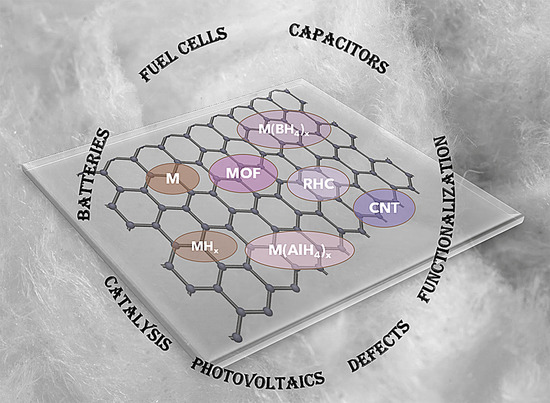
Graphene Supports for Metal Hydride and Energy Storage Applications
Abstract
1. Introduction
2. Overview of Metal Hydrides and Graphene Supports
2.1. Metal-Decorated Graphene
2.2. Mechanistic Insight and Kinetics of H2…Support Interaction
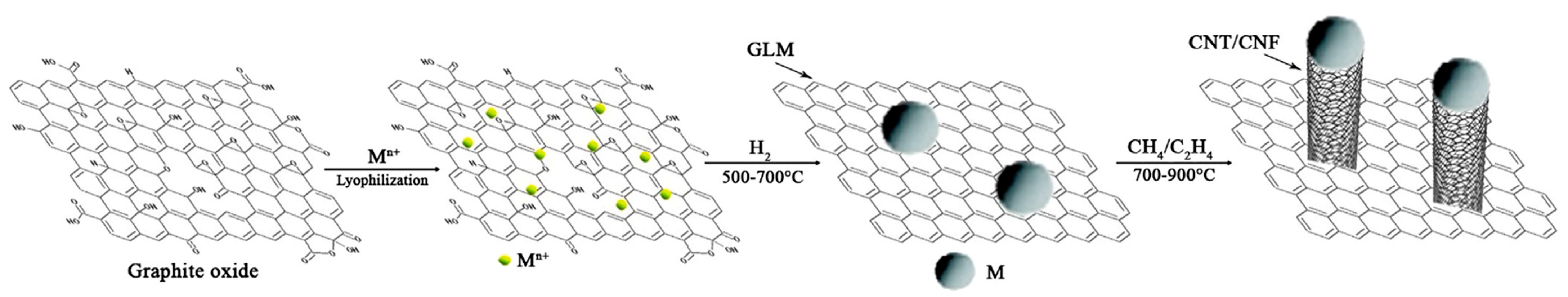
2.3. Manufacturing Techniques
3. Applications of Graphene-Based Hydride Nanocomposites
3.1. Batteries—Battery Electrodes
3.2. (Super)capacitors, Electrochemical Storage
3.3. Solar Cells and Portable Electronic Devices
3.4. Energy Storage
4. Hydrogen Storage Properties of Composites MHx@G
4.1. Binary Hydrides—The Case of MgH2
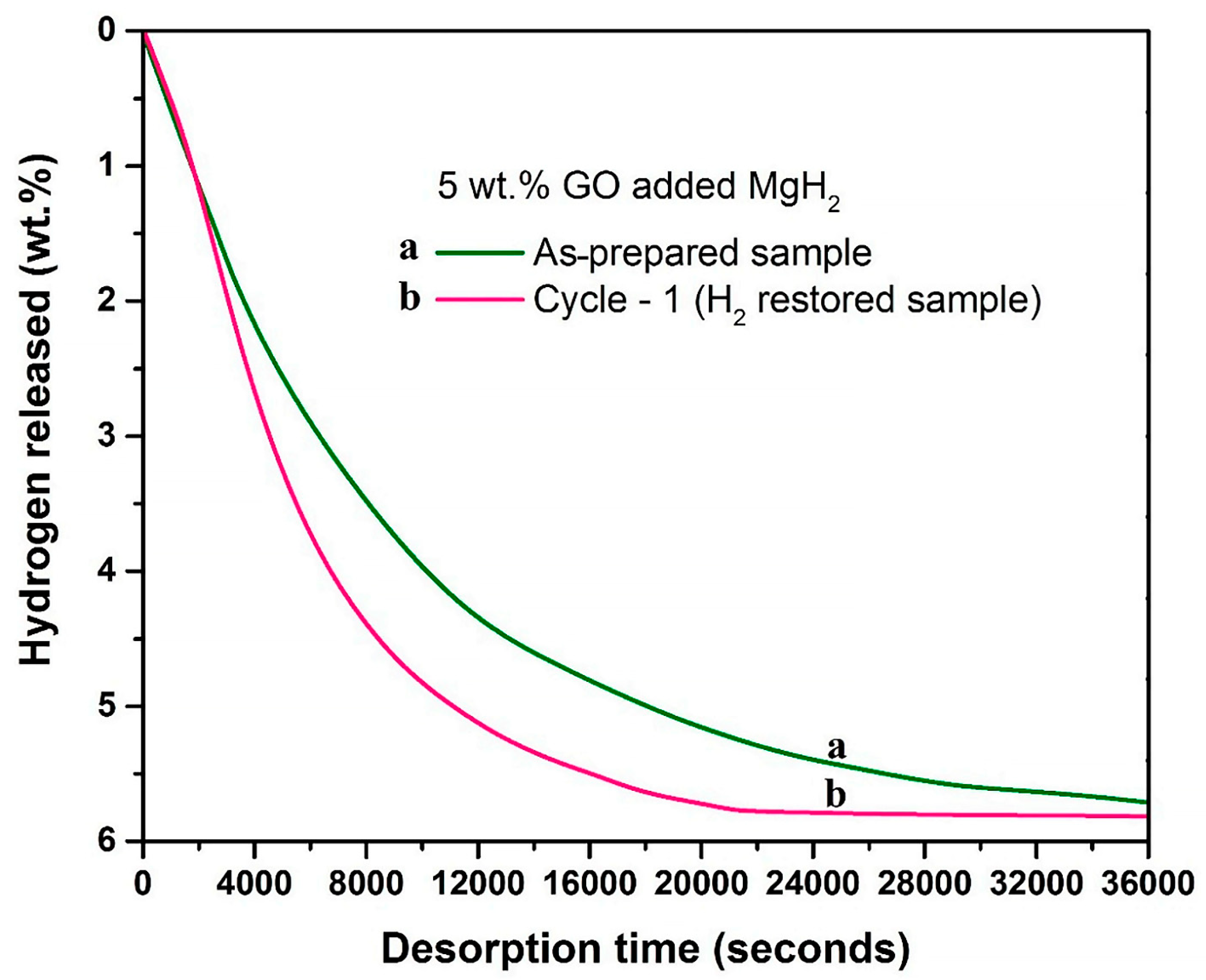
4.1.1. Non-Catalyzed Support
4.1.2. Catalyzed Support
4.2. Complex Hydrides Embedded in Graphene Supports: M(BH4)n, M(AlH4)n
4.3. Reactive Hydride Composites (RHCs) in G Hosts
4.4. Other Hydride Systems Confined in 2D-Graphene
5. Conclusions
6. Current Challenges and Future Outlook
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Yan, S.; Qu, H. Recent progress in magnesium hydride modified through catalysis and nanoconfinement. Int. J. Hydrogen Energy 2018, 43, 1545–1565. [Google Scholar] [CrossRef]
- Comanescu, C. Recent Development in Nanoconfined Hydrides for Energy Storage. Int. J. Mol. Sci. 2022, 23, 7111. [Google Scholar] [CrossRef] [PubMed]
- Sreedhar, I.; Kamani, K.M.; Kamani, B.M.; Reddy, B.M.; Venugopal, A. A Bird’s Eye view on process and engineering aspects of hydrogen storage. Renew. Sustain. Energy Rev. 2018, 91, 838–860. [Google Scholar] [CrossRef]
- So, S.H.; Sung, S.J.; Yang, S.J.; Park, C.R. Where to go for the Development of High-Performance H-2 Storage Materials at Ambient Conditions? Electron. Mater. Lett. 2023, 19, 1–18. [Google Scholar] [CrossRef]
- Comanescu, C. Paving the Way to the Fuel of the Future—Nanostructured Complex Hydrides. Int. J. Mol. Sci. 2023, 24, 143. [Google Scholar] [CrossRef]
- Comanescu, C. Complex Metal Borohydrides: From Laboratory Oddities to Prime Candidates in Energy Storage Applications. Materials 2022, 15, 2286. [Google Scholar] [CrossRef]
- Ding, F.; Yakobson, B.I. Challenges in hydrogen adsorptions: From physisorption to chemisorption. Front. Phys. 2011, 6, 142–150. [Google Scholar] [CrossRef]
- Feng, D.C.; Zhou, D.S.; Zhao, Z.Y.; Zhai, T.T.; Yuan, Z.M.; Sun, H.; Ren, H.P.; Zhang, Y.H. Progress of graphene and loaded transition metals on Mg-based hydrogen storage alloys. Int. J. Hydrogen Energy 2021, 46, 33468–33485. [Google Scholar] [CrossRef]
- Free, Z.; Hernandez, M.; Mashal, M.; Mondal, K. A Review on Advanced Manufacturing for Hydrogen Storage Applications. Energies 2021, 14, 8513. [Google Scholar] [CrossRef]
- Boateng, E.; Chen, A.C. Recent advances in nanomaterial-based solid-state hydrogen storage. Mater. Today Adv. 2020, 6, 100022. [Google Scholar] [CrossRef]
- Jawhari, A.H. Novel Nanomaterials for Hydrogen Production and Storage: Evaluating the Futurity of Graphene/Graphene Composites in Hydrogen Energy. Energies 2022, 15, 9085. [Google Scholar] [CrossRef]
- Arbuzov, A.A.; Mozhzhukhin, S.A.; Volodin, A.A.; Fursikov, P.V.; Tarasov, B.P. Graphene-like nanostructures: Synthesis and use for preparation of catalysts and hydrogen storage composites. Russ. Chem. B+ 2016, 65, 1893–1901. [Google Scholar] [CrossRef]
- Xia, G.; Zhang, B.; Chen, X.; Sun, D.; Guo, Z.; Liang, F.; Yu, X. Graphene for Energy Storage and Conversion: Synthesis and Interdisciplinary Applications. Electrochem. Energy Rev. 2020, 3, 395–430. [Google Scholar] [CrossRef]
- Liu, W.; Setijadi, E.; Crema, L.; Bartali, R.; Laidani, N.; Aguey-Zinsou, K.F.; Speranza, G. Carbon nanostructures/Mg hybrid materials for hydrogen storage. Diam. Relat. Mater. 2018, 82, 19–24. [Google Scholar] [CrossRef]
- Yao, Q.L.; Ding, Y.Y.; Lu, Z.H. Noble-metal-free nanocatalysts for hydrogen generation from boron- and nitrogen-based hydrides. Inorg. Chem. Front. 2020, 7, 3837–3874. [Google Scholar] [CrossRef]
- Tarasov, B.P.; Arbuzov, A.A.; Volodin, A.A.; Fursikov, P.V.; Mozhzhuhin, S.A.; Lototskyy, M.V.; Yartys, V.A. Metal hydride–Graphene composites for hydrogen based Energy storage. J. Alloys Compd. 2022, 896, 162881. [Google Scholar] [CrossRef]
- Aditya, M.V.V.S.; Panda, S.; Tatiparti, S.S.V. Boron from net charge acceptor to donor and its effect on hydrogen uptake by novel Mg-B-electrochemically synthesized reduced graphene oxide. Sci. Rep. 2021, 11, 10995. [Google Scholar] [CrossRef]
- Tan, X.; Tahini, H.A.; Smith, S.C. Conductive Boron-Doped Graphene as an Ideal Material for Electrocatalytically Switchable and High-Capacity Hydrogen Storage. ACS Appl. Mater. Interfaces 2016, 8, 32815–32822. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Guan, C.; He, Z.M.; Lu, Z.S.; Chen, T.; Liu, J.; Tan, X.; Tan, T.T.Y.; Li, C.M. Surface functionalization-enhanced spillover effect on hydrogen storage of Ni-B nanoalloy-doped activated carbon. Int. J. Hydrogen Energy 2011, 36, 13663–13668. [Google Scholar] [CrossRef]
- Wan, L.F.; Cho, E.S.; Marangoni, T.; Shea, P.; Kang, S.Y.; Rogers, C.; Zaia, E.; Cloke, R.R.; Wood, B.C.; Fischer, F.R.; et al. EdgeFunctionalized Graphene Nanoribbon Encapsulation to Enhance Stability and Control Kinetics of Hydrogen Storage Materials. Chem. Mater. 2019, 31, 2960–2970. [Google Scholar] [CrossRef]
- Asefa, T.; Koh, K.; Yoon, C.W. CO2-Mediated H-2 Storage-Release with Nanostructured Catalysts: Recent Progresses, Challenges, and Perspectives. Adv. Energy Mater. 2019, 9, 1158. [Google Scholar] [CrossRef]
- Shevlin, S.A.; Guo, Z.X. Density functional theory simulations of complex hydride and carbon-based hydrogen storage materials. Chem. Soc. Rev. 2009, 38, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.R.; Hussain, T.; Luo, W.; Ahuja, R. Metallized siligraphene nanosheets (SiC7) as high capacity hydrogen storage materials. Nano Res. 2018, 11, 3802–3813. [Google Scholar] [CrossRef]
