Role of the Dilution of the Gd Sublattice in Forming the Scintillation Properties of Quaternary (Gd,Lu)3Al2Ga3O12: Ce Ceramics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Synthesis
2.2. Instrumentation
3. Results and Discussion
3.1. Microstructure Properties
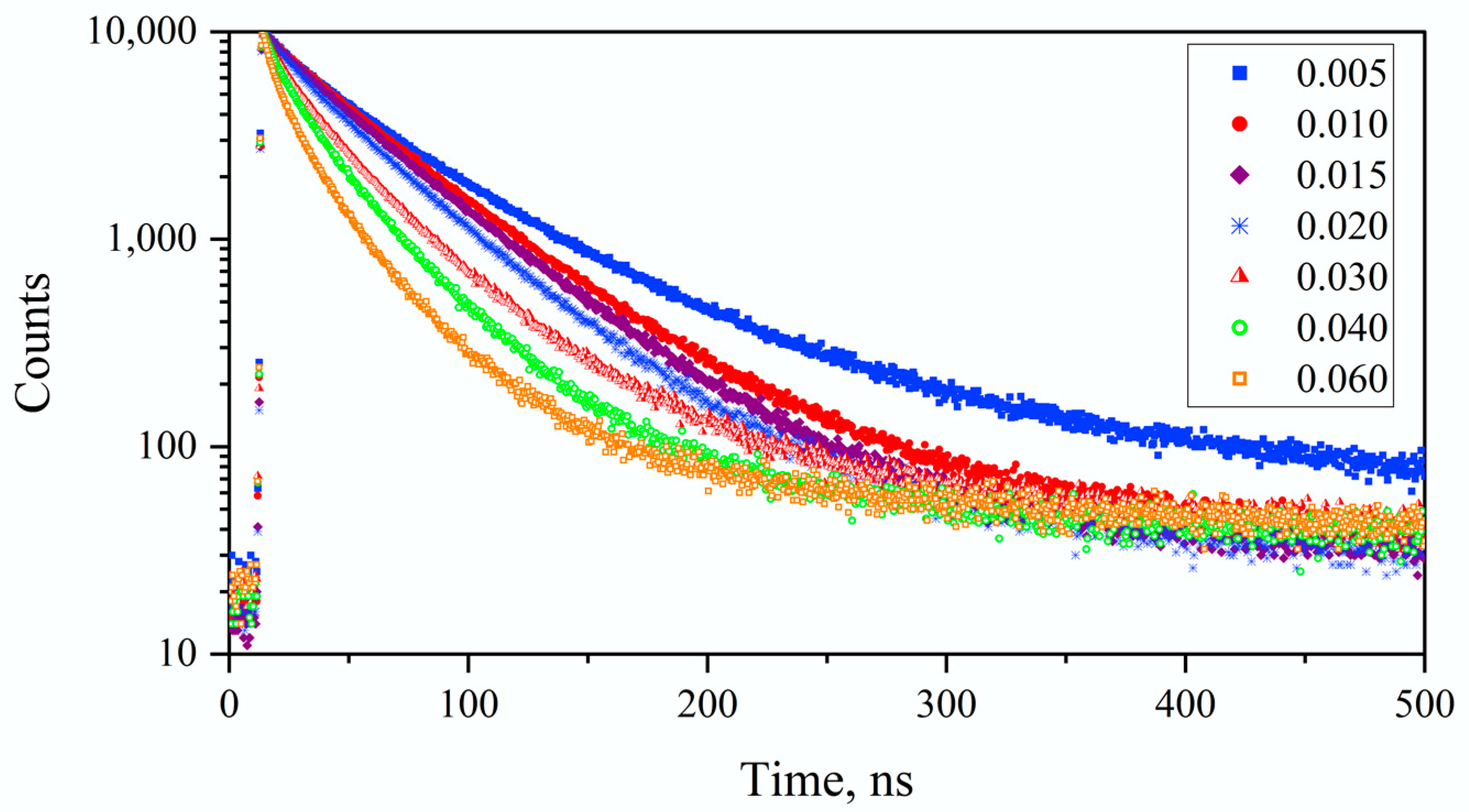
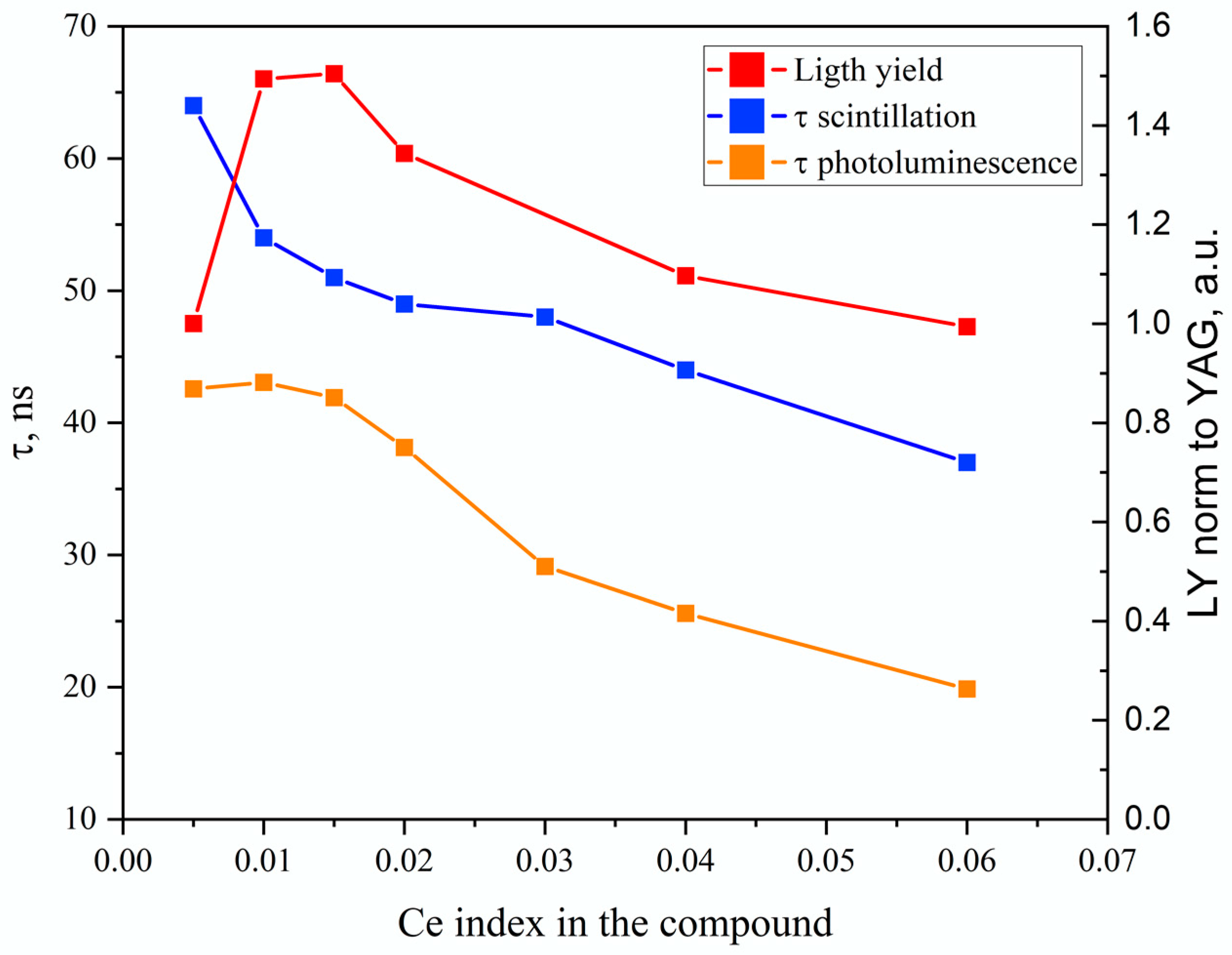
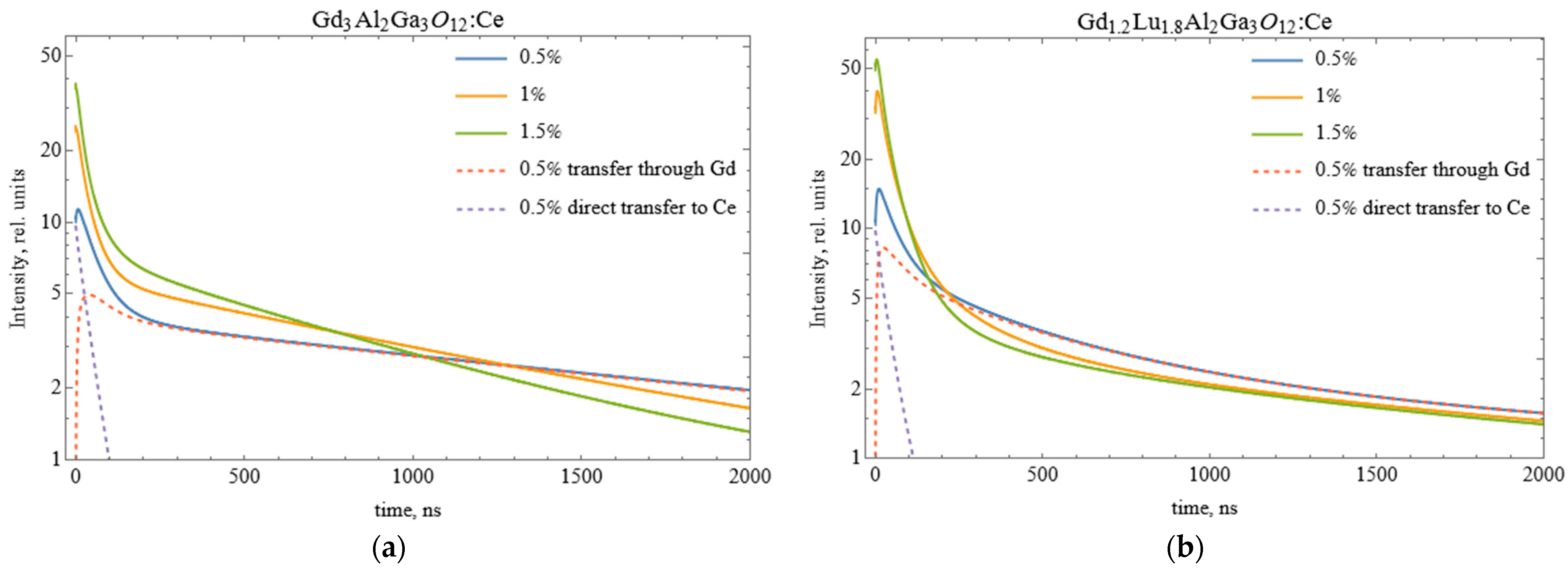
3.2. Results on GAGG:Ce Series
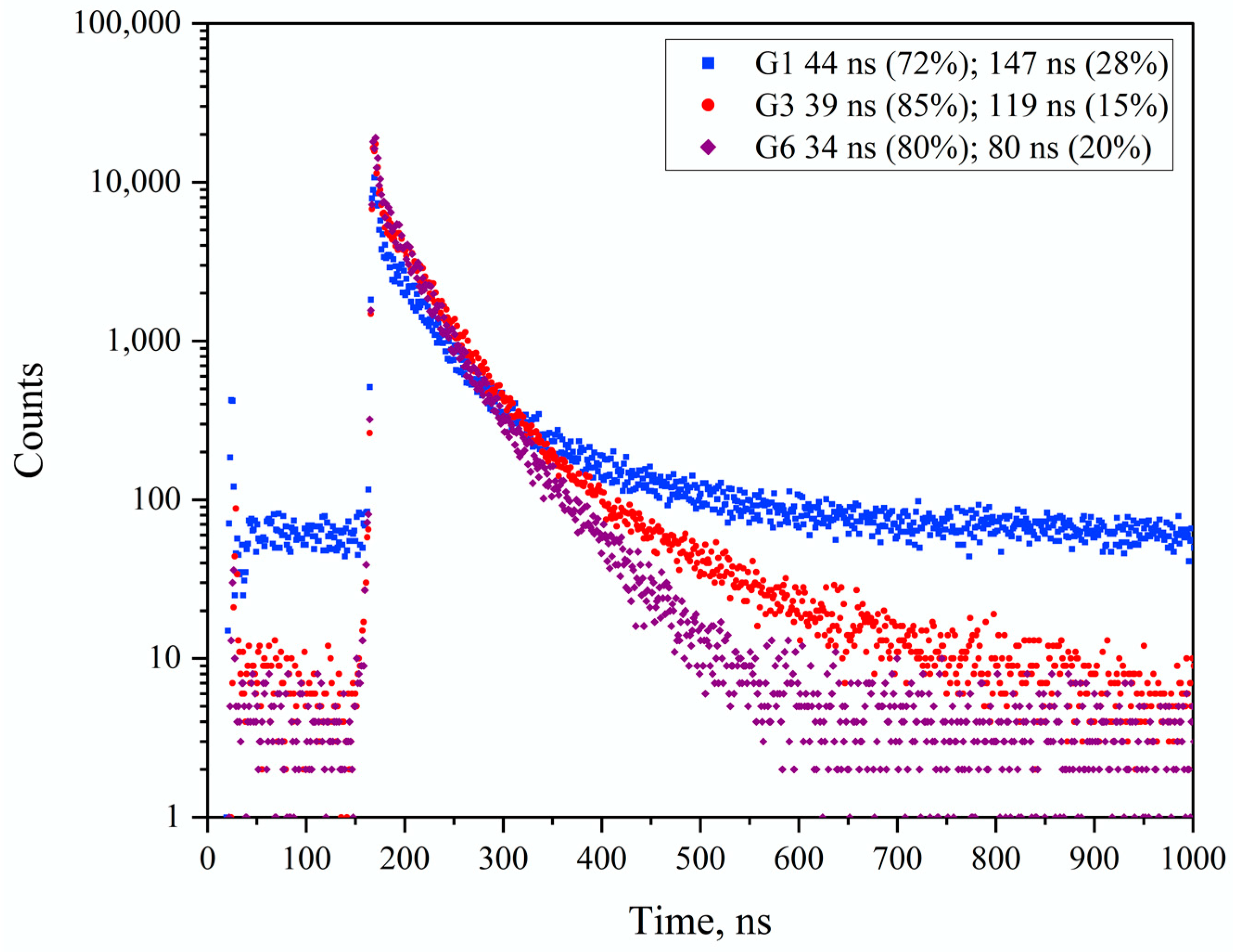
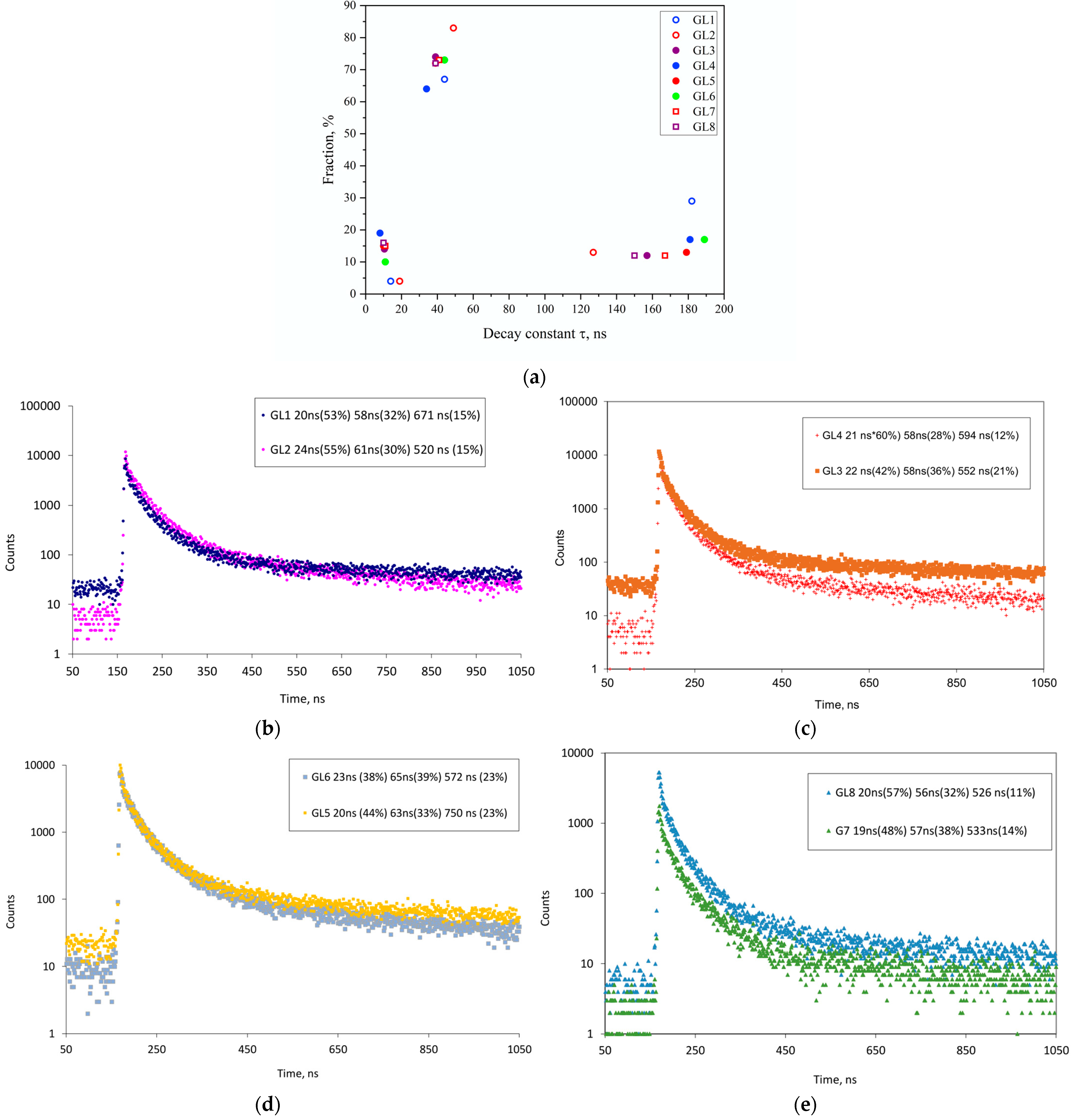
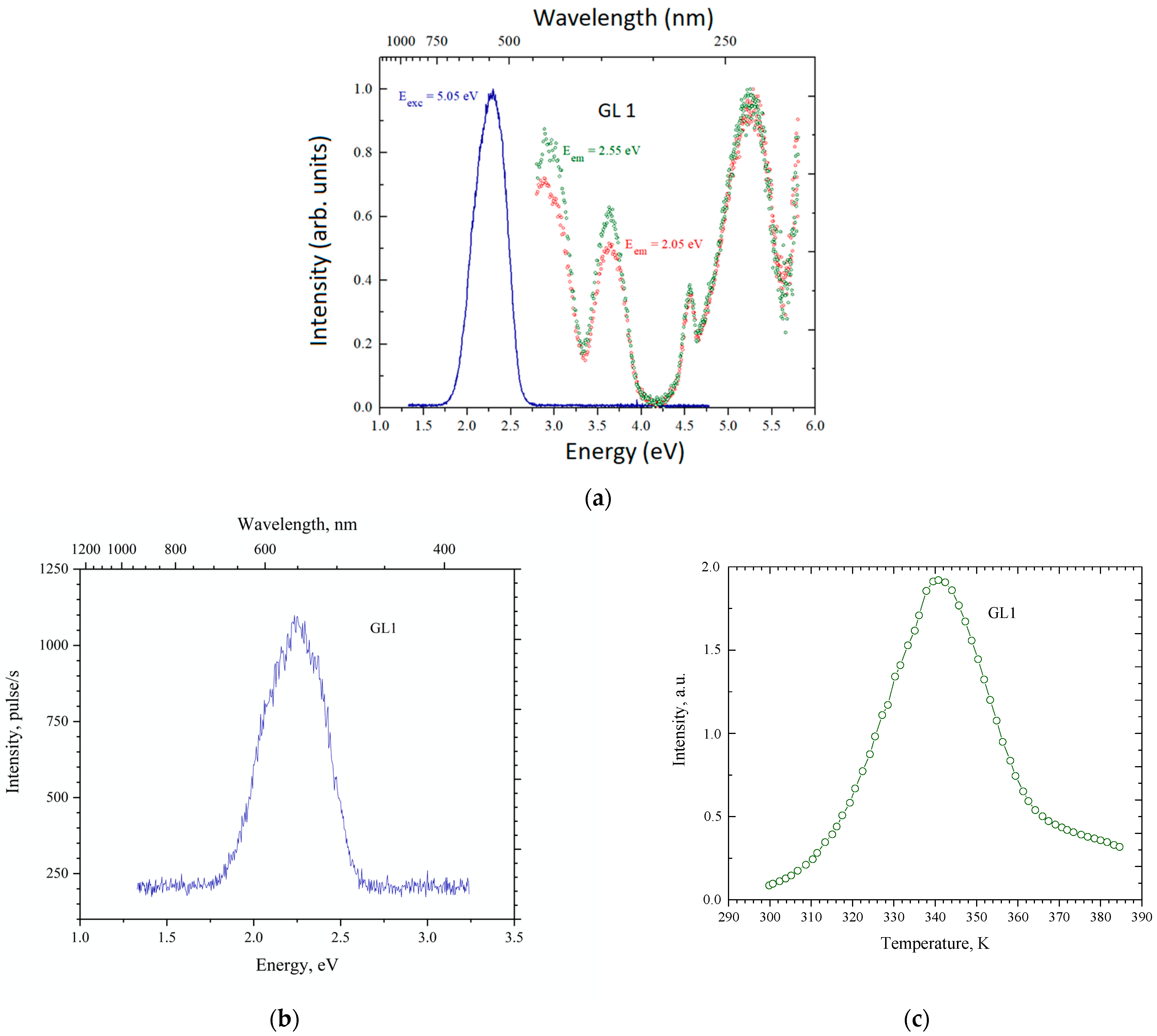
3.3. Results on GLAGG:Ce Series
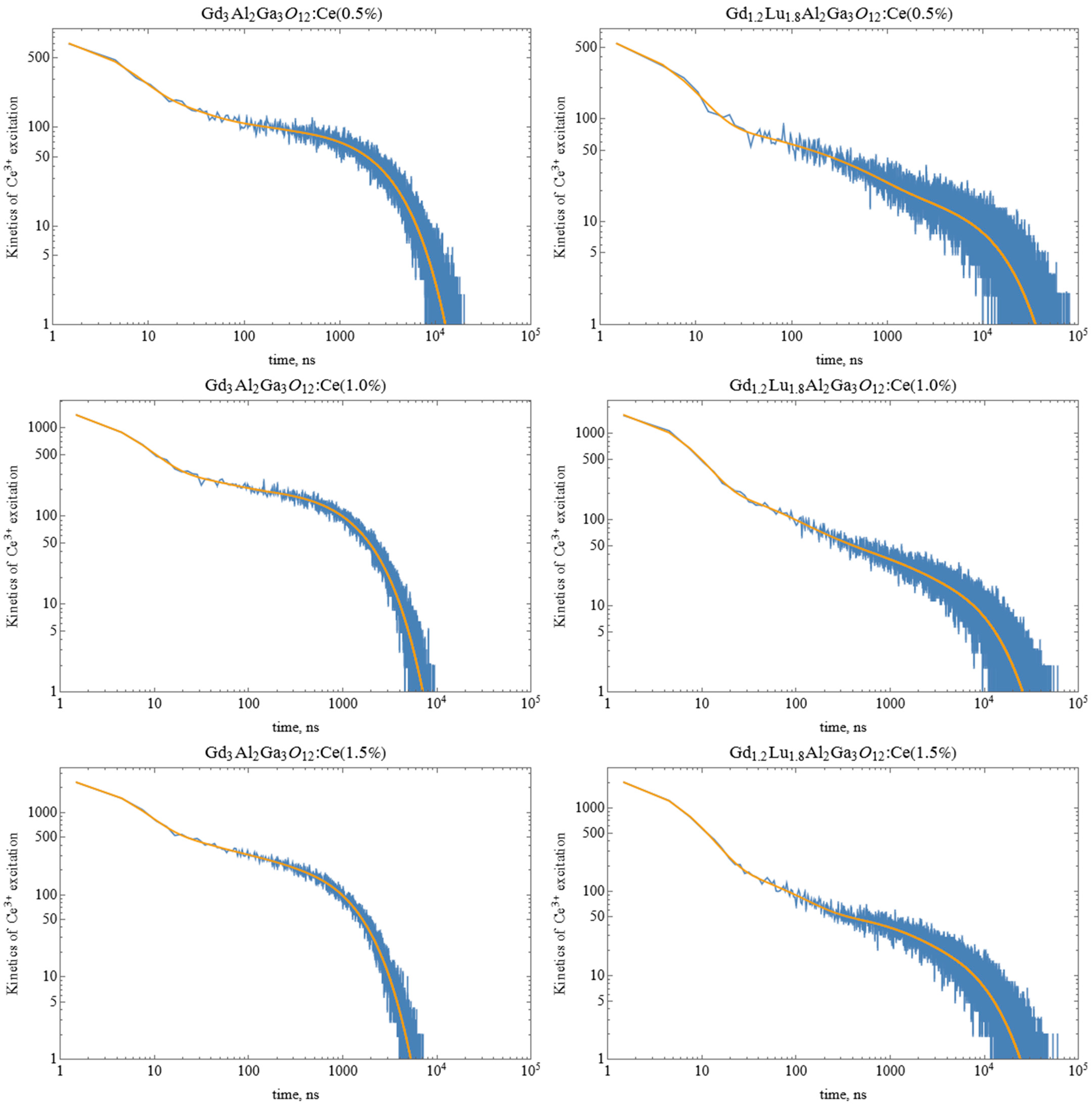
3.4. Monte-Carlo Simulation of Excitation Transfer in GAGG:Ce and GLAGG:Ce
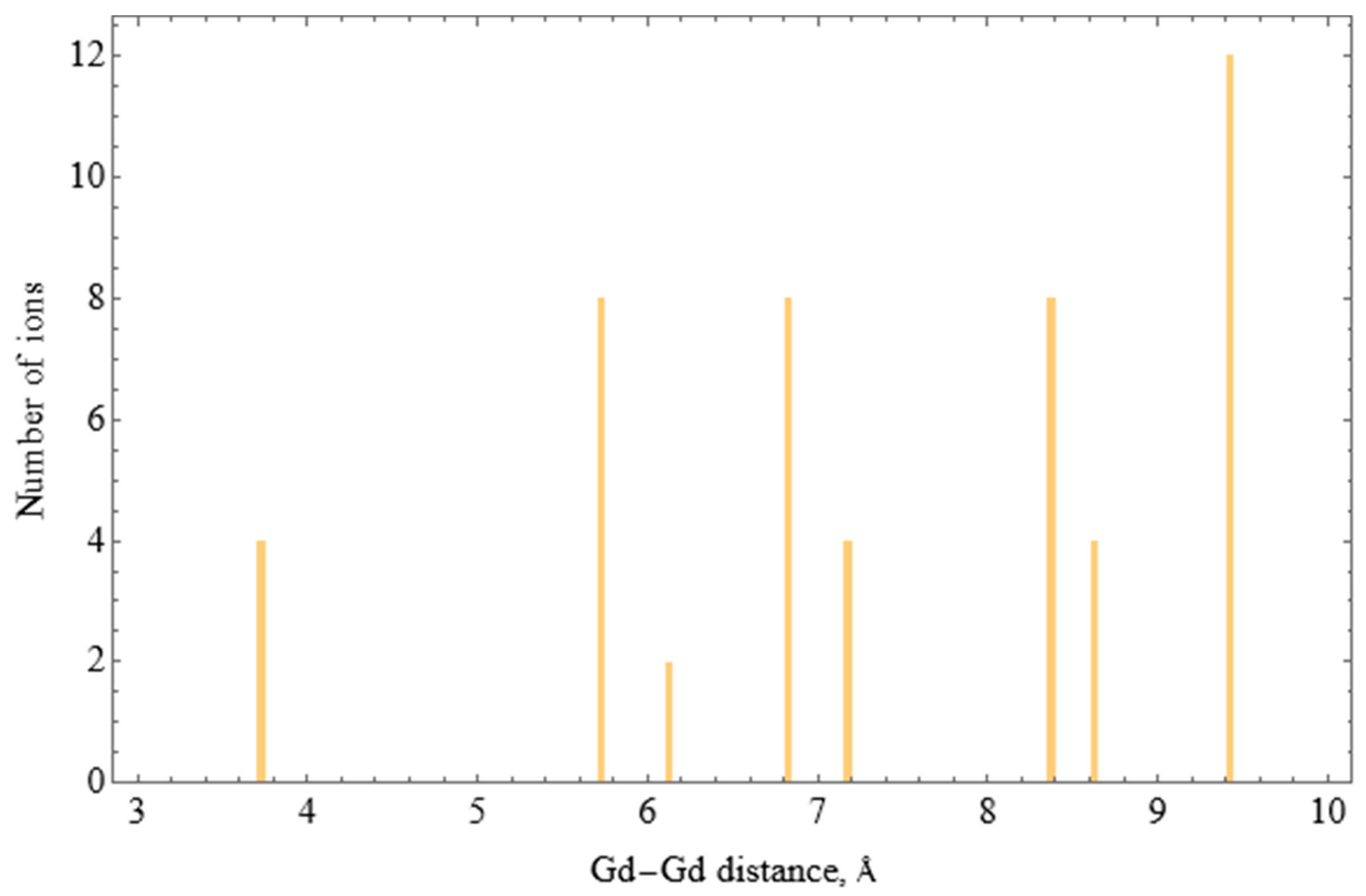
3.5. The Origin of the Slow Component of GLAGG:Ce
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamada, K.; Endo, T.; Tsutumi, K.; Yanagida, T.; Fujimoto, Y.; Fukabori, A.; Yoshikawa, A.; Pejchal, J.; Nikl, M. Composition Engineering in Cerium-Doped (Lu,Gd)3(Ga,Al)5O12 Single-Crystal Scintillators. Cryst. Growth Des. 2011, 11, 4484–4490. [Google Scholar] [CrossRef]
- Kamada, K.; Yanagida, T.; Pejchal, J.; Nikl, M.; Endo, T.; Tsutumi, K.; Fujimoto, Y.; Fukabori, A.; Yoshikawa, A. Scintillator-oriented combinatorial search in Ce-doped (Y,Gd)3(Ga,Al)5O12multicomponent garnet compounds. J. Phys. D Appl. Phys. 2011, 44, 505104. [Google Scholar] [CrossRef]
- Kamada, K.; Yanagida, T.; Endo, T.; Tsutumi, K.; Usuki, Y.; Nikl, M.; Fujimoto, Y.; Fukabori, A.; Yoshikawa, A. 2 inch diameter single crystal growth and scintillation properties of Ce:Gd3Al2Ga3O12. J. Cryst. Growth 2012, 352, 88–90. [Google Scholar] [CrossRef]
- Kamada, K.; Kurosawa, S.; Prusa, P.; Nikl, M.; Kochurikhin, V.V.; Endo, T.; Tsutumi, K.; Sato, H.; Yokota, Y.; Sugiyama, K.; et al. Cz grown 2-in. size Ce:Gd3(Al,Ga)5O12 single crystal; relationship between Al, Ga site occupancy and scintillation properties. Opt. Mater. 2014, 36, 1942–1945. [Google Scholar] [CrossRef]
