CO Oxidation over Metal Oxide (La2O3, Fe2O3, PrO2, Sm2O3, and MnO2) Doped CuO-Based Catalysts Supported on Mesoporous Ce0.8Zr0.2O2 with Intensified Low-Temperature Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterizations of the Support and Catalysts
2.1.1. XRD Analysis
2.1.2. N2 Physisorption Analysis
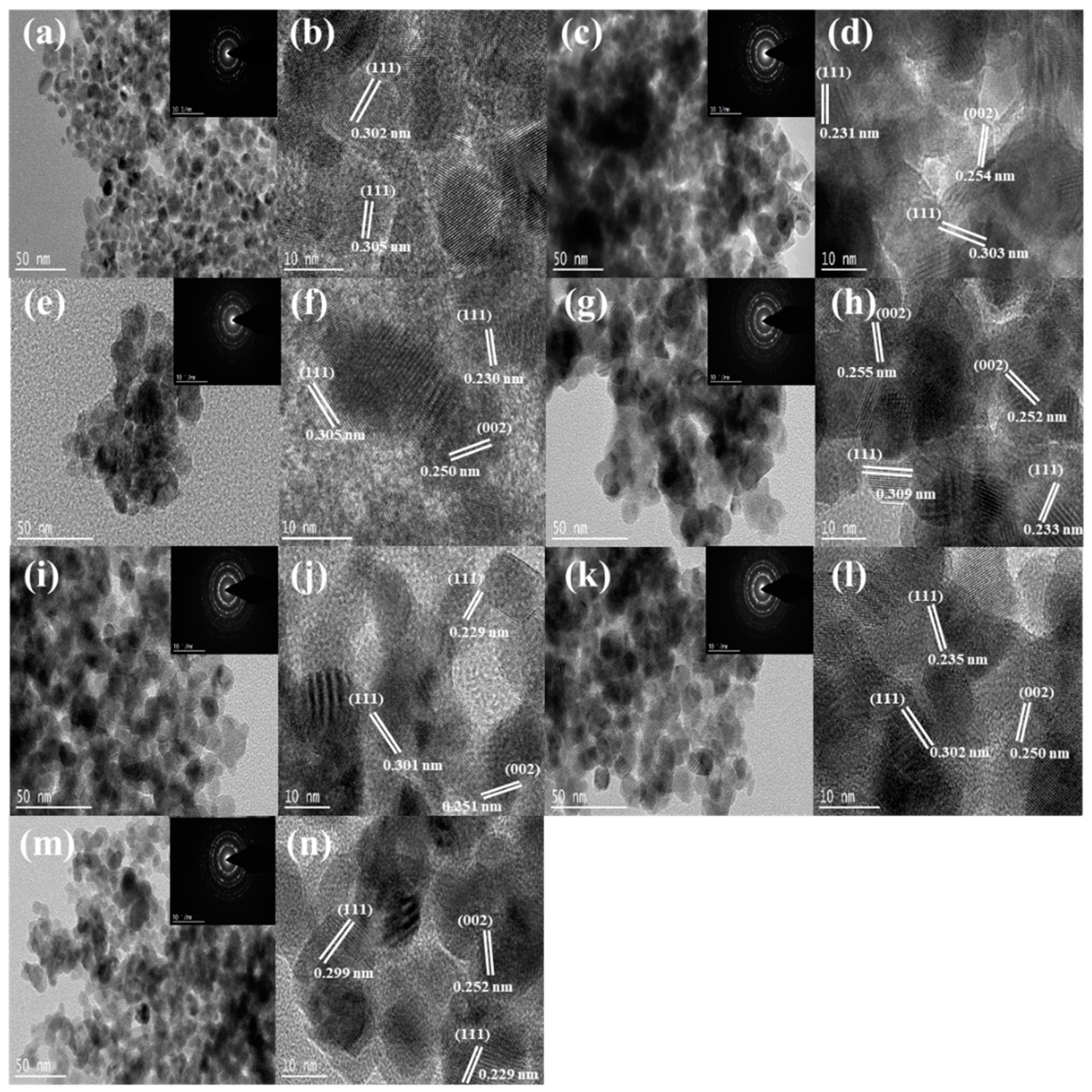
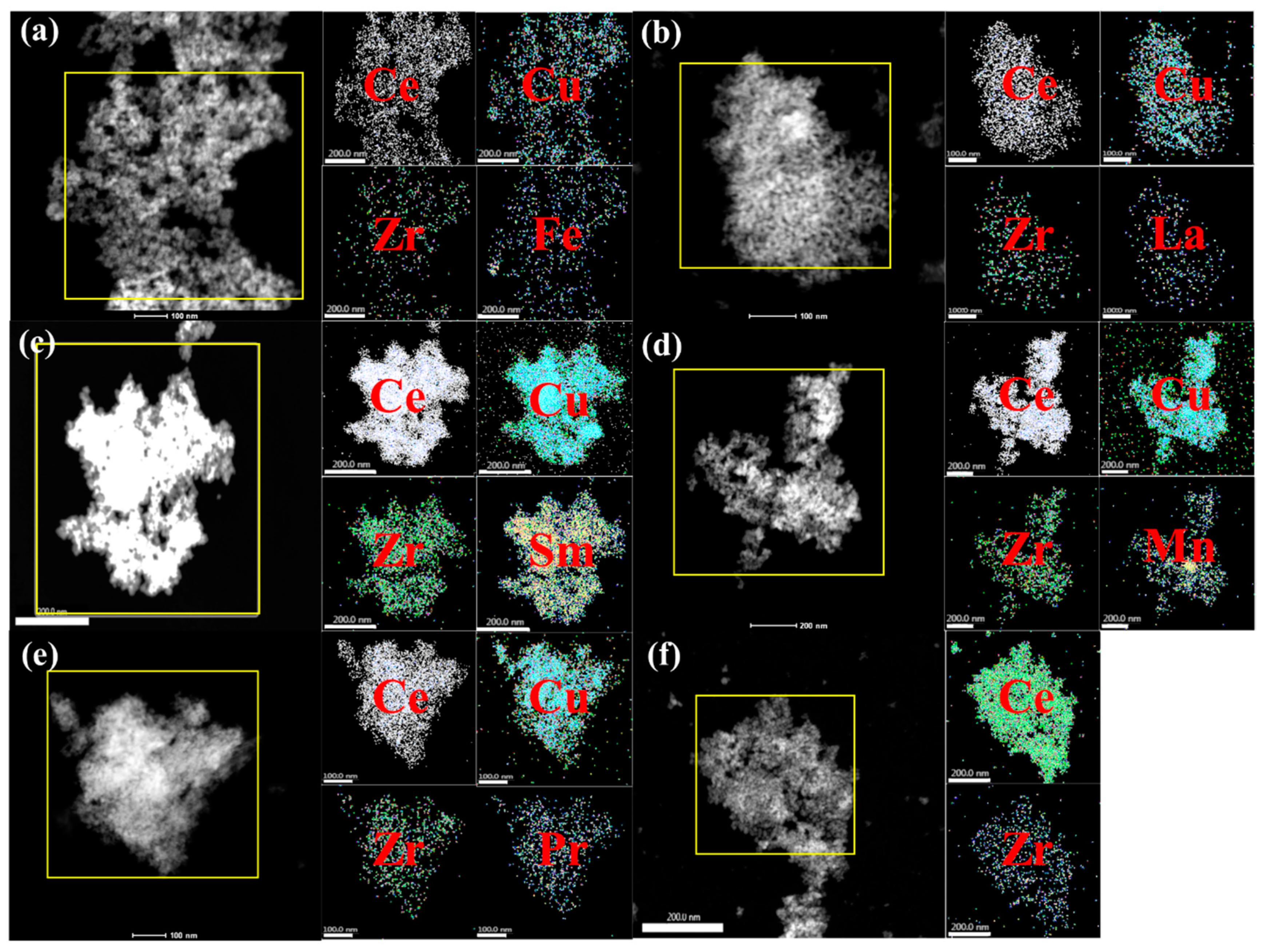
2.1.3. TEM, SAED, STEM, and EDS-Mapping Analyses
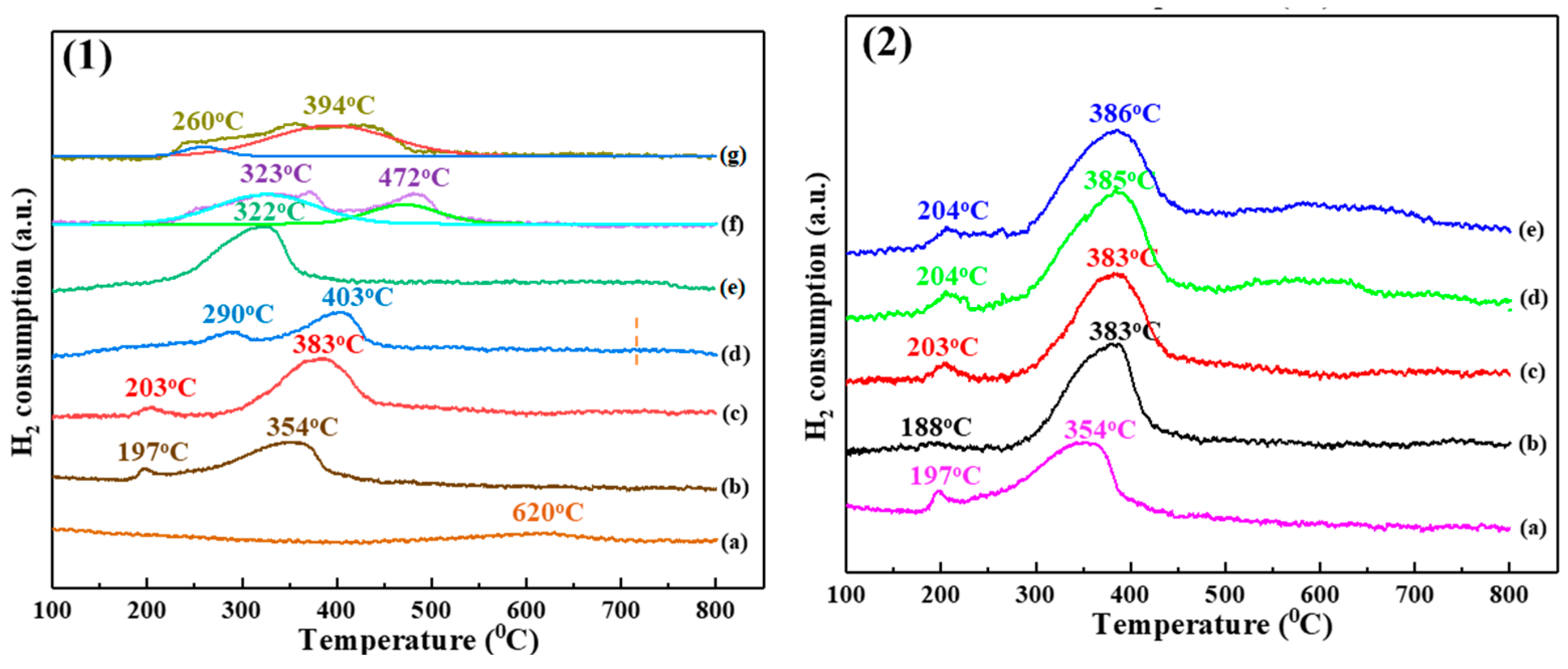
2.1.4. H2-TPR Analysis
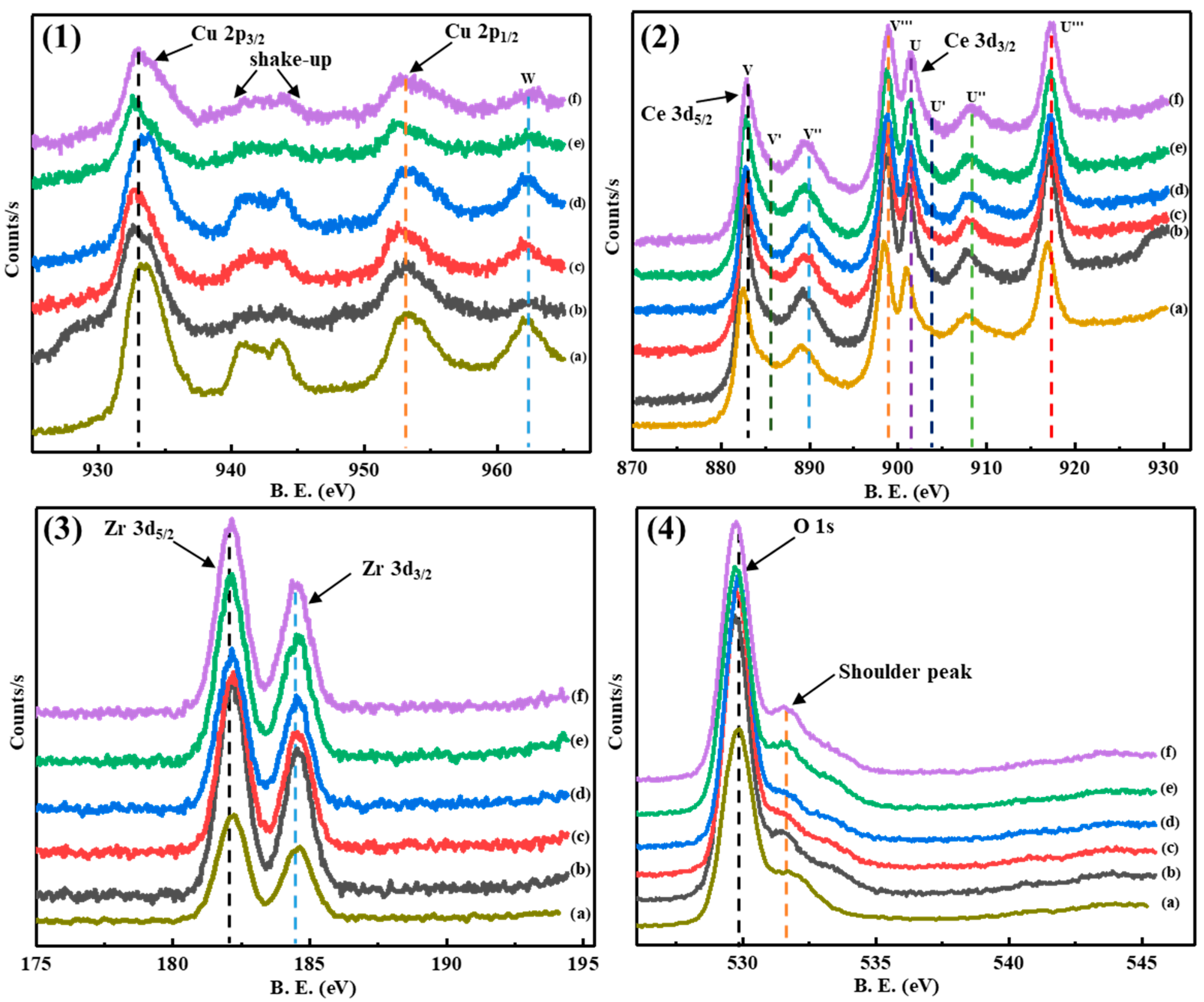
2.1.5. XPS Analysis
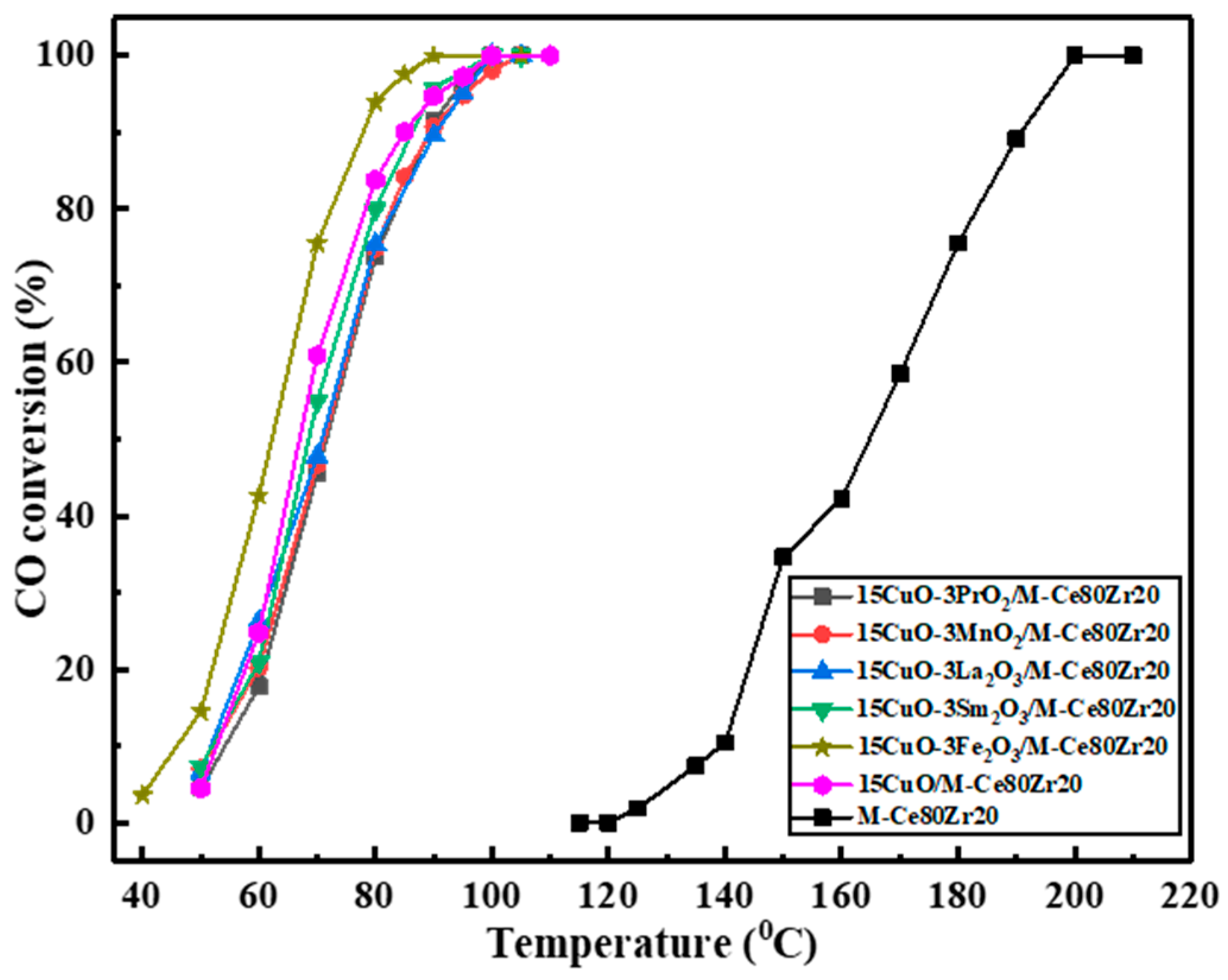
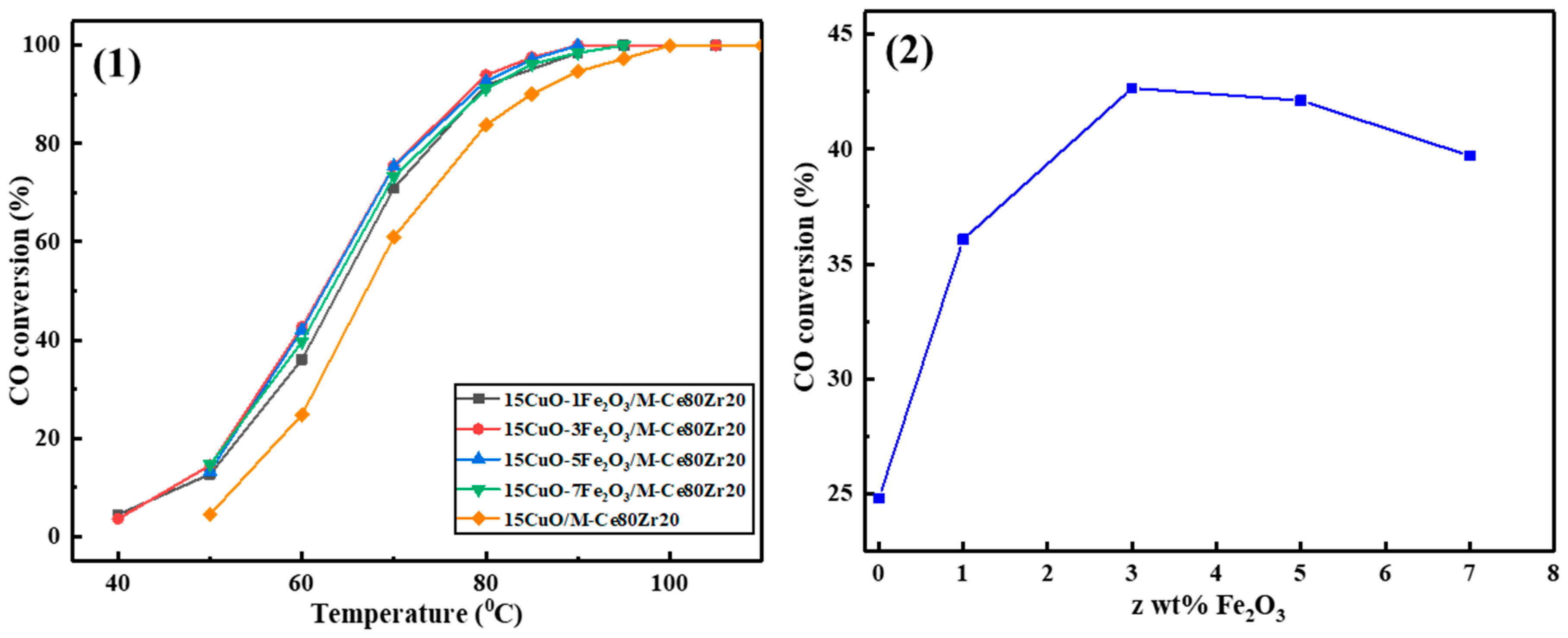
2.2. Catalytic Performance toward CO Oxidation
2.2.1. The Evaluation of the Catalytic Activity during the Process of Screening Catalysts
2.2.2. Long-Term Stability Test
3. Experimental
3.1. Catalyst Preparation
3.2. Catalyst Characterizations
3.3. Catalytic Activity Evaluation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Topacoglu, H.; Katsakoglou, S.; Ipekci, A. Effect of exhaust emissions on carbon monoxide levels in employees working at indoor car wash facilities. Hippokratia 2014, 18, 37. [Google Scholar] [PubMed]
- Hampson, N.B.; Holm, J.R. Suicidal carbon monoxide poisoning has decreased with controls on automobile emissions. Undersea Hyperb. Med. 2015, 42, 159–164. [Google Scholar] [PubMed]
- Green, I.X.; Tang, W.; Neurock, M.; Yates, J.T. Yates, Spectroscopic observation of dual catalytic sites during oxidation of CO on a Au/TiO2 catalyst. Science 2011, 333, 736–739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cheng, D.; Zhu, J. Theoretical study of CO catalytic oxidation on free and defective graphene-supported Au–Pd bimetallic clusters. RSC Adv. 2014, 4, 42554–42561. [Google Scholar] [CrossRef]
- Zou, Z.Q.; Meng, M.; Zha, Y.Q. Surfactant-Assisted Synthesis, Characterizations, and Catalytic Oxidation Mechanisms of the Mesoporous MnOx−CeO2 and Pd/MnOx−CeO2 Catalysts Used for CO and C3H8 Oxidation. J. Phys. Chem. C 2014, 114, 468–477. [Google Scholar] [CrossRef]