- Aydin, S.; Simsek, M. The enhancement of hydrogen storage capacity in Li, Na and Mg-decorated BC3 graphene by CLICH and RICH algorithms. Int. J. Hydrogen Energy 2019, 44, 7354–7370. [Google Scholar] [CrossRef]
- Tokarev, A.; Avdeenkov, A.V.; Langmi, H.; Bessarabov, D.G. Modeling hydrogen storage in boron-substituted graphene decorated with potassium metal atoms. Int. J. Energy Res. 2015, 39, 524–528. [Google Scholar] [CrossRef]
- Ao, Z.M.; Dou, S.X.; Xu, Z.M.; Jiang, Q.G.; Wang, G.X. Hydrogen storage in porous graphene with Al decoration. Int. J. Hydrogen Energy 2014, 39, 16244–16251. [Google Scholar] [CrossRef]
- Costanzo, F.; van Hemert, M.C.; Kroes, G.J. Promoting Effect of Carbon Surfaces on H-2 Dissociation on Al-n Clusters by First Principles Calculations. J. Phys. Chem. C 2014, 118, 513–522. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, H.; Tian, W.Z.; Liu, T.; Wang, Y.Z. Effect of hydrogen adsorption Energy on the electronic and optical properties of Si-modified single-layer graphene with an Al decoration. AIP Adv. 2020, 10, 045012. [Google Scholar] [CrossRef]
- Bakhshi, F.; Farhadian, N. Improvement of hydrogen storage capacity on the palladium-decorated N-doped graphene sheets as a novel adsorbent: A hybrid MD-GCMC simulation study. Int. J. Hydrogen Energy 2019, 44, 13655–13665. [Google Scholar] [CrossRef]
- Ramos-Castillo, C.M.; Reveles, J.U.; Zope, R.R.; de Coss, R. Palladium Clusters Supported on Graphene Monovacancies for Hydrogen Storage. J. Phys. Chem. C 2015, 119, 8402–8409. [Google Scholar] [CrossRef]
- Choudhary, A.; Malakkal, L.; Siripurapu, R.K.; Szpunar, B.; Szpunar, J. First principles calculations of hydrogen storage on Cu and Pd-decorated graphene. Int. J. Hydrogen Energy 2016, 41, 17652–17656. [Google Scholar] [CrossRef]
- Bora, P.L.; Ahmad, R.; Singh, A.K. Remarkable enhancement in hydrogen storage on free-standing Ti3B and BC3 supported Ti-3 clusters. Int. J. Hydrogen Energy 2015, 40, 1054–1061. [Google Scholar] [CrossRef]
- Intayot, R.; Rungnim, C.; Namuangruk, S.; Yodsin, N.; Jungsuttiwong, S. Ti-4-Decorated B/N-doped graphene as a high-capacity hydrogen storage material: A DFT study. Dalton Trans. 2021, 50, 11398–11411. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Castillo, C.M.; Reveles, J.U.; Cifuentes-Quintal, M.E.; Zope, R.R.; de Coss, R. Ti-4- and Ni-4-Doped Defective Graphene Nanoplatelets as Efficient Materials for Hydrogen Storage. J. Phys. Chem. C 2016, 120, 5001–5009. [Google Scholar] [CrossRef]
- Ramos-Castillo, C.M.; Reveles, J.U.; Cifuentes-Quintal, M.E.; Zope, R.R.; de Coss, R. Hydrogen storage in bimetallic Ti-Al sub-nanoclusters supported on graphene. Phys. Chem. Chem. Phys. 2017, 19, 21174–21184. [Google Scholar] [CrossRef]
- Bartali, R.; Speranza, G.; Aguey-Zinsou, K.F.; Testi, M.; Micheli, V.; Canteri, R.; Fedrizzi, M.; Gottardi, G.; Coser, G.; Crema, L.; et al. Efficient hydrogen generation from water using nanocomposite flakes based on graphene and magnesium. Sustain. Energy Fuels 2018, 2, 2516–2525. [Google Scholar] [CrossRef]
- Bouaricha, S.; Dodelet, J.P.; Guay, D.; Huot, J.; Schulz, R. Study of the activation process of Mg-based hydrogen storage materials modified by graphite and other carbonaceous compounds. J. Mater. Res. 2001, 16, 2893–2905. [Google Scholar] [CrossRef]
- Cho, E.S.; Ruminski, A.M.; Liu, Y.S.; Shea, P.T.; Kang, S.Y.; Zaia, E.W.; Park, J.Y.; Chuang, Y.D.; Yuk, J.M.; Zhou, X.W.; et al. Hierarchically Controlled Inside-Out Doping of Mg Nanocomposites for Moderate Temperature Hydrogen Storage. Adv. Funct. Mater. 2017, 27, 1704316. [Google Scholar] [CrossRef]
- Dun, C.; Jeong, S.; Kwon, D.H.; Kang, S.; Stavila, V.; Zhang, Z.L.; Lee, J.W.; Mattox, T.M.; Heo, T.W.; Wood, B.C.; et al. Hydrogen Storage Performance of Preferentially Oriented Mg/rGO Hybrids. Chem. Mater. 2022, 34, 2963–2971. [Google Scholar] [CrossRef]
- Cho, Y.; Kang, S.; Wood, B.C.; Cho, E.S. Heteroatom-Doped Graphenes as Actively Interacting 2D Encapsulation Media for Mg-Based Hydrogen Storage. ACS Appl. Mater. Interfaces 2022, 14, 20823–20834. [Google Scholar] [CrossRef]
- Dong, S.; Lv, E.F.; Wang, J.H.; Li, C.Q.; Ma, K.; Gao, Z.Y.; Yang, W.J.; Ding, Z.; Wu, C.C.; Gates, I.D. Construction of transition metal-decorated boron doped twin-graphene for hydrogen storage: A theoretical prediction. Fuel 2021, 304, 121351. [Google Scholar] [CrossRef]
- Han, D.J.; Kim, S.; Cho, E.S. Revealing the role of defects in graphene oxide in the evolution of magnesium nanocrystals and the resulting effects on hydrogen storage. J. Mater. Chem. A 2021, 9, 9875–9881. [Google Scholar] [CrossRef]
- Du, J.Q.; Lan, Z.Q.; Zhang, H.; Lu, S.X.; Liu, H.Z.; Guo, J. Catalytic enhanced hydrogen storage properties of Mg-based alloy by the addition of reduced graphene oxide supported V2O3 nanocomposite. J. Alloys Compd. 2019, 802, 660–667. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Gupta, B.K.; Tripathi, P.; Veziroglu, A.; Hudson, M.S.L.; Abu Shaz, M.; Srivastava, O.N. Development and Demonstration of Air Stable rGO-EC@AB(5) Type Hydrogenated Intermetallic Hybrid for Hydrogen Fuelled Devices. Adv. Sustain. Syst. 2017, 1, 1700087. [Google Scholar] [CrossRef]
- Cui, R.C.; Yang, C.C.; Li, M.M.; Jin, B.; Ding, X.D.; Jiang, Q. Enhanced high-rate performance of ball-milled MmNi(3.55)Co(0.75)Mn(0.4)Al(0.3) hydrogen storage alloys with graphene nanoplatelets. J. Alloys Compd. 2017, 693, 126–131. [Google Scholar] [CrossRef]
- Morse, J.R.; Zugell, D.A.; Patterson, E.; Baldwin, J.W.; Willauer, H.D. Hydrogenated graphene: Important material properties regarding its application for hydrogen storage. J. Power Sources 2021, 494, 229734. [Google Scholar] [CrossRef]
