Numerical Analysis of Phosphorus Concentration Distribution in a Silicon Crystal during Directional Solidification Process
Abstract
:1. Introduction
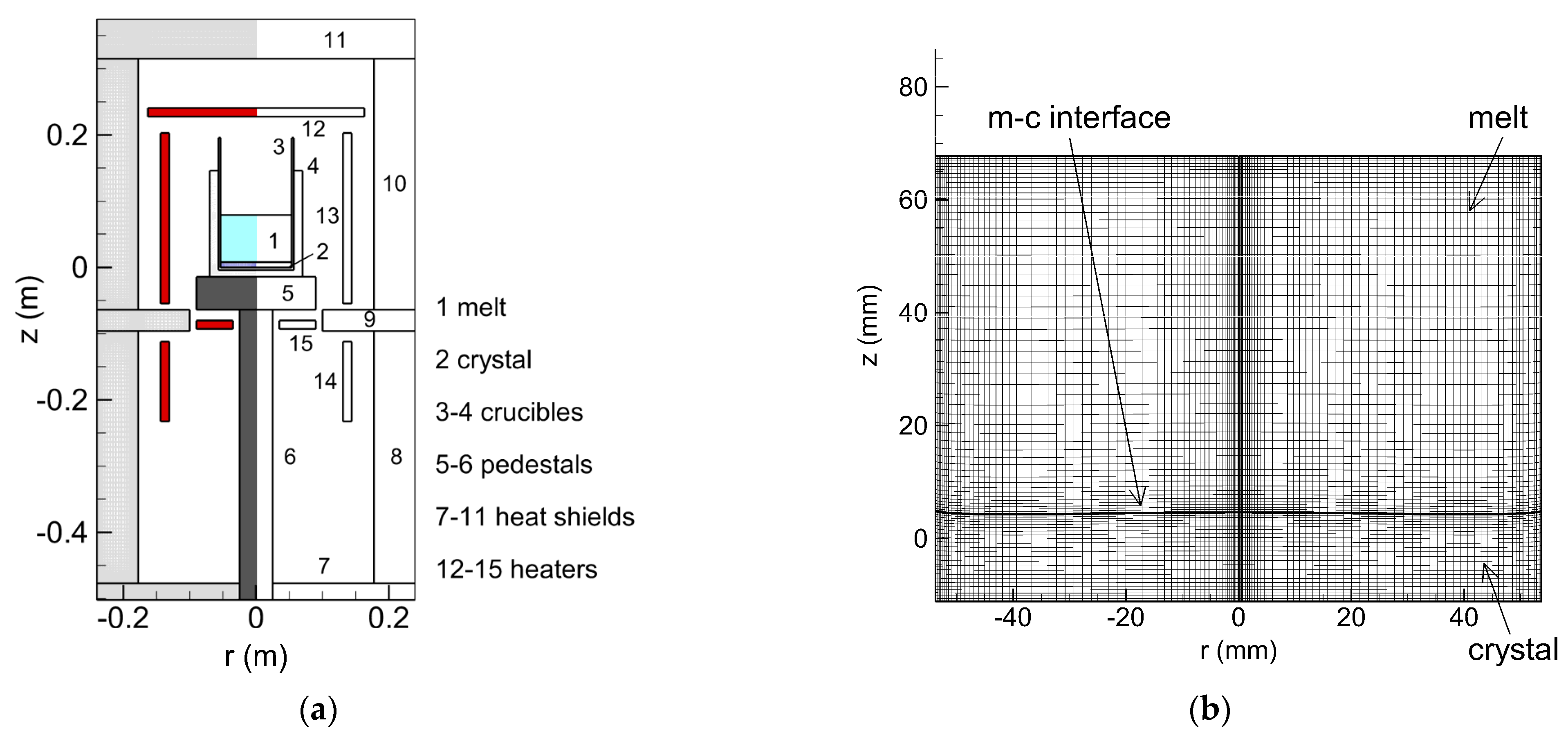
2. Materials and Methods
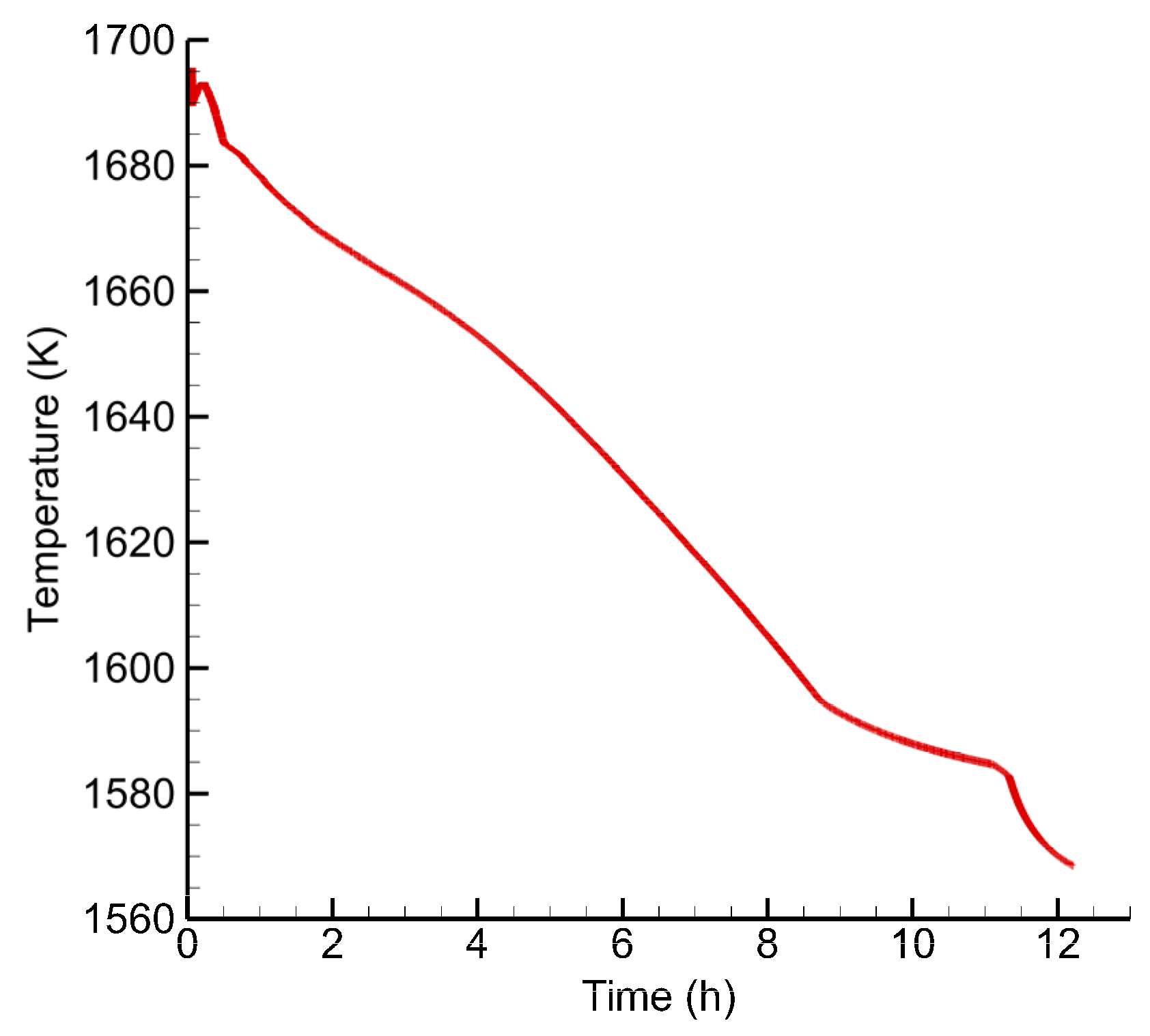
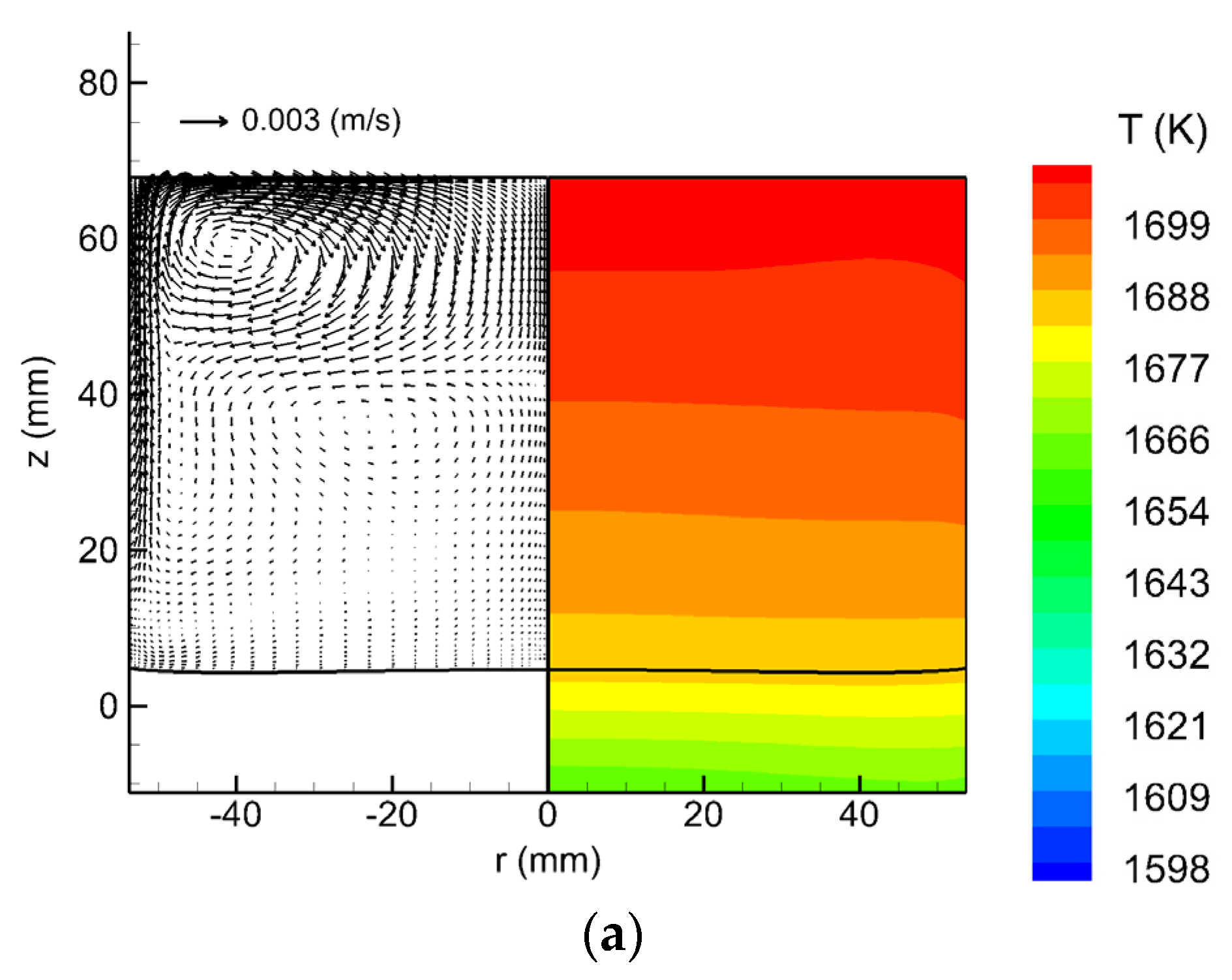
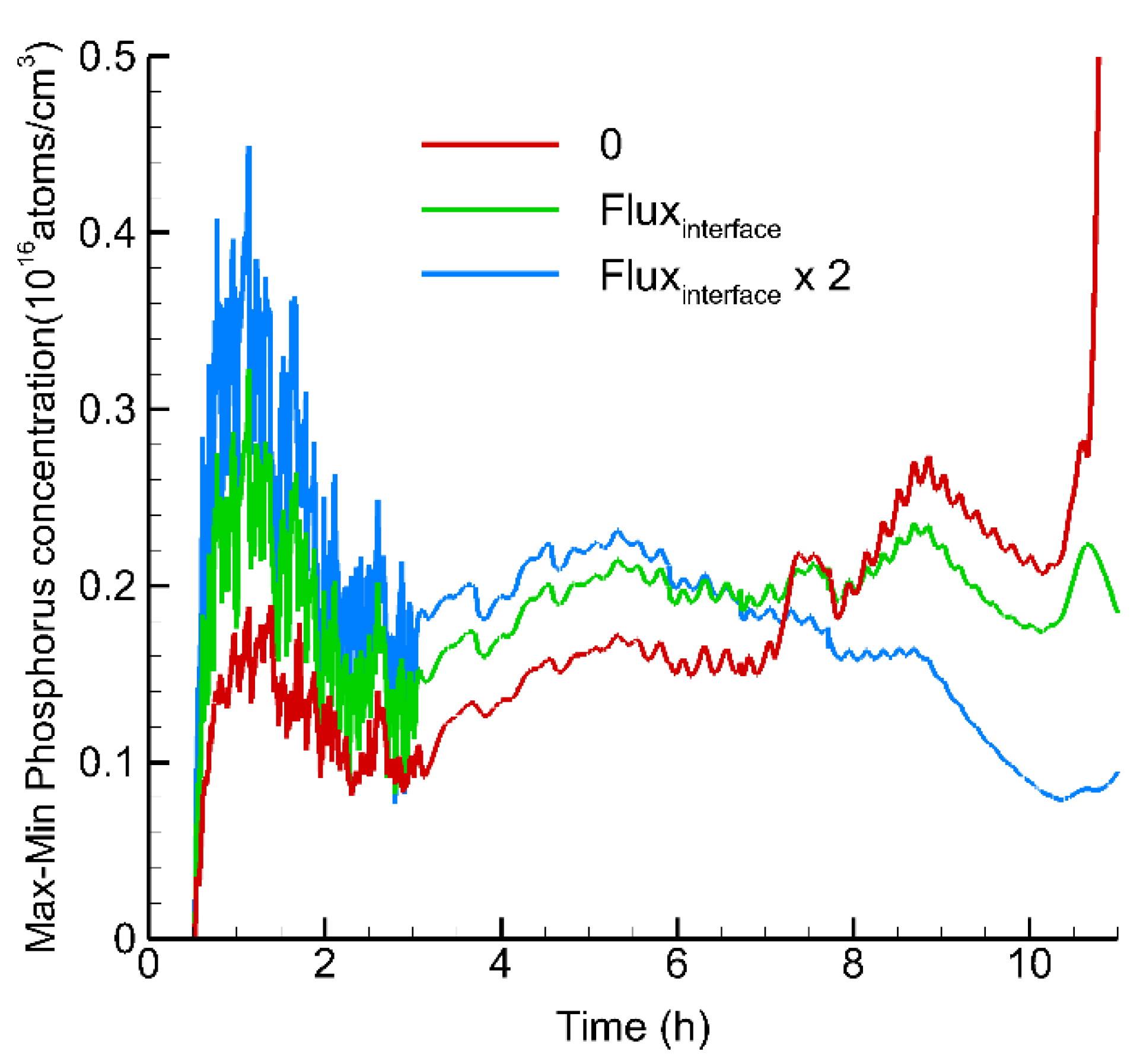
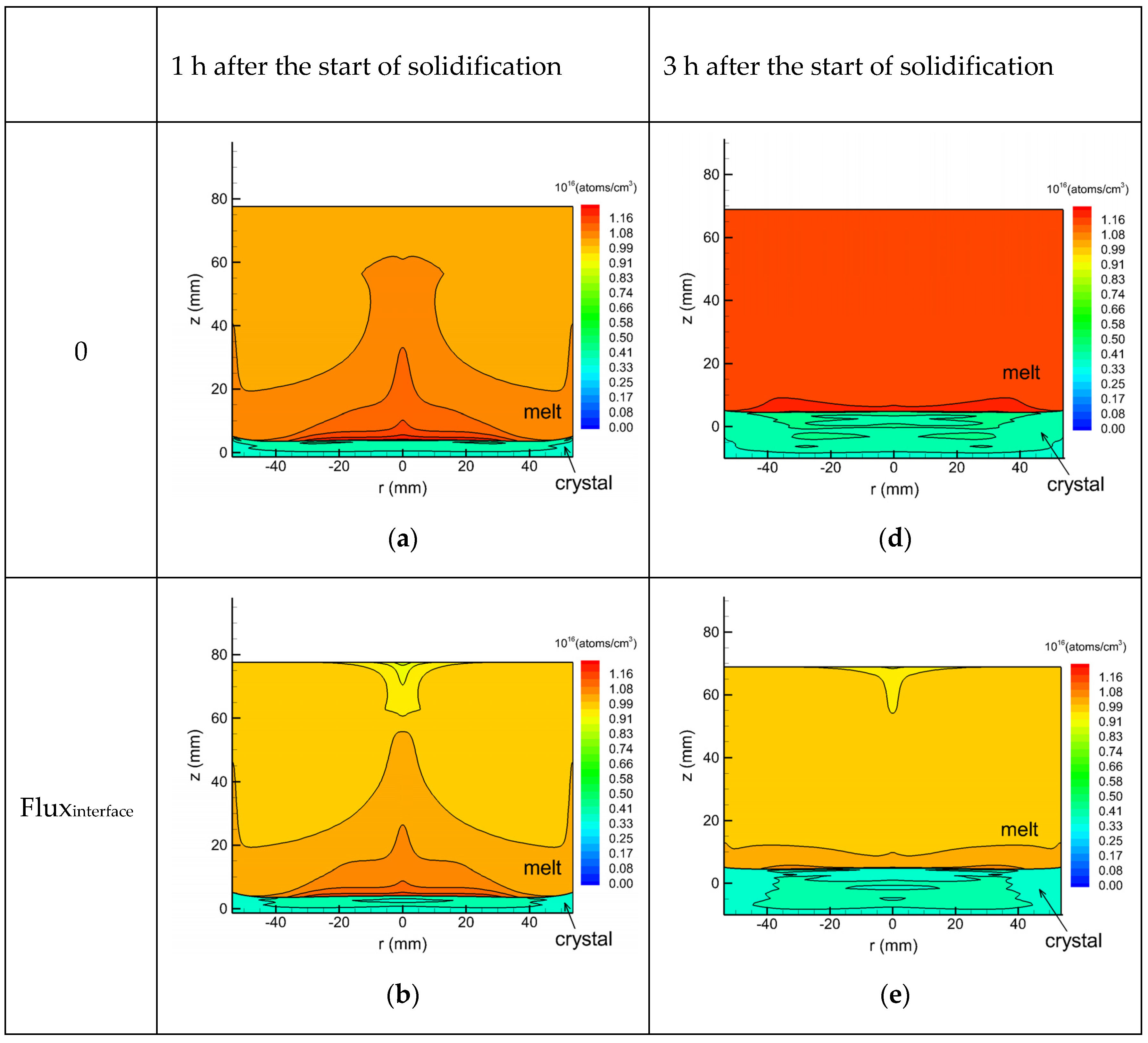
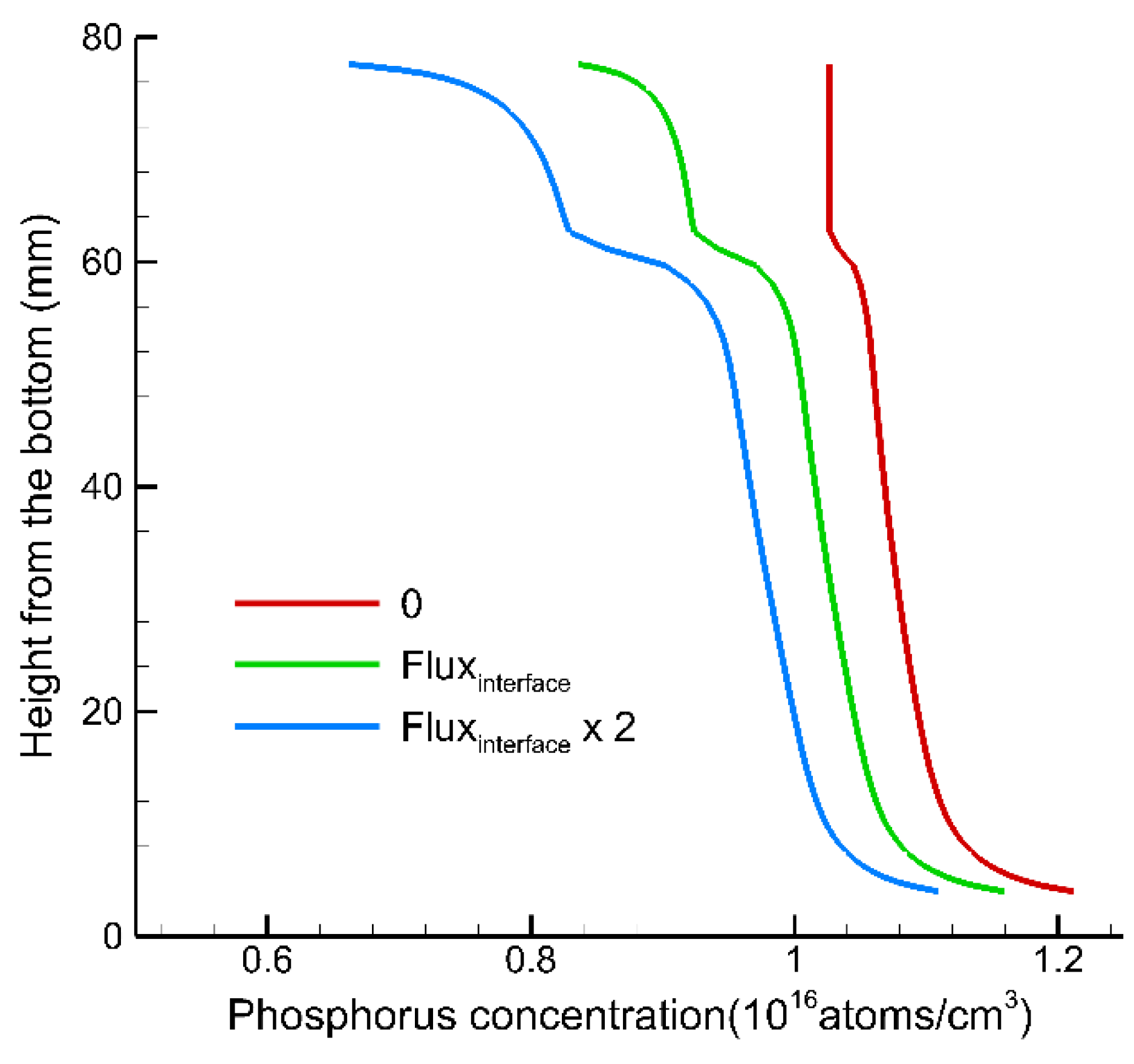
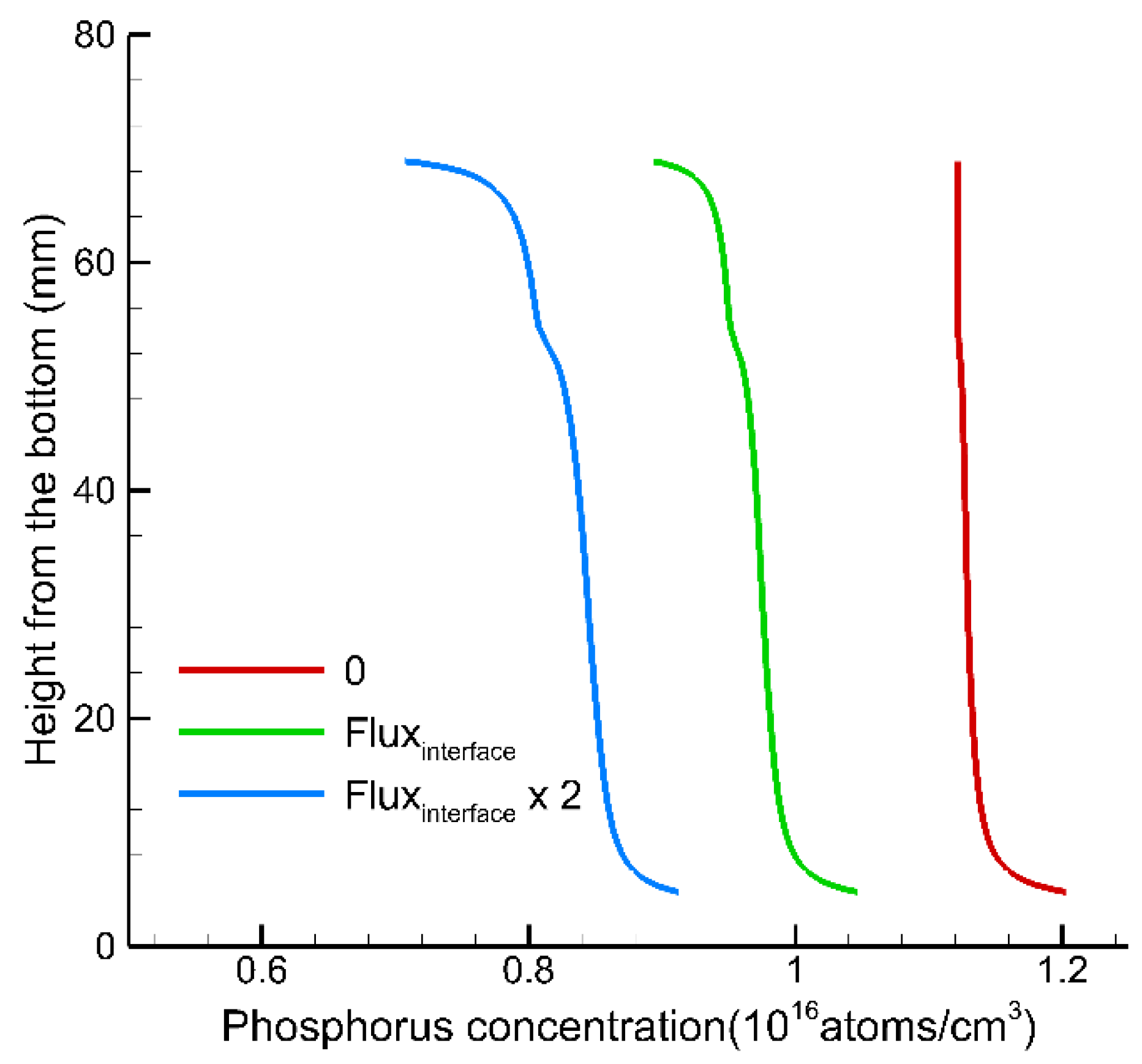
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schindler, F.; Michl, B.; Krenckel, P.; Riepe, S.; Feldmann, F.; Benick, J.; Warta, W.; Schubert, M.C. Efficiency potential of