- Haruta, M.; Yamada, N.; Kobayashi, T.; Iijima, S. Gold catalysts prepared by coprecipitation for low-temperature oxidation of hydrogen and of carbon monoxide. J. Catal. 1989, 115, 301–309. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, Q.; Wang, X.; Ma, K.; Bai, X.; Tan, S.; Ye, T.; Tong, D.; Zheng, L.; Jing, Z.; et al. Enhanced catalytic performance for CO preferential oxidation over CuO catalysts supported on highly defective CeO2 nanocrystals. Appl. Surf. Sci. 2017, 422, 932–943. [Google Scholar] [CrossRef]
- Guo, X.; Li, J.; Zhou, R. Catalytic performance of manganese doped CuO–CeO2 catalysts for selective oxidation of CO in hydrogen-rich gas. Fuel 2016, 163, 56–64. [Google Scholar] [CrossRef]
- Liu, S.; Wei, Z.; Deng, T.; Dong, W.; Zheng, W. Mechanistic Origin of Enhanced CO Catalytic Oxidation over Co3O4/LaCoO3 at Lower Temperature. Chemcatchem 2017, 9, 3102–3106. [Google Scholar] [CrossRef]
- Hu, L.; Sun, K.; Peng, Q.; Xu, B.; Li, Y. Surface active sites on Co3O4 nanobelt and nanocube model catalysts for CO oxidation. Nano Res. 2010, 3, 363–368. [Google Scholar] [CrossRef]
- Gamboa-Rosales, N.K.; Ayastuy, J.L.; Boukha, Z.; Bion, N.; Duprez, D.; Pérez-Omil, J.A.; del Rio, E.; Gutierrez-Ortiz, M.A. Ceria-supported Au–CuO and Au–Co3O4 catalysts for CO oxidation: An 18O/16O isotopic exchange study. Appl. Catal. B Environ. 2015, 168, 87–97. [Google Scholar] [CrossRef]
- Chen, G.; Xu, Q.; Yang, Y.; Li, C.; Huang, T.; Sun, G.; Zhang, S.; Ma, D.; Li, X. Facile and mild strategy to construct mesoporous CeO2–CuO nanorods with enhanced catalytic activity toward CO oxidation. ACS Appl. Mater. Interfaces 2015, 7, 23538–23544. [Google Scholar] [CrossRef] [PubMed]
- Laguna, O.H.; Pérez, A.; Centeno, M.A.; Odriozola, J.A. Synergy between gold and oxygen vacancies in gold supported on Zr-doped ceria catalysts for the CO oxidation. Appl. Catal. B Environ. 2015, 176, 385–395. [Google Scholar] [CrossRef]
