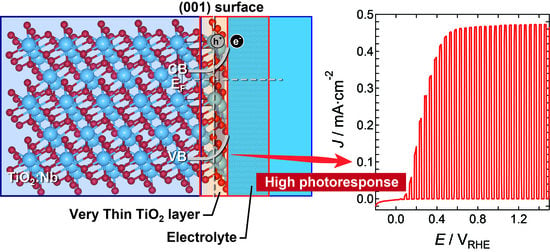
Crystal-Plane Dependence of Nb-Doped Rutile TiO2 Single Crystals on Photoelectrochemical Water Splitting
Abstract
:1. Introduction
2. Results and Discussion
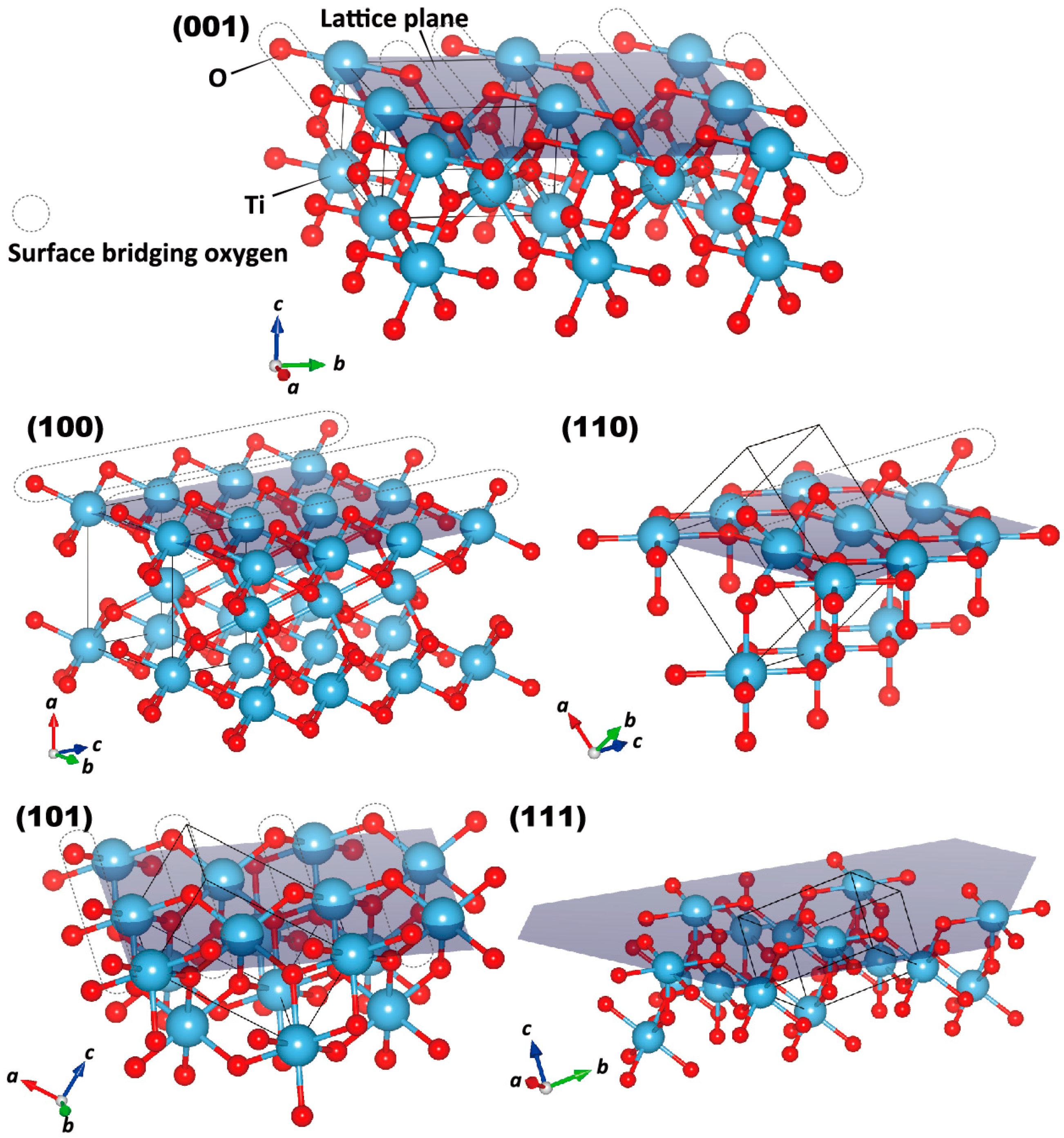
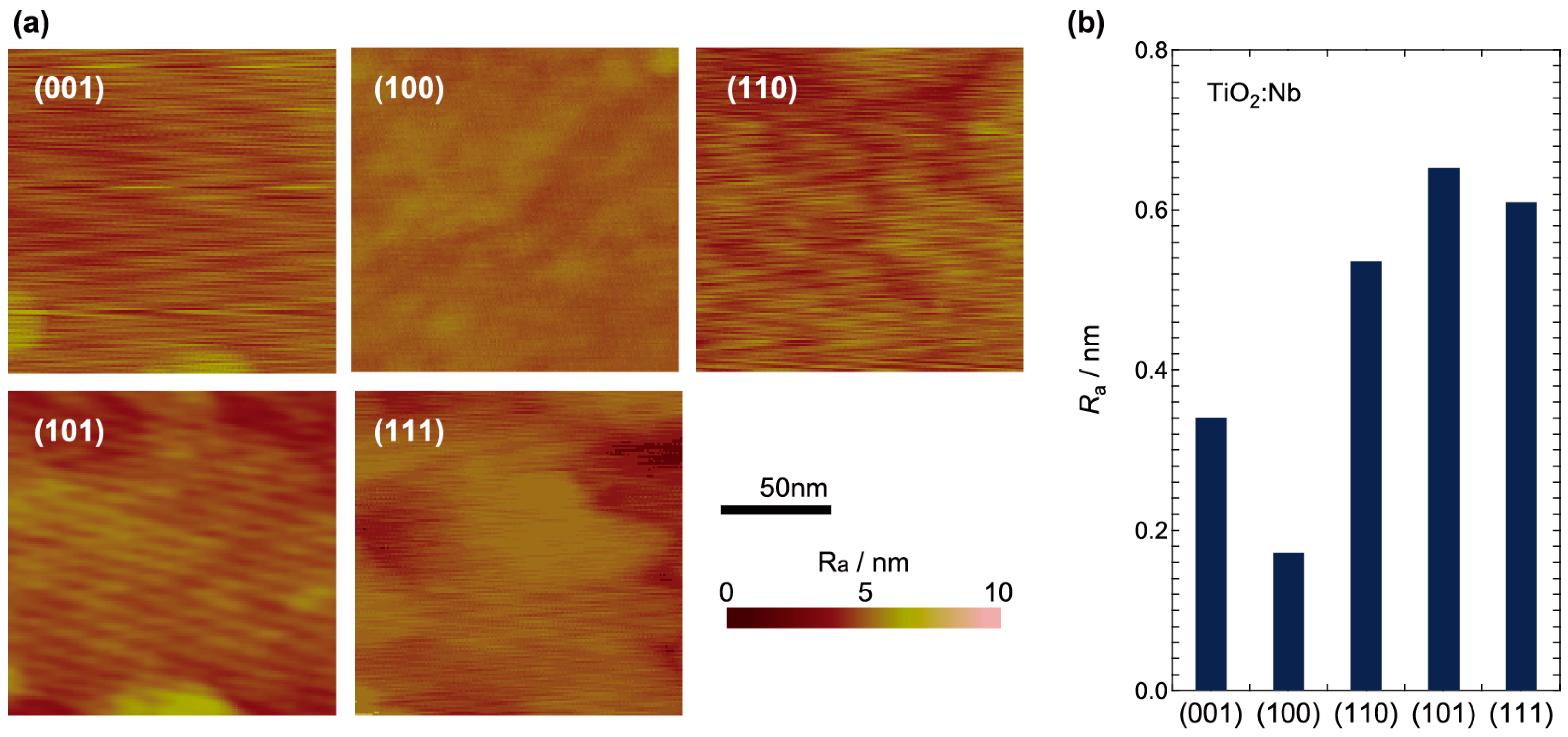
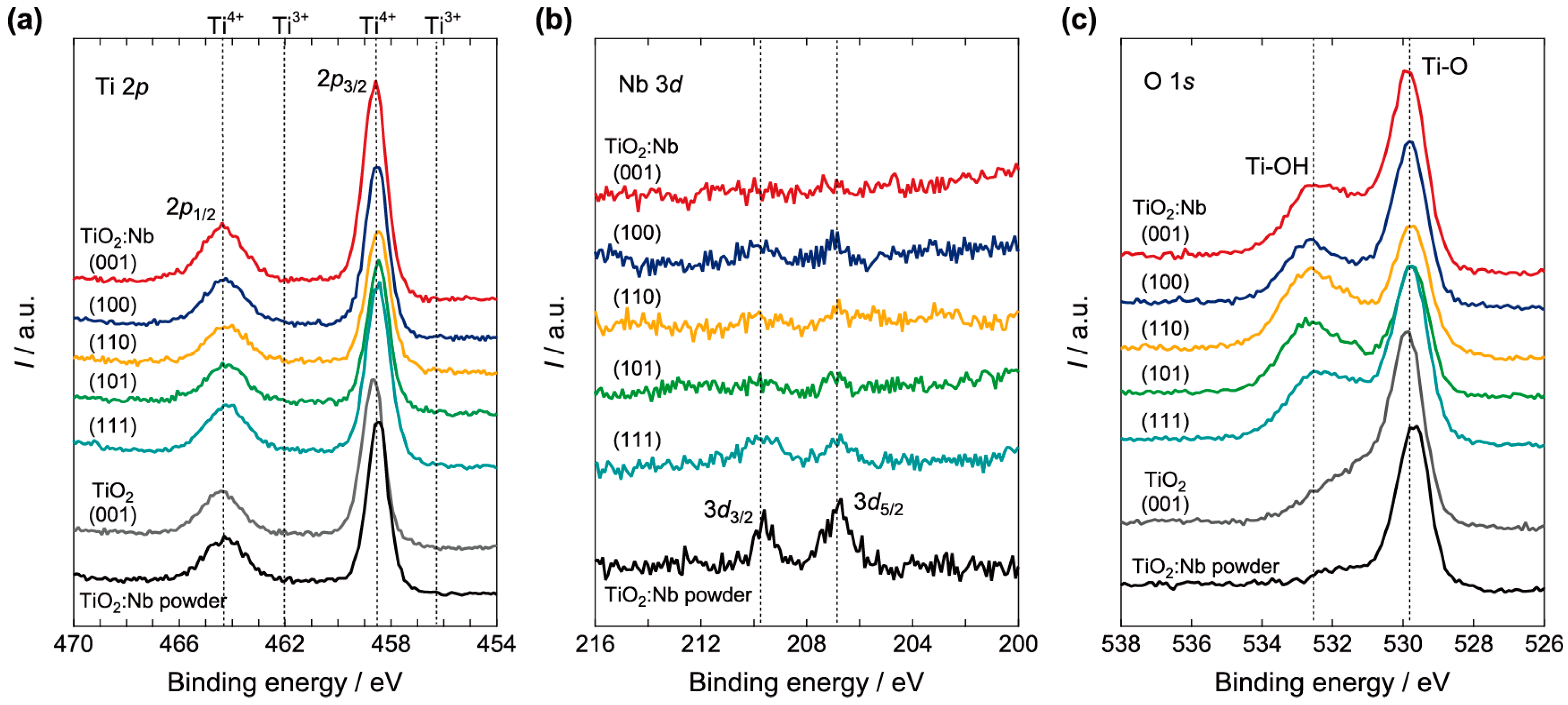
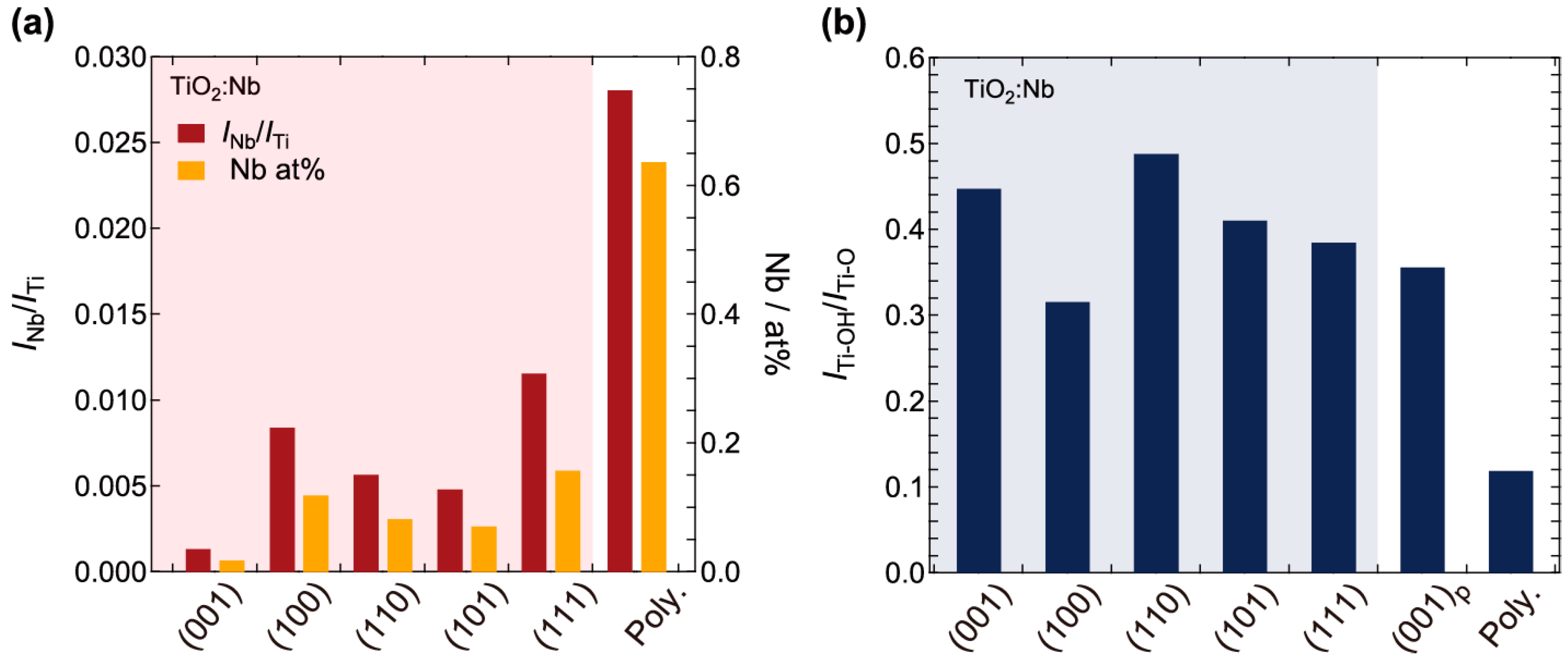
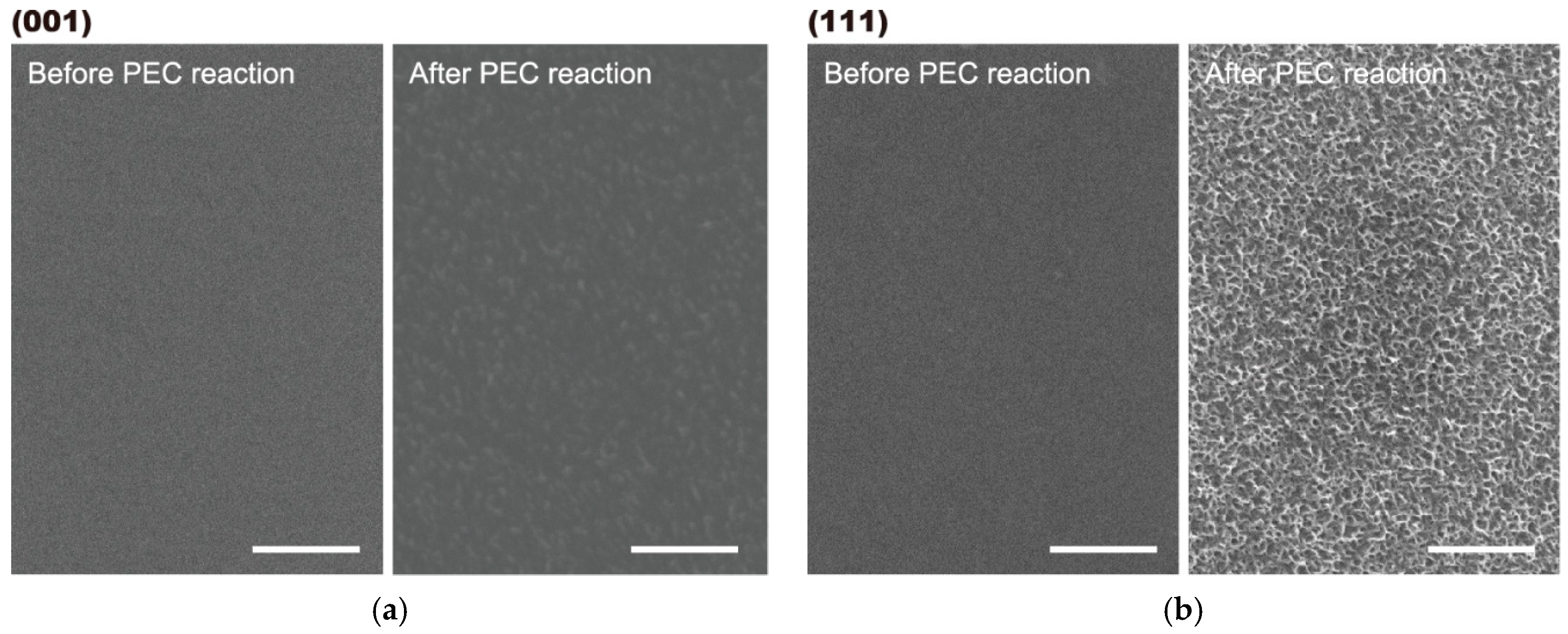
2.1. Surface Analysis
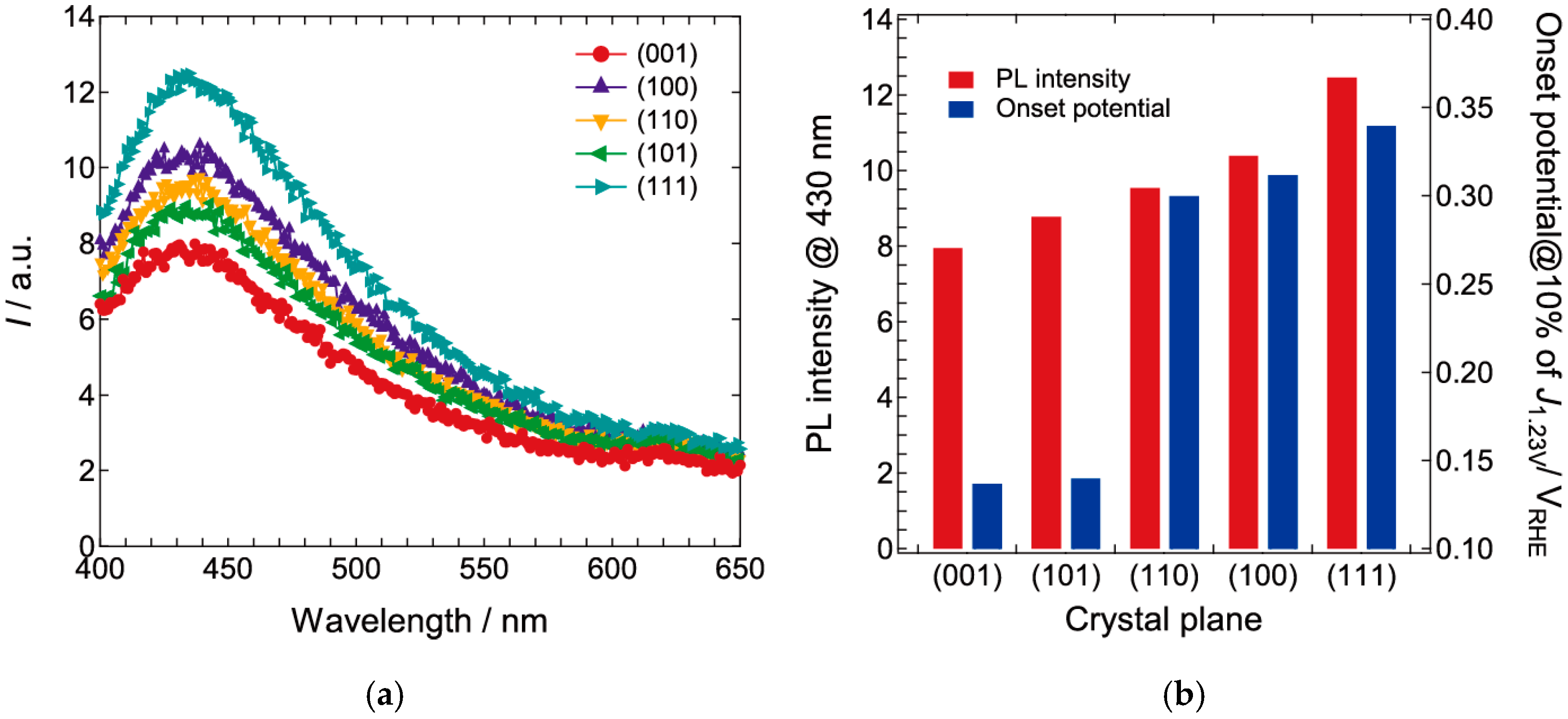
2.2. Photoelectrochemical and Optical Properties
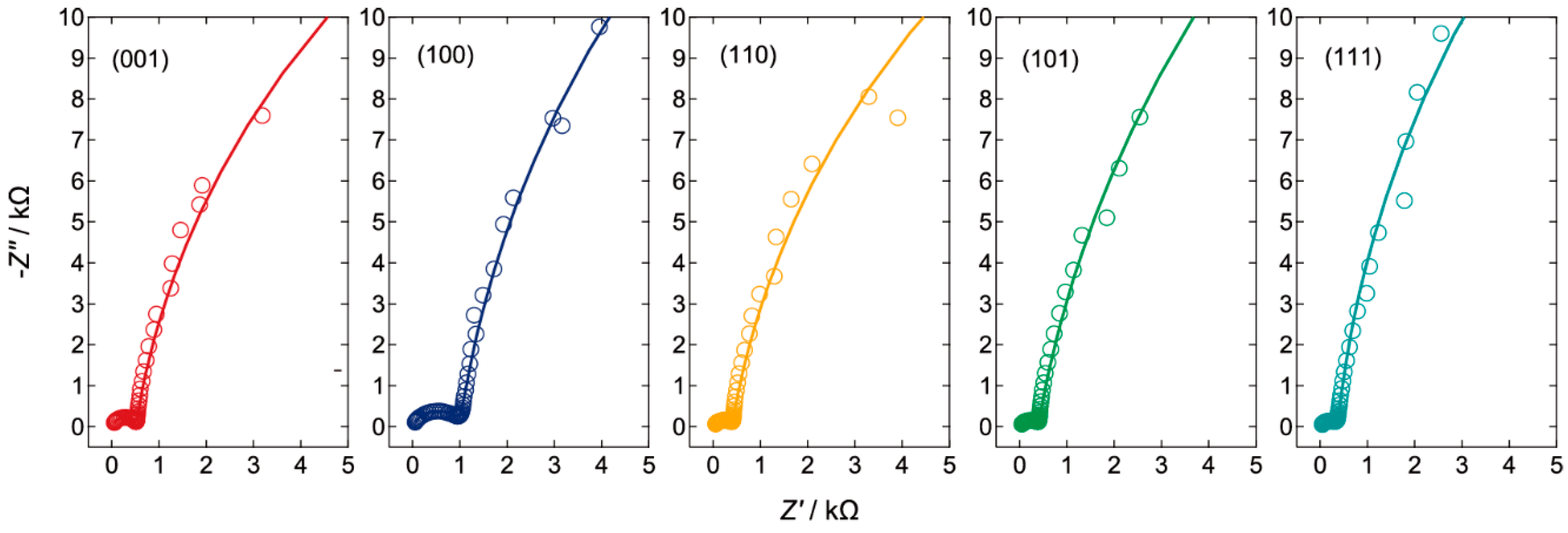
2.3. Electrical Impedance Spectral Analysis of the Transport Property
3. Materials and Methods
3.1. Materials
3.2. Sample Characterization
3.3. PEC Performance Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alexander, B.D.; Kulesza, P.J.; Rutkowska, I.; Solarska, R.; Augustynski, J. Metal oxide photoanodes for solar hydrogen production. J. Mater. Chem. 2008, 18, 2298–2303. [Google Scholar] [CrossRef]
- Nowotny, J.; Bak, T.; Nowotny, M.K.; Sheppard, L.R. Titanium dioxide for solar-hydrogen I. Functional properties. Int. J. Hydrogen Energy 2007, 32, 2609–2629. [Google Scholar] [CrossRef]
- Kronawitter, C.X.; Vayssieres, L.; Shen, S.; Guo, L.; Wheeler, D.A.; Zhang, J.Z.; Antoun, B.R.; Mao, S.S. A perspective on solar-driven water splitting with all-oxide hetero-nanostructures. Energy Environ. Sci. 2011, 4, 3889–3899. [Google Scholar] [CrossRef]
