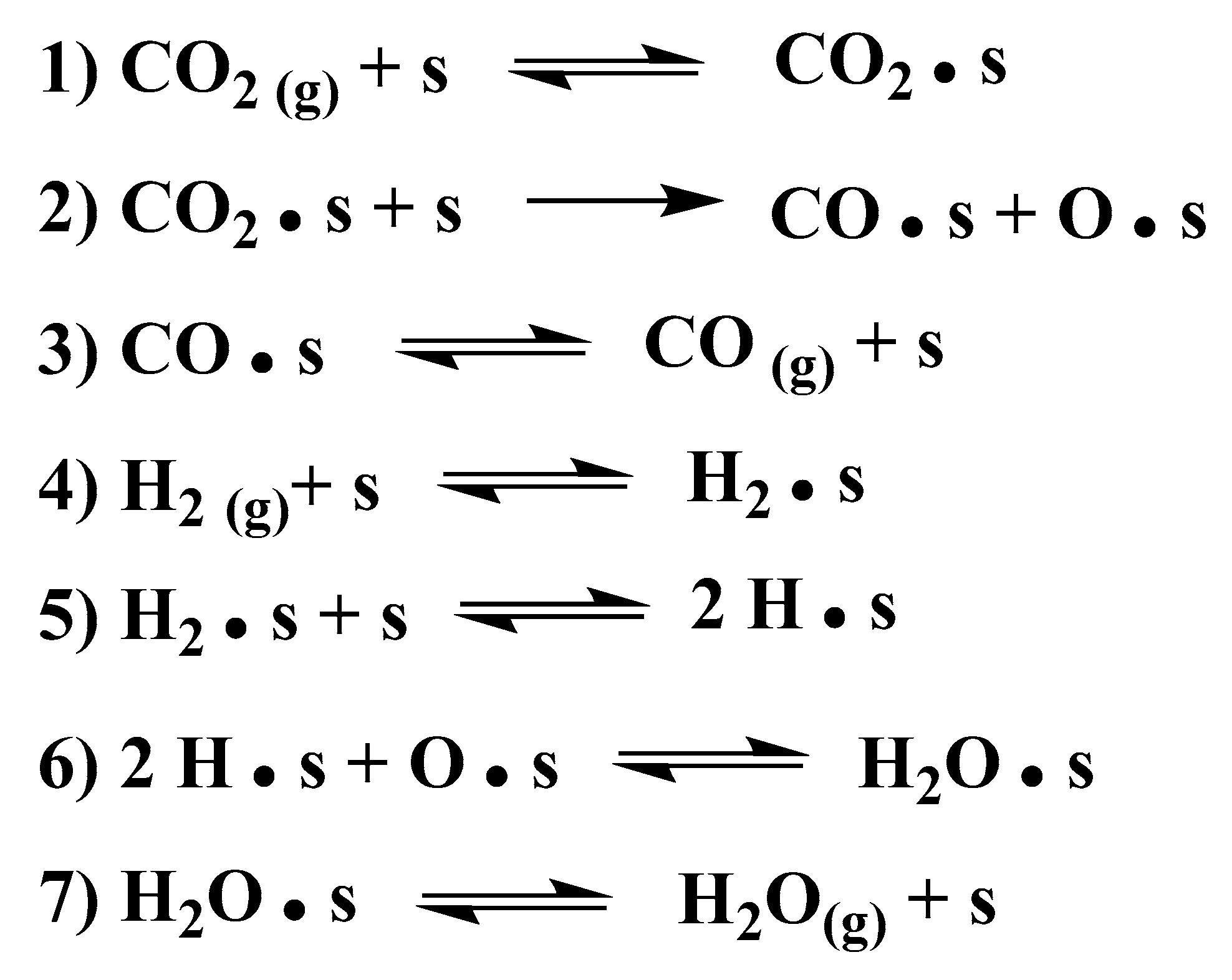1. Introduction
Today’s anthropogenic emissions of carbon dioxide to the atmosphere amount to about 35,000 Tg per year, and the greenhouse effect of these accumulated emissions has been recognized as an alarming hazard to the well-being of modern societies. Although multiple approaches have been considered to mitigate these emissions, recently more emphasis has been placed on the potential synergy of carbon capture and the utilization of these large outflows of carbon dioxide. Among several possibilities, a recent National Academies of Science and Engineering report [
1] on CO
2 waste gas utilization highlights the conversion of CO
2 by hydrogenation into CO and water—the reverse water-gas shift (RWGS) reaction, Equation (1)— as critical, and points to the need for improved catalysts with high stability and durability.
The RWGS reaction can be part of a two-step hydrogenation process for the conversion of CO
2 to valuable products. First, CO
2 is reduced to CO via the RWGS reaction, and second, CO can be converted to either hydrocarbons via the Fischer-Tropsch (FT) process or methanol via CAMERE (CArbon dioxide hydrogenation to form MEthanol via a Reverse WGS reaction) process [
2]. The RWGS reaction is an endothermic reaction (ΔH°
298 K = 41.2 kJ mol
−1), and thus is thermodynamically favorable at higher temperatures.
Noble metals such as Pt [
3,
4,
5] and Pd [
6,
7], and other various metals such as Cu [
8,
9,
10,
11], Ni [
12,
13] and Fe [
14] supported on oxides were reported to be active for the production of CO. Among them, Cu-based materials have been widely studied, and thus have also been investigated in many instances for the RWGS reaction. For example, Cu-Ni/Al
2O
3 [
15], Cu/ZnO [
16], Cu-Zn/Al
2O
3 [
16] and Cu/SiO
2 promoted with potassium [
17] have all shown good RWGS activity. However, Cu materials tend to deactivate by sintering (frittage) at high temperatures (T > 773 K), which are required for high RWGS activity. For high temperature applications, iron can be added as a thermal stabilizer: Chen et al. [
10] showed that adding small amounts of iron to 10% Cu/SiO
2 resulted in stable RWGS activity for 120 h at 873 K and atmospheric pressure, while non-promoted 10% Cu/SiO
2 deactivated rapidly.
Iron oxides (Fe
xO
y) are often used industrially for FT synthesis (473 K–623 K, 1 MPa) [
18,
19] and the high-temperature (623 K–723 K) WGS reaction [
20,
21,
22]. In FT synthesis, alkalized, iron-based catalysts are combined with Cu for reduction promotion. Schulz et al. [
18,
23] showed that the working FT catalysts contain several iron carbide phases and elemental carbon formed after the hydrogen reduction period. Iron oxides and the metallic iron are still present in the catalyst composition, but iron carbide phases are identified as active sites [
23]. The RWGS and WGS reactions are often carried out in conjunction with FT synthesis at a higher temperature regime on iron catalysts, and iron oxide or oxidic amorphous iron phases are known as the active phases for WGS and RWGS [
24,
25,
26].