- Wu, X.J.; Fei, Z.J.; Liu, W.G.; Tan, J.; Wang, G.H.; Xia, D.Q.; Deng, K.; Chen, X.K.; Xiao, D.T.; Wu, S.W.; et al. Adsorption and desorption of hydrogen on/from single-vacancy and double-vacancy graphenes. Nucl. Sci. Tech. 2019, 30, 69. [Google Scholar] [CrossRef]
- Akilan, R.; Vinnarasi, S.; Mohanapriya, S.; Shankar, R. Adsorption of H(2)molecules on B/N-doped defected graphene sheets—A DFT study. Struct. Chem. 2020, 31, 2413–2434. [Google Scholar] [CrossRef]
- Lotfi, R.; Saboohi, Y. A comparative study on hydrogen interaction with defective graphene structures doped by transition metals. Phys. E 2014, 60, 104–111. [Google Scholar] [CrossRef]
- Lototskyy, M.; Sibanyoni, J.M.; Denys, R.V.; Williams, M.; Pollet, B.G.; Yartys, V.A. Magnesium-carbon hydrogen storage hybrid materials produced by reactive ball milling in hydrogen. Carbon 2013, 57, 146–160. [Google Scholar] [CrossRef]
- Ruse, E.; Buzaglo, M.; Pri-Bar, I.; Shunak, L.; Nadiv, R.; Pevzner, S.; Siton-Mendelson, O.; Skripnyuk, V.M.; Rabkin, E.; Regev, O. Hydrogen storage kinetics: The graphene nanoplatelet size effect. Carbon 2018, 130, 369–376. [Google Scholar] [CrossRef]
- Bahuguna, A.; Sasson, Y. Formate-Bicarbonate Cycle as a Vehicle for Hydrogen and Energy Storage. Chemsuschem 2021, 14, 1258–1283. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Heintzman, E.; Price, C. Electrostatic Layer-By-Layer Self-Assembled Graphene/Multi-Walled Carbon Nanotubes Hybrid Multilayers as Efficient ‘All Carbon’ Supercapacitors. J. Nanosci. Nanotechnol. 2016, 16, 4771–4782. [Google Scholar] [CrossRef] [PubMed]
- Ghalami, Z.; Ghoulipour, V.; Khanchi, A. Hydrogen and deuterium adsorption on uranium decorated graphene nanosheets: A combined molecular dynamics and density functional theory study. Curr. Appl. Phys. 2019, 19, 536–541. [Google Scholar] [CrossRef]
- Lang, C.G.; Ouyang, L.Z.; Yang, L.L.; Dai, L.Y.; Wu, D.F.; Shao, H.Y.; Zhu, M. Enhanced hydrogen storage kinetics in Mg@FLG composite synthesized by plasma assisted milling. Int. J. Hydrogen Energy 2018, 43, 17346–17352. [Google Scholar] [CrossRef]
- Lee, J.; Sung, D.; Chung, Y.K.; Bin Song, S.; Huh, J. Unveiling two-dimensional magnesium hydride as a hydrogen storage material via a generative adversarial network. Nanoscale Adv. 2022, 4, 2332–2338. [Google Scholar] [CrossRef]
- Ates, M.; Chebil, A.; Yoruk, O.; Dridi, C.; Turkyilmaz, M. Reliability of electrode materials for supercapacitors and batteries in Energy storage applications: A review. Ionics 2022, 28, 27–52. [Google Scholar] [CrossRef]
- Chaichi, A.; Wang, Y.; Gartia, M.R. Substrate Engineered Interconnected Graphene Electrodes with Ultrahigh Energy and Power Densities for Energy Storage Applications. ACS Appl. Mater. Interfaces 2018, 10, 21235–21245. [Google Scholar] [CrossRef]
- Xia, G.; Zhang, B.; Chen, X.; Sun, D.; Guo, Z.; Liang, F.; Yu, X. Molecular-Scale Functionality on Graphene To Unlock the Energy Capabilities of Metal Hydrides for High-Capacity Lithium-Ion Batteries. ACS Nano 2018, 12, 8177–8186. [Google Scholar] [CrossRef]
- Li, M.M.; Wang, Y.; Yang, C.C.; Jiang, Q. In situ grown Co3O4 nanocubes on N-doped graphene as a synergistic hybrid for applications in nickel metal hydride batteries. Int. J. Hydrogen Energy 2018, 43, 18421–18435. [Google Scholar] [CrossRef]
- Sirisinudomkit, P.; Iamprasertkun, P.; Krittayavathananon, A.; Pettong, T.; Dittanet, P.; Sawangphruk, M. Hybrid Energy Storage of Ni(OH)(2)-coated N-doped Graphene Aerogel//N-doped Graphene Aerogel for the Replacement of NiCd and NiMH Batteries. Sci. Rep. 2017, 7, 1124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xia, G.; Sun, D.; Fang, F.; Yu, X. Magnesium Hydride Nanoparticles Self-Assembled on Graphene as Anode Material for High-Performance Lithium-Ion Batteries. ACS Nano 2018, 12, 3816–3824. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Li, C.; Zhang, X.; Sun, X.Z.; Wang, K.; Ma, Y.W. High Performance Lithium-Ion Hybrid Capacitors Employing Fe3O4-Graphene Composite Anode and Activated Carbon Cathode. ACS Appl. Mater. Interfaces 2017, 9, 17137–17145. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Xu, X.D.; Liu, D.; Li, J.; Wei, Y.H.; Tang, H.L.; Li, J.S.; Li, X.; Xie, Z.Z.; Qu, D.Y. The impacts of nitrogen doping on the electrochemical hydrogen storage in a carbon. Int. J. Energy Res. 2021, 45, 9326–9339. [Google Scholar] [CrossRef]
- Lan, Z.Q.; Zeng, K.; Wei, B.; Li, G.X.; Ning, H.; Guo, J. Nickel-graphene nanocomposite with improved electrochemical performance for La0.7Mg0.3(Ni0.85Co0.15)(3.5) electrode. Int. J. Hydrogen Energy 2017, 42, 12458–12466. [Google Scholar] [CrossRef]
- Xia, G.; Tan, Y.; Chen, X.; Fang, F.; Sun, D.; Li, X.; Guo, Z.; Yu, X. Oxygen-free Layer-by-Layer Assembly of Lithiated Composites on Graphene for Advanced Hydrogen Storage. Adv. Sci. 2016, 4, 1600257. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, Y.; Xu, L.; Zang, L.; Guo, H.; Jiao, L.; Yuan, H.; Wang, Y. Highly Dispersed MgH2 Nanoparticle–Graphene Nanosheet Composites for Hydrogen Storage. ACS Appl. Nano Mater. 2019, 2, 3828–3835. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Xia, G.L.; Chen, J.; Zhang, B.P.; Li, Q.; Yu, X.B. One-step uniform growth of magnesium hydride nanoparticles on graphene. Prog. Nat. Sci.-Mater. 2017, 27, 87–93. [Google Scholar] [CrossRef]
- Jang, M.H.; Park, S.H.; Hong, T.W. Hydrogenation Behaviors of MgHx-Graphene Composites by Reactive Mechanical Grinding. Korean J. Met. Mater. 2016, 54, 288–294. [Google Scholar] [CrossRef]
- Cho, E.S.; Ruminski, A.M.; Aloni, S.; Liu, Y.-S.; Guo, J.; Urban, J.J. Graphene oxide/metal nanocrystal multilaminates as the atomic limit for safe and selective hydrogen storage. Nat. Commun. 2016, 7, 10804. [Google Scholar] [CrossRef]
