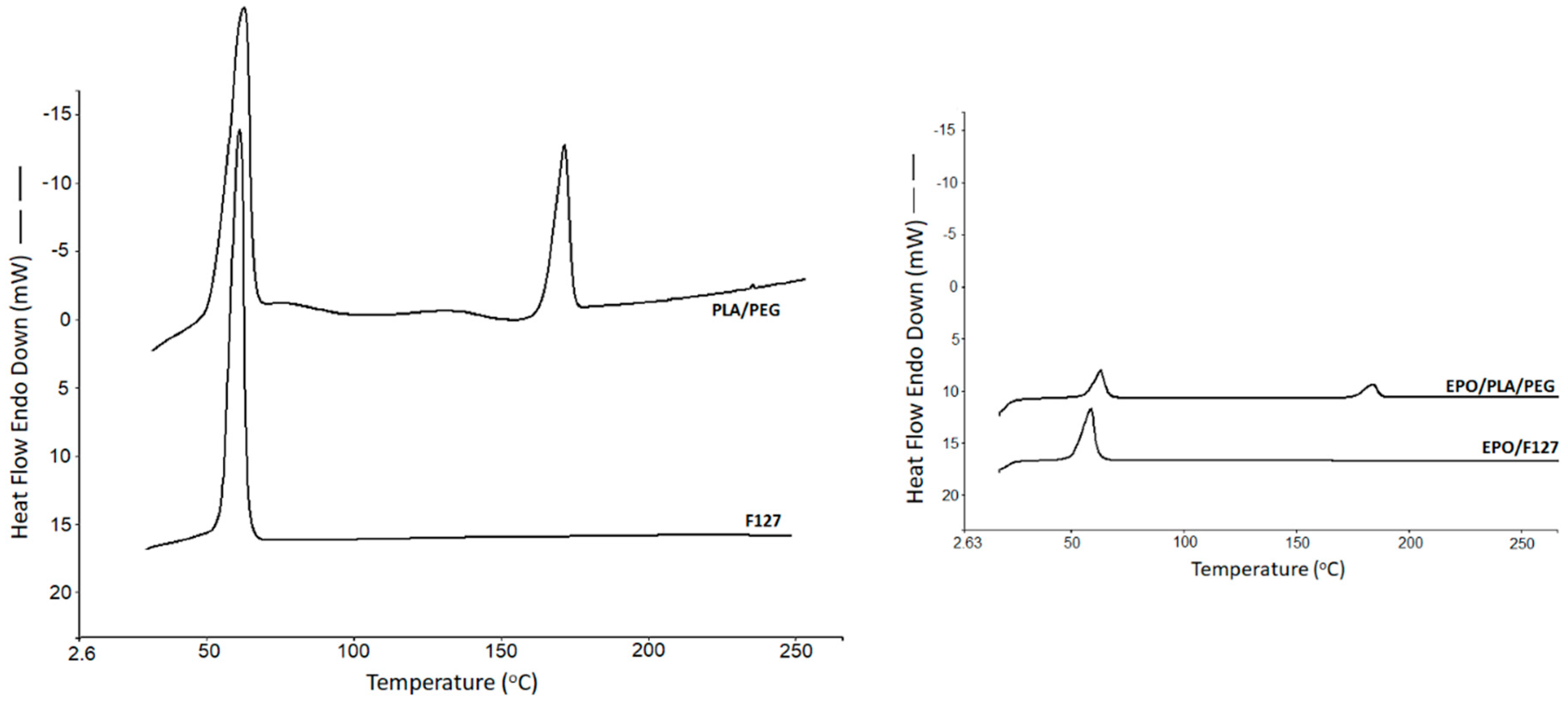
Solid state of the drugs was analyzed via differential scanning calorimetry (DSC) (DCS 4000, Perkin Elmer, Waltham, MA, USA). The study was performed on the chosen bioink formulations, which were utilized for the development of the scaffolds. The crystallinity of the substances used in the formulations was examined using data presented in each of the DSC traces. Samples weighing between 3 and 6 mg were sealed in an aluminium pan and placed in the DSC machine with a scanning rate of 10 °C/min (from 25 to 265 °C) under nitrogen atmosphere.