- Korzhik, M.; Alenkov, V.; Buzanov, O.; Dosovitskiy, G.; Fedorov, A.; Kozlov, D.; Mechinsky, V.; Nargelas, S.; Tamulaitis, G.; Vaitkevičius, A. Engineering of a new single-crystal multi-ionic fast and high-light-yield scintillation material (Gd0.5–Y0.5)3Al2Ga3O12:Ce,Mg. CrystEngComm 2020, 22, 2502–2506. [Google Scholar] [CrossRef]
- Korzhik, M.; Borisevich, A.; Fedorov, A.; Gordienko, E.; Karpyuk, P.; Dubov, V.; Sokolov, P.; Mikhlin, A.; Dosovitskiy, G.; Mechninsky, V.; et al. Uglov, The scintillation mechanisms in Ce and Tb doped (GdxY1-x)Al2Ga3O12 quaternary garnet structure crystalline ceramics. J. Lumin. 2021, 234, 117933. [Google Scholar] [CrossRef]
- Solodovnikov, D.; Weber, M.; Haven, D.; Lynn, K. Single crystal Ce doped scintillator material with garnet structure sensitive to gamma ray and neutron radiation. J. Cryst. Growth 2012, 52, 99–102. [Google Scholar] [CrossRef]
- Chewpraditkul, W.; Pánek, D.; Brůža, P.; Chewpraditkul, W.; Wanarak, C.; Pattanaboonmee, N.; Babin, V.; Bartosiewicz, K.; Kamada, K.; Yoshikawa, A.; et al. Luminescence properties and scintillation response in Ce3+ doped Y2GdAl5-xGaxO12 (x 5 2, 3, 4) single crystals. J. Appl. Phys. 2014, 116, 083505. [Google Scholar] [CrossRef]
- Chewpraditkul, W.; Brůža, P.; Pánek, D.; Pattanaboonmee, N.; Wantong, K.; Chewpraditkul, W.; Babin, V.; Bartosiewicz, K.; Kamada, K.; Yoshikawa, A.; et al. Optical and scintillation properties of Ce3+-doped YGd2Al5xGaxO12(x¼2,3,4) single crystal scintillators. J. Lumin. 2016, 169, 43–50. [Google Scholar] [CrossRef]
- Chewpraditkul, W.; Pattanaboonmee, N.; Chewpraditkul, W.; Sakthong, O.; Yamaji, A.; Kamada, K.; Kurosawa, S.; Yoshikawa, A.; Drozdowski, W.; Witkowski, M.E.; et al. Scintillation Characteristics of Mg2+-Codoped Y0.8Gd2.2(Al5−xGax)O12:Ce Single Crystals. IEEE Trans. Nucl. Sci. 2020, 67, 910–914. [Google Scholar] [CrossRef]
- Cherepy, N.J.; Payne, S.A.; Asztalos, S.J.; Hull, G.; Kuntz, J.D.; Niedermayr, T.; Pimputkar, S.; Roberts, J.J.; Sanner, R.D.; Tillotson, T.M.; et al. Scintillators With Potential to Supersede Lanthanum Bromide. IEEE Trans. Nucl. Sci. 2009, 56, 873–880. [Google Scholar] [CrossRef]