- Pukazhselvan, D.; Shaula, A.L.; Mikhalev, S.M.; Bdikin, I.; Fagg, D.P. Elucidating Evidence for the In Situ Reduction of Graphene Oxide by Magnesium Hydride and the Consequence of Reduction on Hydrogen Storage. Catalysts 2022, 12, 735. [Google Scholar] [CrossRef]
- Han, D.J.; Bang, K.R.; Cho, H.; Cho, E.S. Effect of carbon nanoscaffolds on hydrogen storage performance of magnesium hydride. Korean J. Chem. Eng. 2020, 37, 1306–1316. [Google Scholar] [CrossRef]
- Singh, M.K.; Bhatnagar, A.; Pandey, S.K.; Mishra, P.C.; Srivastava, O.N. Experimental and first principle studies on hydrogen desorption behavior of graphene nanofibre catalyzed MgH2. Int. J. Hydrogen Energy 2017, 42, 960–968. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, X.F.; Mao, C.; Long, C.G.; Chen, J.; Zhou, D.W. Influences and mechanisms of graphene-doping on dehydrogenation properties of MgH2: Experimental and first-principles studies. Energy 2015, 89, 957–964. [Google Scholar] [CrossRef]
- Zhang, L.T.; Chen, L.X.; Xiao, X.Z.; Fan, X.L.; Shao, J.; Li, S.Q.; Ge, H.W.; Wang, Q.D. Fluorographene nanosheets enhanced hydrogen absorption and desorption performances of magnesium hydride. Int. J. Hydrogen Energy 2014, 39, 12715–12726. [Google Scholar] [CrossRef]
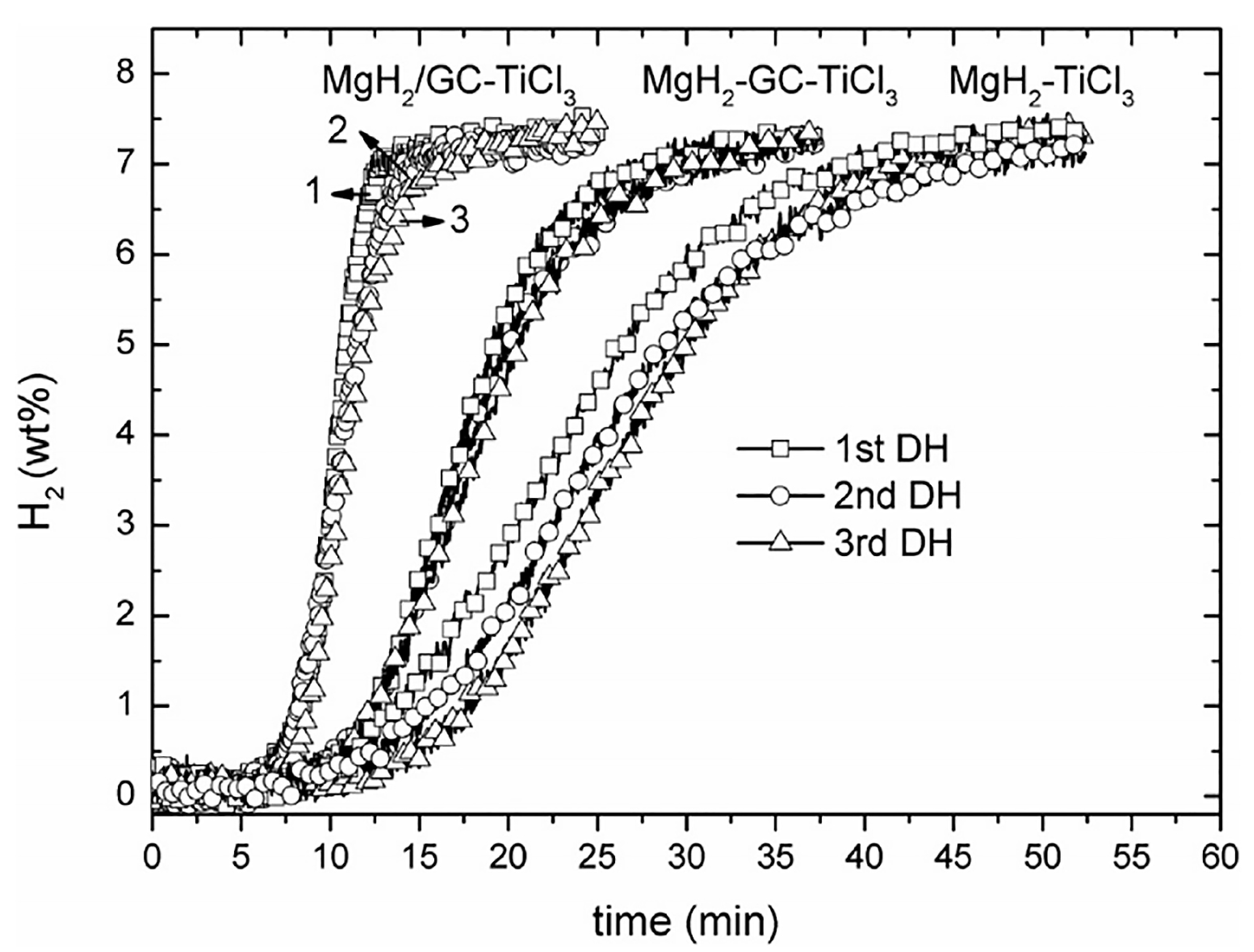
- Verma, S.K.; Bhatnagar, A.; Shukla, V.; Soni, P.K.; Pandey, A.P.; Yadav, T.P.; Srivastava, O.N. Multiple improvements of hydrogen sorption and their mechanism for MgH2 catalyzed through TiH2@Gr. Int. J. Hydrogen Energy 2020, 45, 19516–19530. [Google Scholar] [CrossRef]
- Wang, K.K.; Wu, G.L.; Cao, H.J.; Li, H.L.; Zhao, X.S. Improved reversible dehydrogenation properties of MgH2 by the synergetic effects of graphene oxide-based porous carbon and TiCl3. Int. J. Hydrogen Energy 2018, 43, 7440–7446. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Z.M.; Li, X.J.; Ren, S.Q.; Zhou, S.H.; Zhang, H.M.; Li, Y.; Han, S.M. Improved hydrogen storage properties of MgH2 by nickel@nitrogen-doped carbon spheres. Dalton Trans. 2020, 49, 3495–3502. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, G.; An, C.H.; Li, L.; Qiu, F.Y.; Wang, Y.J.; Jiao, L.F.; Yuan, H.T. Bimetallic NiCo Functional Graphene: An Efficient Catalyst for Hydrogen-Storage Properties of MgH2. Chem.-Asian J. 2014, 9, 2576–2583. [Google Scholar] [CrossRef]
- Zhang, J.; Qu, H.; Yan, S.; Wu, G.; Yu, X.F.; Zhou, D.W. Catalytic effect of nickel phthalocyanine on hydrogen storage properties of magnesium hydride: Experimental and first-principles studies. Int. J. Hydrogen Energy 2017, 42, 28485–28497. [Google Scholar] [CrossRef]
- Xie, X.B.; Chen, M.; Liu, P.; Shang, J.X.; Liu, T. High hydrogen desorption properties of Mg-based nanocomposite at moderate temperatures: The effects of multiple catalysts in situ formed by adding nickel sulfides/graphene. J. Power Sources 2017, 371, 112–118. [Google Scholar] [CrossRef]
- Zhang, J.; Qu, H.; Wu, G.; Song, L.B.; Yu, X.F.; Zhou, D.W. Remarkably enhanced dehydrogenation properties and mechanisms of MgH2 by sequential-doping of nickel and graphene. Int. J. Hydrogen Energy 2016, 41, 17433–17441. [Google Scholar] [CrossRef]
- Zhang, J.G.; Zhu, Y.F.; Zang, X.X.; Huan, Q.Q.; Su, W.; Zhu, D.L.; Li, L.Q. Nickel-decorated graphene nanoplates for enhanced H-2 sorption properties of magnesium hydride at moderate temperatures. J. Mater. Chem. A 2016, 4, 2560–2570. [Google Scholar] [CrossRef]
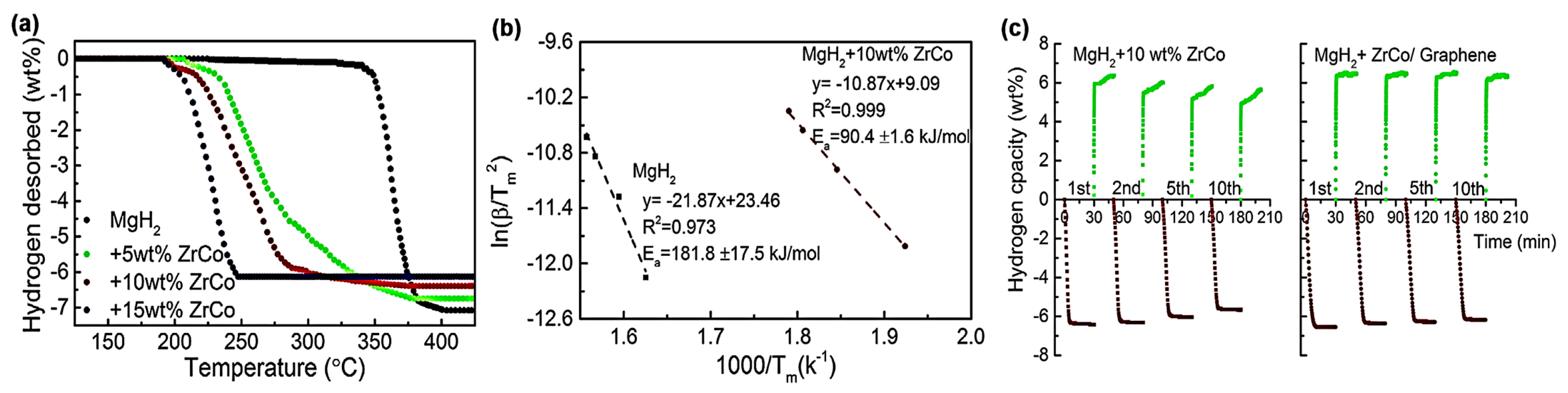
- Zhang, L.T.; Cai, Z.L.; Zhu, X.Q.; Yao, Z.D.; Sun, Z.; Ji, L.; Yan, N.H.; Xiao, B.B.; Chen, L.X. Two-dimensional ZrCo nanosheets as highly effective catalyst for hydrogen storage in MgH2. J. Alloys Compd. 2019, 805, 295–302. [Google Scholar] [CrossRef]
- Leng, H.Y.; Miao, N.; Li, Q. Improved hydrogen storage properties of MgH2 by the addition of KOH and graphene. Int. J. Hydrogen Energy 2020, 45, 28183–28189. [Google Scholar] [CrossRef]
- Li, M.M.; Yang, C.C.; Chen, L.X.; Jiang, Q. Hydrogen storage alloys/reduced graphite oxide: An efficient hybrid electrode with enhanced high-rate dischargeability. Electrochim. Acta 2016, 200, 59–65. [Google Scholar] [CrossRef]
- Huang, H.X.; Yuan, J.G.; Zhang, B.; Zhang, J.G.; Zhu, Y.F.; Li, L.Q.; Wu, Y.; Zhou, S.X. Improvement in the hydrogenation-dehydrogenation performance of a Mg-Al alloy by graphene supported Ni. Int. J. Hydrogen Energy 2020, 45, 798–808. [Google Scholar] [CrossRef]
- Huang, X.T.; Tao, A.P.; Guo, J.; Wei, W.L.; Guo, J.; Lan, Z.Q. Synergistic effect of TiF3@ graphene on the hydrogen storage properties of Mg-Al alloy. Int. J. Hydrogen Energy 2018, 43, 1651–1657. [Google Scholar] [CrossRef]
- Lan, Z.Q.; Sun, Z.Z.; Ding, Y.C.; Ning, H.; Wei, W.L.; Guo, J. Catalytic action of Y2O3@graphene nanocomposites on the hydrogen-storage properties of Mg-Al alloys. J. Mater. Chem. A 2017, 5, 15200–15207. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, L.T.; Yang, X.L.; Zhu, X.Q.; Chen, L.X. The remarkably improved hydrogen storage performance of MgH2 by the synergetic effect of an FeNi/rGO nanocomposite. Dalton Trans. 2020, 49, 4146–4154. [Google Scholar] [CrossRef]
- Liu, G.; Wang, K.F.; Li, J.P.; Wang, Y.J.; Yuan, H.T. Enhancement of hydrogen desorption in magnesium hydride catalyzed by graphene nanosheets supported Ni-CeOx hybrid nanocatalyst. Int. J. Hydrogen Energy 2016, 41, 10786–10794. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Y.J.; Jiao, L.F.; Yuan, H.T. Solid-state synthesis of amorphous TiB2 nanoparticles on graphene nanosheets with enhanced catalytic dehydrogenation of MgH2. Int. J. Hydrogen Energy 2014, 39, 3822–3829. [Google Scholar] [CrossRef]
- Liu, J.C.; Liu, Y.N.; Liu, Z.B.; Ma, Z.L.; Ding, Y.J.; Zhu, Y.F.; Zhang, Y.; Zhang, J.G.; Li, L.Q. Effect of rGO supported NiCu derived from layered double hydroxide on hydrogen sorption kinetics of MgH2. J. Alloys Compd. 2019, 789, 768–776. [Google Scholar] [CrossRef]
