A Critical Review on Engineering of d-Mannitol Crystals: Properties, Applications, and Polymorphic Control
Abstract
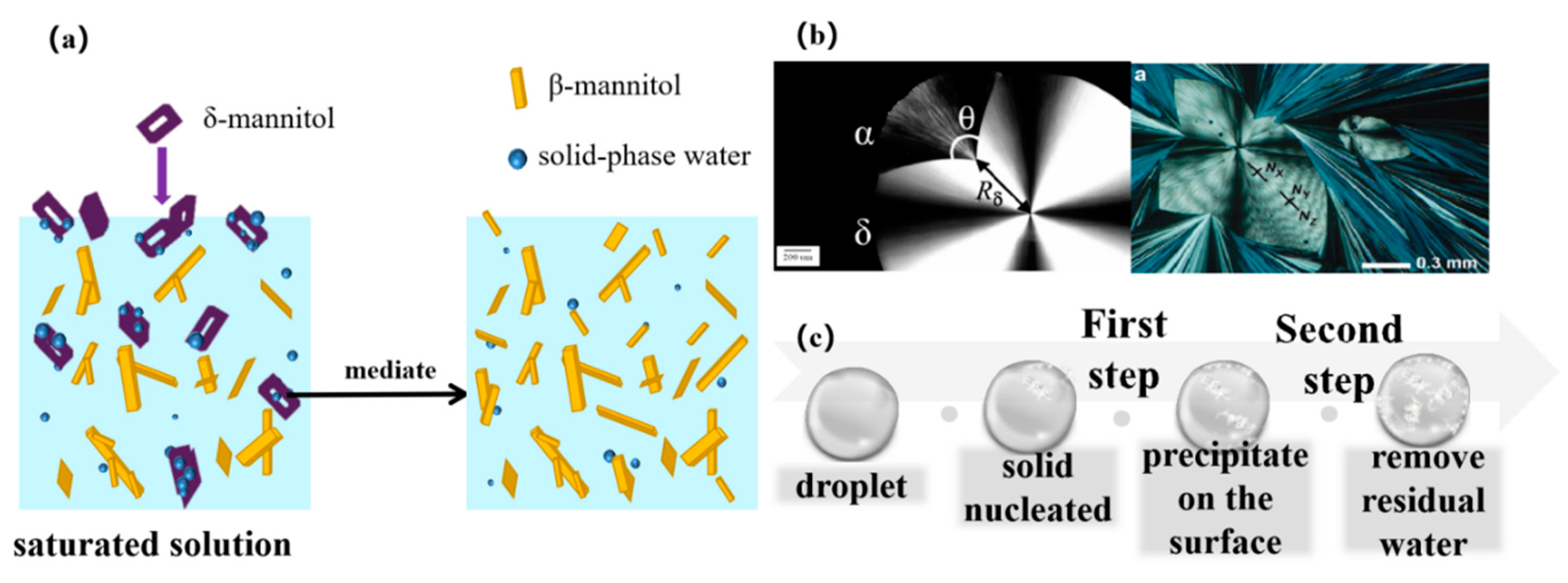
:1. Introduction
2. Physicochemical Properties and Analytical Techniques of d-Mannitol Crystals
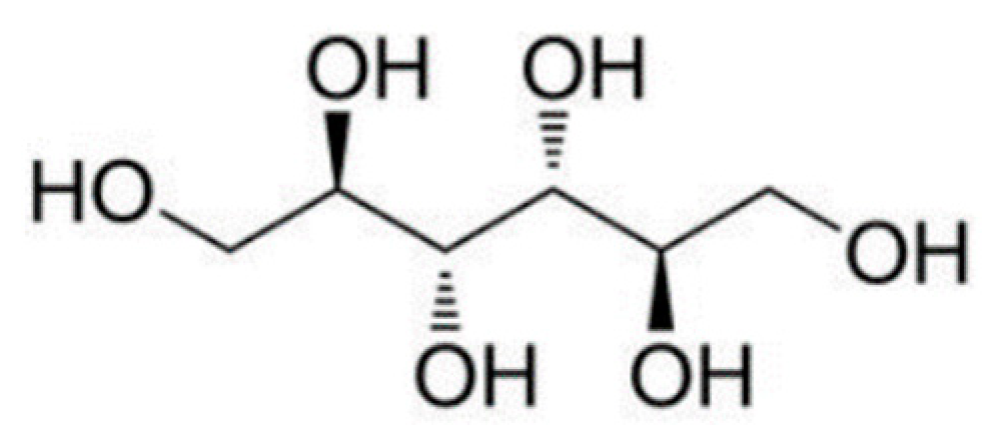
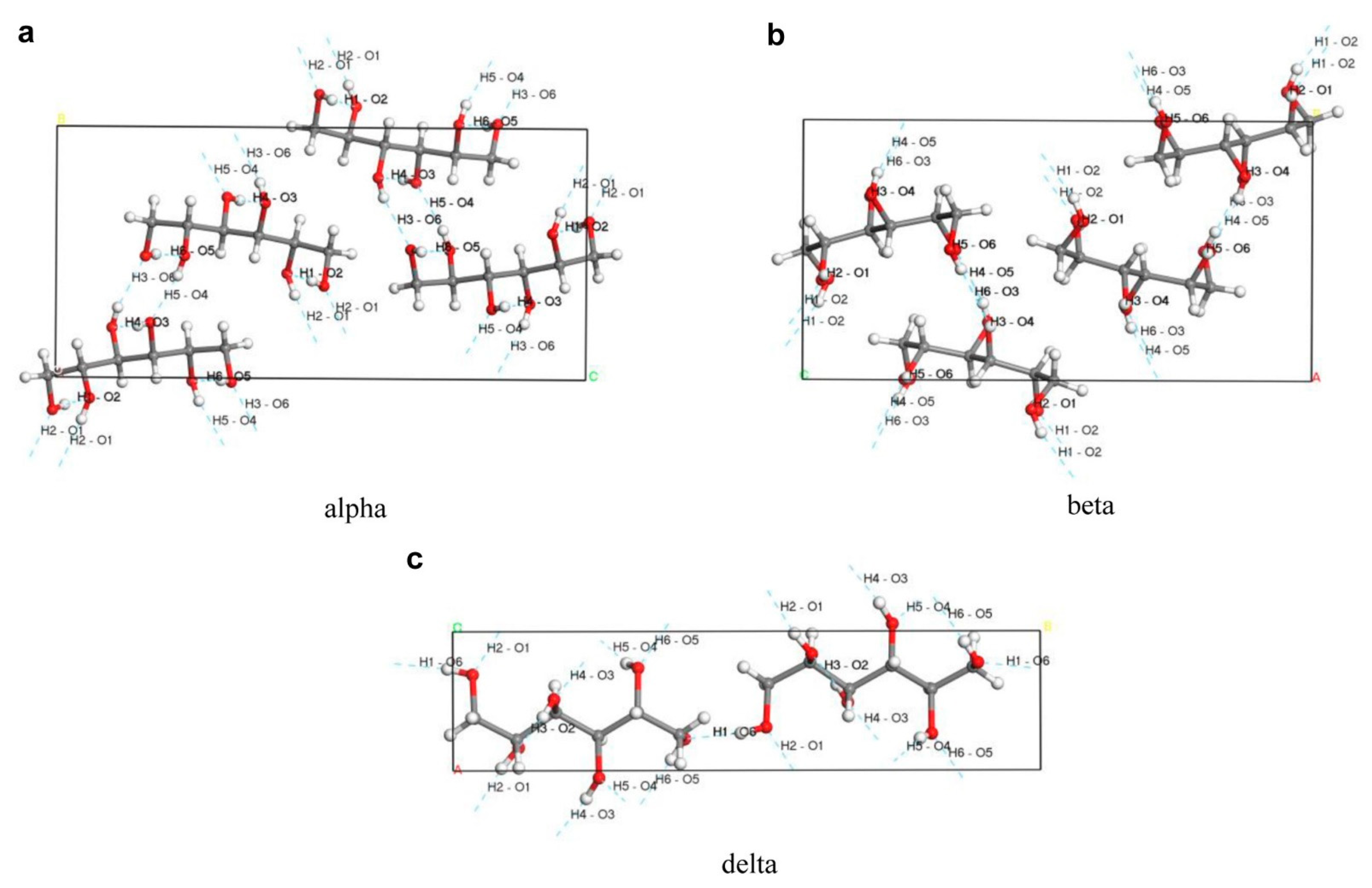
2.1. Physicochemical Properties of d-Mannitol Crystals
| Mark | Literature | Space Group | a/nm | b/nm | c/nm | β/(°) | v/nm3 | z | Dx/kg·m−3 |
|---|---|---|---|---|---|---|---|---|---|
| β | Marwick, 1931 [16] | P212121 | 0.865 | 1.690 | 0.556 | – | 203 ① | 4 | 1.488 |
| β | Rye, 1952 ② [20] | P212121 | 0.865 | 1.690 | 0.556 | – | 203 ① | 4 | 1.488 |
| β | Berman, 1968 [21] | P212121 | 0.8672 | 1.6875 | 0.5560 | – | – | 4 | 1.487 |
| β | Walter-Levy, 1968 [22] | P212121 | 0.8672 | 1.689 | 0.5549 | – | – | 4 | 1.489 |
| β | Kaminsky, 1997 [23] | P212121 | 0.8694 (7) | 1.6902 (8) | 0.5549 (6) | – | 815 | 4 | 1.490 (2) |
| β | R. Fronczek, 2003 [18] | P212121 | 0.55381 (10) | 0.8580 (2) | 1.6795 (5) | – | 789.0 (3) | 4 | 1.516 |
| α | Walter-Levy, 1968 [22] | P212121 | 0.8839 | 1.8778 | 0.4896 | – | – | 4 | 1.470 |
| K | Kim, 1968 [24] | P212121 | 0.8942 | 1.8798 | 0.4893 | – | – | 4 | 1.471 |
| α | R. Fronczek, 2003 [18] | P212121 | 0.48653 (10) | 0.8873 (2) | 1.8739 (3) | – | 809.0 (3) | 4 | 1.496 |
| δ | Walter-Levy, 1968 [22] | P21 or P21/m | 0.5095 | 1.8254 | 0.4919 | 118.36 | – | 2 | 1.501 |
| δ | R. Fronczek, 2003 [18] | P21 | 0.4899 (2) | 1.8268 (6) | 0.5043 (2) | 118.39 (2) | 397.0 (3) | 2 | 1.524 |
| δ | Botez, 2003 [17] | P21 | 0.508941 (5) | 1.82504 (2) | 0.491702 (5) | 118.303 | 402.103 | 2 | 1.377 ③ |
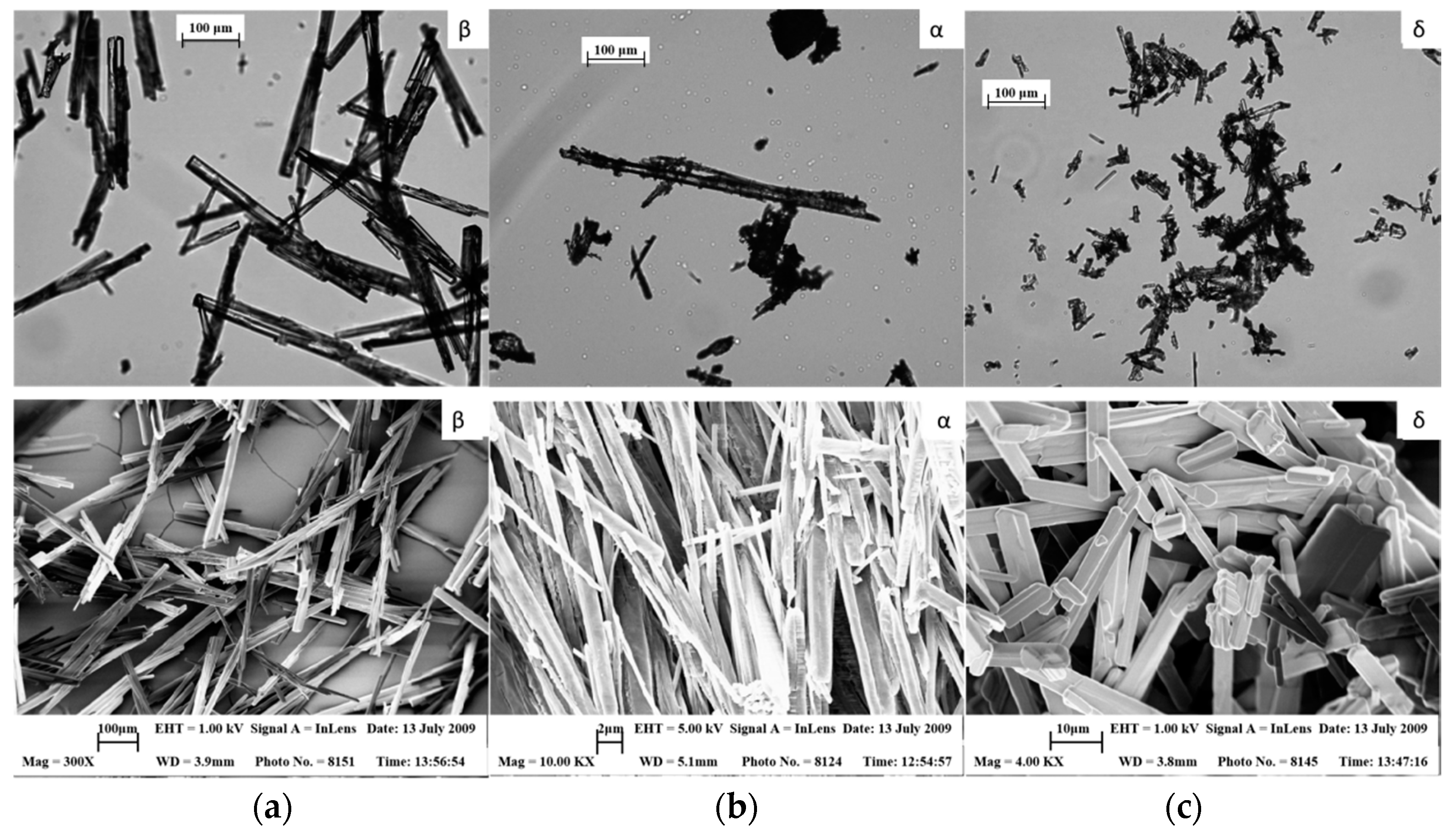
2.2. Analytical Techniques of d-Mannitol Polymorphisms
3. Polymorphic Control during the Preparation of Mannitol Crystals
3.1. Recrystallization
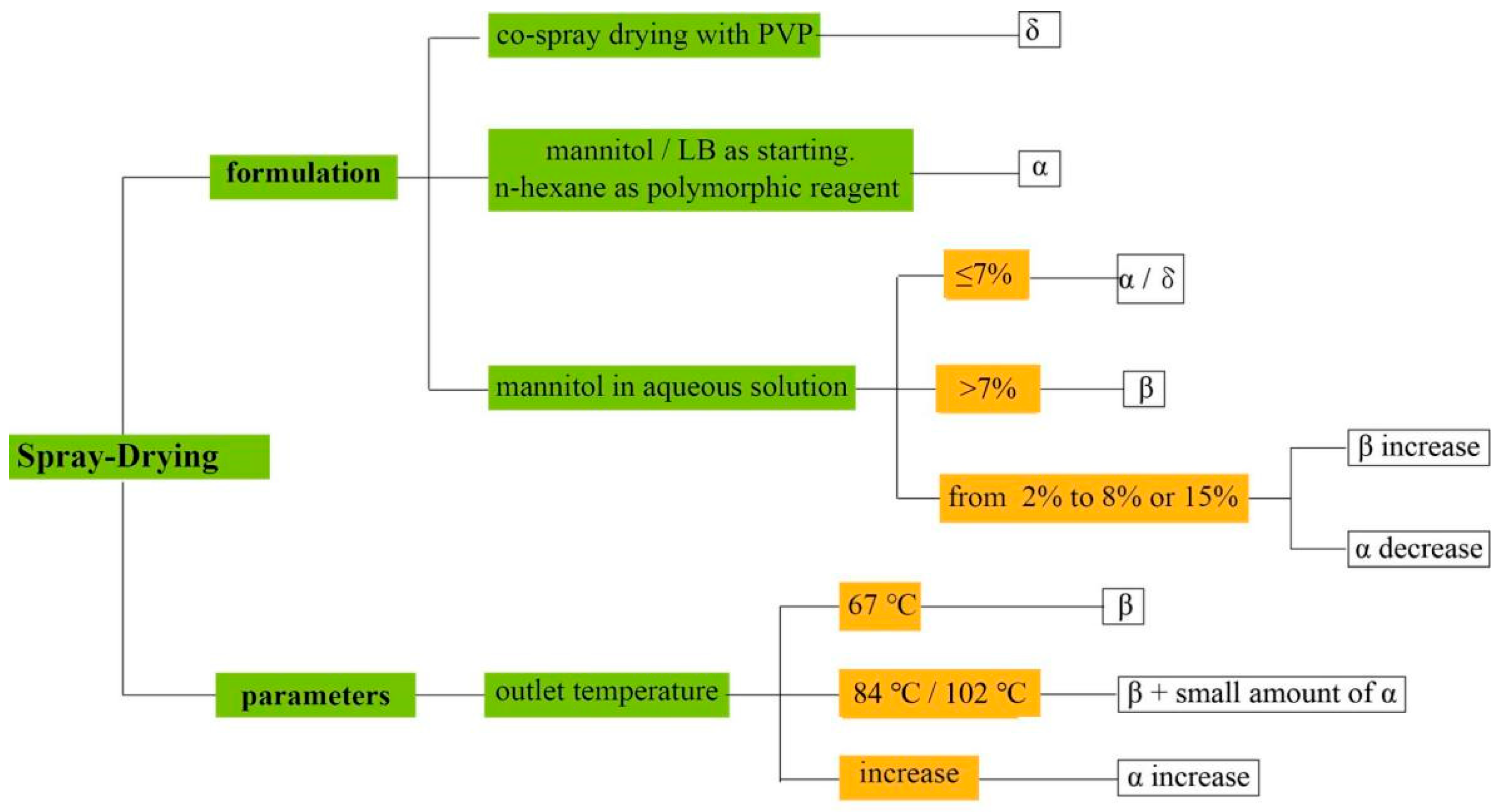
3.2. Spray Drying
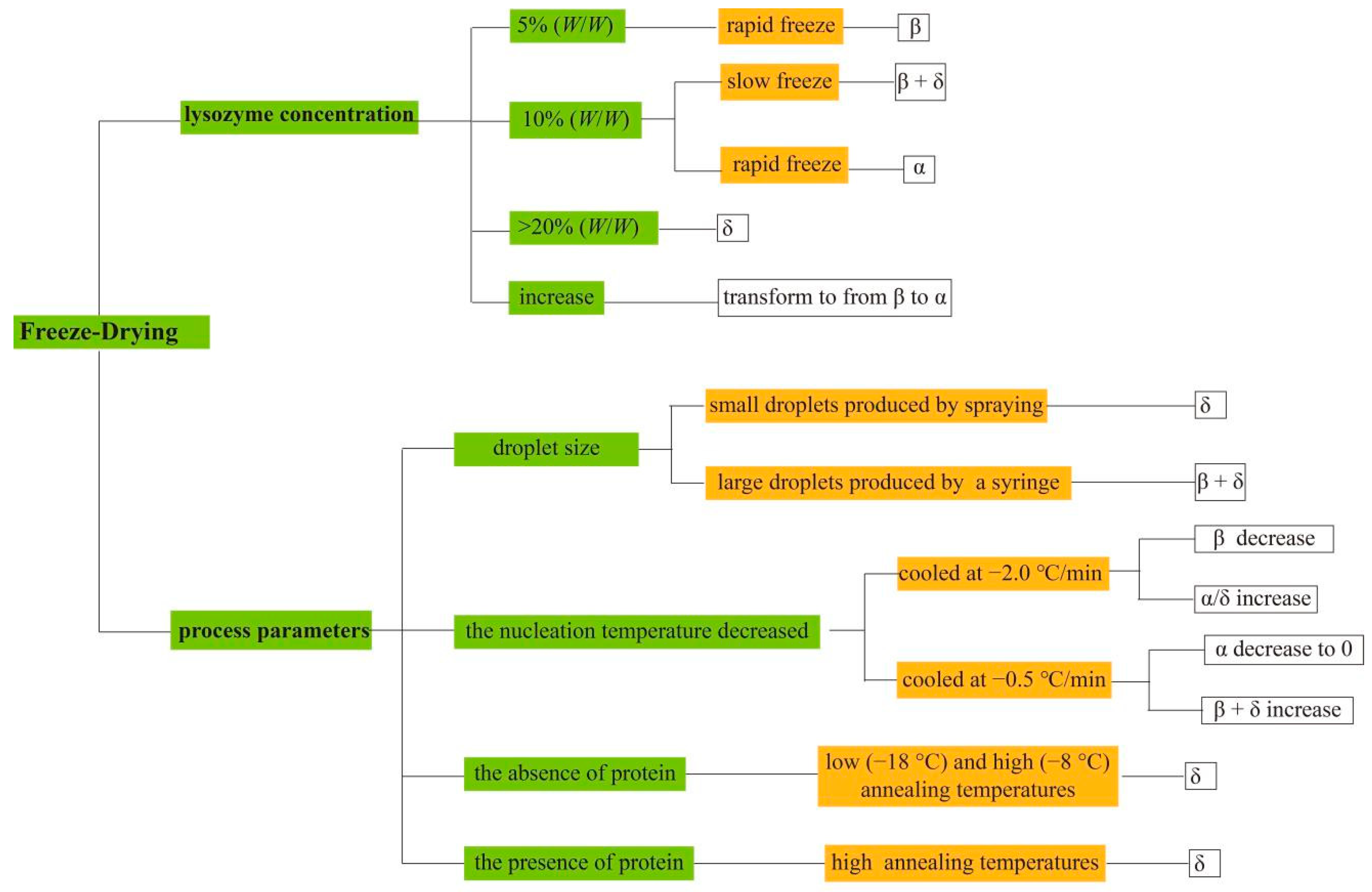
3.3. Freeze Drying
4. Kinetic and Thermodynamic Factors in Polymorphic Control of Mannitol
4.1. Crystal Growth Kinetic Factors
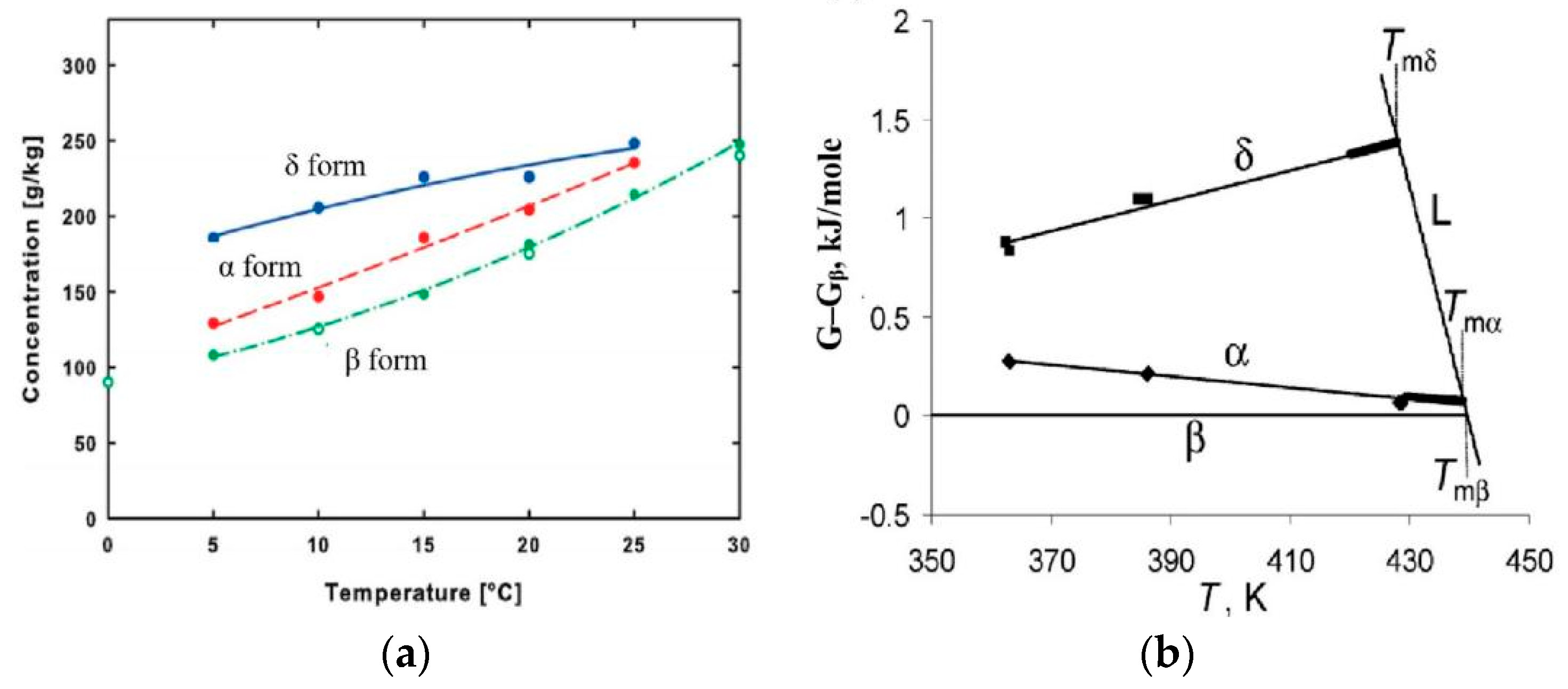
4.2. Thermodynamics Factors
4.3. Competition between Kinetic and Thermodynamic Factors
5. Pharmaceutical Applications of d-Mannitol Crystals
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thakral, S.; Sonje, J.; Suryanarayanan, R. Anomalous behavior of mannitol hemihydrate: Implications on sucrose crystallization in colyophilized systems. Int. J. Pharm. 2020, 587, 119629. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, W.; Yu, D.; Bo, Y.; Li, J. Enhanced carbon and nitrogen removal performance of simultaneous anammox and denitrification (SAD) with mannitol addition treating saline wastewater. J. Chem. Technol. Biotechnol. 2019, 94, 377–388. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, W.L.; Wu, H.; Guang, C.; Mu, W.M. Mannitol: Physiological functionalities, determination methods, biotechnological production, and applications. Appl. Microbiol. Biotechnol. 2020, 104, 6941–6951. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Meng, Q.; Mu, W.; Zhang, T. Recent advances in the applications and biotechnological production of mannitol. J. Funct. Foods 2017, 36, 404–409. [Google Scholar] [CrossRef]