- Min, K.; Min, W.S.; Chang, H.L. Catalytic carbon monoxide oxidation over CoOx/CeO2 composite catalysts. Appl. Catal. A Gen. 2003, 251, 143–156. [Google Scholar]
- Moretti, E.; Storaro, L.; Talon, A.; Lenarda, M.; Riello, P.; Frattini, R.; Mdm, D.Y.; Jimenez-Lopez, A.; Rodriguez-Castellon, E.; Ternero, F.; et al. Effect of thermal treatments on the catalytic behaviour in the CO preferential oxidation of a CuO–CeO2–ZrO2 catalyst with a flower-like morphology. Appl. Catal. B Environ. 2011, 102, 627–637. [Google Scholar] [CrossRef]
- Narula, C.K.; Haack, L.P.; Chun, W.; Jen, H.W.; Graham, G.W. Single-Phase PrOy− ZrO2 Materials and Their Oxygen Storage Capacity: A Comparison with Single-Phase CeO2−ZrO2, PrOy− CeO2, and PrO y− CeO2− ZrO2 Materials. Phys. Chem. B 1999, 103, 3634–3639. [Google Scholar] [CrossRef]
- Wang, J.A.; Chen, L.F.; Valenzuela, M.A.; Montoya, A.; Salmones, J.; Del Angel, P. Rietveld refinement and activity of CO oxidation over Pd/Ce0.8Zr0.2O2 catalyst prepared via a surfactant-assisted route. Appl. Surf. Sci. 2004, 230, 34–43. [Google Scholar] [CrossRef]
- Si, R.; Zhang, Y.W.; Xiao, C.X.; Li, S.J.; Lin, B.X.; Kou, Y.; Yan, C.H. Non-Template Hydrothermal Route Derived Mesoporous Ce0.2Zr0.8O2 Nanosized Powders with Blue-Shifted UV Absorption and High CO Conversion Activity. ChemInform 2004, 6, 1056–1063. [Google Scholar] [CrossRef]
- Wang, S.P.; Zhang, T.Y.; Su, Y.; Wang, S.R.; Zhang, S.M.; Zhu, B.L.; Wu, S.H. An Investigation of Catalytic Activity for CO Oxidation of CuO/CexZr1–xO2 Catalysts. Catal. Lett. 2008, 121, 70–76. [Google Scholar] [CrossRef]
- Cui, Y.; Lian, X.; Xu, L.; Chen, M.; Yang, B.; Wu, C.-E.; Li, W.; Huang, B.; Hu, X. Designing and Fabricating Ordered Mesoporous Metal Oxides for CO2 Catalytic Conversion: A Review and Prospect. Materials 2019, 12, 276. [Google Scholar] [CrossRef]