- Zhou, D.M.; Cui, K.X.; Zhou, Z.W.; Liu, C.R.; Zhao, W.; Li, P.; Qu, X.H. Enhanced hydrogen-storage properties of MgH2 by Fe-Ni catalyst modified three-dimensional graphene. Int. J. Hydrogen Energy 2021, 46, 34369–34380. [Google Scholar] [CrossRef]
- Liu, J.C.; Ma, Z.L.; Liu, Z.B.; Tang, Q.K.; Zhu, Y.F.; Lin, H.J.; Zhang, Y.; Zhang, J.G.; Liu, Y.; Li, L.Q. Synergistic effect of rGO supported Ni3Fe on hydrogen storage performance of MgH2. Int. J. Hydrogen Energy 2020, 45, 16622–16633. [Google Scholar] [CrossRef]
- Luo, S.C.; Li, S.J.; Liu, Y.N.; Zhang, J.G.; Zhu, Y.F.; Zhang, Y.; Lin, H.J.; Li, L.Q. Synergistically tuned hydrogen storage thermodynamics and kinetics of Mg-Al alloys by Cu formed in situ mechanochemically. J. Alloys Compd. 2019, 806, 370–377. [Google Scholar] [CrossRef]
- Peng, D.D.; Ding, Z.M.; Zhang, L.; Fu, Y.K.; Wang, J.S.; Li, Y.; Han, S.M. Remarkable hydrogen storage properties and mechanisms of the shell-core MgH2@carbon aerogel microspheres. Int. J. Hydrogen Energy 2018, 43, 3731–3740. [Google Scholar] [CrossRef]
- Tian, M.; Shang, C.X. Mg-based composites for enhanced hydrogen storage performance. Int. J. Hydrogen Energy 2019, 44, 338–344. [Google Scholar] [CrossRef]
- Shukla, V.; Bhatnagar, A.; Verma, S.K.; Pandey, A.P.; Vishwakarma, A.K.; Srivastava, P.; Yadav, T.P.; Srivastava, O.N. Simultaneous improvement of kinetics and thermodynamics based on SrF2 and SrF2@Gr additives on hydrogen sorption in MgH2. Mater. Adv. 2021, 2, 4277–4290. [Google Scholar] [CrossRef]
- Song, M.C.; Zhang, L.T.; Zheng, J.G.; Yu, Z.D.; Wang, S.N. Constructing graphene nanosheet-supported FeOOH nanodots for hydrogen storage of MgH2. Int. J. Miner. Met. Mater. 2022, 29, 1464–1473. [Google Scholar] [CrossRef]
- Tarasov, B.P.; Arbuzov, A.A.; Mozhzhuhin, S.A.; Volodin, A.A.; Fursikov, P.V.; Lototskyy, M.V.; Yartys, V.A. Hydrogen storage behavior of magnesium catalyzed by nickel-graphene nanocomposites. Int. J. Hydrogen Energy 2019, 44, 29212–29223. [Google Scholar] [CrossRef]
- Zhang, B.P.; Xia, G.L.; Chen, W.; Gu, Q.F.; Sun, D.L.; Yu, X.B. Controlled-Size Hollow Magnesium Sulfide Nanocrystals Anchored on Graphene for Advanced Lithium Storage. ACS Nano 2018, 12, 12741–12750. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.D.; Ma, Z.N.; Wu, D.H.; Zhang, X.; Jing, Y.; Zhou, Z. Computational study of catalytic effect of C3N4 on H-2 release from complex hydrides. Int. J. Hydrogen Energy 2015, 40, 8897–8902. [Google Scholar] [CrossRef]
- Yu, H.Z.; Du, A.J.; Song, Y.; Searles, D.J. Graphyne and Graphdiyne: Versatile Catalysts for Dehydrogenation of Light Metal Complex Hydrides. J. Phys. Chem. C 2013, 117, 21643–21650. [Google Scholar] [CrossRef]
- Gasnier, A.; Luguet, M.; Pereira, A.G.; Troiani, H.; Zampieri, G.; Gennari, F.C. Entanglement of N-doped graphene in resorcinolformaldehyde: Effect over nanoconfined LiBH4 for hydrogen storage. Carbon 2019, 147, 284–294. [Google Scholar] [CrossRef]
- Gasnier, A.; Amica, G.; Juan, J.; Troiani, H.; Gennari, F.C. N-Doped Graphene-Rich Aerogels Decorated with Nickel and Cobalt Nanoparticles: Effect on Hydrogen Storage Properties of Nanoconfined LiBH4. J. Phys. Chem. C 2020, 124, 115–125. [Google Scholar] [CrossRef]
- Ye, J.K.; Xia, G.L.; Yu, X.B. In-situ constructed destabilization reaction of LiBH4 wrapped with graphene toward stable hydrogen storage reversibility. Mater. Today Energy 2021, 22, 100885. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, W.; Zhang, Y.; Zhao, X.; Wen, P.; Ma, D. Fe3O4 Nanoclusters Highly Dispersed on a Porous Graphene Support as an Additive for Improving the Hydrogen Storage Properties of LiBH4. RSC Adv. 2018, 8, 19353–19361. [Google Scholar] [CrossRef]
- Gasnier, A.; Gennari, F.C. Graphene Entanglement in a Mesoporous Resorcinol-Formaldehyde Matrix Applied to the Nanoconfinement of LiBH4 for Hydrogen Storage. RSC Adv. 2017, 7, 27905–27912. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Zhang, W.; Ren, Z.; Huang, Z.; Hu, J.; Gao, M.; Pan, H.; Liu, Y. Nano-synergy enables highly reversible storage of 9.2 wt% hydrogen at mild conditions with lithium borohydride. Nano Energy 2021, 83, 105839. [Google Scholar] [CrossRef]
- Xia, Y.P.; Wei, S.; Huang, Q.; Li, J.Q.; Cen, X.H.; Zhang, H.Z.; Chu, H.L.; Sun, L.X.; Xu, F.; Huang, P.R. Facile synthesis of NiCo2O4-anchored reduced graphene oxide nanocomposites as efficient additives for improving the dehydrogenation behavior of lithium alanate. Inorg. Chem. Front. 2020, 7, 1257–1272. [Google Scholar] [CrossRef]
- Palade, P.; Comanescu, C.; Radu, C. Synthesis of Nickel and Cobalt Ferrite-Doped Graphene as Efficient Catalysts for Improving the Hydrogen Storage Kinetics of Lithium Borohydride. Materials 2023, 16, 427. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.T.; Ding, X.L.; Zhang, Q.G. Self-Printing on Graphitic Nanosheets with Metal Borohydride Nanodots for Hydrogen Storage. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Q.; Xia, G.L.; Zhang, J.; Guo, Z.P.; Yu, X.B. Graphene-tailored molecular bonds for advanced hydrogen and lithium storage performance. Energy Storage Mater. 2019, 17, 178–185. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, X.; Li, H.-W.; Huang, Z.; Hu, J.; Gao, M.; Pan, H.; Liu, Y. Titanium Hydride Nanoplates Enable 5 Wt% of Reversible Hydrogen Storage by Sodium Alanate below 80 °C. Research 2021, 2021, 9819176. [Google Scholar] [CrossRef]
- Kumar, L.H.; Rao, C.V.; Viswanathan, B. Catalytic effects of nitrogen-doped graphene and carbon nanotube additives on hydrogen storage properties of sodium alanate. J. Mater. Chem. A 2013, 1, 3355–3361. [Google Scholar] [CrossRef]