- Yokoyama, H.; Kobayashi, H.; Hasegawa, J.; Fukuoka, A. Selective Dehydration of Mannitol to Isomannide over H beta Zeolite. ACS Catal. 2017, 7, 4828–4834. [Google Scholar] [CrossRef]
- Wagner, C.M.; Pein, M.; Breitkreutz, J. Roll compaction of granulated mannitol grades and the unprocessed crystalline delta-polymorph. Powder Technol. 2015, 270, 470–475. [Google Scholar] [CrossRef]
- Raithore, S.; Peterson, D.G. Effects of polyol type and particle size on flavor release in chewing gum. Food Chem. 2018, 253, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Vanhoorne, V.; Almey, R.; De Beer, T.; Vervaet, C. Delta-mannitol to enable continuous twin-screw granulation of a highly dosed, poorly compactable formulation. Int. J. Pharm. 2020, 583, 119374. [Google Scholar] [CrossRef] [PubMed]
- Shu, T.; Suzuki, H.; Hironaka, K.; Ito, K. Studies of Rapidly Disintegrating Tablets in the Oral Cavity Using Co-ground Mixtures of Mannitol with Crospovidone. Chem. Pharm. Bull. 2002, 50, 193–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, B.C.; Racine, F.M. Biotechnological production of mannitol and its applications. Appl. Microbiol. Biotechnol. 2011, 89, 879–891. [Google Scholar] [CrossRef]
- Shakiba, S.; Mansouri, S.; Selomulya, C.; Woo, M.W. Time scale based analysis of in-situ crystal formation in droplet undergoing rapid dehydration. Int. J. Pharm. 2019, 560, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Dierks, T.M.; Korter, T.M. Origins of the Relative Stabilities of Anhydrous and Hydrated d-Mannitol Crystals. J. Phys. Chem. A 2016, 120, 6629–6636. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Grant, D.J.W. Crystal structures of drugs: Advances in determination, prediction and engineering. Nat. Rev. Drug Discov. 2004, 3, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Poornachary, S.K.; Parambil, J.V.; Chow, P.S.; Tan, R.B.H.; Heng, J.Y.Y. Nucleation of Elusive Crystal Polymorphs at the Solution-Substrate Contact Line. Cryst. Growth Des. 2013, 13, 1180–1186. [Google Scholar] [CrossRef]
- Su, W.; Jia, N.; Li, H.; Hao, H.; Li, C. Polymorphism of D-mannitol: Crystal structure and the crystal growth mechanism. Chin. J. Chem. Eng. 2017, 25, 358–362. [Google Scholar] [CrossRef]
- Marwick, T.C. An X-Ray Study of Mannitol, Dulcitol, and Mannose. Nature 1931, 127, 11–12. [Google Scholar] [CrossRef]
- Botez, C.E.; Stephens, P.W.; Nunes, C.; Suryanarayanan, R. Crystal structure of anhydrous delta-D-mannitol. Powder Diffr. 2003, 18, 214–218. [Google Scholar] [CrossRef] [Green Version]
- Fronczek, F.R.; Kamel, H.N.; Slattery, M. Three polymorphs (alpha, beta and delta) of D-mannitol at 100 K. Acta Crystallogr. Sect. C-Cryst. Struct. Commun. 2003, 59, O567–O570. [Google Scholar] [CrossRef]