- Kikugawa, M.; Yamazaki, K.; Shinjoh, H. Characterization and catalytic activity of CuO/TiO2-ZrO2 for low temperature CO oxidation. Appl. Catal. A Gen. 2017, 547, 199–204. [Google Scholar] [CrossRef]
- Zhong, Z.; Ho, J.; Teo, J.; Shen, S.; Gedanken, A. Synthesis of Porous α-Fe2O3 Nanorods and Deposition of Very Small Gold Particles in the Pores for Catalytic Oxidation of CO. Chem. Mater. 2007, 19, 69–73. [Google Scholar] [CrossRef]
- Fu, Z.D.; Ye, Q.; Cheng, S.Y.; Wang, D. Catalytic Oxidation of CO over Ag-Doped Manganese Oxide Catalysts: Preparation and Catalytic Activity. Adv. Mater. Res. 2015, 1089, 133–136. [Google Scholar] [CrossRef]
- Dongsheng, Q.I.A.O.; Guanzhong, L.U.; Yun, G.U.O.; Yanqin, W.A.N.G.; Yanglong, G.U.O. Effect of water vapor on the CO and CH4 catalytic oxidation over CeO2-MOx (M= Cu, Mn, Fe, Co, and Ni) mixed oxide. J. Rare Earths 2010, 28, 742–746. [Google Scholar]
- Li, Y.; Zhang, X.; Long, E.; Li, H.; Wu, D.; Cai, L.; Gong, M.; Chen, Y. Influence of CeO2 and La2O3 on properties of palladium catalysts used for emission control of natural gas vehicles. J. Nat. Gas. Chem. 2009, 18, 415–420. [Google Scholar] [CrossRef]
- Duan, D.; Hao, C.; Shi, W.; Wang, H.; Sun, Z. Sm2O3/Co3O4 catalysts prepared by dealloying for low-temperature CO oxidation. RSC Adv. 2018, 8, 11289–11295. [Google Scholar] [CrossRef]
- Wang, J.B.; Shih, W.H.; Huang, T.J. Study of Sm2O3-doped CeO2/Al2O3-supported copper catalyst for CO oxidation. Appl. Catal. A Gen. 2000, 203, 191–199. [Google Scholar] [CrossRef]
- Zhao, Z.J.; Wang, R.Y.; Zhao, Q.L.; Wang, E.P.; Su, H.Q.; Zeng, S.H. CuO/CeO2 and CuO/PrO2-CeO2 Catalysts for Preferential Oxidation of CO. Adv. Mater. Res. 2013, 773, 601–605. [Google Scholar] [CrossRef]
- Zhang, X.B.; Ma, K.Y.; Zhang, L.H.; Yong, G.P.; Dai, Y.; Liu, S.M. Effect of precipitation method and Ce doping on the catalytic activity of copper manganese oxide catalysts for CO oxidation. Chinese. J. Chem. Phys. 2011, 24, 97. [Google Scholar] [CrossRef]