- Osterloh, F.E. Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting. Chem. Soc. Rev. 2013, 42, 2294–2320. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Hagino, A.; Nakamura, T.; Tsuchiya, T.; Sayama, K. WO3 nanosponge photoanodes with high applied bias photon-to-current efficiency for solar hydrogen and peroxydisulfate production. J. Mater. Chem. A 2016, 4, 17809–17818. [Google Scholar] [CrossRef]
- Dang, H.X.; Hahn, N.T.; Park, H.S.; Bard, A.J.; Mullins, C.B. Nanostructured Ta3N5 films as visible-light active photoanodes for water oxidation. J. Phys. Chem. C 2012, 116, 19225–19232. [Google Scholar] [CrossRef]
- Nakajima, T.; Lee, C.-Y.; Yang, Y.; Schmuki, P. N-Doped lepidocrocite nanotubular arrays: Hydrothermal formation from anodic TiO2 nanotubes and enhanced visible light photoresponse. J. Mater. Chem. A 2013, 1, 1860–1866. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, C.; Lin, T.; Yin, H.; Chen, P.; Wan, D.; Xu, F.; Huang, F.; Lin, J.; Xie, X.; et al. Visible-light photocatalytic, solar thermal and photoelectrochemical properties of aluminium-reduced black titania. Energy Environ. Sci. 2013, 6, 3007–3014. [Google Scholar] [CrossRef]