- Su, W.; Zhang, Y.; Liu, J.; Ma, M.; Guo, P.; Liu, X.; Wang, H.; Li, C. Molecular Dynamic Simulation of D-Mannitol Polymorphs in Solid State and in Solution Relating With Spontaneous Nucleation. J. Pharm. Sci. 2020, 109, 1537–1546. [Google Scholar] [CrossRef] [PubMed]
- Rye, A.; Sörum, H.; Stenhagen, E.; Finsnes, E.; Sörensen, J.S.; Sörensen, N.A. Crystalline Modifications of D-Mannitol. Acta Chem. Scand. 1952, 6, 1128–1129. [Google Scholar] [CrossRef]
- Berman, H.M.; Jeffrey, G.A.; Rosenstein, R.D. The crystal structures of the alpha and beta forms of D-mannitol. Acta Crystallographica. Sect. B Struct. Crystallogr. Cryst. Chem. 1968, 24, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Walter-Levy, L. The crystalline varieties of D-mannitol. Acad. Sci. Paris Ser. C. 1968, 267, 1779. [Google Scholar]
- Kaminsky, W.; Glazer, A.M. WCrystal optics of D-mannitol, C6H14O6: Crystal growth, structure, basic physical properties, birefringence, optical activity, Faraday effect, electro-optic effects and model calculations. Z. Für Krist.-Cryst. Mater. 1997, 212, 283–296. [Google Scholar] [CrossRef]
- Kim, H.S.; Jeffrey, G.A.; Rosenstein, R.D. The crystal structure of the K form of D-mannitol. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1968, 24, 1449–1455. [Google Scholar] [CrossRef]
- Cornel, J.; Kidambi, P.; Mazzotti, M. Precipitation and Transformation of the Three Polymorphs of D-Mannitol. Ind. Eng. Chem. Res. 2010, 49, 5854–5862. [Google Scholar] [CrossRef]
- Benetti, A.A.; Bianchera, A.; Buttini, F.; Bertocchi, L.; Bettini, R. Mannitol Polymorphs as Carrier in DPIs Formulations: Isolation Characterization and Performance. Pharmaceutics 2021, 13, 1113. [Google Scholar] [CrossRef]
- Cares-Pacheco, M.G.; Vaca-Medina, G.; Calvet, R.; Espitalier, F.; Letourneau, J.J.; Rouilly, A.; Rodier, E. Physicochemical characterization of D-mannitol polymorphs: The challenging surface energy determination by inverse gas chromatography in the infinite dilution region. Int. J. Pharm. 2014, 475, 69–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fedorov, A.Y.; Rychkov, D.A. Comparison of different computational approaches for unveiling the high-pressure behavior of organic crystals at a molecular level. Case study of tolazamide polymorphs. J. Struct. Chem. 2020, 61, 1356–1366. [Google Scholar] [CrossRef]
- Cruz-Cabeza, A.J.; Reutzel-Edens, S.M.; Bernstein, J. Facts and fictions about polymorphism. Chem. Soc. Rev. 2015, 44, 8619–8635. [Google Scholar] [CrossRef]
- Yu, L.; Huang, J.; Jones, K.J. Measuring Free-Energy Difference between Crystal Polymorphs through Eutectic Melting. J. Phys. Chem. B 2005, 109, 19915–19922. [Google Scholar] [CrossRef] [PubMed]
- Ho, R.; Wilson, D.A.; Heng, J.Y.Y. Crystal Habits and the Variation in Surface Energy Heterogeneity. Cryst. Growth Des. 2009, 9, 4907–4911. [Google Scholar] [CrossRef] [Green Version]