Extensive research on iron-based catalysts has been reported mainly on the WGS reaction over decades [
20]. Chromium is a structural promoter that helps prevent the iron from sintering at high temperature. A more recent survey on Cr-free, Fe-based WGS catalysts shows the current strong interest in this topic [
27]. However, the studies on RWGS reactions over iron-based catalysts are much less frequent. Fishman et al. [
28] synthesized hematite nano-sheets to obtain a 28% CO
2 conversion at 783 K, and hematite nanowires to obtain a 50% CO
2 conversion at a very high temperature of 1023 K. Hematite was reduced to magnetite during the reaction. The catalytic behavior over time on stream and the stability of the CO production were, however, not investigated. Fe nanoparticles have also shown good stability and activity (35% CO
2 conversion, >85% CO selectivity) in RWGS by Kim et al. [
29], yet no kinetic parameters were determined, and the mechanism was not discussed.
Two principal mechanisms of the WGS (or RWGS) reaction have been investigated extensively: The “redox mechanism” and the “associative” mechanism [
30,
31]. Different catalysts may lead to a different reaction pathway. The redox mechanism was suggested to be active for the WGS reaction over iron catalysts promoted with chromium [
32]. A distinguishing feature of the redox mechanism is that products can be generated in the absence of both reactants. The catalyst is first reduced by the adsorbed H
2, and is subsequently oxidized by CO
2 (in RWGS) or H
2O (in WGS). The associative mechanism was proposed to be dominant in the WGS reaction over iron oxide catalysts [
33]. In this mechanism, both reactants are adsorbed on the catalyst surface at the same time to create products. Several carbon-containing intermediates, including formate, carbonate, carbonyl and carboxyl species, have been proposed. In a previous report in alumina-supported iron catalysts [
14], we showed that the redox mechanism is the only pathway for RWGS over Fe/γ-Al
2O
3, and the predominant pathway over Fe-K/γ-Al
2O
3. The addition of the potassium promoter activates a secondary pathway for CO formation, which is probably the associative pathway.
In the present report, unsupported Fe3O4-derived catalyst is identified as a highly active, selective and stable catalyst for the reverse water-gas shift reaction at temperatures between 723 K and 753 K. The characterization of surface composition, bulk properties, and the evaluation of the CO production specific rate showed that the working catalyst is constructed during the H2-activation and the period of reaction conditions. Quantitative gas-switching experiments in combination with isotopic switching experiments allowed the redox and associative reaction pathway to be differentiated. The catalysts appear to be highly stable under the reaction conditions investigated.
2. Results and Discussion
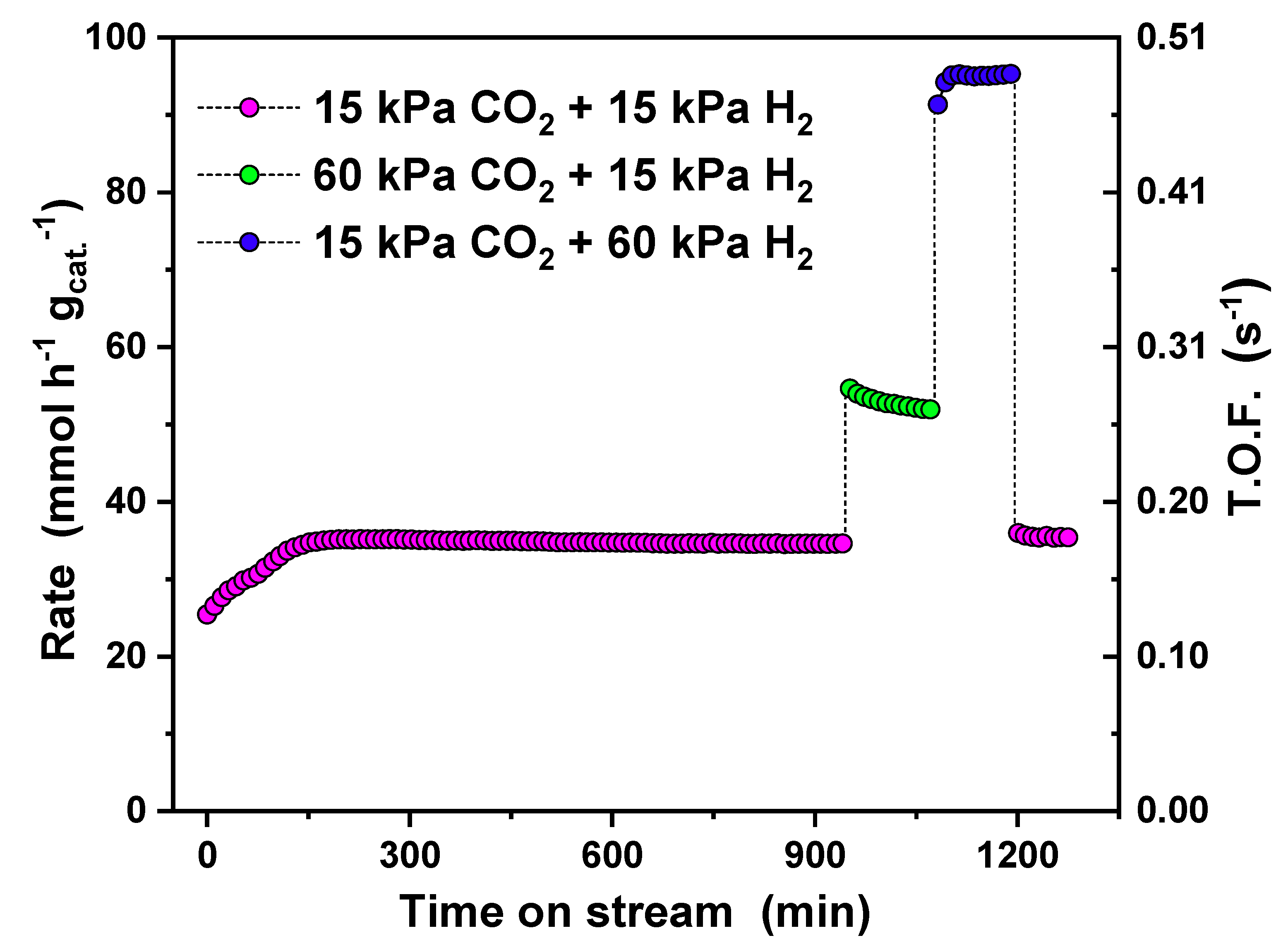
The catalytic CO formation rates on the Fe
3O
4 catalyst with various H
2 to CO
2 ratios (
Figure 1) show that after an induction period of ~120 min, the catalyst produced CO at a steady rate of 35.1 mmol h
−1 g
cat. −1 at 753 K with 12.5% CO
2 conversion. The selectivity to CO was greater than 99% under equimolar CO
2 and H
2. After 950 min, the partial pressure of CO
2 was raised to 60 kPa, while the partial pressure of H
2 was kept constant. The rate increased to 54.6 mmol h
−1 g
cat.−1 with 4.4% CO
2 conversion. Deactivation also occurred during this period: Starting at a deactivation rate of 3.71 mmol h
−1 g
cat.−1 per h, this rate gradually decreased to 0.23 mmol h
−1 g
cat.−1 per h. When the partial pressure of H
2 was 60 kPa, and the CO
2 was switched back to 15 kPa, the CO formation rate increased first to 91.3 mmol h
−1 g
cat.−1 and gradually stabilized to a value of 95.3 mmol h
−1 g
cat.−1 with 33.7% CO
2 conversion. The final rate of the catalyst reactivation was about 0.50 mmol h
−1 g
cat.−1 per h. It should be noted that the differential condition (see Equation (8)) was used to determine reaction rates, and preferred for the investigation of the kinetic properties of our materials. Under higher concentration of H
2 (15 kPa CO
2 + 60 kPa H
2), high CO
2 conversion (>12%) could lead to small errors in the estimation of the reaction rate and the reaction order. The trend observed in
Figure 1, however, should not be affected by this approximation.