- Yi, Z.; Ye, J.; Kikugawa, N.; Kako, T.; Ouyang, S.; Stuart-Williams, H.; Yang, H.; Cao, J.; Luo, W.; Li, Z.; et al. An orthophosphate semiconductor with photooxidation properties under visible-light irradiation. Nat. Mater. 2010, 9, 559–564. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.-Q.; Fu, X.; Zhang, N.; Xu, Y.-J. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef]
- Pinaud, B.A.; Vesborg, P.C.K.; Jaramillo, T.F. Effect of film morphology and thickness on charge transport in Ta3N5/Ta photoanodes for solar water splitting. J. Phys. Chem. C 2012, 116, 15918–15924. [Google Scholar] [CrossRef]
- Seki, M.; Yamahara, H.; Tabata, H. Enhanced photocurrent in Rh-substituted α-Fe2O3 thin films grown by pulsed laser deposition. Appl. Phys. Express 2012, 5, 115801-1–115801-3. [Google Scholar] [CrossRef]
- Nakajima, T.; Nakamura, T.; Tsuchiya, T. The significant effect of heterojunction quality on photoelectrochemical water splitting in bilayer photoelectrodes: RbxWO3 thin films on RbLaNb2O7 layers. Phys. Chem. Chem. Phys. 2014, 16, 26901–26908. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Zhao, Y.; Li, A.; Wang, S.; Wang, Z.; Yang, J.; Wang, Y.; Liu, T.; Chen, R.; Zhu, J.; et al. Enhancing charge separation on high symmetry SrTiO3 exposed with anisotropic facets for photocatalytic water splitting. Energy Environ. Sci. 2016, 9, 2463–2469. [Google Scholar] [CrossRef]