- Cares-Pacheco, M.G.; Calvet, R.; Vaca-Medina, G.; Rouilly, A.; Espitalier, F. Inverse gas chromatography a tool to follow physicochemical modifications of pharmaceutical solids: Crystal habit and particles size surface effects. Int. J. Pharm. 2015, 494, 113–126. [Google Scholar] [CrossRef] [Green Version]
- Ho, R.; Naderi, M.; Heng, J.Y.Y.; Williams, D.R.; Thielmann, F.; Bouza, P.; Keith, A.R.; Thiele, G.; Burnett, D.J. Effect of Milling on Particle Shape and Surface Energy Heterogeneity of Needle-Shaped Crystals. Pharm. Res. 2012, 29, 2806–2816. [Google Scholar] [CrossRef]
- Yoshinari, T.; Forbes, R.T.; York, P.; Kawashima, Y. The improved compaction properties of mannitol after a moisture-induced polymorphic transition. Int. J. Pharm. 2003, 258, 121–131. [Google Scholar] [CrossRef]
- Su, W.; Liu, J.; Wang, H.; Li, C.; Jia, N. Thermodynamic study of three anhydrous polymorphs of d-mannitol in different binary solvent mixtures from T = (258.15 to 278.15) K. J. Chem. Thermodyn. 2020, 141, 105680. [Google Scholar] [CrossRef]
- Feng, H.; Bondi, R.W., Jr.; Anderson, C.A.; Drennen, J.K.I.; Igne, B. Investigation of the Sensitivity of Transmission Raman Spectroscopy for Polymorph Detection in Pharmaceutical Tablets. Appl. Spectrosc. 2017, 71, 1856–1867. [Google Scholar] [CrossRef]
- Auer, M.E.; Griesser, U.J.; Sawatzki, J. Qualitative and quantitative study of polymorphic forms in drug formulations by near infrared FT-Raman spectroscopy. J. Mol. Struct. 2003, 661, 307–317. [Google Scholar] [CrossRef]
- Lee, Y.; Wu, J.X.; Yang, M.; Young, P.M.; van den Berg, F.; Rantanen, J. Particle size dependence of polymorphism in spray-dried mannitol. Eur. J. Pharm. Sci. 2011, 44, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Kaialy, W.; Hussain, T.; Alhalaweh, A.; Nokhodchi, A. Towards a More Desirable Dry Powder Inhaler Formulation: Large Spray-Dried Mannitol Microspheres Outperform Small Microspheres. Pharm. Res. 2014, 31, 60–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Da, M. Evaluation of integrated Raman-DSC technology in early pharmaceutical development: Characterization of polymorphic systems. J. Pharm. Biomed. 2013, 86, 92–99. [Google Scholar] [CrossRef]
- Burger, A.; Henck, J.-O.; Hetz, S.; Rollinger, J.M.; Weissnicht, A.A.; Stöttner, H. Energy/Temperature Diagram and Compression Behavior of the Polymorphs of d-Mannitol. J. Pharm. Sci. 2000, 89, 457–468. [Google Scholar] [CrossRef]
- Roberts, S.N.C.; Williams, A.C.; Grimsey, I.M.; Booth, S.W. Quantitative analysis of mannitol polymorphs. FT-Raman spectroscopy. J. Pharm. Biomed. Anal. 2002, 28, 1135–1147. [Google Scholar] [CrossRef]
- Guimarães, T.F.; Lanchote, A.D.; da Costa, J.S.; Viçosa, A.L.; de Freitas, L.A.P. A multivariate approach applied to quality on particle engineering of spray-dried mannitol. Adv. Powder Technol. 2015, 26, 1094–1101. [Google Scholar] [CrossRef]
- Cao, W.; Xie, Y.; Krishnan, S.; Lin, H.; Ricci, M. Influence of Process Conditions on the Crystallization and Transition of Metastable Mannitol Forms in Protein Formulations During Lyophilization. Pharm. Res. 2013, 30, 131–139. [Google Scholar] [CrossRef]