- Xu, L.; Lian, X.; Chen, M.; Cui, Y.; Wang, F.; Li, W.; Huang, B. CO2 methanation over CoNi bimetal-doped ordered mesoporous Al2O3 catalysts with enhanced low-temperature activities. Int. J. Hydrogen. Energ. 2018, 43, 17172–17184. [Google Scholar] [CrossRef]
- Cao, J.L.; Wang, Y.; Zhang, T.Y.; Wu, S.H.; Yuan, Z.Y. Preparation, characterization and catalytic behavior of nanostructured mesoporous CuO/Ce0.8Zr0.2O2 catalysts for low-temperature CO oxidation. Appl. Catal. B Environ. 2008, 78, 120–128. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Lin, R.; Luo, M.F.; Zhong, Y.J.; Yan, Z.L.; Liu, G.Y.; Liu, W.P. Comparative study of CuO/Ce0.7 Sn0.3O2, CuO/CeO2 and CuO/SnO2 catalysts for low-temperature CO oxidation. Appl. Catal. A Gen. 2003, 255, 331–336. [Google Scholar]
- Radlik, M.; Adamowska, M.; Łamacz, A.; Krztoń, A.; Da Costa, P.; Turek, W. Study of the surface evolution of nitrogen species on CuO/CeZrO2 catalysts. Reactn. Kinets. Mech. Catal. 2013, 109, 43–56. [Google Scholar] [CrossRef]
- Luo, M.F.; Ma, J.M.; Lu, J.Q.; Song, Y.P.; Wang, Y.J. High-surface area CuO–CeO2 catalysts prepared by a surfactant-templated method for low-temperature CO oxidation. J. Catal. 2007, 246, 52–59. [Google Scholar] [CrossRef]
- Luo, M.F.; Zhong, Y.J.; Yuan, X.X.; Zheng, X.M. TPR and TPD studies of CuOCeO2 catalysts for low temperature CO oxidation. Appl. Catal. A Gen. 1997, 162, 121–131. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Ioannides, T. Selective CO oxidation over CuO-CeO2 catalysts prepared via the urea–nitrate combustion method. Appl. Catal. A Gen. 2003, 244, 155–167. [Google Scholar] [CrossRef]
- Sukonket, T.; Khan, A.; Saha, B.; Ibrahim, H.; Tantayanon, S.; Kumar, P.; Idem, R. Influence of the catalyst preparation method, surfactant amount, and steam on CO2 reforming of CH4 over 5Ni/Ce0.6Zr0.4O2 catalysts. Energ. Fuel. 2011, 25, 864–877. [Google Scholar] [CrossRef]