- Seeley, Z.; Cherepy, N.; Payne, S. Homogeneity of Gd-based garnet transparent ceramic scintillators for gamma spectroscopy. J. Cryst. Growth 2013, 379, 79–83. [Google Scholar] [CrossRef]
- Cherepy, N.; Payne, S.A.; Seeley, Z.; Cohen, P.C.; Andreaco, M.S.; Schmand, M.J. Transparent Ceramic Garnet Scintillator Detector for Positron Emission Tomography. U.S. Patent 10,000,698, 19 June 2018. [Google Scholar]
- Nargelas, S.; Talochka, Y.; Vaitkevičius, A.; Dosovitskiy, G.; Buzanov, O.; Vasil’Ev, A.; Malinauskas, T.; Korzhik, M.; Tamulaitis, G. Influence of matrix composition and its fluctuations on excitation relaxation and emission spectrum of Ce ions in (GdxY1-x)3Al2Ga3O12:Ce scintillators. J. Lumin. 2022, 242, 118590. [Google Scholar] [CrossRef]
- Lecoq, P.; Morel, C.; O Prior, J.; Visvikis, D.; Gundacker, S.; Auffray, E.; Križan, P.; Turtos, R.M.; Thers, D.; Charbon, E.; et al. Roadmap toward the 10 ps time-of-flight PET challenge. Phys. Med. Biol. 2020, 65, 21RM01. [Google Scholar] [CrossRef] [PubMed]
- Prusa, P.; Kamada, K.; Yoshikawa, A.; Kucera, M.; Nikl, M. (Lu,Gd)3(Al,Ga)5O12:Ce Scintillator—A Short History. XXXVI Days of Radiation Protection Book of Presentations and Posters; International Nuclear Information System: Poprad, Slovakia, 2014; p. 1171. [Google Scholar]
- Kamada, K.; Yoshikawa, A.; Endo, T.; Tsutsumi, K.; Shoji, Y.; Kurosawa, S.; Yokota, Y.; Prusa, P.; Nikl, M. Growth of 2-inch size Ce:doped Lu2Gd1Al2Ga3O12:Ce single crystal by the Czochralski method and their scintillation properties. J. Cryst. Growth 2015, 410, 14–17. [Google Scholar] [CrossRef]
- Witkiewicz-Lukaszek, S.; Gorbenko, V.; Zorenko, T.; Sidletskiy, O.; Gerasymov, I.; Fedorov, A.; Yoshikawa, A.; Mares, J.A.; Nikl, M.; Zorenko, Y. Development of composite Scintillators based on Single crystalline films and crystals of Ce3+ doped (Lu,Gd)3(Al,Ga)5O12 mixed garnet compounds. Cryst. Growth Des. 2018, 18, 1834–1842. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Z.; Dai, J.; Chen, H.; Shi, Y.; Kou, H.; Kučerková, R.; Beitlerová, A.; Nikl, M.; Li, J. Fabrication and optical properties of Ce doped Lu3Ga3Al2O12 scintillation ceramics. Opt. Mater. 2018, 85, 121–126. [Google Scholar] [CrossRef]