p- and n-type high performance multi-crystalline silicon. Energy Procedia 2015, 77, 633–638. [Google Scholar] [CrossRef]
- Benick, J.; Richter, A.; Müller, R.; Hauser, H.; Feldmann, F.; Krenckel, P.; Riepe, S.; Schindler, F.; Schubert, M.C.; Hermle, M.; et al. High-efficiency n-type HP mc silicon solar cells. IEEE J. Photovolt. 2017, 7, 1171–1175. [Google Scholar] [CrossRef]
- Meemongkolkiat, V.; Nakayashiki, K.; Rohatgi, A.; Crabtree, G.; Nickerson, J.; Jester, T.L. Resistivity and lifetime variation along commercially grown Ga- and B-Doped Czochralski Si ingots and its effect on light-induced degradation and performance of solar cells. Prog. Photovolt. Res. Appl. 2006, 14, 125. [Google Scholar] [CrossRef]
- Richter, A.; Benick, J.; Feldmann, F.; Fell, A.; Hermle, M. n-Type Si solar cells with passivating electron contact: Identifying sources for efficiency limitations by wafer thickness and resistivity variation. Sol. Energy Mater. Sol. Cells 2017, 173, 96–105. [Google Scholar] [CrossRef]
- Rehman, A.U.; Lee, S.H. Advancements in n-type base crystalline silicon solar cells and their emergence in the photovoltaic industry. Sci. World J. 2013, 2013, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Solanki, C.S. Solar Photovoltaics: Fundamentals, Technologies and Application, 3rd ed.; PHI Learning Pvt. Ltd.: New Delhi, India, 2015. [Google Scholar]