- Li, W.; Bai, Y.; Liu, W.; Liu, C.; Yang, Z.; Feng, X.; Lu, X.; Chan, K.-Y. Single-crystalline and reactive facets exposed anatase TiO2 nanofibers with enhanced photocatalytic properties. J. Mater. Chem. 2011, 21, 6718–6724. [Google Scholar] [CrossRef]
- Ye, L.; Mao, J.; Liu, J.; Jiang, Z.; Peng, T.; Zan, L. Synthesis of anatase TiO2 nanocrystals with {101}, {001} or {010} single facets of 90% level exposure and liquid phase photocatalytic reduction and oxidation activity orders. J. Mater. Chem. A 2013, 1, 10532–10537. [Google Scholar] [CrossRef]
- Ballestas-Barrientos, A.; Li, X.; Yick, S.; Masters, A.F.; Maschmeyer, T. Optimised heterojunctions between [100]-oriented rutile TiO2 arrays and {001} faceted anatase nanodomains for enhanced photoelectrochemical activity. Sustain. Energy Fuels 2018, 2, 1463–1473. [Google Scholar] [CrossRef]
- Hitosugi, T.; Ueda, A.; Nakao, S.; Yamada, N.; Furubayashi, Y.; Hirose, Y.; Shimada, T.; Hasegawa, T. Fabrication of highly conductive Ti1−xNbxO2 polycrystalline films on glass substrates via crystallization of amorphous phase grown by pulsed laser deposition. Appl. Phys. Lett. 2007, 90, 212106-1–212106-3. [Google Scholar] [CrossRef]
- Tucker, R.T.; Beckers, N.A.; Fleischauer, M.D.; Brett, M.J. Electron beam deposited Nb-doped TiO2 toward nanostructured transparent conductive thin films. Thin Solid Films 2012, 525, 28–34. [Google Scholar] [CrossRef]