- Liu, C.J.; Ye, J.; Jiang, J.; Pan, Y. Progresses in the preparation of coke resistant Ni-based catalyst for steam and CO2 reforming of methane. Chemcatchem 2011, 3, 529–541. [Google Scholar] [CrossRef]
- Du, X.; Zhang, D.; Shi, L.; Gao, R.; Zhang, J. Morphology dependence of catalytic properties of Ni/CeO2 nanostructures for carbon dioxide reforming of methane. J. Phys. Chem. C 2012, 116, 10009–10016. [Google Scholar] [CrossRef]
- Reddy, B.M.; Khan, A.; Yamada, Y.; Kobayashi, T.; Loridant, S.; Volta, J.C. Surface characterization of CeO2/SiO2 and V2O5/CeO2/SiO2 catalysts by Raman, XPS, and other techniques. J. Phys. Chem. B 2002, 106, 10964–10972. [Google Scholar] [CrossRef]
- Galtayries, A.; Sporken, R.; Riga, J.; Blanchard, G.; Caudano, R. XPS comparative study of ceria/zirconia mixed oxides: powders and thin film characterisation. J. Electron. Spectrosc. 1998, 88, 951–956. [Google Scholar] [CrossRef]
- Reddy, B.M.; Khan, A. Nanosized CeO2–SiO2, CeO2–TiO2, and CeO2–ZrO2 mixed oxides: Influence of supporting oxide on thermal stability and oxygen storage properties of ceria. Catal. Surv. Asia 2005, 9, 155–171. [Google Scholar] [CrossRef]
- Milt, V.G.; Spretz, R.; Ulla, M.A.; Lombardo, E.A.; Fierro, J.G. The nature of active sites for the oxidation of methane on La-based perovskites. Catal. Lett. 1996, 42, 57–63. [Google Scholar] [CrossRef]
- Uwamino, Y.; Ishizuka, T.; Yamatera, H. X-ray photoelectron spectroscopy of rare-earth compounds. J. Electron. Spectrosc. 1984, 34, 67–78. [Google Scholar] [CrossRef]
- Sarma, D.D.; Rao, C.N.R. XPES studies of oxides of second-and third-row transition metals including rare earths. J. Electron. Spectrosc. 1980, 20, 25–45. [Google Scholar] [CrossRef]
- Yadav, R.S.; Havlica, J.; Masilko, J.; Kalina, L.; Wasserbauer, J.; Hajdúchová, M.; Enev, V.; Kuřitka, I.; Kožáková, Z. Impact of Nd3+ in CoFe2O4 spinel ferrite nanoparticles on cation distribution, structural and magnetic properties. J. Magn. Magn. Mater. 2016, 399, 109–117. [Google Scholar] [CrossRef]
