Comparative Study of the Characteristics and Activities of Pd/γ-Al2O3 Catalysts Prepared by Vortex and Incipient Wetness Methods
Abstract
:1. Introduction
2. Results
2.1. Catalyst Preparation
2.2. Catalyst Characterization
2.2.1. Pulse CO Chemisorption
2.2.2. Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES)
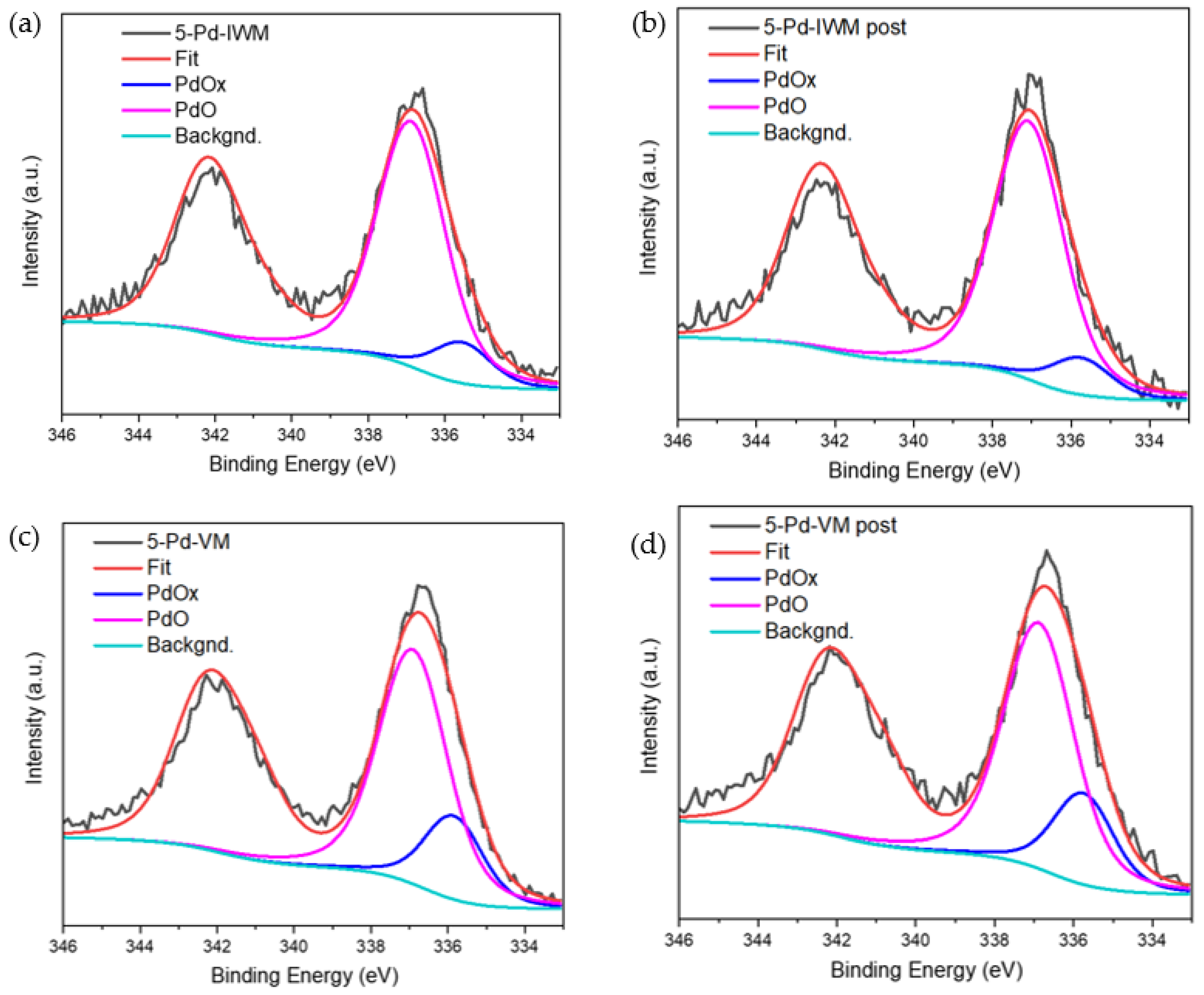
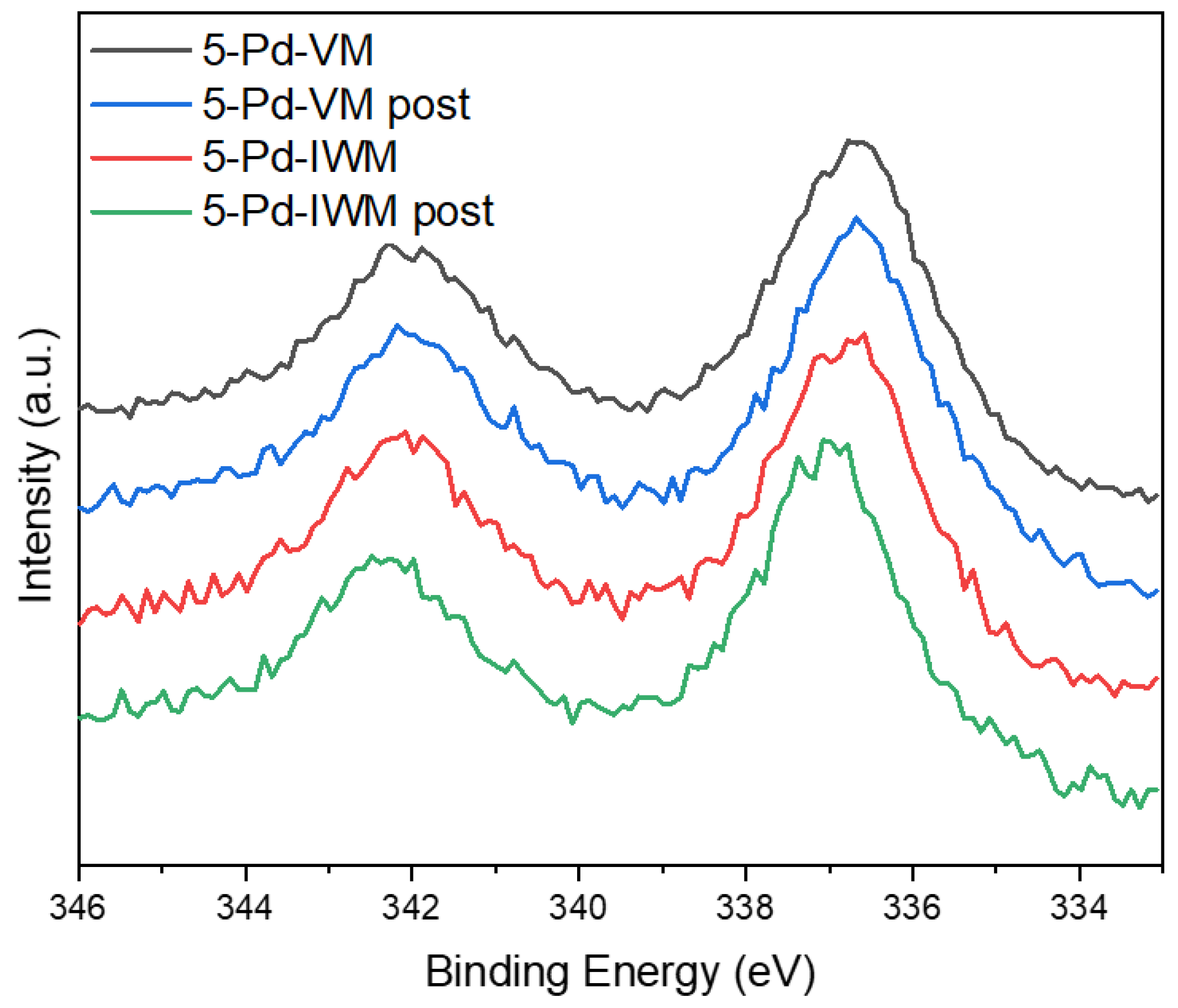