- Trenczek-Zając, A.; Radecka, M.; Rekas, M. Photoelectrochemical properties of Nb-doped titanium dioxide. Physica B Condens. Matter 2007, 399, 55–59. [Google Scholar] [CrossRef]
- Yang, M.; Jha, H.; Liu, N.; Schmuki, P. Increased photocurrent response in Nb-doped TiO2 nanotubes. J. Mater. Chem. 2011, 21, 15205–15208. [Google Scholar] [CrossRef]
- Baur, W.H.; Khan, A.A. Rutile-type compounds. VI. SiO2, GeO2 and a comparison with other rutile-type structures. Acta Crystallogr. B 1971, 27, 2133–2139. [Google Scholar] [CrossRef]
- Philippin, G.; Delhalle, J.; Mekhalif, Z. Comparative study of the monolayers of CH3–(CH2)n–SiCl3 and CH3–(CH2)n–PO(OH)2, n = 4 and 13, adsorbed on polycrystalline titanium substrates. Appl. Surf. Sci. 2003, 212–213, 530–536. [Google Scholar] [CrossRef]
- Kačiulis, S.; Mattogno, G.; Napoli, A.; Bemporad, E.; Ferrari, F.; Montenero, A.; Gnappi, G. Surface analysis of biocompatible coatings on titanium. J. Electron Spectrosc. 1998, 95, 61–69. [Google Scholar] [CrossRef]
- Nakajima, T.; Nakamura, T.; Shinoda, K.; Tsuchiya, T. Rapid formation of black titania photoanodes: Pulsed laser-induced oxygen release and enhanced solar water splitting efficiency. J. Mater. Chem. A 2014, 2, 6762–6771. [Google Scholar] [CrossRef]
- Krishna, V.; Bai, W.; Han, Z.; Yano, A.; Thakur, A.; Georgieva, A.; Tolley, K.; Navarro, J.; Koopman, B.; Moudgil, B. Contaminant-activated visible light photocatalysis. Sci. Rep. 2018, 8, 1894-1–1894-11. [Google Scholar] [CrossRef]