- Li, Y.T.; Fang, F.; Fu, H.L.; Qiu, J.M.; Song, Y.; Li, Y.S.; Sun, D.L.; Zhang, Q.G.; Ouyang, L.Z.; Zhu, M. Carbon nanomaterial-assisted morphological tuning for thermodynamic and kinetic destabilization in sodium alanates. J. Mater. Chem. A 2013, 1, 5238–5246. [Google Scholar] [CrossRef]
- Do, H.W.; Kim, H.; Cho, E.S. Enhanced hydrogen storage kinetics and air stability of nanoconfined NaAlH4 in graphene oxide framework. RSC Adv. 2021, 11, 32533–32540. [Google Scholar] [CrossRef]
- Huang, Y.K.; Shao, H.X.; Zhang, Q.Y.; Zang, L.; Guo, H.N.; Liu, Y.F.; Jiao, L.F.; Yuan, H.T.; Wang, Y.J. Layer-by-layer uniformly confined Graphene-NaAlH4 composites and hydrogen storage performance. Int. J. Hydrogen Energy 2020, 45, 28116–28122. [Google Scholar] [CrossRef]
- Qian, Z.; Hudson, M.S.L.; Raghubanshi, H.; Scheicher, R.H.; Pathak, B.; Araujo, C.M.; Blomqvist, A.; Johansson, B.; Srivastava, O.N.; Ahuja, R. Excellent Catalytic Effects of Graphene Nanofibers on Hydrogen Release of Sodium alanate. J. Phys. Chem. C 2012, 116, 10861–10866. [Google Scholar] [CrossRef]
- Wang, J.; Ebner, A.D.; Prozorov, T.; Zidan, R.; Ritter, J.A. Effect of graphite as a co-dopant on the dehydrogenation and hydrogenation kinetics of Ti-doped sodium aluminum hydride. J. Alloys Compd. 2005, 395, 252–262. [Google Scholar] [CrossRef]
- Zheng, H.Y.; Ding, Z.Q.; Xie, Y.J.; Li, J.F.; Huang, C.K.; Cai, W.T.; Liu, H.Z.; Guo, J. Cerium hydride generated during ball milling and enhanced by graphene for tailoring hydrogen sorption properties of sodium alanate. Int. J. Hydrogen Energy 2021, 46, 4168–4180. [Google Scholar] [CrossRef]
- Xu, L.Y.; Ge, Q.F. Effect of defects and dopants in graphene on hydrogen interaction in graphene-supported NaAlH4. Int. J. Hydrogen Energy 2013, 38, 3670–3680. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Xia, G.L.; Zhang, J.; Sun, D.L.; Guo, Z.P.; Yu, X.B. Graphene-Tailored Thermodynamics and Kinetics to Fabricate Metal Borohydride Nanoparticles with High Purity and Enhanced Reversibility. Adv. Energy Mater. 2018, 8, 1702975. [Google Scholar] [CrossRef]
- Jeong, S.; Heo, T.W.; Oktawiec, J.; Shi, R.; Kang, S.Y.; White, J.L.; Schneemann, A.; Zaia, E.W.; Wan, L.F.; Ray, K.G.; et al. A Mechanistic Analysis of Phase Evolution and Hydrogen Storage Behavior in Nanocrystalline Mg(BH4)2 within Reduced Graphene Oxide. ACS Nano 2020, 14, 1745–1756. [Google Scholar] [CrossRef] [PubMed]
- White, J.L.; Strange, N.A.; Sugar, J.D.; Snider, J.L.; Schneemann, A.; Lipton, A.S.; Toney, M.F.; Allendorf, M.D.; Stavila, V. Melting of Magnesium Borohydride under High Hydrogen Pressure: Thermodynamic Stability and Effects of Nanoconfinement. Chem. Mater. 2020, 32, 5604–5615. [Google Scholar] [CrossRef]
- Wang, Z.M.; Tao, S.; Li, J.J.; Deng, J.Q.; Zhou, H.Y.; Yao, Q.R. The Improvement of Dehydriding the Kinetics of NaMgH3 Hydride via Doping with Carbon Nanomaterials. Metal 2017, 7, 9. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Pandey, S.K.; Shahi, R.R.; Hudson, M.S.L.; Shaz, M.A.; Srivastava, O.N. Synthesis, characterization and hydrogen sorption studies of mixed sodium-potassium alanate. Cryst. Res. Technol. 2013, 48, 520–531. [Google Scholar] [CrossRef]
- Varin, R.A.; Bidabadi, A.S.; Polanski, M.; Biglari, M.; Stobinski, L. The effects of filamentary Ni, graphene and lithium amide (LiNH2) additives on the dehydrogenation behavior of mechano-chemically synthesized crystalline manganese borohydride (Mn(BH4)(2)) and its solvent filtration/extraction. Mater. Res. Bull. 2018, 100, 394–406. [Google Scholar] [CrossRef]
- Li, N.; Du, Y.; Feng, Q.P.; Huang, G.W.; Xiao, H.M.; Fu, S.Y. A Novel Type of Battery-Supercapacitor Hybrid Device with Highly Switchable Dual Performances Based on a Carbon Skeleton/Mg2Ni Free-Standing Hydrogen Storage Electrode. ACS Appl. Mater. Interfaces 2017, 9, 44828–44838. [Google Scholar] [CrossRef]
- Du, Y.; Li, N.; Zhang, T.L.; Feng, Q.P.; Du, Q.; Wu, X.H.; Huang, G.W. Reduced Graphene Oxide Coating with Anticorrosion and Electrochemical Property-Enhancing Effects Applied in Hydrogen Storage System. ACS Appl. Mater. Interfaces 2017, 9, 28980–28989. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.G.; Zhu, Y.F.; Lin, H.J.; Liu, Y.N.; Zhang, Y.; Li, S.Y.; Ma, Z.L.; Li, L.Q. Metal Hydride Nanoparticles with Ultrahigh Structural Stability and Hydrogen Storage Activity Derived from Microencapsulated Nanoconfinement. Adv. Mater. 2017, 29, 1700760. [Google Scholar] [CrossRef] [PubMed]
- Puszkiel, J.; Gasnier, A.; Amica, G.; Gennari, F. Tuning LiBH4 for Hydrogen Storage: Destabilization, Additive, and Nanoconfinement Approaches. Molecules 2020, 25, 163. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.L.; Tan, Y.B.; Wu, F.L.; Fang, F.; Sun, D.L.; Guo, Z.P.; Huang, Z.G.; Yu, X.B. Graphene-wrapped reversible reaction for advanced hydrogen storage. Nano Energy 2016, 26, 488–495. [Google Scholar] [CrossRef]
- Chou, C.C.; Chen, B.H.; Lee, D.J. Hydrogen storage in a chemical hydride fuel system containing ammonia borane and Ni-Co/r-GO catalyst. Enregy Procedia 2014, 61, 142–145. [Google Scholar] [CrossRef]
- Li, J.L.; Ren, X.Y.; Lv, H.; Wang, Y.Y.; Li, Y.F.; Liu, B. Highly efficient hydrogen production from hydrolysis of ammonia borane over nanostructured Cu@CuCoOx supported on graphene oxide. J. Hazard. Mater. 2020, 391, 122199. [Google Scholar] [CrossRef]
- Champet, S.; van den Berg, J.; Szczesny, R.; Godula-Jopek, A.; Gregory, D.H. Nano-inclusion in one step: Spontaneous icetemplating of porous hierarchical nanocomposites for selective hydrogen release. Sustain. Energy Fuels 2019, 3, 396–400. [Google Scholar] [CrossRef]
- Liu, X.R.; Wu, Y.F.; Wang, S.M.; Li, Z.N.; Guo, X.M.; Ye, J.H.; Jiang, L.J. Current progress and research trends on lithium amidoborane for hydrogen storage. J. Mater. Sci. 2020, 55, 2645–2660. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, S.; Hashmi, S.A.R.; Kim, K.H. MXenes: Emerging 2D materials for hydrogen storage. Nano Energy 2021, 85, 105989. [Google Scholar] [CrossRef]