With 60 kPa of H2 and 15 kPa of CO2 in the feed, a small amount of CH4—the only side-product—was produced at the rate of 1.35 mmol h−1 gcat.−1, reducing the CO selectivity from near 100% to 98.6%. Methane production implies that C–H bond formation is facilitated at higher partial pressure of H2. There was no further C–C chain growth under this reaction condition, indicating that the FT synthesis was not active over the working catalyst. The effect of H2 partial pressure on the CO formation rate was much higher than the effect of CO2, implying a higher reaction order on H2 than CO2. The catalyst showed overall high stability in 1300 min, and the final reactivation rate in excess H2 was higher than the deactivation rate under the excess CO2 condition. This indicates that this Fe3O4-derived catalyst is easy to regenerate in a very short period (<100 min), making the catalyst attractive in industrial use for long-term application.
The activation of the catalyst was carried out by a pretreatment under reducing conditions (H
2 gas). The bulk structure of the catalyst after the pretreatment can be identified. Based on the body-centered cubic (BCC) structure of α-Fe (JCPDS PDF 00-006-0696) after the pretreatment (
Figure S1), the surface density of Fe atoms on the Fe(110) surface can be calculated as 1.297 × 10
19 Fe atoms m
−2. Assuming all Fe atoms on Fe(110) were active sites, the observed CO formation rates can be converted to turnover frequency (TOF). This is reported in
Figure 1 based on the measured CO formation rates, atomic surface density, and BET surface area (2.52 m
2/g for Fe
3O
4) of the pristine catalyst. The turnover frequency of this catalyst under the equimolar condition was as high as 0.18 s
−1 (P
tot = 1 bar, T = 753 K, F
tot. = 75 sccm, GHSV = 4.5 × 10
4 cm
3 h
−1 g
cat.−1).
The stable reaction rates observed after the initial break-in period at 753 K allow for the determination of kinetic parameters without having to model deactivation profiles. The kinetic parameters, including reaction orders with respect to CO
2 and H
2, and measured activation energies (E
meas) over Fe
3O
4 under near equimolar CO
2 and H
2 (~1:1), and in H
2 excess (2:1, 4:1, and 9:1)—see
Table 1— indicate that CO formation rates have a higher dependence on H
2 partial pressure (order of ~0.8) than CO
2 partial pressure (order of ~0.33) under equimolar composition. In general, rate orders depend on reaction conditions: Increasing the H
2 partial pressure increases the order on CO
2 to 0.39 and decreases the rate order on H
2 to 0.72. At a ratio of H
2:CO
2 near 4:1, the reaction orders still show the same trend: An increasing dependence on CO
2 (order of 0.43) and decreasing dependence on H
2 (order of 0.31). In a high excess of H
2 (H
2:CO
2 = ~9:1), the reaction rate over Fe
3O
4 was of the order 1.30 with respect to CO
2, and was independent of H
2 pressure. The activation energies (E
meas) also depend on the H
2:CO
2 partial pressure ratios; that is, different reaction pathways may occur under these conditions. This behavior is not unique to iron catalysts. Similar reaction orders were also observed by Ginés et al. [
34] in the same regime of P
H2/P
CO2 < 3 (CO
2 order ≈ 0.3, H
2 order ≈ 0.8) on the CuO/ZnO/Al
2O
3 catalyst, and by Kim et al. [
3] on Pt/Al
2O
3 catalysts (CO
2 order = 0.32, H
2 order = 0.70). It was also suggested by Ginés et al. [
34] that different reaction pathways should be existed for P
H2/P
CO2 < 3 and P
H2/P
CO2 > 3 regions.
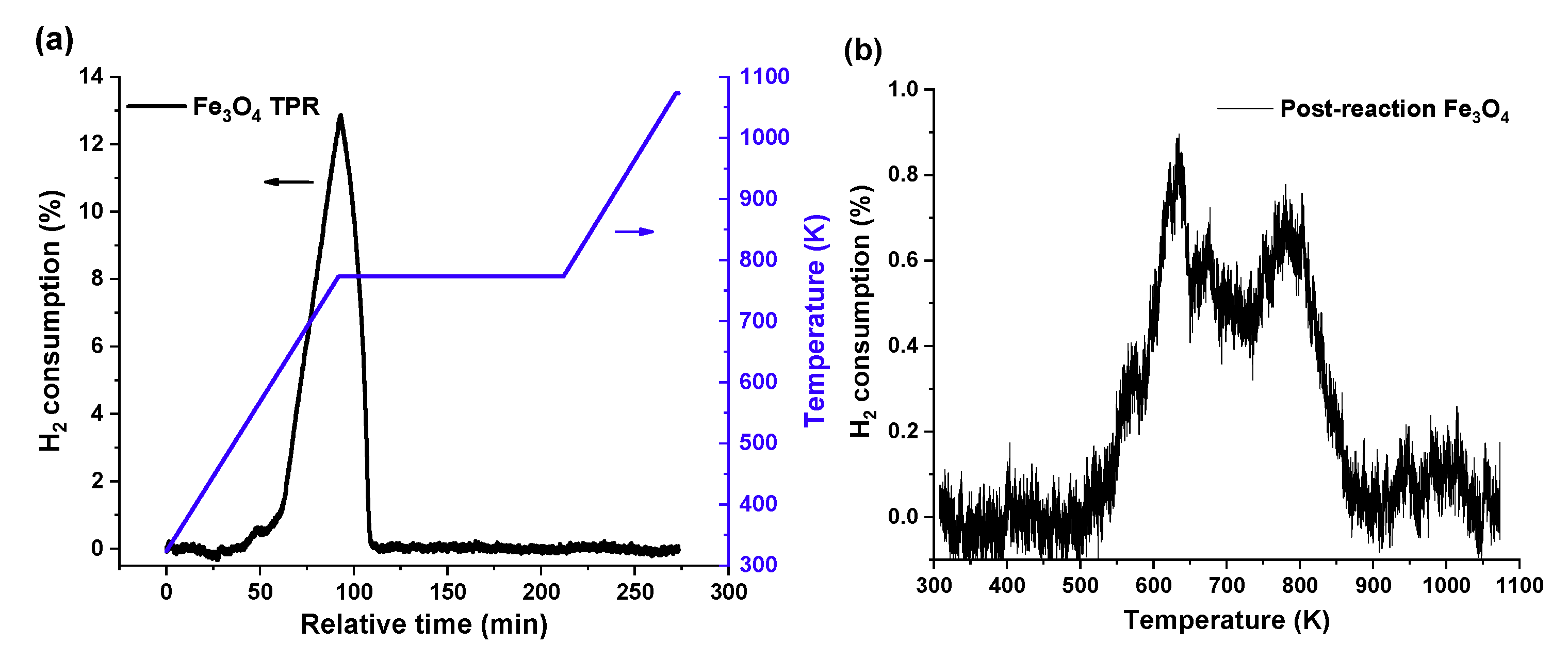
Figure 2 presents the temperature-programmed reduction (TPR) profiles of (a) the fresh Fe
3O
4 sample and (b) the post-reaction Fe
3O
4 sample. In
Figure 2a, the fresh Fe
3O
4 was heated to 753 K in a hydrogen atmosphere, kept for 2 h at these conditions, and then heated to 1,073 K at the rate of 5 K/min. The small peak observed in the TPR trace at about 563 K is assigned to an impurity of hematite present in the initial sample of magnetite (Fe
3O
4), but not detected in the XRD pattern, as shown in the report by Jozwiak et al. [
35]. The following broader and asymmetric peak suggests a two-step reduction process that has been previously postulated in literature [
36], as the following: (1)
and (2)
. These two steps can be deconvoluted into two peaks located at ~ 688 K and 773 K in the TPR traces. After the 2 h reduction period at 773 K, there was no further H
2 consumption at higher temperatures. That is, the sample, after the reduction pretreatment used in our activation protocol, has been converted into metallic iron. This result is also consistent with the XRD pattern in
Figure S1, which shows that α-Fe was the crystal formed after the reduction pretreatment of the Fe
3O
4 sample in the microreactor.