- Hu, J.; Dong, Y.; Ng, W.K.; Pastorin, G. Preparation of drug nanocrystals embedded in mannitol microcrystals via liquid antisolvent precipitation followed by immediate (on-line) spray drying. Adv. Powder Technol. 2018, 29, 957–963. [Google Scholar] [CrossRef]
- Su, W.; Hao, H.; Glennon, B.; Barrett, M. Spontaneous Polymorphic Nucleation of D-Mannitol in Aqueous Solution Monitored with Raman Spectroscopy and FBRM. Cryst. Growth Des. 2013, 13, 5179–5187. [Google Scholar] [CrossRef]
- Park, Y.; Hong, M.; Koo, J.Y.; Lee, M.; Lee, J.; Moon, D.J.; Sohn, S.H.; Joo, T.; Lim, W.T.; Lim, H.; et al. Reverse Anti-solvent Crystallization Process for the Facile Synthesis of Zinc Tetra(4-pyridyl) porphyrin Single Crystalline Cubes. Sci. Rep. 2017, 7, 2582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaialy, W.; Momin, M.N.; Ticehurst, M.D.; Murphy, J.; Nokhodchi, A. Engineered mannitol as an alternative carrier to enhance deep lung penetration of salbutamol sulphate from dry powder inhaler. Colloids Surf. B Biointerfaces 2010, 79, 345–356. [Google Scholar] [CrossRef]
- Penha, F.M.; Gopalan, A.; Meijlink, J.C.; Ibis, F.; Eral, H.B. Selective Crystallization of d-Mannitol Polymorphs Using Surfactant Self-Assembly. Cryst. Growth Des. 2021, 21, 3928–3935. [Google Scholar] [CrossRef] [PubMed]
- Telang, C.; Suryanarayanan, R.; Yu, L. Crystallization of D-mannitol in binary mixtures with NaCl: Phase diagram and polymorphism. Pharm. Res. 2003, 20, 1939–1945. [Google Scholar] [CrossRef]
- Vanhoorne, V.; Van Bockstal, P.-J.; Van Snick, B.; Peeters, E.; Monteyne, T.; Gomes, P.; De Beer, T.; Remon, J.P.; Vervaet, C. Continuous manufacturing of delta mannitol by cospray drying with PVP. Int. J. Pharm. 2016, 501, 139–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdullahi, H.; Neoptolemou, P.; Burcham, C.L.; Vetter, T. Single droplets to particles-size, shape, shell thick-ness and porosity analyses using X-ray computed tomography. Chem. Eng. Sci. 2021, 245, 116879. [Google Scholar] [CrossRef]
- Zhang, F.; Ngoc, N.; Tay, B.H.; Lau, R.; Shao, Y.-H.; IEEE. Roughness-controlled self-assembly of nanorod-coverd mannitol/LB Agar microparticles by polymorphic transformation for pulmonary drug delivery. In Proceedings of the 2015 IEEE 15th International Conference On Nanotechnology (IEEE-Nano), Rome, Italy, 27–30 July 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 1505–1508. [Google Scholar] [CrossRef]
- Lu, W.; Wang, S.; Lin, R.; Yang, X.; Cheng, Z.; Liu, W. Unveiling the importance of process parameters on droplet shrinkage and crystallization behaviors of easily crystalline material during spray drying. Dry. Technol. 2022, 40, 326–336. [Google Scholar] [CrossRef]
- Kramek-Romanowska, K.; Odziomek, M.; Sosnowski, T.R.; Gradon, L. Effects of Process Variables on the Properties of Spray-Dried Mannitol and Mannitol/Disodium Cromoglycate Powders Suitable for Drug Delivery by Inhalation. Ind. Eng. Chem. Res. 2011, 50, 13922–13931. [Google Scholar] [CrossRef]