2.2.3. X-ray Photoelectron Spectroscopy (XPS)
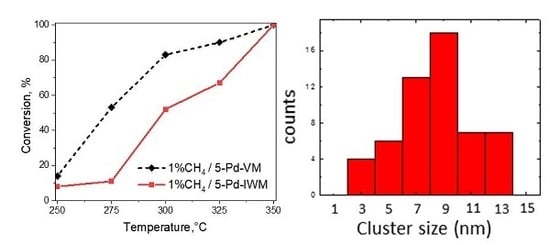
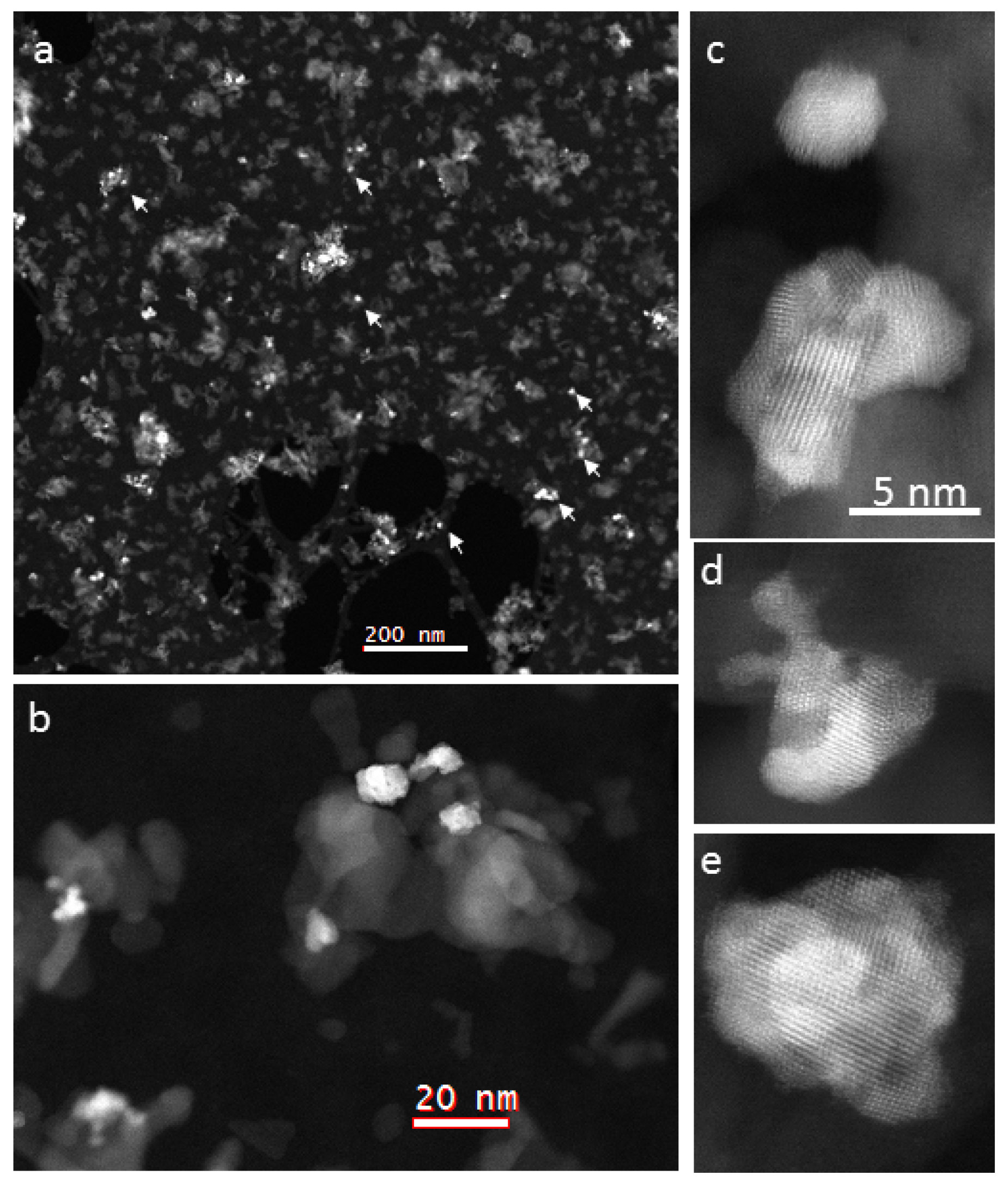
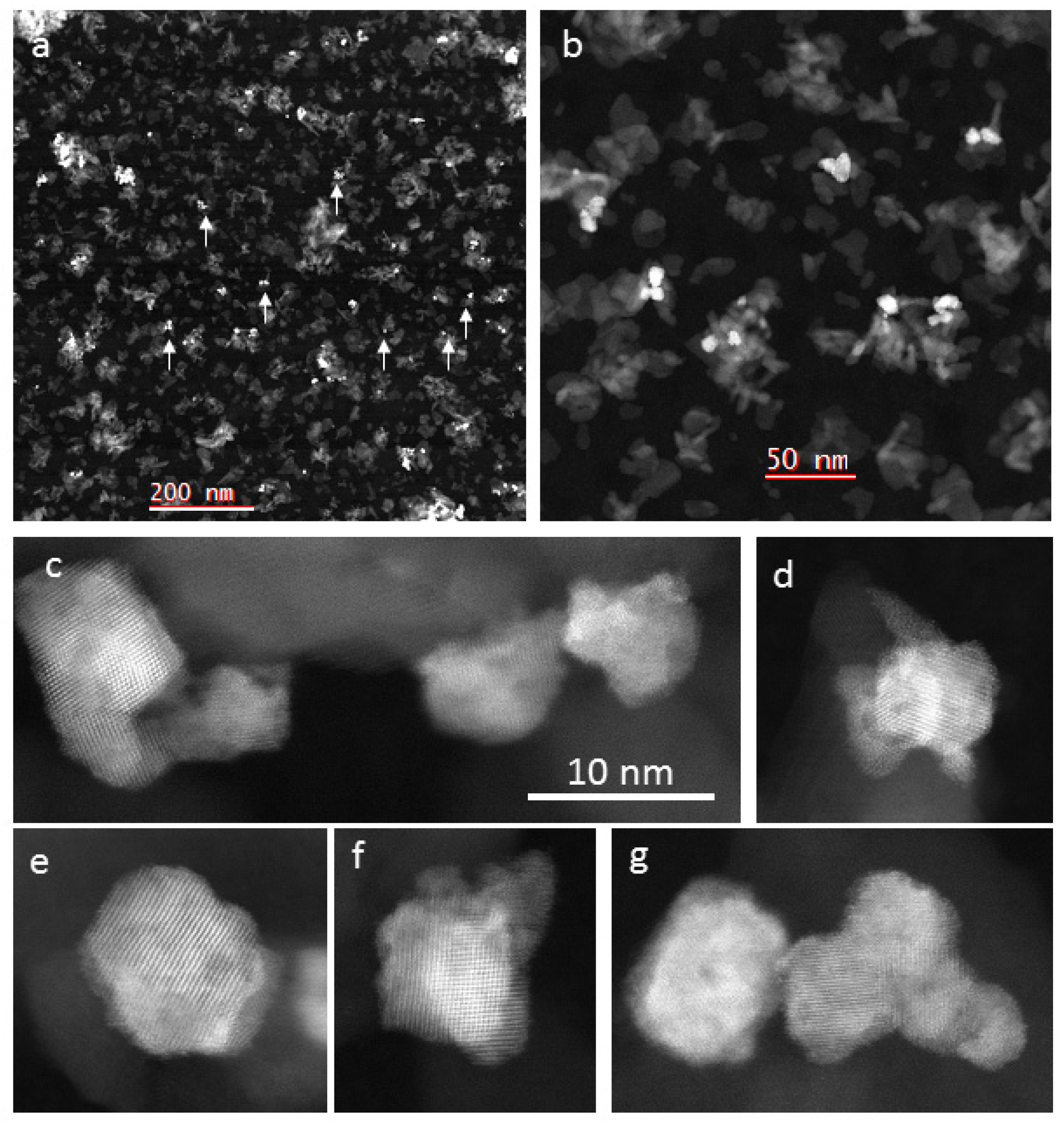
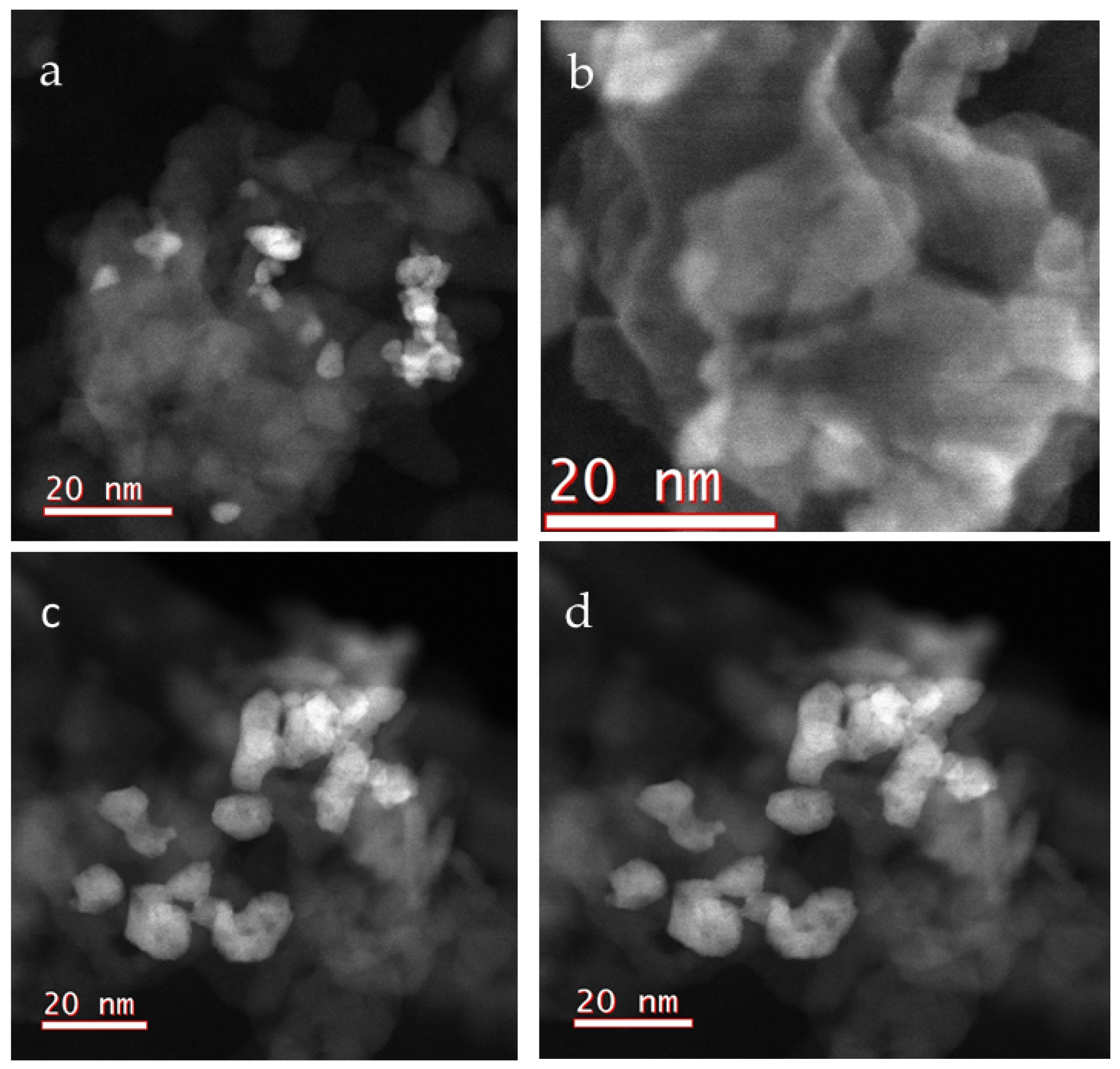
2.2.4. Scanning Transmission Electron Microscopy (STEM)
- (i)
- The crystallite sizes are 3–13 nm in 5-Pd-VM and 3–17 nm in 5-Pd-IWM;
- (ii)
- The catalyst 5-Pd-VM contains more smaller particles 3–5 nm than 5-Pd-IWM;
- (iii)
- The catalyst 5-Pd-IWM contains bigger particles 15–17 nm and these are absent in 5-Pd-VM;
- (iv)
- The particle size distribution was narrower (2–14 nm) in 5-Pd-VM than 5-Pd-IWM (2–18 nm).