- Chewpraditkul, W.; Pattanaboonmee, N.; Chewpraditkul, W.; Kamada, K.; Yoshikawa, A.; Nikl, M. Luminescence and scintillation response of YGd2Al2Ga3O12:Ce and LuGd2Al2Ga3O12:Ce scintillators. Radiat. Meas. 2016, 90, 153–156. [Google Scholar] [CrossRef]
- Chewpraditkul, W.; Pattanaboonmee, N.; Chewpraditkul, W.; Szczesniak, T.; Moszynski, M.; Kamada, K.; Yoshikawa, A.; Kucerkova, R.; Nikl, M. Optical and scintillation properties of LuGd2Al2Ga3O12:Ce, Lu2GdAl2Ga3O12:Ce, and Lu2YAl2Ga3O12:Ce single crystals: A comparative study. Nucl. Instr. Methods Phys. Res. A 2021, 1004, 165381. [Google Scholar] [CrossRef]
- Glodo, J.; Wang, Y.; Shawgo, R.; Brecher, C.; Hawrami, R.H.; Tower, J.; Shah, K.S. New Developments in Scintillators for security Applications. Phys. Porc. 2017, 90, 285–290. [Google Scholar] [CrossRef]
- Available online: https://www.dynasil.com/product-category/scintillators/scintillation-detectors/gamma-neutron-ceramic-scintillation-detector-glugag/ (accessed on 15 June 2022).
- Babin, V.; Herman, P.; Kucera, M.; Nikl, M.; Zazubovich, S. Effect of Mg2+ co-doping on the photo- and thermally stimulated luminescence of the (Lu,Gd)3(Ga,Al)5O12:Ce epitaxial films. J. Lumin. 2019, 215, 116608. [Google Scholar] [CrossRef]
- Lecoq, P.; Gektin, A.; Korzhik, M. Inorganic Scintillators for Detector Systems: Physical Principles and Crystal Engineering, 2nd ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Khanin, V.; Venevtsev, I.; Chernenko, K.; Pankratov, V.; Klementiev, K.; van Swieten, T.; van Bunningen, A.J.; Vrubel, I.; Shendrik, R.; Ronda, C.; et al. Exciton interaction with Ce3+ and Ce4+ ions in (LuGd)3(Ga,Al)5O12 ceramics. J. Lumin. 2021, 237, 118150. [Google Scholar] [CrossRef]