- Maas, S.G.; Schaldach, G.; Walzel, P.E.; Urbanetz, N.A. Tailoring dry powder inhaler performance by modifying carrier surface topography by spray drying. At. Spray 2010, 20, 763–774. [Google Scholar] [CrossRef]
- Littringer, E.M.; Mescher, A.; Schroettner, H.; Achelis, L.; Walzel, P.; Urbanetz, N.A. Spray dried mannitol carrier particles with tailored surface properties—The influence of carrier surface roughness and shape. Eur. J. Pharm. Biopharm. 2012, 82, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.G.; Schaldach, G.; Littringer, E.M.; Mescher, A.; Griesser, U.J.; Braun, D.E.; Walzel, P.E.; Urbanetz, N.A. The impact of spray drying outlet temperature on the particle morphology of mannitol. Powder Technol. 2011, 213, 27–35. [Google Scholar] [CrossRef]
- Mönckedieck, M.; Kamplade, J.; Fakner, P.; Urbanetz, N.; Walzel, P.; Steckel, H.; Scherließ, R. Dry powder inhaler performance of spray dried mannitol with tailored surface morphologies as carrier and salbutamol sulphate. Int. J. Pharm. 2017, 524, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Takada, A.; Nail, S.L.; Yonese, M. Influence of Ethanol on Physical State of Freeze-Dried Mannitol. Pharm. Res. 2009, 26, 1112–1120. [Google Scholar] [CrossRef]
- Nakagawa, K.; Murakami, W.; Hatanaka, T. Redistribution of Protein Biological Activity in a Freeze-Dried Cake. Dry. Technol. 2013, 31, 102–111. [Google Scholar] [CrossRef]
- Kim, A.I.; Akers, M.J.; Nail, S.L. The physical state of mannitol after freeze-drying: Effects of mannitol concentration, freezing rate, and a noncrystallizing cosolute. J. Pharm. Sci. 1998, 87, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Grohganz, H.; Lee, Y.-Y.; Rantanen, J.; Yang, M. The influence of lysozyme on mannitol polymorphism in freeze-dried and spray-dried formulations depends on the selection of the drying process. Int. J. Pharm. 2013, 447, 224–230. [Google Scholar] [CrossRef]
- Pajander, J.P.; Matero, S.; Sloth, J.; Wan, F.; Rantanen, J.; Yang, M. Raman Mapping of Mannitol/Lysozyme Particles Produced Via Spray Drying and Single Droplet Drying. Pharm. Res. 2015, 32, 1993–2002. [Google Scholar] [CrossRef]
- Chakkittakandy, R.; Corver, J.A.W.M.; Planken, P.C.M. Terahertz spectroscopy to identify the polymorphs in freeze-dried mannitol. J. Pharm. Sci. 2010, 99, 932–940. [Google Scholar] [CrossRef]
- Nakagawa, K.; Murakami, W.; Andrieu, J.; Vessot, S. Freezing step controls the mannitol phase composition heterogeneity. Chem. Eng. Res. Des. 2009, 87, 1017–1027. [Google Scholar] [CrossRef]
- Liao, X.; Krishnamurthy, R.; Suryanarayanan, R. Influence of Processing Conditions on the Physical State of Mannitol—Implications in Freeze-Drying. Pharm. Res. 2007, 24, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; O’Reilly Beringhs, A.; Kim, R.; Zhang, W.; Patel, S.M.; Bogner, R.H.; Lu, X. Impact of formulation on the quality and stability of freeze-dried nanoparticles. Eur. J. Pharm. Biopharm. 2021, 169, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, J.; Subash, M.; Thorat, B.N. Drying induced polymorphic transformation of pharmaceutical in-gredients: A critical review of recent progresses and challenges. Dry Technol. 2021. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Barrett, P.; Hsiao, G.; Carr, A.; Glennon, B. In Situ Monitoring of Polymorphic Transitions. Org. Process Res. Dev. 2003, 7, 977–982. [Google Scholar] [CrossRef]