As soon as CO
2 was fed into the reactor, the surface of the catalyst was partially oxidized. This is known from the results of
Figure 2b that illustrates the presence of two significant peaks in the TPR profile for the post-reaction catalysts (at 630 K and 780 K, respectively). The location of these two predominant peaks shows good agreement with the results of
Figure 2a and results reported elsewhere [
35,
37]. There is at least a two-step reduction at ~630 K and 780 K, implying the coexistence of different oxidation states of iron on the post-reaction sample due to the partial oxidation from CO
2. For this post-reaction sample, the H
2 concentration in the effluent stream decreased by only a small amount (less than 1%), suggesting that the consumption of the H
2 feed would not affect the reduction rate during the TPR reaction.
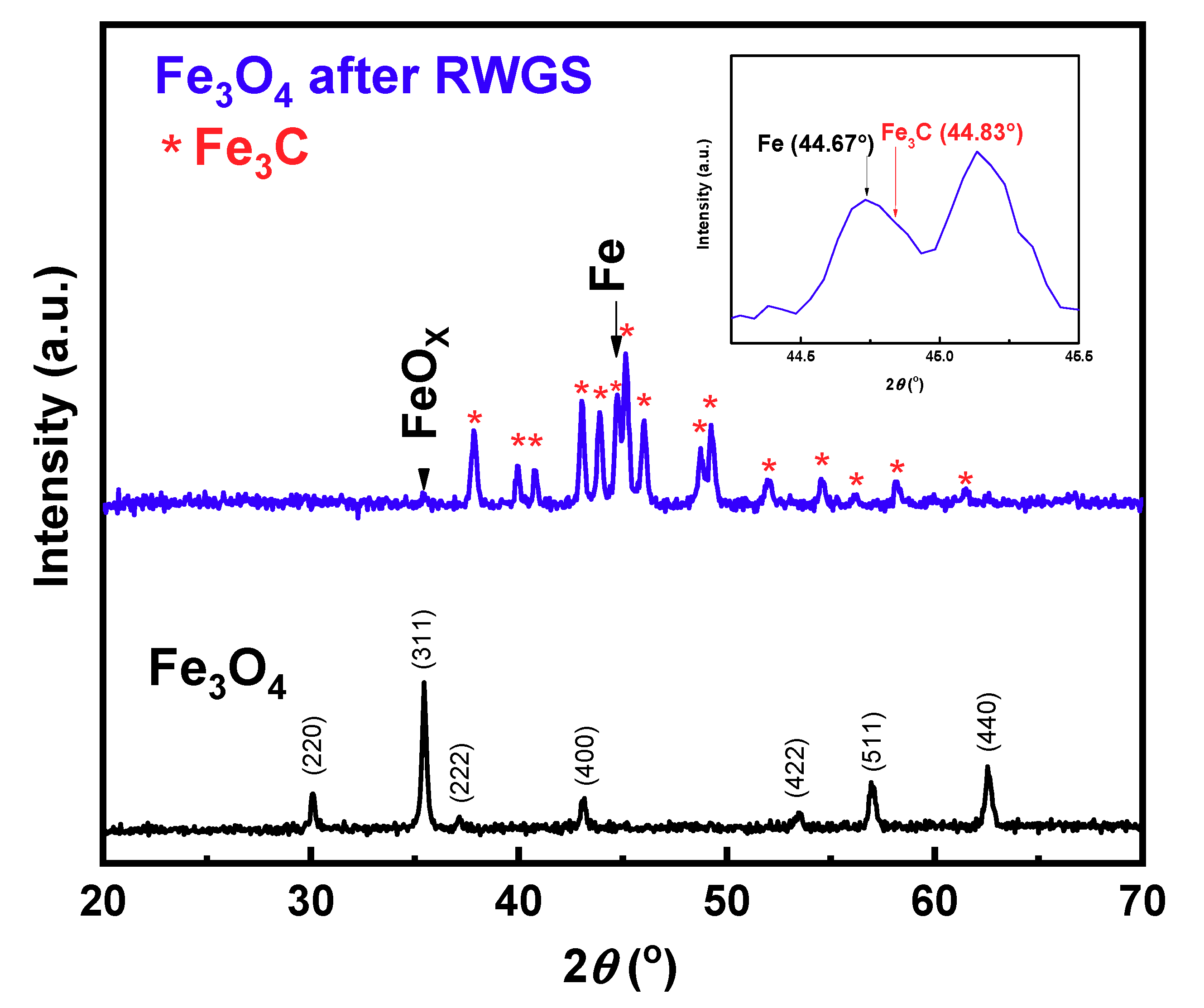
Figure 3 displays the diffraction pattern of fresh Fe
3O
4 and the catalyst after the RWGS reaction (post-reaction Fe
3O
4). The 2θ degree peak positions in fresh Fe
3O
4 were 30.15°, 35.45°, 37.15°,43.15°, 53.50°, 56.95° and 62.55°, which are all consistent with magnetite (JCPDS PDF 01-071-6336). The post-reaction Fe
3O
4 shows a very different XRD pattern: This pattern was composed of metallic iron (α-Fe, 44.67°, shown in the inset of
Figure 3), iron carbide (Fe
3C), and a small peak of FeO
X (35.47°).
Iron oxides can be converted directly into carbides in a reducing and carburizing atmosphere [
38], and the carbon source of the Fe
3C production can be either from impurities in the fresh Fe
3O
4 sample or due to reaction with the product CO, after the RWGS reaction as indicated by Equation (2) [
39]:
The bulk composition of the catalyst after the reaction is also consistent with the TPR results in
Figure 2a,b. During TPR, the amount of H
2 consumption of magnetite relative to the amount of H
2 consumption after the reaction was 12:1, therefore the iron species in the catalyst has been changed into a more reduced chemical state after the pretreatment and RWGS reaction. The reduction was mainly caused by the pretreatment, while the following CO
2/H
2 reaction shifted the metallic iron back to a slightly more oxidized state; a combination of iron carbide, metallic iron and some iron oxides.