2.9. Drug Release Study from the Scaffold
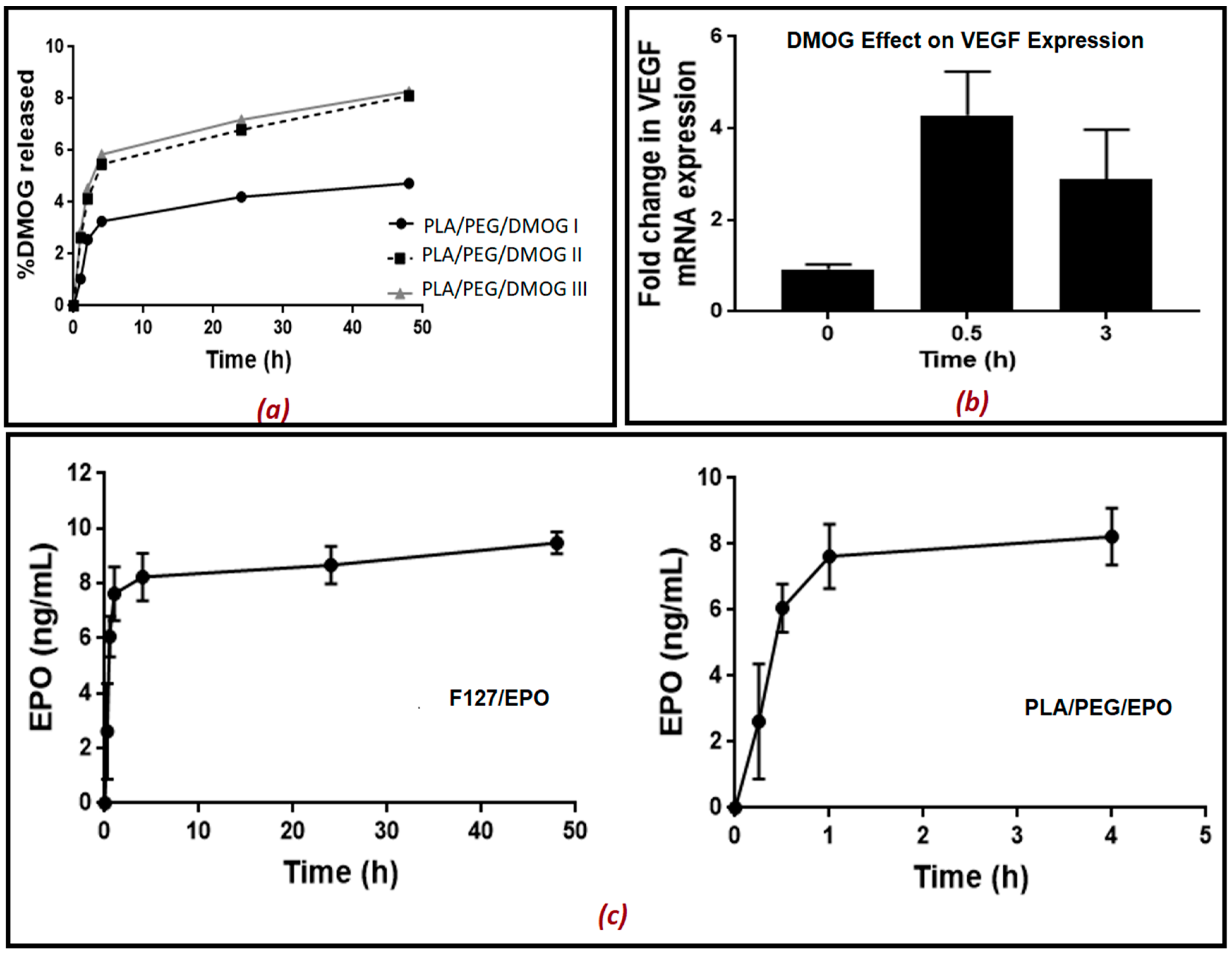
The scaffolds were placed into PBS (500 µL). The PBS containing the released drug was removed at different time intervals (5, 15, 30 min, 1, 2, 4, 24 and 48 h) and replaced with fresh PBS each time. The aliquots removed were kept at −20 °C until further analysis. Rat aortic endothelial cells (RAECs) were seeded into 24-well plates and cultured until approximately 80% confluency in 21% O2. 100 µL of the aliquots with the released drug at different time intervals was added on RAECs and left for 2 h. As DMOG is a HIF-1α inducer, its release from the scaffold into PBS solution was quantitatively measured indirectly using a bioassay for measuring HIF-1α. In another set of experiments, DMOG was measured indirectly by measuring the gene expression of VEGF using real-time qPCR. EPO release from the scaffold in PBS solution was also quantitatively measured directly using an ELISA kit for EPO.
2.10. Real-Time qPCR
Treated cells were lysed using TRIzol (Invitrogen/ Life Technologies, Dartford, UK) and RNA was extracted and purified as described previously [
22]. RNA quality and concentration were determined using a NanoDrop ND-1000 (NanoDrop Technologies). Reverse transcription and real-time quantitative PCR (qPCR) for VEGF and β2-microglobulin (a housekeeping gene), were carried out on RNA samples using Taqman gene expression assays (Applied Biosystems/Life Technologies, Dartford, UK) as previously reported [
23]. For gene expression quantification, the comparative threshold cycle (ΔΔCt) method was used following Applied Biosystems/Life Technologies′ guidelines. Results were normalized to β2-microglobulin expression and expressed as arbitrary units using one of the untreated samples as a calibrator as specified in the figure legend.
2.11. HIF-1α Enzyme-Linked Immunosorbent Assay (ELISA)