- Lee, K.H. Axial Distribution of Gallium in Silicon Crystals for Photovoltaic Applications. Jpn. J. Appl. Phys. 2007, 46, 2831. [Google Scholar] [CrossRef]
- Wang, J.H.; Im, J.I. Resistivity distribution in bulk growth of silicon single crystals. Jpn. J. Appl. Phys. 2004, 43, 4079. [Google Scholar] [CrossRef]
- Forster, M.; Dehestru, B.; Thomas, A.; Fourmond, E.; Einhaus, R.; Cuevas, A.; Lemiti, M. Compensation engineering for uniform n-type silicon ingots. Sol. Energy Mater. Sol. Cells 2013, 111, 146–152. [Google Scholar] [CrossRef]
- Buchovska, I.; Dropka, N.; Kayser, S.; Kiessling, F.M. The influence of travelling magnetic field on phosphorus distribution in n-type multi-crystalline silicon. J. Cryst. Growth 2019, 507, 299–306. [Google Scholar] [CrossRef]
- Dupret, F.; Nicodeme, P.; Ryclmans, Y.; Wouters, P.; Crochet, M.J. Global modelling of heat transfer in crystal growth furnaces. Int. J. Heat Mass Transf. 1990, 33, 1849. [Google Scholar] [CrossRef]
- Liu, L.J.; Nakano, S.; Kakimoto, K. Dynamic simulation of temperature and iron distributions in a casting process for crystalline silicon solar cells with a global model. J. Cryst. Growth 2006, 292, 515. [Google Scholar] [CrossRef]