Besides bulk property information obtained from XRD, XPS analyses were conducted to characterize the surface composition of the initial fresh Fe
3O
4 and the change of the catalyst after the RWGS reaction.
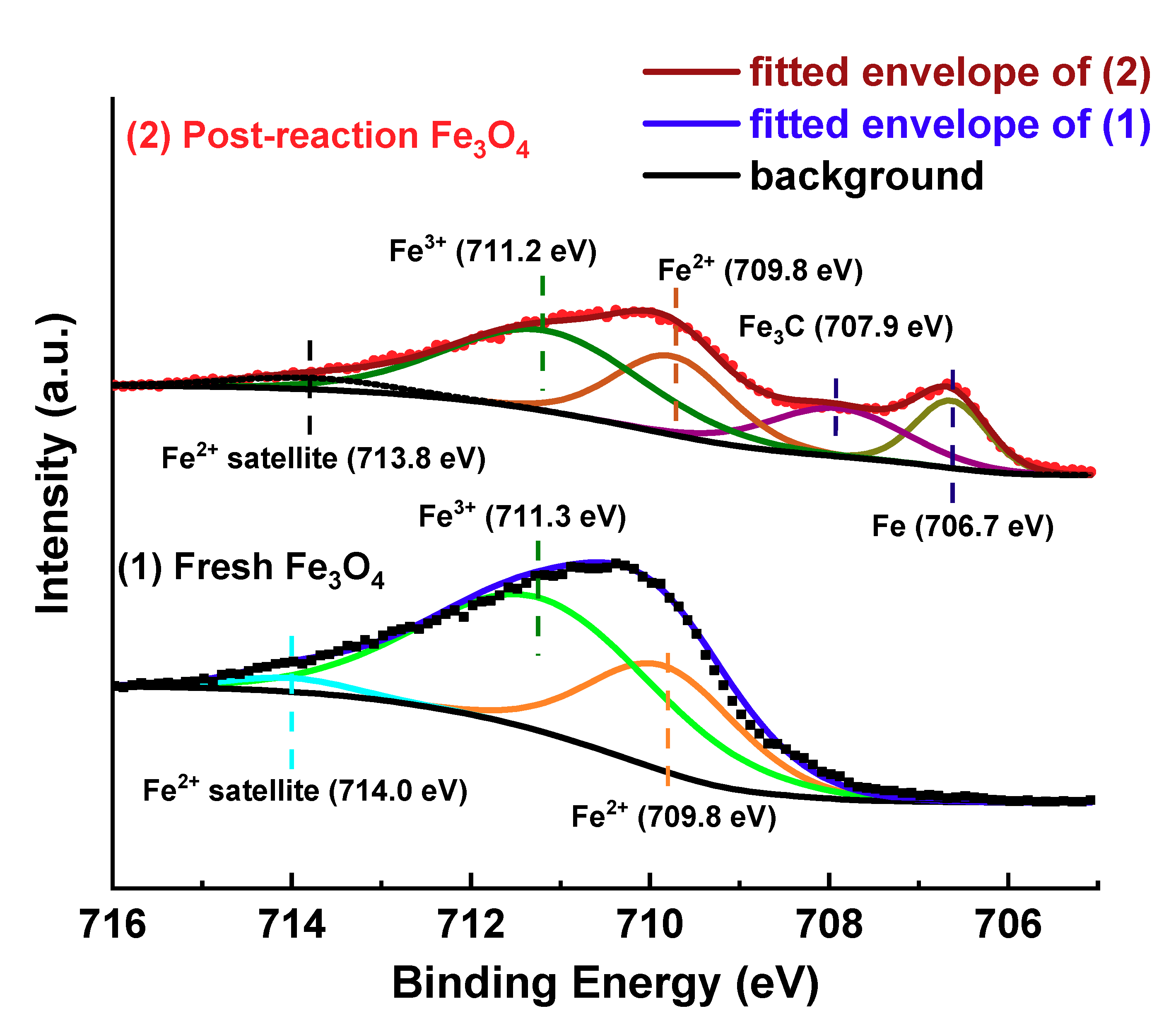
Figure 4 shows the XPS spectra, peak deconvolutions and the fitting envelopes for the Fe 2p
3/2 spectra of Fe
3O
4 and post-reaction Fe
3O
4.
Atomic percent contributions are calculated from the fitted peaks of Fe 2p
3/2 due to its larger intensity (Area of Fe 2p
3/2:Fe 2p
1/2 = 2:1). The Fe 2p
3/2 spectra were fitted over the range of 705–722 eV. The spectra between 716–722 eV were not shown in the figure for clarity. In this range, there were only small Fe
3+ satellite peaks located at 719.2 eV and 719.4 eV for both the fresh and post-reaction Fe
3O
4 samples, respectively, although the area of the satellite peaks was still included in the corresponding components when calculating the relative atomic percentage. The fits, including the binding energy, full width at half maximum (FWHM), and the relative iron composition, are summarized in
Table 2. The fitted XPS spectrum of fresh Fe
3O
4 was composed of doublets for Fe
2+ at 709.8 eV and Fe
3+ at 711.3 eV. Fe
3O
4 has an inverse spinel structure which can be written as Fe
3+TET[Fe
2+Fe
3+]
OCTO
4, with one Fe
3+ on a tetrahedral site, and Fe
2+ and the other Fe
3+ distributed on octahedral sites. Therefore, the theoretical relative composition of Fe
2+/Fe
3+ is 0.5, which is close to the area fitted and the calculated relative composition in our fresh Fe
3O
4 (Fe
2+:Fe
3+ = 34.8/65.2 = 0.53). The Fe
3+ peak has a larger FWHM than Fe
2+. This is as expected because the electronic configuration of Fe
2+ is 3d
6, while that of Fe
3+ is 3d
5, that is, Fe
2+ will have a longer life time compared to Fe
3+; and therefore the FWHM of the Fe
2+ peak should be smaller than the Fe
3+ peak [
40]. Additionally, the Fe
3+ peaks can be attributed to two different structures, octahedral Fe
3+ and tetrahedral Fe
3+, a factor that will also lead to broader Fe
3+ peaks.
After the RWGS reaction, the spectrum was fitted using four different components corresponding to metallic Fe (706.7 eV), Fe
3C (707.9 eV), Fe
2+ (709.8 eV) and Fe
3+ (711.2 eV). The peak locations of Fe
2+ and Fe
3+ were the same or very close to the fresh sample, indicating that there was only a small surface charging effect with the flood gun on. The binding energies of the components are in agreement with literature results [
38,
40,
41,
42]. In terms of the atomic percentages, the overall peak area was re-allocated to a more reduced regime after the RWGS reaction. The Fe
2+ decreased from 34.8% to 27.5%, the Fe
3+ decreased from 65.2% to 43.9%, while there were two components formed: Fe (12.2%) and Fe
3C (16.4%). The shift of the spectra was due to the H
2 reduction pretreatment before operating the RWGS reaction, and the flow of H
2/CO
2 reactants through the system would balance each other to make the catalyst partially oxidized or reduced. Though the sample surface could be oxidized by the air during the transportation from the reactor to the XPS analysis chamber, the result from XPS still can confirm the reduction of the surface during the reaction, since new crystal structures such as Fe and Fe
3C are detected by XRD.