- Khanin, V.M.; Rodnyi, P.A.; Wieczorek, H.; Ronda, C.R. Electron Traps in Gd3Ga3Al2O12:Ce Garnets Doped with Rare-Earth Ions. Tech. Phys. Lett. 2017, 43, 439–442. [Google Scholar] [CrossRef]
- Khanin, V.M.; Vrubel, I.I.; Polozkov, R.G.; Venevtsev, I.D.; Rodnyi, P.A.; Tukhvatulina, T.; Chernenko, K.; Drozdowski, W.; Witkowski, M.E.; Makowski, M.; et al. Complex Garnets: Microscopic Parameters Characterizing Afterglow. J. Phys. Chem. C 2019, 123, 22725–22734. [Google Scholar] [CrossRef]
- Schauer, P.; Lalinský, O.; Kučera, M.; Lučeničová, Z.; Hanuš, M. Effect of Mg co-doping on cathodoluminescence properties of LuGAGG:Ce single crystalline garnet films. Opt. Mater. 2017, 72, 359–366. [Google Scholar] [CrossRef]
- Rodnyi, P.A.; Venevtsev, I.D.; Khanin, V.M. Thermal quenching of luminescence in (Lu,Gd,Y)3(Ga, Al)5O12:Ce complex garnet ceramics at high and low temperatures. Phys. Complex Syst. Saint Petersburg Russia 2021, 2, 1–5. [Google Scholar] [CrossRef]
- Babin, V.; Chernenko, K.; Kučera, M.; Nikl, M.; Zazubovich, S. Photostimulated luminescence and defects creation processes in Ce- doped epitaxial films of multicomponent Lu3-xGdxGayAl5-yO12 garnets. J. Lumin. 2016, 179, 487–495. [Google Scholar] [CrossRef]
- Korzhik, M.; Alenkov, V.; Buzanov, O.; Fedorov, A.; Dosovitskiy, G.; Grigorjeva, L.; Mechinsky, V.; Sokolov, P.; Tratsiak, Y.; Zolotarjovs, A.; et al. Nanoengineered Gd3Al2Ga3O12 scintillation materials with disordered garnet structure for novel detectors of ionizing radiation. Cryst. Res. Technol. 2019, 54, 1800172. [Google Scholar] [CrossRef]
- Dosovitskiy, G.; Dubov, V.; Karpyuk, P.; Volkov, P.; Tamulaitis, G.; Borisevich, A.; Vaitkevičius, A.; Prikhodko, K.; Kutuzov, L.; Svetogorov, R.; et al. Activator segregation and micro-luminescence properties in GAGG: Ce ceramics. J. Lumin. 2021, 236, 118140. [Google Scholar] [CrossRef]
- Gordienko, E.; Fedorov, A.; Radiuk, E.; Mechinsky, V.; Dosovitskiy, G.; Vashchenkova, E.; Kuznetsova, D.; Retivov, V.; Korjik, M.; Sandu, R. Synthesis of crystalline Ce-activated garnet phosphor powders and technique to characterize their scintillation light yield. Opt. Mater. 2018, 78, 312–318. [Google Scholar] [CrossRef]