HIF-1α was measured using a commercial ELISA kit (R&D systems/ Biotechne, UK) following the manufacturers′ instructions. Endothelial cells were lysed in 80 μL lysis buffer (25 mmol/L Tris HCl pH 7.6, 0.1% SDS, 1% deoxycholate, 1% NP40, 0.5 mol/L EDTA, 40 mmol/L EGTA and protease inhibitors). Lysates were then centrifuged at 11,000× g for 15 min at 4 °C and the supernatant was collected. Protein concentrations were quantified using a BCA reagent kit (Pierce Biotechnology, Rockford, IL, USA) Results are expressed as pg/mg protein.
2.12. Statistical Analysis
All values were evaluated by one-way analysis of variance followed by Bonferroni′s multiple comparison tests (GraphPad Prism 7). Significant differences were assumed at p < 0.05.
4. Conclusions
The optimized bioink formulations represent an intriguing alternative for 3D bioprinting materials when loaded with drugs. In this paper, we successfully exploited the use of emerging 3D bioprinting techniques for the development and optimization of novel bioinks of biomaterials like F127 and PLA. As a result, a novel composite bioink from PLA/PEG has been synthesized and characterized. All developed bioinks exhibited excellent printability and bioink properties indicated by printing/plotting fidelity. Moreover, the 3D printed constructs showed homogenous distribution of the drugs in the scaffolds without compromising the mechanical and thermal properties of the scaffolds. Further experiments are ongoing to confirm the final interaction between the components used in the formulations and further optimise it for the higher release of the drugs from the scaffolds. Moreover, the bio-functional benefits of the materials used in the formulations were evidenced by the sustained release of the model drugs and VEGF. The current studies demonstrate the exciting potential of our developed semi-solid formulations as a robust, and reliable bioink for 3D printing in biomedical applications.