- Kakimoto, K.; Liu, L.J.; Nakano, S. Analysis of temperature and impurity distributions in a unidirectional-solidification process for multi-crystalline silicon of solar cells by a global model. Mater. Sci. Eng. B 2006, 134, 269. [Google Scholar] [CrossRef]
- Liu, L.; Nakano, S.; Kakimoto, K. Investigation of oxygen distribution in electromagnetic CZ-Si melts with a transverse magnetic field using 3D global modeling. J. Cryst. Growth 2007, 299, 48. [Google Scholar] [CrossRef]
- Togawa, S.; Huang, X.; Izunome, K.; Terashima, K.; Kimura, S. Oxygen transport analysis in Czochralski silicon melt by considering the oxygen evaporation from the melt surface. J. Crystal Growth 1995, 148, 70. [Google Scholar] [CrossRef]
- Zulehner, W.; Huber, D. Crystals-Growth, Properties and Applications; Grabmaier, J., Ed.; Springer: Berlin/Heidelberg, Germany, 1988; Volume 8, p. 1. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakano, S.; Liu, X.; Han, X.-F.; Kakimoto, K. Numerical Analysis of Phosphorus Concentration Distribution in a Silicon Crystal during Directional Solidification Process. Crystals 2021, 11, 27. https://doi.org/10.3390/cryst11010027
Nakano S, Liu X, Han X-F, Kakimoto K. Numerical Analysis of Phosphorus Concentration Distribution in a Silicon Crystal during Directional Solidification Process. Crystals. 2021; 11(1):27. https://doi.org/10.3390/cryst11010027
Chicago/Turabian StyleNakano, Satoshi, Xin Liu, Xue-Feng Han, and Koichi Kakimoto. 2021. "Numerical Analysis of Phosphorus Concentration Distribution in a Silicon Crystal during Directional Solidification Process" Crystals 11, no. 1: 27. https://doi.org/10.3390/cryst11010027
APA StyleNakano, S., Liu, X., Han, X.-F., & Kakimoto, K. (2021). Numerical Analysis of Phosphorus Concentration Distribution in a Silicon Crystal during Directional Solidification Process. Crystals, 11(1), 27. https://doi.org/10.3390/cryst11010027