- Auffray, E.; Augulis, R.; Fedorov, A.; Dosovitskiy, G.; Grigorjeva, L.; Gulbinas, V.; Koschan, M.; Lucchini, M.; Melcher, C.; Nargelas, S.; et al. Excitation Transfer Engineering in Ce-Doped Oxide Crystalline Scintillators by Codoping with Alkali-Earth Ions. Phys. Stat. Sol. 2018, 215, 1700798–1700808. [Google Scholar] [CrossRef]
- Dosovitskiy, G.; Fedorov, A.; Mechinsky, V.; Borisevich, A.; Tret’Jak, E.; Korjik, M. Persistent luminescence in powdered and ceramic polycrystalline Gd3Al2Ga3O12:Ce. IOP Conf. Ser. Mater. Sci. Eng. 2017, 169, 012014. [Google Scholar] [CrossRef]
- Yokota, M.; Tanimoto, O. Effects of Diffusion on Energy Transfer by Resonance. J. Phys. Soc. Jpn. 1967, 22, 779–784. [Google Scholar] [CrossRef]
- Weber, M.J. Multiphonon Relaxation of Rare-Earth Ions in Yttrium Orthoaluminate. Phys. Rev. B 1973, 8, 54–64. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Cryst. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Onderisinova, Z.; Kucera, M.; Hanus, M.; Nikl, M. Temperature-dependent nonradiative energy transfer from Gd3+ to Ce3+ ions in co-doped LuAG:Ce,Gd garnet scintillators. J. Lumin. 2015, 167, 106–113. [Google Scholar] [CrossRef]
- De Vries, A.J.; Kiliaan, H.S.; Blasse, G. An investigation of energy migration in luminescent diluted Gd3+ system. J. Solid State Chem. 1986, 65, 190–198. [Google Scholar] [CrossRef]
- Barandiarán, Z.; Seijo, L. Quantum chemical analysis of the bond lengths in fn and fn−1d1 states of Ce3+, Pr3+, Pa4+, and U4+ defects in chloride hosts. J. Chem. Phys. 2003, 119, 3785–3790. [Google Scholar] [CrossRef]
- Setlur, A.A.; Shiang, J.J.; Vess, C.J. Transition from Long-Range to Short-Range Energy Transfer through Donor Migration in Garnet Hosts. J. Phys. Chem. C 2011, 115, 3475–3480. [Google Scholar] [CrossRef]
- Omelkov, S.; Kirm, M.; Pustovarov, V.; Isaenko, L. Energy transfer in pure and rare-earth doped SrAlF5 crystals. IOP Conf. Ser. Mater. Sci. Eng. 2010, 15, 012011. [Google Scholar] [CrossRef] [Green Version]