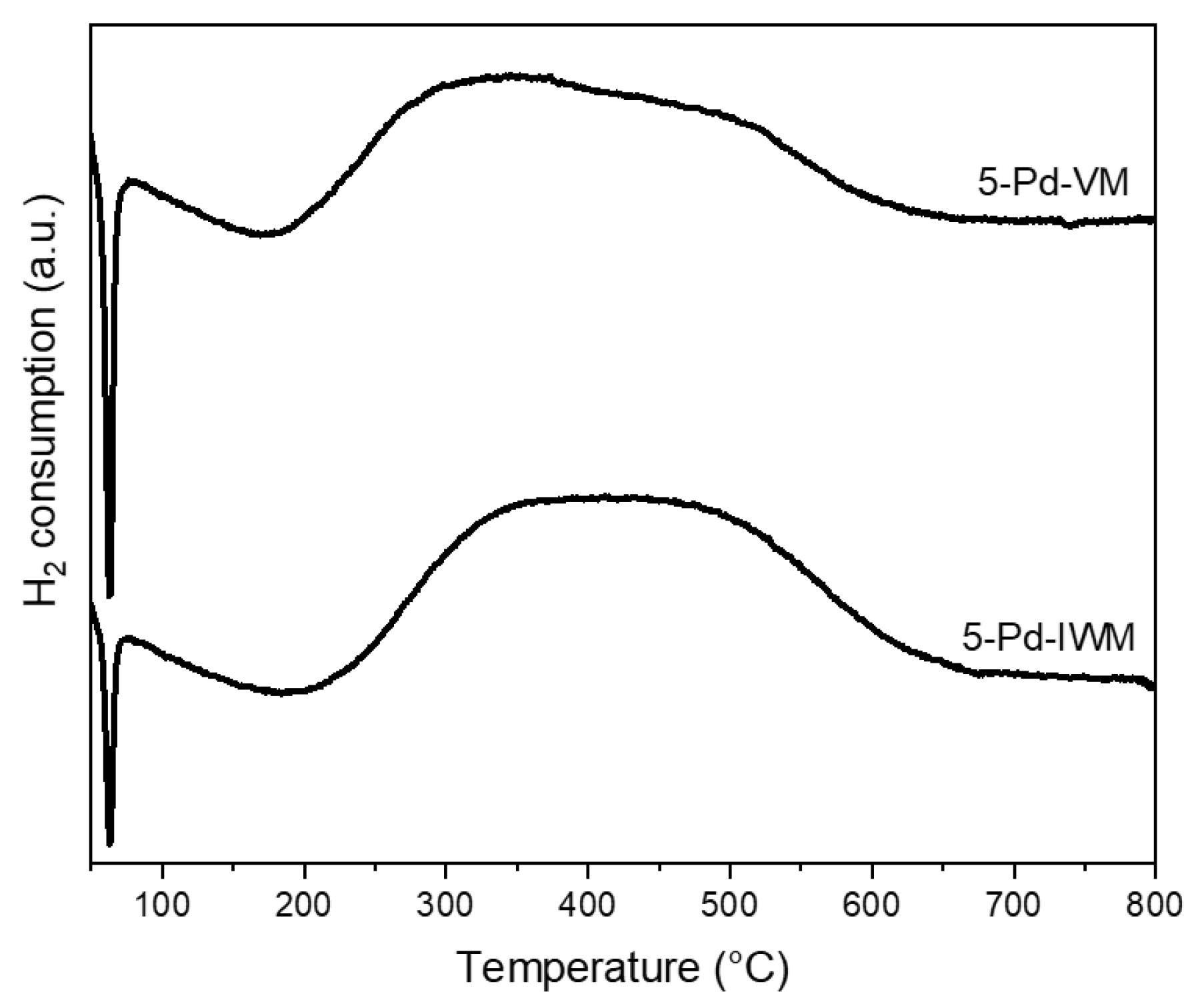
2.2.5. Temperature Programmed Reduction