- Shtukenberg, A.G.; Cui, X.; Freudenthal, J.; Gunn, E.; Camp, E.; Kahr, B. Twisted Mannitol Crystals Establish Homologous Growth Mechanisms for High-Polymer and Small-Molecule Ring-Banded Spherulites. J. Am. Chem. Soc. 2012, 134, 6354–6364. [Google Scholar] [CrossRef]
- Tao, J.; Yu, L. Kinetics of Cross-Nucleation between Polymorphs. J. Phys. Chem. B 2006, 110, 7098–7101. [Google Scholar] [CrossRef] [PubMed]
- Har, C.L.; Fu, N.; Chan, E.S.; Tey, B.T.; Chen, X.D. In situ crystallization kinetics and behavior of mannitol during droplet drying. Chem. Eng. J. 2018, 354, 314–326. [Google Scholar] [CrossRef]
- Rychkov, D.A.; Arkhipov, S.G.; Boldyreva, E.V. Simple and efficient modifications of well known techniques for reliable growth of high-quality crystals of small bioorganic molecules. J. Appl. Crystallogr. 2014, 47, 1435–1442. [Google Scholar] [CrossRef] [Green Version]
- Drebushchak, V.A.; McGregor, L.; Rychkov, D.A. Cooling rate “window” in the crystallization of metacetamol form II. J. Therm. Anal. Calorim. 2017, 127, 1807–1814. [Google Scholar] [CrossRef]
- Pinto, J.T.; Zellnitz, S.; Guidi, T.; Schiaretti, F.; Schroettner, H.; Paudel, A. Spray-Congealing and Wet-Sieving as Alternative Processes for Engineering of Inhalation Carrier Particles: Comparison of Surface Properties, Blending and In Vitro Performance. Pharm. Res. 2021, 38, 1107–1123. [Google Scholar] [CrossRef]
- Hertel, N.; Birk, G.; Scherliess, R. Performance tuning of particle engineered mannitol in dry powder inhalation formulations. Int. J. Pharm. 2020, 586, 119592. [Google Scholar] [CrossRef]
- Lyu, F.; Liu, J.J.; Zhang, Y.; Wang, X.Z. Combined control of morphology and polymorph in spray drying of mannitol for dry powder inhalation. J. Cryst. Growth 2017, 467, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Hertel, N.; Birk, G.; Scherliess, R. Particle engineered mannitol for carrier-based inhalation—A serious alternative? Int. J. Pharm. 2020, 577, 118901. [Google Scholar] [CrossRef] [PubMed]
- Lute, S.V.; Dhenge, R.M.; Salman, A.D. Twin Screw Granulation: Effects of Properties of Primary Powders. Pharmaceutics 2018, 10, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Megarry, A.; Taylor, A.; Gholami, A.; Wikstrom, H.; Tajarobi, P. Twin-screw granulation and high-shear granulation: The influence of mannitol grade on granule and tablet properties. Int. J. Pharm. 2020, 590, 119890. [Google Scholar] [CrossRef] [PubMed]