- Shi, L.J.; Liu, X.J.; Tuo, Y.X.; Xu, J.; Li, P.; Han, Y.F. Graphene CNT composite as catalyst support for microwave-assisted hydrogen releasing from liquid organic hydride. Int. J. Hydrogen Energy 2017, 42, 17403–17413. [Google Scholar] [CrossRef]
- Ventura-Espinosa, D.; Carretero-Cerdan, A.; Baya, M.; Garcia, H.; Mata, J.A. Catalytic Dehydrogenative Coupling of Hydrosilanes with Alcohols for the Production of Hydrogen On-demand: Application of a Silane/Alcohol Pair as a Liquid Organic Hydrogen Carrier. Chem.-Eur. J. 2017, 23, 10815–10821. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Wang, X.H.; Liu, H.Z.; Gao, S.C.; Wang, Y.Y.; Li, S.Q.; Yan, M. Enhanced hydrogen desorption/absorption properties of magnesium hydride with CeF3@Gn. Int. J. Hydrogen Energy 2020, 45, 4754–4764. [Google Scholar] [CrossRef]
- Li, Q.Y.; Qiu, S.Y.; Wu, C.Z.; Lau, T.; Sun, C.H.; Jia, B.H. Computational Investigation of MgH2/Graphene Heterojunctions for Hydrogen Storage. J. Phys. Chem. C 2021, 125, 2357–2363. [Google Scholar] [CrossRef]
- Sigal, A.; Rojas, M.I.; Leiva, E.P.M. Interferents for hydrogen storage on a graphene sheet decorated with nickel: A DFT study. Int. J. Hydrogen Energy 2011, 36, 3537–3546. [Google Scholar] [CrossRef]
- Singh, S.; Bhatnagar, A.; Shukla, V.; Vishwakarma, A.K.; Soni, P.K.; Verma, S.K.; Shaz, M.A.; Sinha, A.S.K.; Srivastava, O.N. Ternary transition metal alloy FeCoNi nanoparticles on graphene as new catalyst for hydrogen sorption in MgH2. Int. J. Hydrogen Energy 2020, 45, 774–786. [Google Scholar] [CrossRef]
- Al-Msrhad, T.M.H.; Devrim, Y.; Uzundurukan, A.; Budak, Y. Investigation of hydrogen production from sodium borohydride by carbon nano tube-graphene supported PdRu bimetallic catalyst for PEM fuel cell application. Int. J. Energy Res. 2021, 46, 4156–4173. [Google Scholar] [CrossRef]

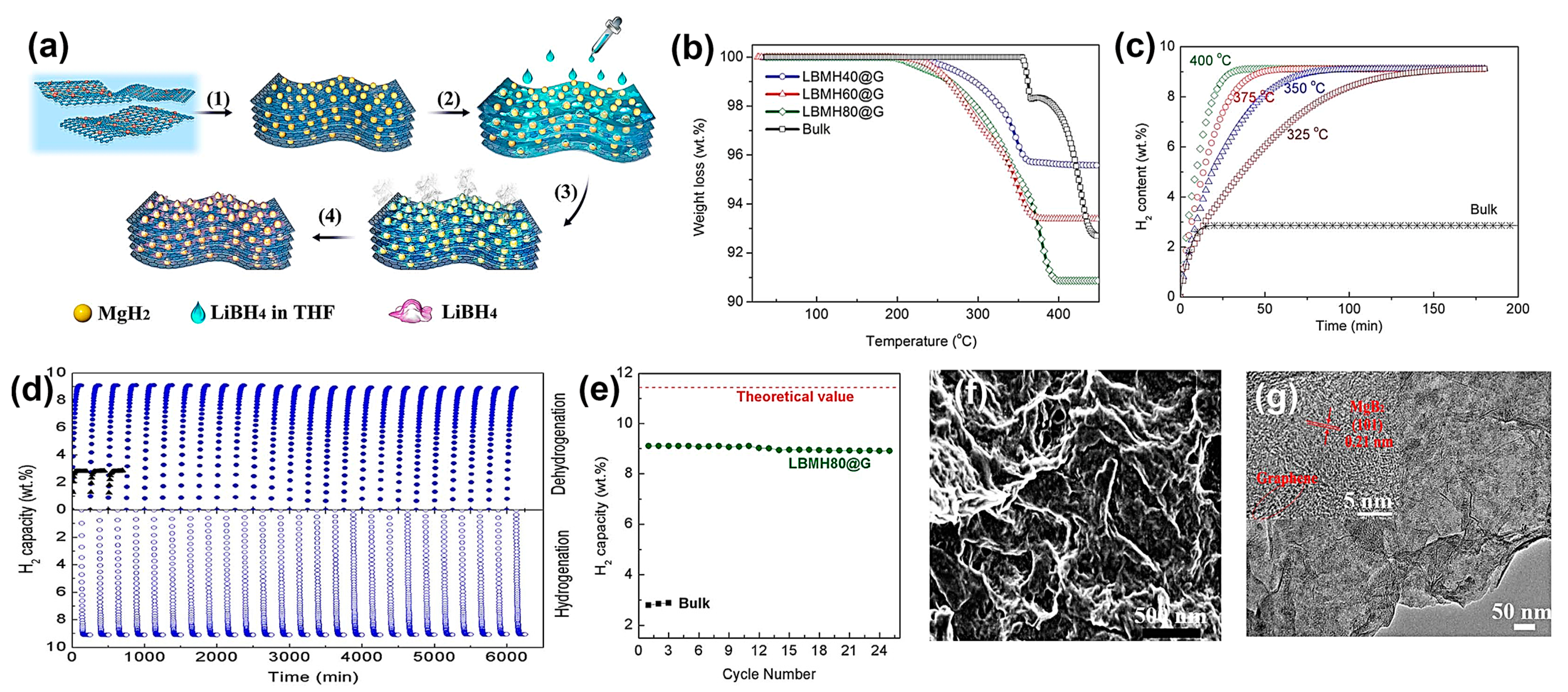
| Hydrogen Storing Species | Graphene Dopant | Nanocomposite | Observations | Ref. |
|---|---|---|---|---|
| Mg | 5 wt.% NiS | Mg—5 wt.% NiS/rGO (by HPMR, hydrogen plasma metal reaction) | ball milling Mg and NiS/rGO, the NiS-catalyzed support was prepared by co-reduction of Ni2+—impregnated GO support; des: 3.7 wt.% H2 in 10 min and 4.5 wt.% H2 in 60 min; Ea, abs = 44.47 kJ/mol; Ea, des = 63.02 kJ/mol | [81] |
| MgH2 | Ni | MgH2@Ni/G | (MgH2 + 10 wt.%G + 10 wt.%Ni) composites were investigated from a combined DFT and experimental viewpoint; des. onset at 339.5 °C | [82] |
| MgH2 | Ni | MgH2@Ni–Gn | nickel-decorated graphene support (Ni–Gn) afforded abs: 6.28 wt.%, 100 min, 373 K, and des: 5.73 wt.%, 1800 s, 523 K | [83] |
| MgH2 | ZrCo | MgH2@10 wt.% ZrCo/G | The ZrCo dopant was present as 2D nanosheets; des: 6.3 wt.% H2, 5 min, 300 °C, Ea,des = 90.4 kJ/mol; abs: 4.4 wt.% H2, 10 min, 120 °C, 3 MPa H2, Ea,ads = 57.6 kJ/mol. | [84] |
| MgH2 | KOH | MgH2 + KOH/graphene | KMgH3, MgO formed in-situ; Ea = 109.89 kJ/mol; 5.43 wt.% H2 reversibly. Graphene provides more H diffusion channels and better disperses the catalysts | [85] |
| Alloys HSAs | – | (LaCeY)(NiMnCoAl)5@rGO | HSAs = hydrogen storage alloys; retention rate 51.25% at a discharge current density 3000 mA/g | [86] |
| Mg–Al alloy | Ni | Mg90Al10-x (80 wt.%Ni@Gn) | Mg–Al alloy in graphene–supported Ni; x = 0,4, 8, 12 wt.%; for x = 8, a: 5.11 wt.% in 400 s, 523 K; d: 5.81 wt.%, 1800 s, 573 K | [87] |
| Mg–Al alloy | TiF3 | Mg–Al alloy–TiF3@G | Mg–Al-M (M = G, TiF3, and TiF3@G) composites; Ea = 139.8 kJ/mol for TiF3@G support; Mg–Al–TiF3@G released 5.41 wt.% at 350 °C | [88] |
| Mg–Al alloy | Y2O3 | Mg–Al alloy—Y2O3@G | Mg-Al-Y2O3@rGO reversibly store/release H2 at 250 °C; with 5 wt.% of Y2O3@rGO loading, Mg–Al composite had Ea,release = 145.9 (vs. 162.6 kJ/mol for pristine Mg–Al alloy). Ea,uptake = 54.3 kJ/mol for Y2O3 catalyzed G. | [89] |
| MgH2 | FeNi | MgH2@FeNi/rGO | 5 wt.% FeNi/rGO modified MgH2 released 6.5 wt.% H2 at 300 °C (onset 230 °C); uptake: 5.4 wt.%, 20 min, 125 °C, 32 bar H2 | [90] |
| MgH2 | Ni–CeOx | MgH2–Ni–CeOx/GNS | MgH2 catalyzed by Ni–CeOx/GNS; graphene nanosheets supported nanoscale Ni&CeOx (x = 1.69) | [91] |
| MgH2 | TiB2 | MgH2–TiB2/GNS | MgH2 catalyzed by TiB2/GNS | [92] |
| MgH2 | NiCu | MgH2–NiCu/rGO | MgH2 catalyzed by NiCu/rGO from double layer hydroxide | [93] |
| MgH2 | Fe–Ni | MgH2 + 10 wt.% Fe–LiCo 3D G | MgH2@Fe–Ni/G, namely MgH2 + 10 wt.% Fe–LiCo 3D G (3D-graphene) | [94] |
| MgH2 | NiFe–LDH | MgH2–Ni3Fe/rGO | NiFe–LDH (layered double hydroxide precursor)/GO yield (Ni3Fe/rGO) active catalyst | [95] |
| MgH2 | Al, Cu | Mg90Al10–Cu@G | MgH2→Mg–Al alloys with Cu introduced in situ: Mg90Al10–Cu@G nanoplates | [96] |
| MgH2 | – | MgH2@CA microspheres | abs: 6.2 wt.% H2 within 5 min at 275 °C; des: 4.9 wt.% H2 within 100 min at 350 °C; Ea,des = 114.8 kJ/mol. | [97] |
| MgH2 | TiC | MgH2–TiC@G | desorption at 180 °C by the plasma carbon–modified MgH2/TiC containing FLGS (few layer graphene sheets) | [98] |
| MgH2 | SrF2 and SrF2 | MgH2–SrF2(SrF2)@Gr | MgH2 cat. by SrF2 and SrF2@Gr additives | [99] |
| MgH2 | FeCoNi | MgH2@FeCoNi NPs/G | MgH2@FeCoNi NPs/G as new catalyst | [4] |
| MgH2 | FeOOH | MgH2@FeOOH nanodots/G | MgH2@FeOOH nanodots NDs @G, release H2 at 229.8 °C (ΔT = 106.8 °C lower than pristine MgH2), showing good cycling stability (over 20 cycles, 98.5% of initial capacity maintained, while also reducing the activation energy Ea) | [100] |
| Mg | Ni | Mg/MgH2 + Ni/GLM | Mg@Ni/G nanocomposites with 5–60 wt.% Ni loading: Mg/MgH2 + Ni/GLM (graphene-like material) | [101] |
| MgS | – | MH2@G, MgS@G | MgS@G prepared by reaction: MH2@G + S → MgS@G + H2; for advanced Li-storage | [102] |