- Idriss, H. Surface reactions of uranium oxide powder, thin films and single crystals. Surf. Sci. Rep. 2010, 65, 67–109. [Google Scholar] [CrossRef]
- Das, C.; Roy, P.; Yang, M.; Jha, H.; Schmuki, P. Nb doped TiO2 nanotubes for enhanced photoelectrochemical water-splitting 2011, 3, 3094–3096. Nanoscale 2011, 3, 3094–3096. [Google Scholar] [CrossRef]
- Ramamoorthy, M.; Vanderbilt, D.; King-Smith, R.D. First-principle calculations of the energetics of stoichiometric TiO2 surfaces. Phys. Rev. B 1994, 49, 16721–16727. [Google Scholar] [CrossRef]
- Lowekamp, J.B.; Rohrer, G.S.; Hotsenpiller, P.A.M.; Bolt, J.D.; Farneth, W.E. Anisotropic photochemical reactivity of bulk TiO2 crystals. J. Phys. Chem. B 1998, 102, 7323–7327. [Google Scholar] [CrossRef]
- Ohno, T.; Sarukawa, K.; Matsumura, M. Crystal faces of rutile and anatase TiO2 particles and their roles in photocatalytic reactions. New J. Chem. 2002, 26, 1167–1170. [Google Scholar]
- Haisch, C.; Günnemann, C.; Melchers, S.; Fleisch, M.; Schneider, J.; Emeline, A.V.; Bahnemann, D.W. Irreversible surface changes upon n-type doping—A photoelectrochemical study on rutile single crystals. Electrochim. Acta 2018, 280, 278–289. [Google Scholar]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Harris, L.A.; Wilson, R.H. Aging effects in single crystal reduced rutile anodes. J. Electrochem. Soc. 1976, 123, 1010–1015. [Google Scholar] [CrossRef]