- Tikhonov, A.N. Solution of incorrectly formulated problems and the regularization method. Sov. Math. 1963, 4, 1035–1038. [Google Scholar]
- Korzhik, M.; Tamulaitis, G.; Vasil’ev, A.N. Physics of Fast Processes in Scintillators; Springer International Publishing: Cham, Switzerland, 2020; Chapter 5.7. [Google Scholar]
- Kanai, T.; Satoh, M.; Miura, I. Characteristics of a Nonstoichiometric Gd3+δ(Al,Ga)5−δO12:Ce Garnet Scintillator. J. Am. Ceram. Soc. 2008, 91, 456–462. [Google Scholar] [CrossRef]
- Liu, S.; Mares, J.A.; Babin, V.; Hu, C.; Kou, H.; D’Ambrosio, C.; Li, J.; Pan, Y.; N Nikl, M. Composition and properties tailoring in Mg2+codoped non-stoichiometric LuAG:Ce,Mg scintillation ceramics. J. Eur. Ceram. Soc. 2017, 37, 1689–1694. [Google Scholar] [CrossRef]
- Korzhik, M.V. Physics of Scintillators on a Base of Oxide Single Crystals; BSU: Minsk, Belarus, 2003; p. 263. ISBN 985-485-061-7. [Google Scholar]
- Belsky, A.N.; Auffray, E.; Lecoq, P.; Dujardin, C.; Garnier, N.; Candibano, H.; Pedrini, C.; Petrosyan, A.G. Progress in the development of LuAlO3-based scintillators. IEEE Trans. Nucl. Sci. 2001, 48, 1095–1100. [Google Scholar] [CrossRef]
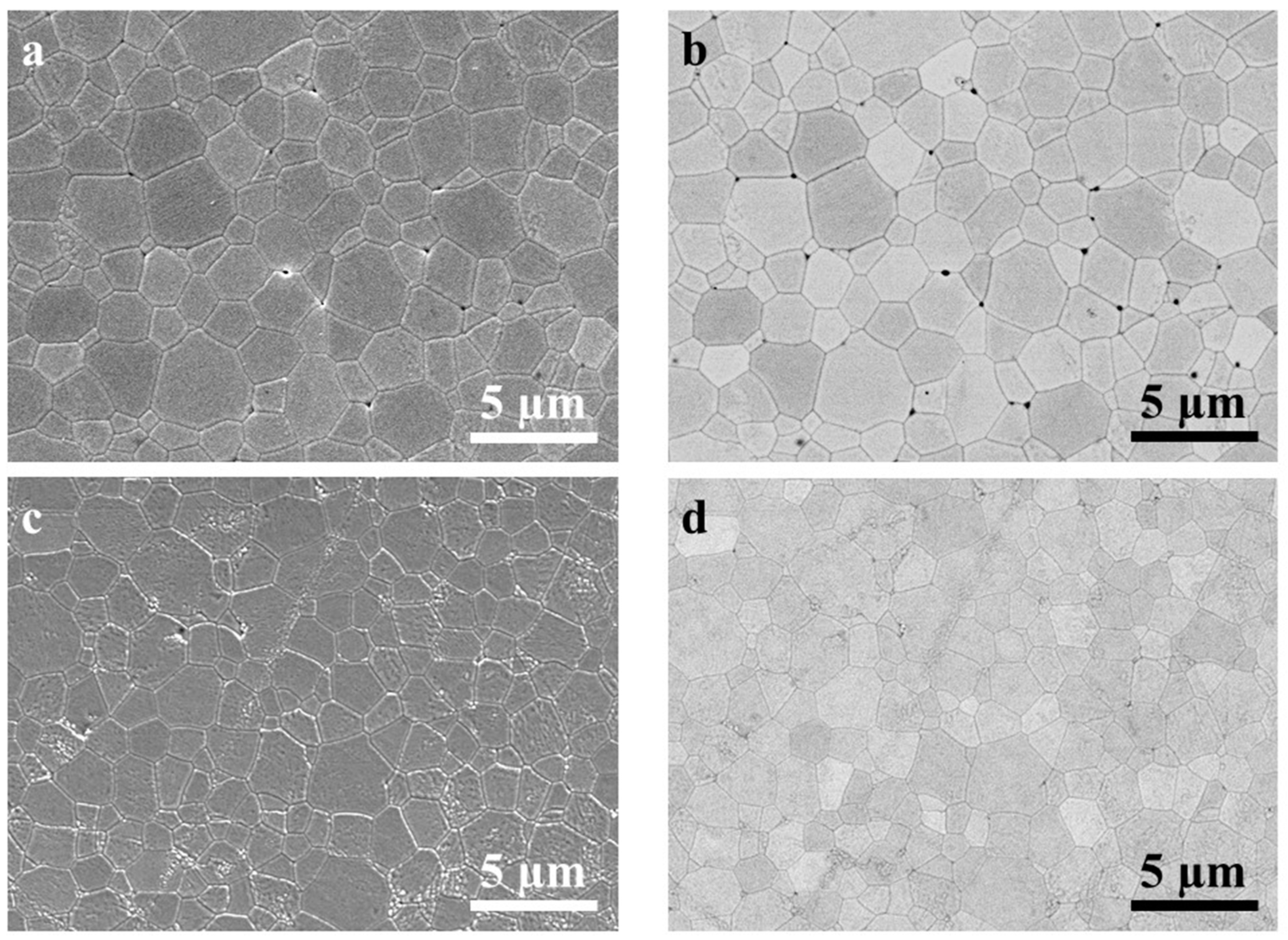


| GAGG Series | GLAGG Series |
|---|---|
| Gd3−xAl2Ga3O12:Cex (x = 0.005 or 0.167 at.%) (G1) | Gd1.195Lu1.79+xAl2Ga3O12:Ce (x = 0.015 or 0.5 at.%) (GL1) |
| Gd3−xAl2Ga3O12:Cex (x = 0.01 or 0.33 at.%) (G2) | Gd1.493Lu1.492+xAl2Ga3O12:Ce (x = 0.015 or 0.5 at.%) (GL2) |
| Gd3−xAl2Ga3O12:Cex (x = 0.015 or 0.5 at.%) (G3) | Gd1.2Lu1.77+xAl2Ga3O12:Ce (x = 0.03 or 1 at.%) (GL3) |
| Gd3−xAl2Ga3O12:Cex (x = 0.02 or 0.67 at.%) (G4) | Gd1.2Lu1.77+xAl2Ga3O12:Ce (x = 0.03 or 1 at.%) + 20 ppm (Mg), (GL4) |
| Gd3−xAl2Ga3O12:Cex (x = 0.03 or 1 at.%) (G5) | Gd1.26Lu1.77+xAl2Ga3O12:Ce (x = 0.03 or 1 at.%)(GL5); the super-stochiometric additive of Gd |
| Gd3−xAl2Ga3O12:Cex (x = 0.04 or 1.33 at.%) (G6) | Gd1.26Lu1.77+xAl2Ga3O12:Ce (x = 0.03 or 1 at.%) + 20 ppm (Mg) (GL6); the super-stochiometric additive of Gd |