- Ohrem, H.L.; Schornick, E.; Kalivoda, A.; Ognibene, R. Why is mannitol becoming more and more popular as a pharmaceutical excipient in solid dosage forms? Pharm. Dev. Technol. 2014, 19, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Al-khattawi, A.; Mohammed, A.R. Compressed orally disintegrating tablets: Excipients evolution and formulation strategies. Expert Opin. Drug Del. 2013, 10, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Sano, S.; Iwao, Y.; Kimura, S.; Itai, S. Preparation and evaluation of swelling induced-orally disintegrating tablets by microwave irradiation. Int. J. Pharm. 2011, 416, 252–259. [Google Scholar] [CrossRef]
- Sano, S.; Iwao, Y.; Noguchi, S.; Kimura, S.; Itai, S. Design and evaluation of microwave-treated orally disintegrating tablets containing polymeric disintegrant and mannitol. Int. J. Pharm. 2013, 448, 132–141. [Google Scholar] [CrossRef]
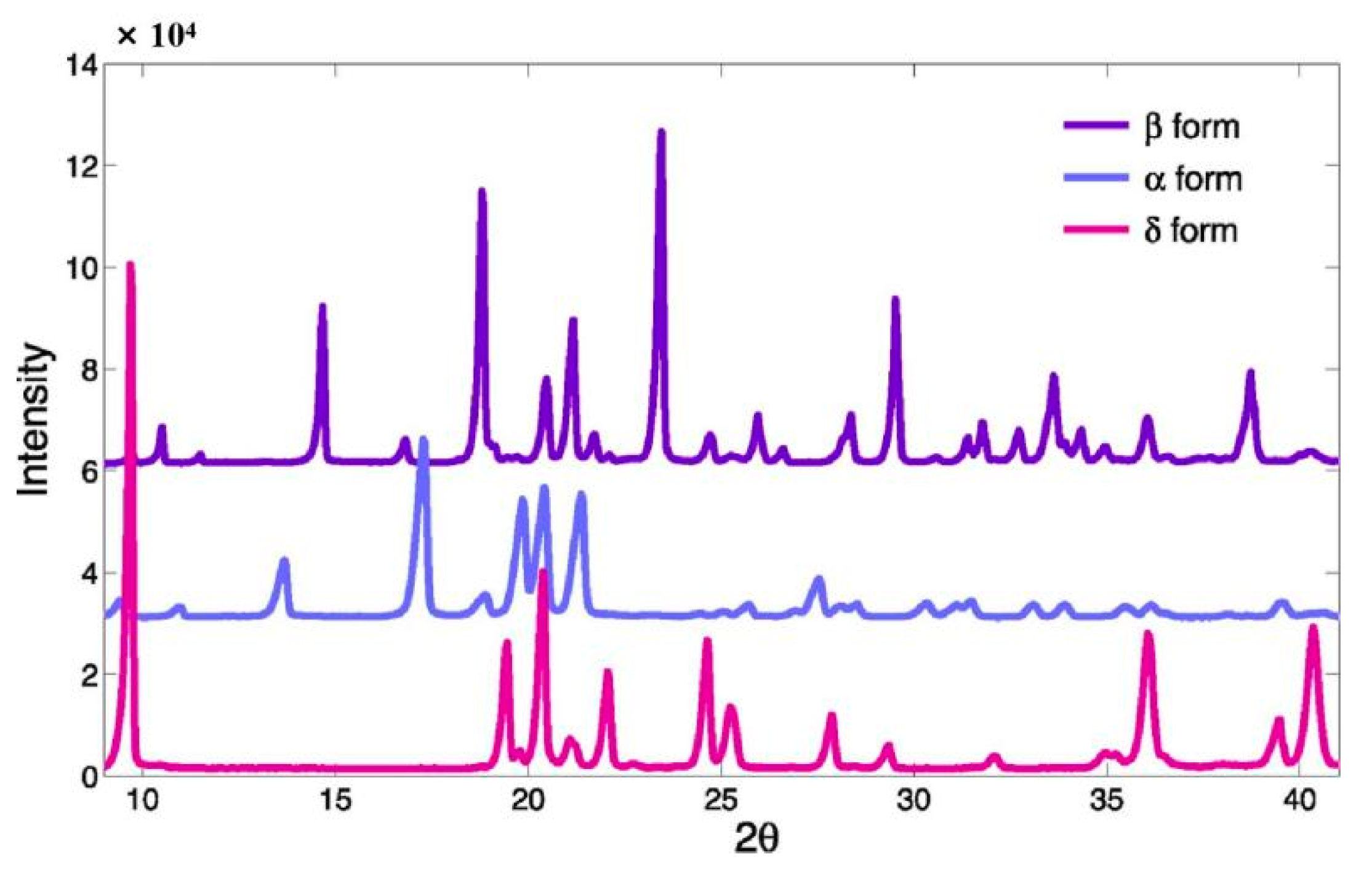

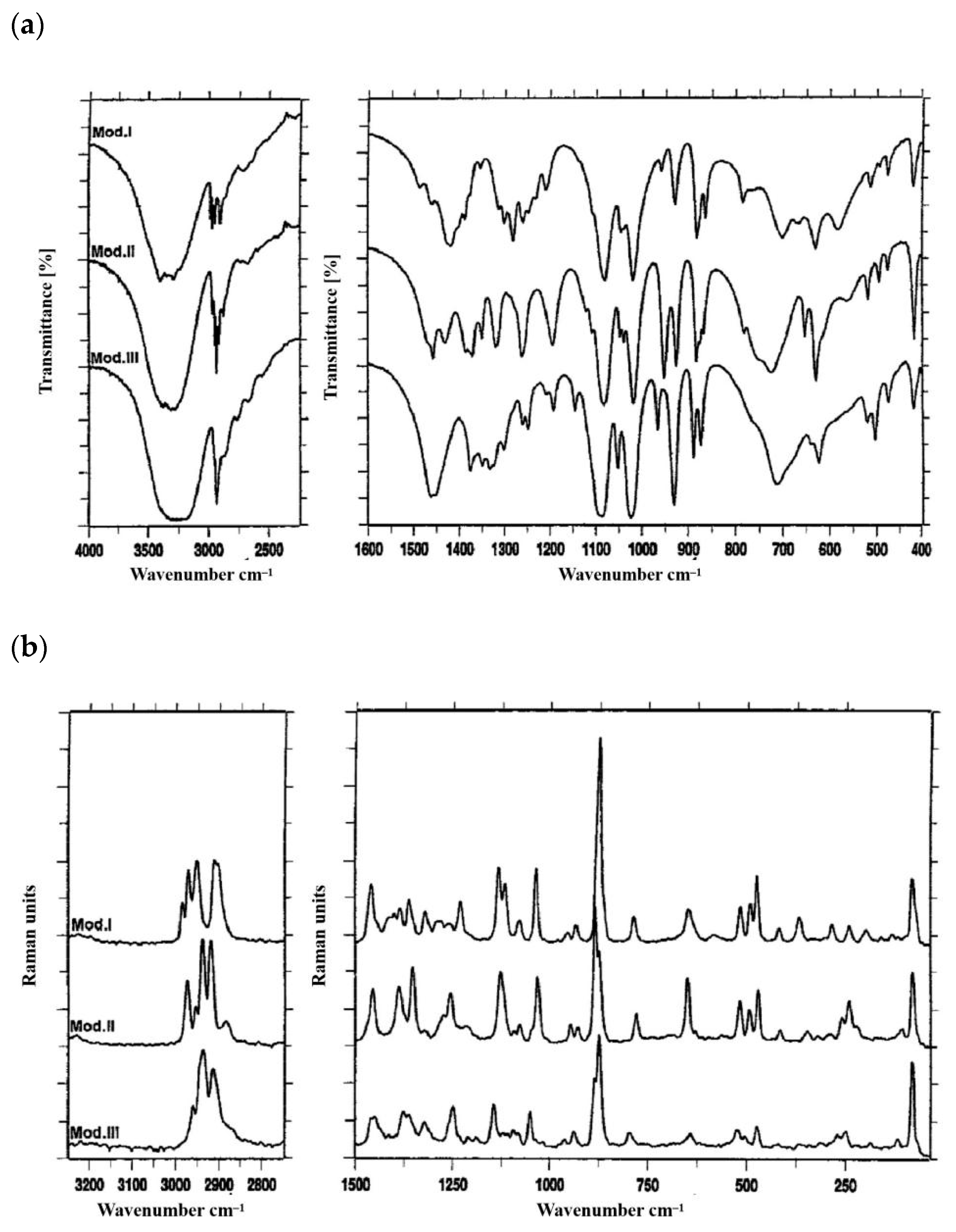


| Characteristics | Mannitol Polymorphs | Ref. | ||
|---|---|---|---|---|
| β | α | δ | ||
| Crystal structure | P212121 symmetry | P21 | [25] | |
| Morphology | needle-like | monoclinic | [25] | |
| Thermodynamic stability | β > α > δ | [19] | ||
| Molecular conformational energy | β > α > δ | [19] | ||
| Solubility in water | δ > α > β | [25] | ||
| Solubility in methanol–water and ethanol–water | δ > α > β | [35] | ||
| hydrogen bonding force | β > α > δ | [19] | ||
| compactability and tabletability | δ > α≈β | [10] | ||
| Dosage Form | Factor | Polymorphs | Morphology | Increase | Ref | ||
|---|---|---|---|---|---|---|---|
| β | α | δ | |||||
| DPI | Concentration of NH₄HCO₃ | — | ↑ | — | Became irregular | FPF | [78] |
| Presence of mannitol | —— | Became rough | decompose API aggregates | [79] | |||
| Tablet | Wet granulation process | —— | Porous structure | compressibility | [10] | ||
| ↓ | ↑ | — | Spherical particles to needle-like | tensile strength | [80] | ||
| ↑ | — | ↓ | Many needle-shaped primary crystals | tensile strength | [81] | ||
| ODTs | Microwave | ↑ | — | ↓ | Primary β crystals | hardness and disintegration time | [84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Liu, J.; Hu, A.; Nie, T.; Cheng, Z.; Liu, W. A Critical Review on Engineering of d-Mannitol Crystals: Properties, Applications, and Polymorphic Control. Crystals 2022, 12, 1080. https://doi.org/10.3390/cryst12081080
Yang Y, Liu J, Hu A, Nie T, Cheng Z, Liu W. A Critical Review on Engineering of d-Mannitol Crystals: Properties, Applications, and Polymorphic Control. Crystals. 2022; 12(8):1080. https://doi.org/10.3390/cryst12081080
Chicago/Turabian StyleYang, Yuxin, Jia Liu, Anna Hu, Ting Nie, Zeneng Cheng, and Wenjie Liu. 2022. "A Critical Review on Engineering of d-Mannitol Crystals: Properties, Applications, and Polymorphic Control" Crystals 12, no. 8: 1080. https://doi.org/10.3390/cryst12081080
APA StyleYang, Y., Liu, J., Hu, A., Nie, T., Cheng, Z., & Liu, W. (2022). A Critical Review on Engineering of d-Mannitol Crystals: Properties, Applications, and Polymorphic Control. Crystals, 12(8), 1080. https://doi.org/10.3390/cryst12081080