- Hong, L.Y.; Yong, Q.W.; Shao, M.Z.; Li, S.L.; Xi, L.C.; Shi, Y.L.; Rui, J.Y.; Yao, M.H.; Ning, L. Low-Temperature Preparation of Superparamagnetic CoFe2O4 Microspheres with High Saturation Magnetization. Nanoscale Res. Lett. 2010, 5, 1817–1821. [Google Scholar]
- Li, J.; Zhu, P.; Zuo, S.; Huang, Q.; Zhou, R. Influence of Mn doping on the performance of CuO-CeO2 catalysts for selective oxidation of CO in hydrogen-rich streams. Appl. Catal. A Gen. 2010, 381, 261–266. [Google Scholar] [CrossRef]
- Rosso, J.J.; Hochella, M.F., Jr. Natural Iron and Manganese Oxide Samples by XPS. Surf. Sci. Spectra 1996, 4, 253–265. [Google Scholar] [CrossRef]
- Stranick, M.A. Mn2O3 by XPS. Surf. Sci. Spectra 1999, 6, 39–46. [Google Scholar] [CrossRef]
- Cao, J.L.; Wang, Y.; Yu, X.L.; Wang, S.R.; Wu, S.H.; Yuan, Z.Y. Mesoporous CuO–Fe2O3 composite catalysts for low-temperature carbon monoxide oxidation. Appl. Catal. B Environ. 2008, 79, 26–34. [Google Scholar] [CrossRef]
- Said, A.E.A.A.; El-Wahab, M.M.A.; Goda, M.N. Synthesis and characterization of pure and (Ce, Zr, Ag) doped mesoporous CuO-Fe2O3 as highly efficient and stable nanocatalysts for CO oxidation at low temperature. Appl. Surf. Sci. 2016, 390, 649–665. [Google Scholar] [CrossRef]

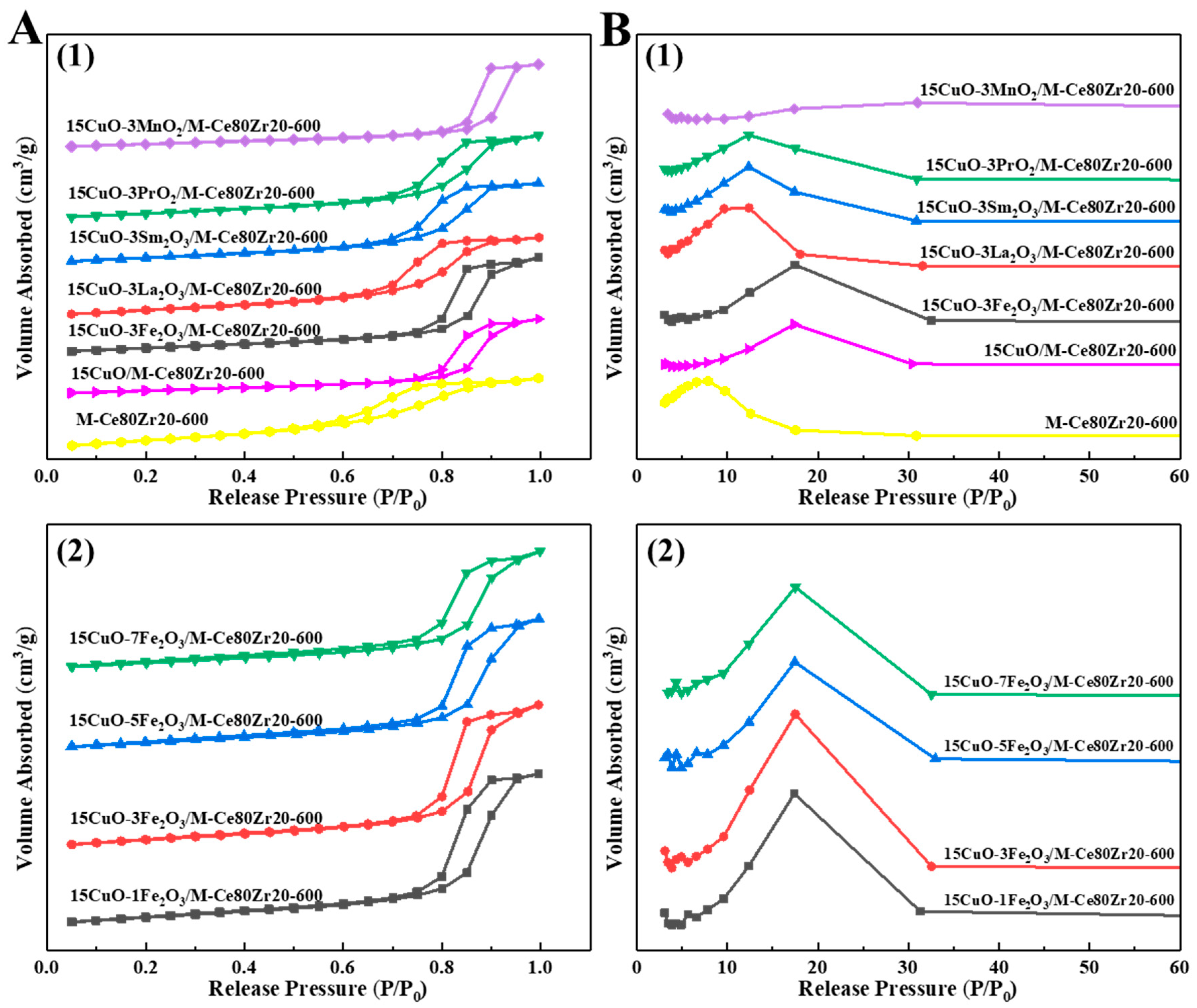



| Samples | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) | Isotherm Type |
|---|---|---|---|---|
| M-Ce80Zr20 | 68.9 | 0.12 | 7.9 | IV H2 |
| 15CuO/M-Ce80Zr20 | 55.2 | 0.13 | 9.6 | IV H2 |
| 15CuO-3Mn2O3/M-Ce80Zr20 | 31.1 | 0.13 | 13.0 | IV H2 |
| 15CuO-3PrO2/M-Ce80Zr20 | 48.4 | 0.14 | 12.3 | IV H2 |
| 15CuO-3Sm2O3/M-Ce80Zr20 | 48.9 | 0.14 | 12.3 | IV H2 |
| 15CuO-3La2O3/M-Ce80Zr20 | 54.8 | 0.14 | 12.4 | IV H2 |
| 15CuO-3Fe2O3/M-Ce80Zr20 | 43.8 | 0.16 | 17.5 | IV H2 |
| 15CuO-1Fe2O3/M-Ce80Zr20 | 44.5 | 0.17 | 17.4 | IV H2 |