- Tsujiko, A.; Kisumi, T.; Magari, Y.; Murakoshi, K.; Nakato, Y. Selective formation of nanoholes with (100)-face walls by photoetching of n-TiO2 (rutile) electrodes, accompanied by increases in water-oxidation photocurrent. J. Phys. Chem. B 2000, 104, 4873–4879. [Google Scholar] [CrossRef]
- Imanishi, A.; Okamura, T.; Ohashi, N.; Nakamura, R.; Nakato, Y. Mechanism of water photooxidation reaction at atomically flat TiO2 (rutile) (110) and (100) surfaces: Dependence on solution pH. J. Am. Chem. Soc. 2007, 129, 11569–11578. [Google Scholar] [CrossRef]
- Hu, Y.H. A highly efficient photocatalyst—hydrogenated black TiO2 for the photocatalytic splitting of water. Angew. Chem. Int. Ed. 2012, 51, 12410–12412. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.; Ling, Y.; Tang, Y.; Yang, X.; Fitzmorris, R.C.; Wang, C.; Zhang, J.Z.; Li, Y. Hydrogen-treated TiO2 nanowire arrays for photoelectrochemical water splitting. Nano Lett. 2011, 11, 3026–3033. [Google Scholar] [CrossRef]
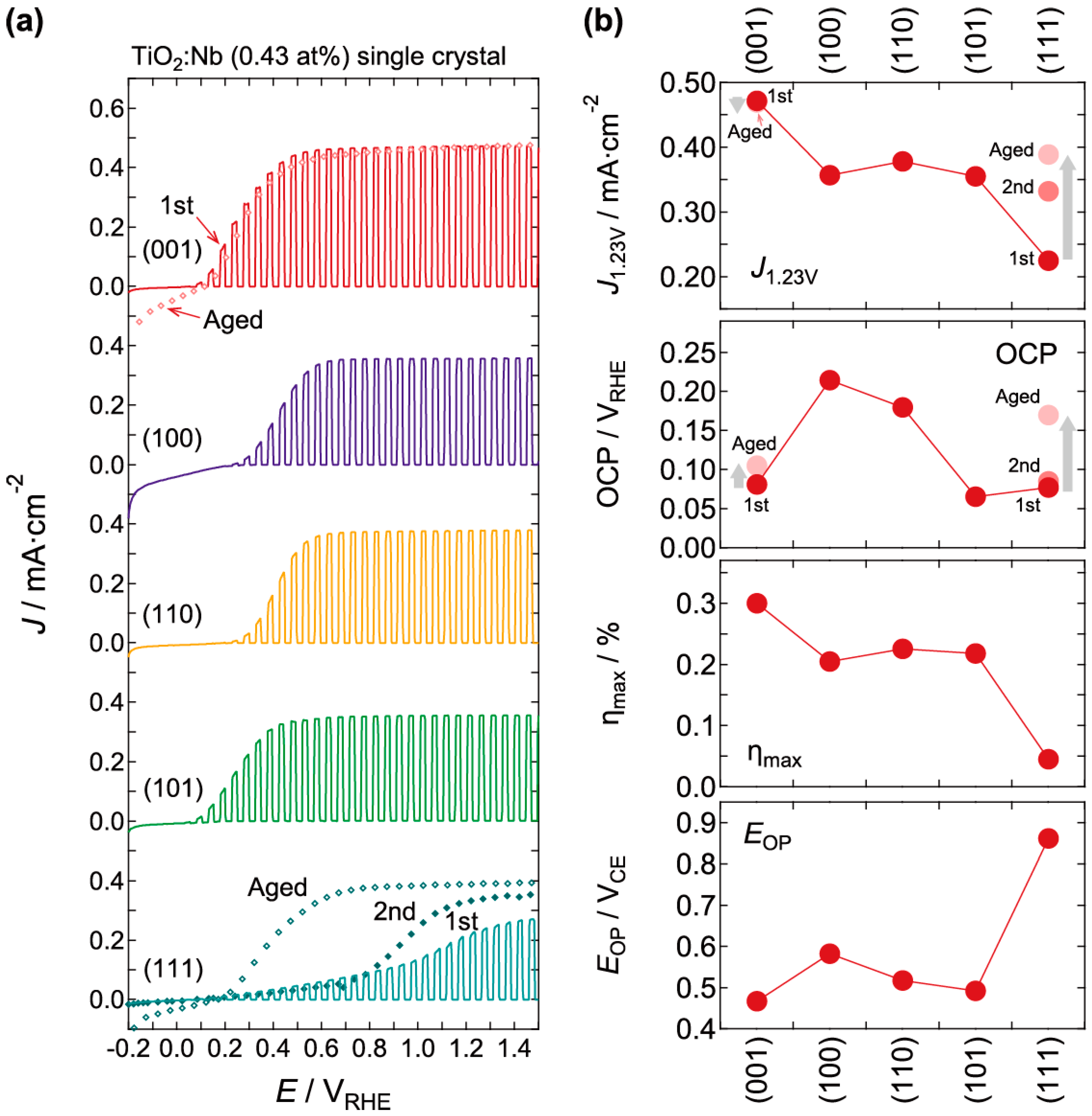


| Lattice Plane | J0.55V/mA/cm2 | JIPCE/mA/cm2 |
|---|---|---|
| (001) | 0.444 | 0.393 |
| (100) | 0.314 | 0.316 |
| (110) | 0.342 | 0.345 |
| (101) | 0.339 | 0.329 |
| (111) | 0.057 | 0.290 |