The XPS analyses indicate that the active catalyst consisted of a mixture of metallic Fe, Fe
3C, Fe
2+ and Fe
3+; however, it cannot establish the relative contributions of these components to the observed rate of the RWGS reaction. To determine if the iron carbide formed in our reaction can catalyze the RWGS reaction, reaction rates over pure Fe
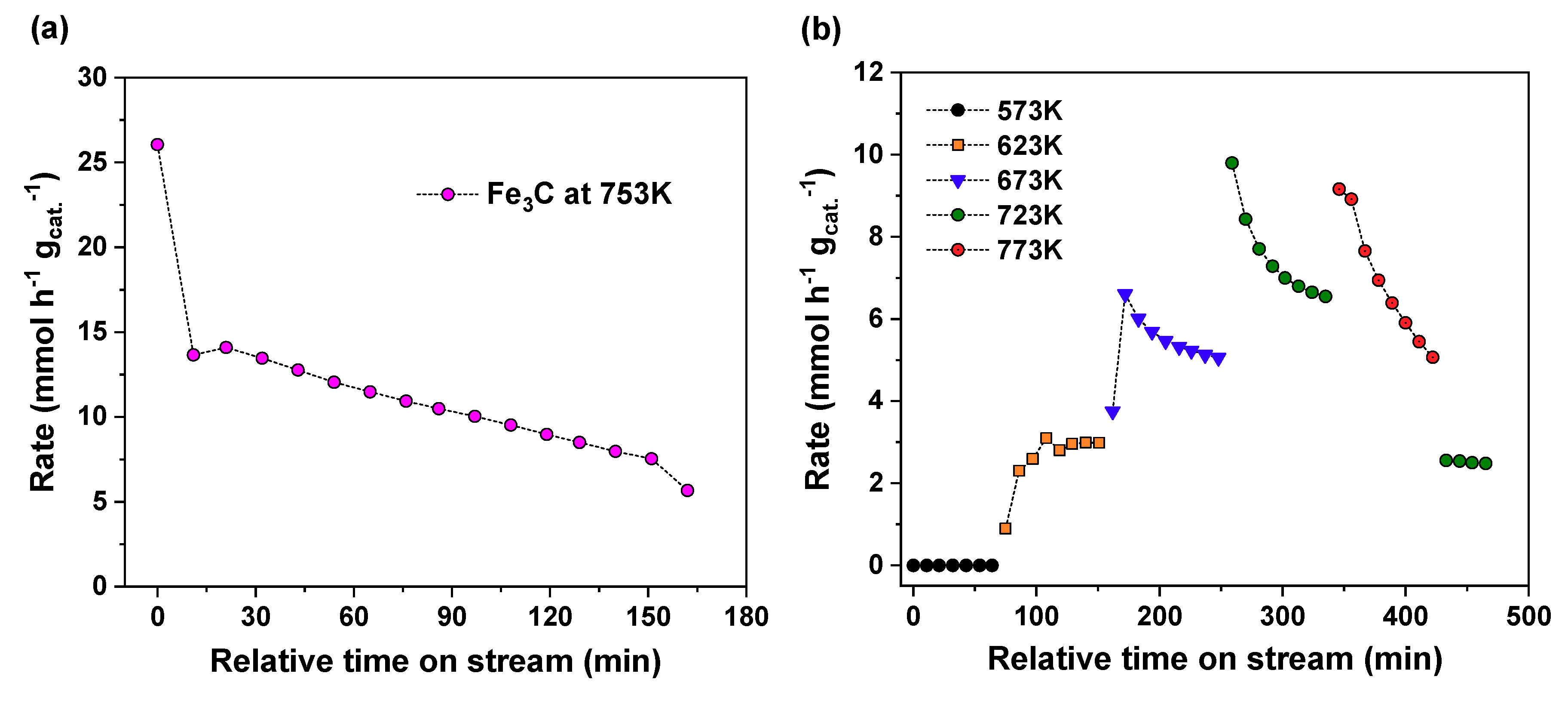
3C were measured (
Figure 5). In
Figure 5a, Fe
3C showed an initial CO formation rate of 26.0 mmol h
−1 g
−1, but dropped 48% to near 13.5 mmol h
−1 g
−1 in 10 min, and then further down to 5.7 mmol h
−1 g
−1 in 160 min at 753 K. This is clearly different from the properties of the Fe
3O
4-derived catalyst in
Figure 1, since the Fe
3O
4-derived catalyst displayed high stability for at least 1300 min. To evaluate the fast deactivation shown in
Figure 5a and remove the initial reduction effect during the ramping by hydrogen, another measurement was carried out with respect to temperature, (see
Figure 5b). This experiment was conducted flowing a CO
2/H
2/He gas mixture in the same relative concentration used in the standard activity test during the ramping procedure from room temperature to 773 K. At 573 K, the material did not catalyze the formation of CO; however, when the temperature was increased to 623 K, the catalyst immediately showed catalytic rates in the range of 2.60 mmol h
−1 g
−1 to 2.99 mmol h
−1 g
−1. After 1 h of the reaction, the rate remained at quasi-steady state at this low temperature (623 K).
The CO formation rates were 6.60 mmol h−1 g−1, 9.80 mmol h−1 g−1, and 9.16 mmol h−1 g−1, as the temperature was increased to 673 K, 723 K and 773 K, respectively. The formation rate at 773 K was not higher than that at the lower temperature because of rapid deactivation at this temperature. Faster deactivation rates were observed at higher temperatures: The average deactivation rates were 1.36 mmol h−1 g−1 per h, 2.57 mmol h−1 g−1 per h and 3.50 mmol h−1 g−1 per h.
After reacting at 773 K, the temperature was reduced to 723 K to monitor the reaction rate and compare to the previous value. Much lower rates (2.55 mmol h
−1 g
−1) were observed than in the previous measurement at the same temperature (723 K), indicating that there was an irreversible change in the catalyst structure or composition. At higher temperatures, the reverse reaction of Equation (2), where Fe
3C reacts with CO
2 and forms Fe and CO, is more favorable than the Fe
3C formation [
39]. Therefore, the initial CO formation rate was probably due to the formation of CO from decomposition, but quickly dropped since it is harder to convert the metallic iron back to Fe
3C at higher temperatures. The iron carbide catalyst only showed steady CO production at 623 K. This explains why the deactivation at 723 K over Fe
3C is very different from the steady-state magnetite catalyst reported in
Figure 1, which showed a very stable CO formation rate at 753 K. This observation suggests that the operating temperature of RWGS in this study was not an environment conducive for a stable iron carbide for CO production. In addition, the iron carbide (Fe
5C
2 or Fe
3C) is normally considered to be the active phase of iron for hydrocarbon production [
43,
44], and iron oxide is the active phase for WGS and RWGS [
25]. Several reports have suggested that the stability of the iron catalyst in either FT synthesis [
45] or RWGS [
29] can be related to an iron carbide layer. Davis [
45] suggested that catalyst composition and reaction condition will define the existence of the pseudo-equilibrium layer of iron carbide to ensure a very slow deactivation condition. Kim et al. [
29] concluded that the stability of the catalyst could have originated from migration of C and O into the catalyst bulk, forming iron oxide and iron carbide, which likely prevented the nanoparticles on the surface from agglomerating. Based on the XPS results and Fe
3C catalytic tests, the iron carbide of the working catalyst is less likely to be the main active site for CO production, but is an important species to provide stability in the overall catalytic performance.