| 15CuO-5Fe2O3/M-Ce80Zr20 | 39.5 | 0.14 | 17.4 | IV H2 |
| 15CuO-7Fe2O3/M-Ce80Zr20 | 37.3 | 0.13 | 17.5 | IV H2 |
| Samples | Cu 2p3/2 | O 1s | Ce 3d5/2 | Zr 3d5/2 |
|---|---|---|---|---|
| 15CuO/M-Ce80Zr20 | 933.3 | 529.8 | 882.5 | 181.3 |
| 15CuO-3PrO2/M-Ce80Zr20 | 933.7 | 529.5 | 882.4 | 181.4 |
| 15CuO-3Fe2O3/M-Ce80Zr20 | 933.1 | 529.6 | 882.5 | 181.5 |
| 15CuO-3MnO2/M-Ce80Zr20 | 933.4 | 529.8 | 882.6 | 181.4 |
| 15CuO-3La2O3/M-Ce80Zr20 | 933.2 | 529.8 | 882.7 | 181.4 |
| 15CuO-3Sm2O3/M-Ce80Zr20 | 933.2 | 529.8 | 882.5 | 181.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Xu, L.; Chen, M.; Lv, C.; Lian, X.; Wu, C.-e.; Yang, B.; Miao, Z.; Wang, F.; Hu, X. CO Oxidation over Metal Oxide (La2O3, Fe2O3, PrO2, Sm2O3, and MnO2) Doped CuO-Based Catalysts Supported on Mesoporous Ce0.8Zr0.2O2 with Intensified Low-Temperature Activity. Catalysts 2019, 9, 724. https://doi.org/10.3390/catal9090724
Cui Y, Xu L, Chen M, Lv C, Lian X, Wu C-e, Yang B, Miao Z, Wang F, Hu X. CO Oxidation over Metal Oxide (La2O3, Fe2O3, PrO2, Sm2O3, and MnO2) Doped CuO-Based Catalysts Supported on Mesoporous Ce0.8Zr0.2O2 with Intensified Low-Temperature Activity. Catalysts. 2019; 9(9):724. https://doi.org/10.3390/catal9090724
Chicago/Turabian StyleCui, Yan, Leilei Xu, Mindong Chen, Chufei Lv, Xinbo Lian, Cai-e Wu, Bo Yang, Zhichao Miao, Fagen Wang, and Xun Hu. 2019. "CO Oxidation over Metal Oxide (La2O3, Fe2O3, PrO2, Sm2O3, and MnO2) Doped CuO-Based Catalysts Supported on Mesoporous Ce0.8Zr0.2O2 with Intensified Low-Temperature Activity" Catalysts 9, no. 9: 724. https://doi.org/10.3390/catal9090724
APA StyleCui, Y., Xu, L., Chen, M., Lv, C., Lian, X., Wu, C.-e., Yang, B., Miao, Z., Wang, F., & Hu, X. (2019). CO Oxidation over Metal Oxide (La2O3, Fe2O3, PrO2, Sm2O3, and MnO2) Doped CuO-Based Catalysts Supported on Mesoporous Ce0.8Zr0.2O2 with Intensified Low-Temperature Activity. Catalysts, 9(9), 724. https://doi.org/10.3390/catal9090724