| LATTICE PLANE | Rs/Ω | Rsc/Ω | CPEsc | Rct/kΩ | CPEH | ||
|---|---|---|---|---|---|---|---|
| Csc/nFsn−1 | n | CH/μFsn−1 | n | ||||
| (001) | 15.7 | 496.3 | 51.9 | 0.914 | 42.3 | 3.34 | 0.918 |
| (100) | 11.8 | 853.4 | 242 | 0.785 | 55.9 | 3.81 | 0.926 |
| (110) | 17.2 | 385.7 | 363 | 0.800 | 44.0 | 4.34 | 0.912 |
| (101) | 16.5 | 377.2 | 355 | 0.802 | 60.5 | 4.35 | 0.908 |
| (111) | 9.66 | 368.0 | 783 | 0.751 | 58.7 | 2.84 | 0.947 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakajima, T.; Nakamura, T.; Tsuchiya, T. Crystal-Plane Dependence of Nb-Doped Rutile TiO2 Single Crystals on Photoelectrochemical Water Splitting. Catalysts 2019, 9, 725. https://doi.org/10.3390/catal9090725
Nakajima T, Nakamura T, Tsuchiya T. Crystal-Plane Dependence of Nb-Doped Rutile TiO2 Single Crystals on Photoelectrochemical Water Splitting. Catalysts. 2019; 9(9):725. https://doi.org/10.3390/catal9090725
Chicago/Turabian StyleNakajima, Tomohiko, Takako Nakamura, and Tetsuo Tsuchiya. 2019. "Crystal-Plane Dependence of Nb-Doped Rutile TiO2 Single Crystals on Photoelectrochemical Water Splitting" Catalysts 9, no. 9: 725. https://doi.org/10.3390/catal9090725
APA StyleNakajima, T., Nakamura, T., & Tsuchiya, T. (2019). Crystal-Plane Dependence of Nb-Doped Rutile TiO2 Single Crystals on Photoelectrochemical Water Splitting. Catalysts, 9(9), 725. https://doi.org/10.3390/catal9090725