| Hydrogen Storing Species | Graphene Dopant | Nanocomposite | Observations | Ref. |
|---|---|---|---|---|
| LiBH4, LiAlH4, NaAlH4 | (C3N4) | (Li, Na)XH4 (X = B/Al)/C3N4 | LiBH4, LiAlH4, NaAlH4/C3N4 studied by DFT; support offers suitable adsorption site for AlHx/BHx (x = 3, 2, 1) | [103] |
| LiBH4, LiAlH4, NaAlH4 | – | (Li, Na)XH4 (X = B/Al)/G | LiBH4, LiAlH4, NaAlH4/Graphene and Graphdiyne; DFT study shows strong support interaction due to well-defined pore structure | [104] |
| LiBH4 | N | LiBH4–N-doped G/MC | LiBH4 in 10 at%N-doped Graphene/resorcinol formaldehyde; impregnation degree: 30, 50 and 70 vol%; XRD diffraction 2θ = 12.6° that can be attributed to Li–B–N(G)–H interaction | [105] |
| LiBH4 | Ni/Co | LiBH4–Ni/Co NPs–N-doped G aerogels | LiBH4 catalyzed by NiCo NPs–N-doped G aerogels; Co-decorated, des.: 8 wt.% H2 at 325 °C (1st cycle; with 1 wt.% at 226 °C). Ni-decorated, des: 8 wt.% H2, | [106] |
| LiBH4 | – | LiBH4–G | LiBH4 wrapped in G | [107] |
| LiBH4 | Fe3O4 | LiBH4–Fe3O4/porous G | LiBH4 catalyzed by (Fe3O4 dispersed on porous G) | [108] |
| LiBH4 | – | LiBH4–(mesoporous resorcinol-formaldehyde/G) | LiBH4 nanoconfined in (mesoporous resorcinol-formaldehyde/G) entangled supports; des: 13 wt.% at 400 °C; recharging at 400 °C, 5 h, 60 bar H2; 6 wt.% reversible storage capacity; | [109] |
| LiBH4 | Ni nanocrystals (2–4 nm) | LiBH4/G | LiBH4 (5–10 nm)–Ni(2–4 nm)/G, affording 9.2 wt.% H2 due to by-passing B2H6 and B12H122– anion formation | [110] |
| LiAlH4 | NiCo2O4 | LiAlH4–NiCo2O4 @ rGO | LiAlH4 dehydrogenation by NiCo2O4 nanorods@rGO nanocomposites by ball milling; LiAlH4 + 7 wt.% NiCo2O4@rGO des. onset at 62.7 °C (in total 6.28 wt.% H2); 4.0 wt.% hydrogen within 20 min at 150 °C (isothermal) | [111] |
| LiBH4 | NiFe2O4 | LiBH4-graphene-NiFe2O4 (Ar) | EA = 127 kJ/mol (vs. 170 kJ/mol for pristine LiBH4); after 5 a/d cycles. H2 storage was ~6.14 wt.%; des. onset at 349 °C | [112] |
| NaBH4 | – | nano-NaBH4@GNs | Ultrasmall (6–10 nm) nano-NaBH4@GNs by MFSP (mechanical-force-driven self-printing, a technique similar to 3D printing), for scalable fabrication of 0D complex hydrides in 2D supports; ~5 wt.% stable H2 storage capacity | [113] |
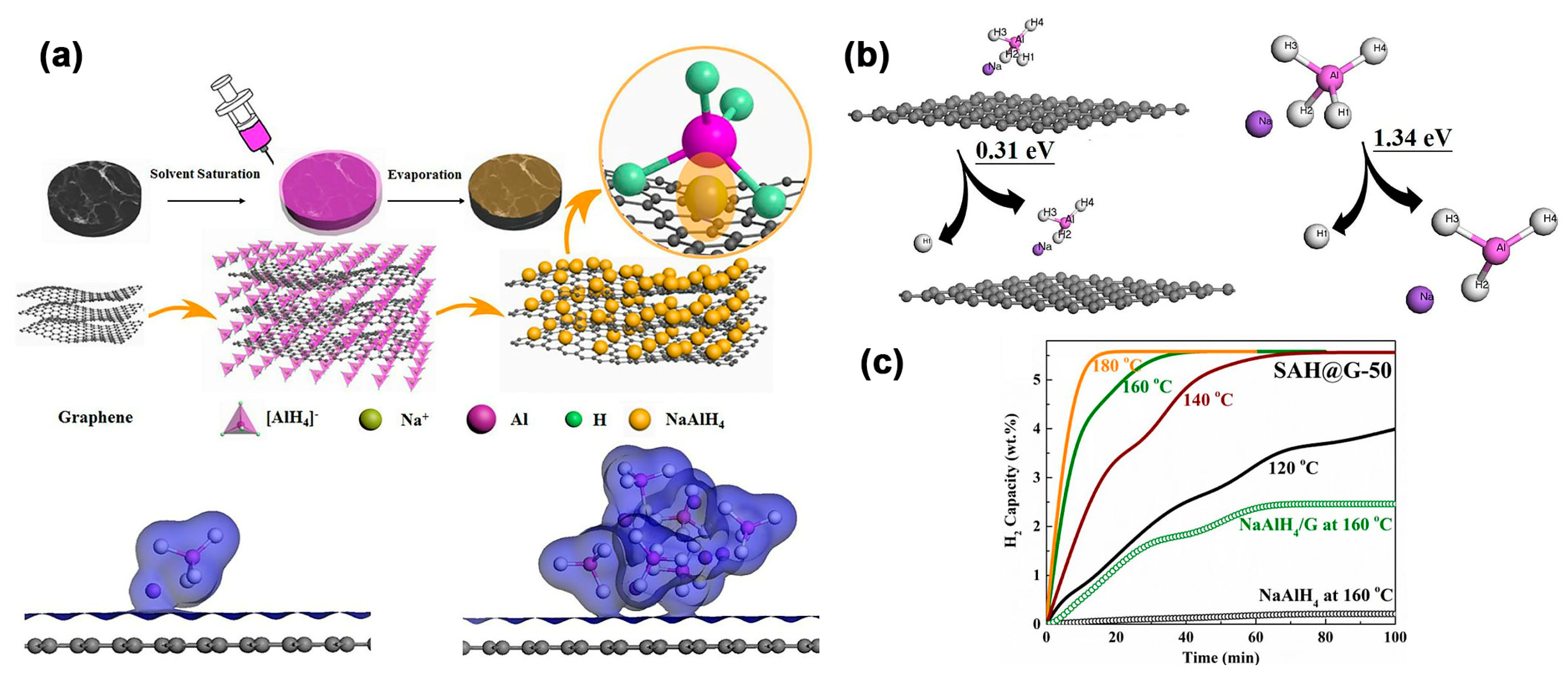
| NaAlH4 | – | NaAlH4@G | NaAlH4 (12 nm)@G–50; G weakens Al–H bonds of NaAlH4; des.: 5.6 wt.% at 300 °C; 3.8 wt.% (120 °C); Ea = 68.23 kJ/mol (vs. 128 kJ/mol for bulk) | [114] |
| NaAlH4 | NP–TiH2 | NaAlH4– 7 wt.% NP–TiH2@ G | NaAlH4 catalyzed by 7 wt.% NP–TiH2@G as active catalyst; 5 wt.% H2 is achieved reversibly, with onset below 80 °C; TiH2 (~50 nm lateral, ~15 nm thick) produced my metathesis TiCl4 + LiH in THF with G support | [115] |
| NaAlH4 | N. CNTs | NaAlH4@N-doped G/CNTs | NaAlH4@N-doped G and CNTs (produced by NH3 treatment of G, 600 °C, 30 min); 1.8 wt.% reversible H2 storage | [116] |
| NaAlH4 | – | NaAlH4/GNs | NaAlH4/GNs graphene nanosheets, C60 fullerenes and MC mesoporous carbon; NaAlH4–support interactions revealed by FE–SEM and 27Al solid state NMR. | [117] |
| NaAlH4 | – | NaAlH4@rGO | NaAlH4 @rGO framework, GOF with NaAlH4@GOF (1 M), desorbing 1.01 wt.% (20.0 wt.% NaAlH4), 16.6% of bulk NaAlH4 due to oxygen functional groups in the GOF reduced by BH4−., | [118] |
| NaAlH4 | TiO2 | TiO2–NaAlH4@G | Layer-by-layer TiO2–NaAlH4@G composites with 90% high loading (des. peak at 191.6 °C vs. 286.5 °C for bulk NaAlH4) | [119] |
| NaAlH4 | – | NaAlH4@G | NaAlH4@G nanofibers | [120] |
| NaAlH4 | Al, TiCl3 | Al, Ti-doped NaAlH4@G | Ti-doped NaAlH4@G: NaAlH4 co-doped 2 mol% TiCl3, 10 mol% G, 5 mol% Al (ball milling) | [121] |
| NaAlH4 | CeH2.51 | NaAlH4@FLG/Ce (CeH2.51) | NaAlH4@FLG/Ce (CeH2.51); onset at 85 °C, 5.06 wt.% H2 at 200 °C; 4.91 wt.% reversible after 8 a/d cycles. Ce activated G surface. | [122] |
| NaAlH4 | Heteroatoms | NaAlH4@dopant/G | NaAlH4@dopant/G (dopant: S-, N-, vacancies, B, N, O-, P-, F-, HO-), investigated by means of DFT | [123] |
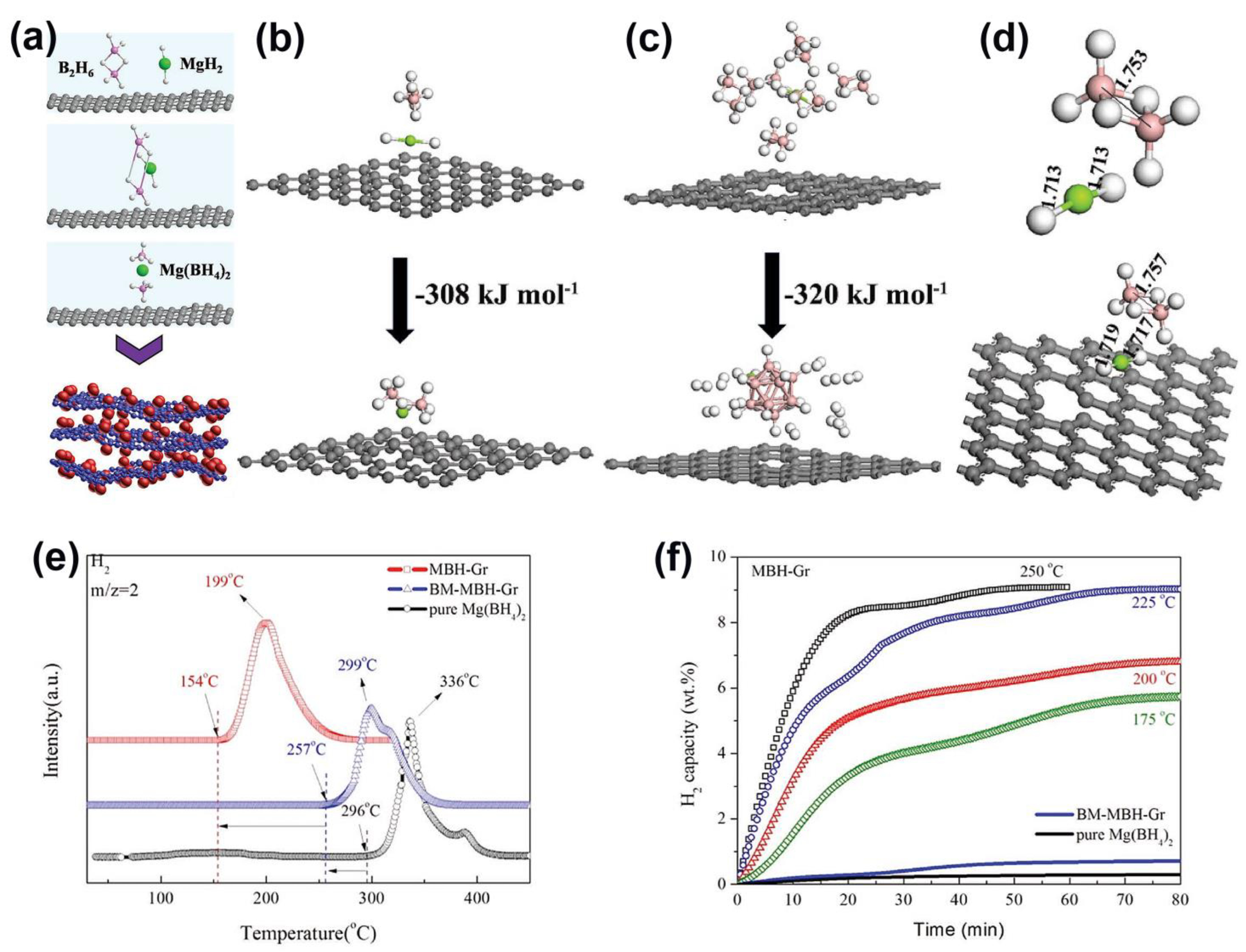
| Mg(BH4)2 | – | Mg(BH4)2@G | Mg(BH4)2@G synthesized by [MgH2@G + B2H6]; it was shown that G weakens the Mg–H and B–H bonds, affording a desorption of H2 starting at 154 °C (onset) and up to 225 °C (end) | [124] |
| Mg(BH4)2 | – | Mg(BH4)2@rGO | Mg(BH4)2@rGO (1–4 wt.%), investigation of mechanism and phase evolution | [125] |
| Mg(BH4)2 | – | Mg(BH4)2@G | Mg(BH4)2@G; the 3 polymorphs of Mg were produced from MgBu2 by tuning reaction conditions (11.2, 10.3, and 9.9 wt % H for the γ, β, and α phases) | [126] |
| NaMgH3 | – | NaMgH3 @GO | NaMgH3 @GO and NaMgH3 @ MWCNTs | [127] |
| K2NaAlH6 | – | K2NaAlH6–GS | GS graphene sheets as catalysts for sodium potassium alanate K2NaAlH6 | [128] |
| Mn(BH4)2 | Ni, LiNH2 | Mn(BH4)2@(Ni, LiNH2)/G | Mn(BH4)2@G (Ni, LiNH2 additives) by solvent infiltration/extraction | [129] |
| Mg2Ni | – | Mg2Ni–rGO/MWCNTs | Mg2Ni–rGO/MWCNTs for battery—supercapacitor hybrid device (high discharge capacity 644 mAh/g) | [130] |
| Mg2Ni | – | Mg2Ni/rGO | rGO/Mg2Ni—enhanced cycling stability | [131] |
| Mg2NiH4 | – | Mg2NiH4@GS | Mg2NiH4@GS (surface graphene nanosheets) | [132] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Comanescu, C. Graphene Supports for Metal Hydride and Energy Storage Applications. Crystals 2023, 13, 878. https://doi.org/10.3390/cryst13060878
Comanescu C. Graphene Supports for Metal Hydride and Energy Storage Applications. Crystals. 2023; 13(6):878. https://doi.org/10.3390/cryst13060878
Chicago/Turabian StyleComanescu, Cezar. 2023. "Graphene Supports for Metal Hydride and Energy Storage Applications" Crystals 13, no. 6: 878. https://doi.org/10.3390/cryst13060878
APA StyleComanescu, C. (2023). Graphene Supports for Metal Hydride and Energy Storage Applications. Crystals, 13(6), 878. https://doi.org/10.3390/cryst13060878