| Gd3−xAl2Ga3O12:Cex (x = 0.06 or 2 at.%) (G7) | Gd1.2Lu1.755+xAl2Ga3O12:Ce (x = 0.045 or 1.5 at.%) (GL7) |
| Gd1.26Lu1.755+xAl2Ga3O12:Ce (x = 0.045 or 1.5 at.%) (GL8); the super-stochiometric additive of Gd |
| Sample | GL1 | GL2 | GL3 | GL4 | GL5 | GL6 | GL7 | GL8 |
|---|---|---|---|---|---|---|---|---|
| LY relative to YAG:Ce with grinded surfaces (20,600 ph/MeV) | 1.45 | 1.31 | 1.18 | 1.13 | 1.11 | 1 | 1.13 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korzhik, M.; Retivov, V.; Bondarau, A.; Dosovitskiy, G.; Dubov, V.; Kamenskikh, I.; Karpuk, P.; Kuznetsova, D.; Smyslova, V.; Mechinsky, V.; et al. Role of the Dilution of the Gd Sublattice in Forming the Scintillation Properties of Quaternary (Gd,Lu)3Al2Ga3O12: Ce Ceramics. Crystals 2022, 12, 1196. https://doi.org/10.3390/cryst12091196
Korzhik M, Retivov V, Bondarau A, Dosovitskiy G, Dubov V, Kamenskikh I, Karpuk P, Kuznetsova D, Smyslova V, Mechinsky V, et al. Role of the Dilution of the Gd Sublattice in Forming the Scintillation Properties of Quaternary (Gd,Lu)3Al2Ga3O12: Ce Ceramics. Crystals. 2022; 12(9):1196. https://doi.org/10.3390/cryst12091196
Chicago/Turabian StyleKorzhik, Mikhail, Vasilii Retivov, Alexei Bondarau, Georgiy Dosovitskiy, Valery Dubov, Irina Kamenskikh, Petr Karpuk, Daria Kuznetsova, Valentina Smyslova, Vitaly Mechinsky, and et al. 2022. "Role of the Dilution of the Gd Sublattice in Forming the Scintillation Properties of Quaternary (Gd,Lu)3Al2Ga3O12: Ce Ceramics" Crystals 12, no. 9: 1196. https://doi.org/10.3390/cryst12091196
APA StyleKorzhik, M., Retivov, V., Bondarau, A., Dosovitskiy, G., Dubov, V., Kamenskikh, I., Karpuk, P., Kuznetsova, D., Smyslova, V., Mechinsky, V., Pustovarov, V., Tavrunov, D., Tishchenko, E., & Vasil’ev, A. (2022). Role of the Dilution of the Gd Sublattice in Forming the Scintillation Properties of Quaternary (Gd,Lu)3Al2Ga3O12: Ce Ceramics. Crystals, 12(9), 1196. https://doi.org/10.3390/cryst12091196