Gas-switching experiments, in which H
2 and CO
2 are flown on and off, were used to distinguish and quantify contributions from redox and associative reaction pathways [
3,
14,
46]. In the simplest form of the redox mechanism, gas-phase CO
2 adsorbs on a reduced site to form CO and an oxidized site (Equation (3)), which can then be reduced by gas phase H
2 to reform the reduced site (Equation (4)). The simplest redox cycle can be described as follows:
A simplified associative pathway can be described generally by Equation (5). CO
2 and H
2 adsorb on the catalyst surface to form a carbon-containing intermediate (i.e. formate, carbonate, or bicarbonate), which then decomposes in the presence of H
2 to form CO and H
2O.
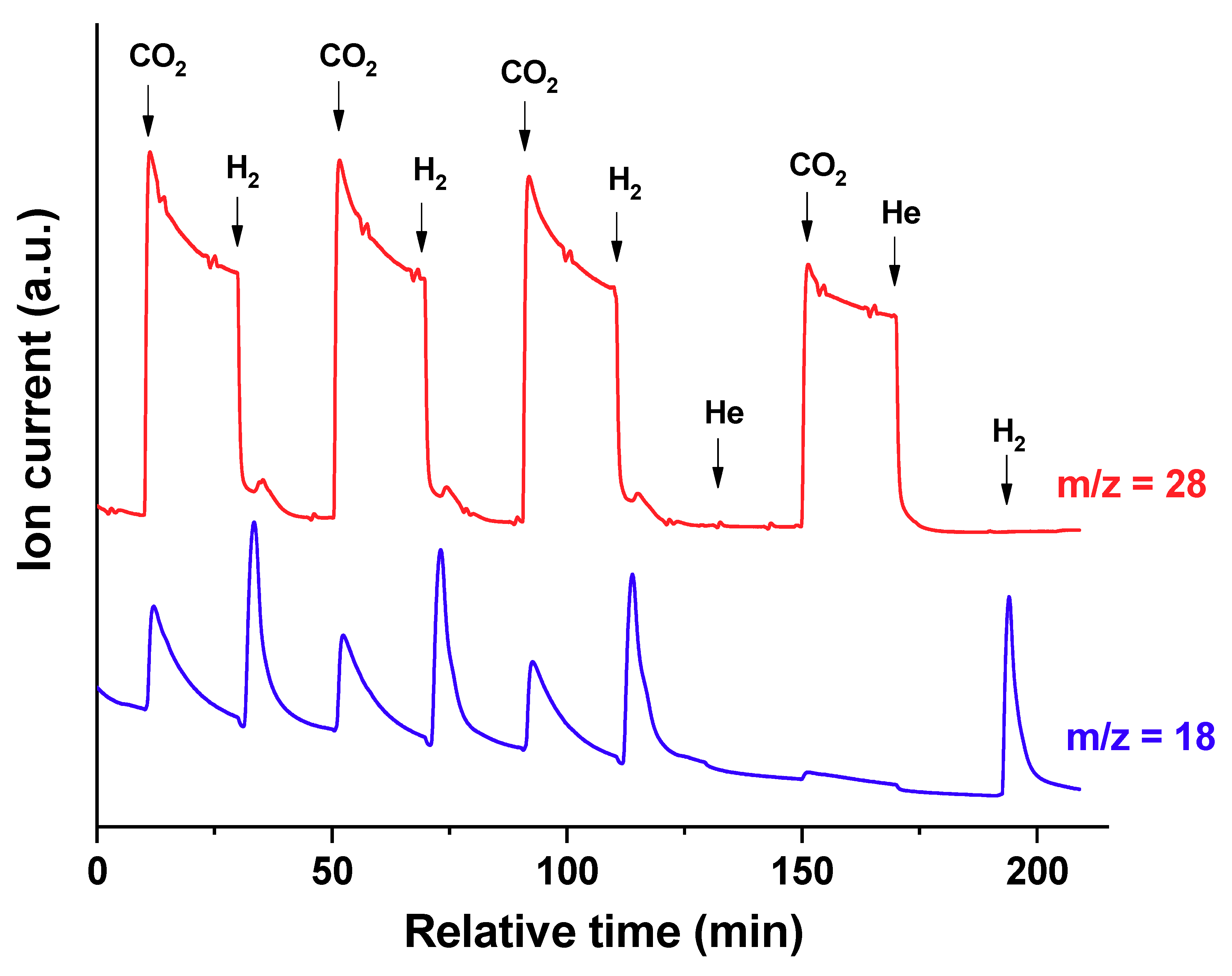
CO and H
2O were the main products formed during gas-switching experiment (
Figure 6). In the first three cycles, CO was formed when switching from H
2 to CO
2, and a very small amount of CO was formed when switching from CO
2 to H
2 at 30 min, 70 min and 110 min, respectively. When the catalyst was purged 20 min with helium before switching from CO
2 to H
2, CO was not formed, and H
2O was produced at 195 min. Water was formed when switching from H
2 to CO
2 and when switching from CO
2 to H
2. After flowing H
2 and purging the reactor with He for 20 min, only a negligible amount of H
2O was formed upon the admission of CO
2 (at 150 min).
In
Figure 6, the fact that CO was formed when the reduced form of the Fe
3O
4 catalyst was contacted with CO
2, (even after the purge with He to decrease the concentration of any surface H
2), is evidence of a redox pathway. During the first 125 min of gas-switching experiments, H
2O was produced during flows of only CO
2 or only H
2. This differs from what is expected in the traditional redox cycle, in which H
2O is only produced during the H
2 feeding period (see Equation (4)). However, after flowing H
2 and purging the reactor with He for 20 min, the admission of CO
2 only produced negligible amounts of water.
Table 3 summarizes the estimated initial rates of CO production on the Fe
3O
4 catalyst during each segment of the gas-switching experiments. The CO production rates were calculated from the initial slopes of the concentration vs. time data in
Figure 6, essentially modeling the system as a batch reactor (Equation (6)).
It is observed (
Table 3) that the rate after switch from H
2 to CO
2 fluctuated between 2.74 µmol L
−1 s
−1 g
cat.−1 and 2.98 µmol L
−1 s
−1 g
cat.−1 in the first three periods of CO
2 admission, and it decreased after the He purge. The rate after switching from CO
2 to H
2 decreased in the first three periods, and it was zero (with no CO produced) during the last admission of CO
2 after the He purge. The decrease of the CO initial rate after switching from CO
2 to H
2, especially when equal to zero after the purge, raises doubts about the existence of residual CO
2 during the first three admissions of H
2 in the switching experiment. As a control, when the gas was switched from CO
2 to H
2, the CO
2 gas did not exit from the surface very quickly (see
Figure S2). Therefore, a small amount of CO can be produced by the residual CO
2 with the available reduced sites; evidence of this interpretation in the detection of very small peaks after the H
2 admissions (
Figure 6). The negligible CO production (relative time = 34 min, 74 min, and 114 min, respectively) after the H
2 admissions should not be considered evidence of the associative mechanism. In summary, the CO formation upon switching from H
2 to CO
2 is evidence consistent with the redox mechanism, while the small contribution of CO production upon switching from CO
2 to H
2 was suppressed by the confirmation of the helium purge. Thus, from the gas-switching experiment, only the redox pathway is active on our Fe
3O
4-derived catalyst.
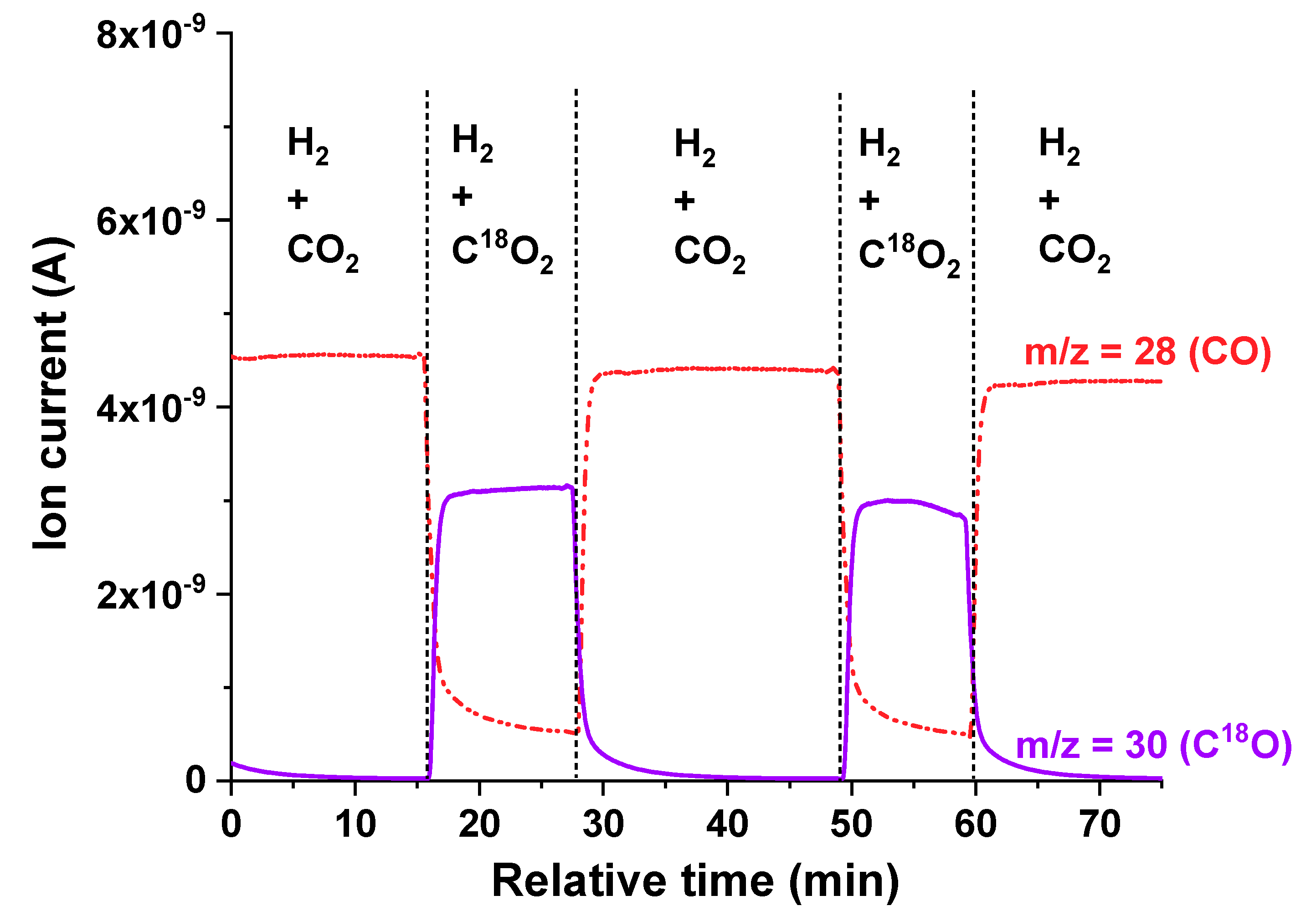
Isotopic experiments were conducted to gain insight into the mechanism of the reaction. The isotopic C
18O
2 to the CO
2 switching experiment is shown in
Figure 7. Here it can be seen that C
18O (m/z = 30) formed and CO (m/z = 28) decreased when the gas (CO
2/H
2) was switched to C
18O
2/H
2. CO can be only formed from CO
2, and not from the lattice oxygen.
The gas-switching experiments with CO
2 and H
2 led us to conclude that a redox pathway is active on the Fe
3O
4-derived catalyst. A model that can present a redox reaction pathway for this catalyst is given in
Scheme 1. It includes the adsorption of both of the reactants, CO
2 and H
2. In the surface redox mechanism, the dissociation of CO
2 at the catalyst surface (step 2 in
Scheme 1) is known to be the RDS [
8,
30,
34]. Evidence for H
2 dissociation (step 5 in
Scheme 1) was observed when H
2/D
2 mixtures were fed to the catalyst in the presence of CO
2 (see
Figure S3).
HD formation was observed to occur quickly, since the amount of CO2 to CO conversion decreased on the same time scale when switching the concentration from H2/D2 (7.5 kPa/7.5 kPa) to H2 (7.5 kPa), indicating that H2 dissociation is reversible and not rate limiting.
An additional gas switching experiment was conducted to monitor the H
2O production (
Figure S4). The amount of H
2O produced during H
2 flow periods was consistent between each cycle (
Figure S4a), that is, the adsorbed O·s species formed upon CO
2 reduction are stable at these reaction conditions. However, the amount of H
2O produced during the period of CO
2 flow decreased as the purge time in helium increased. After only a five min purge, the amount of H
2O produced during the period of CO
2 flow was much greater than that produced following a 20 min purge in helium, and the rate fitted from the initial slope of this region dropped dramatically (see
Table S1). This suggests that H* atoms from the catalyst surface appeared to desorb (as H
2) during the He purge. This was a slow process, because even following a 20 min purge, there were enough H* atoms on the sample to form small amounts of H
2O when CO
2 was administered. Both gas-switching experiments (
Figure 6 and
Figure S4) suggest that the redox mechanism should be the dominant reaction pathway for this Fe
3O
4-derived catalyst during the CO
2 hydrogenation at our reaction conditions (753 K, 1 atm).