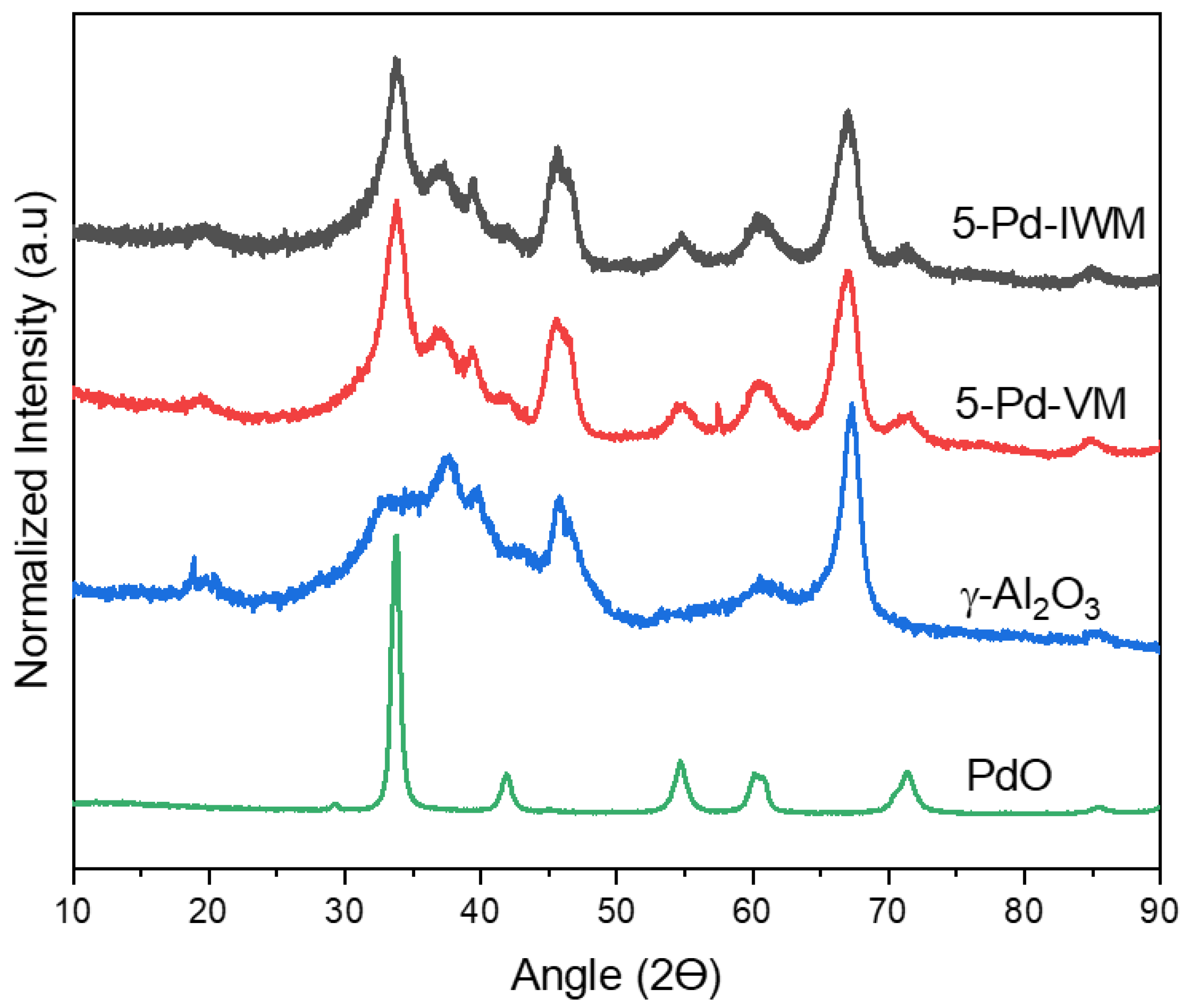
2.2.6. X-ray Diffraction
2.3. Activity Studies
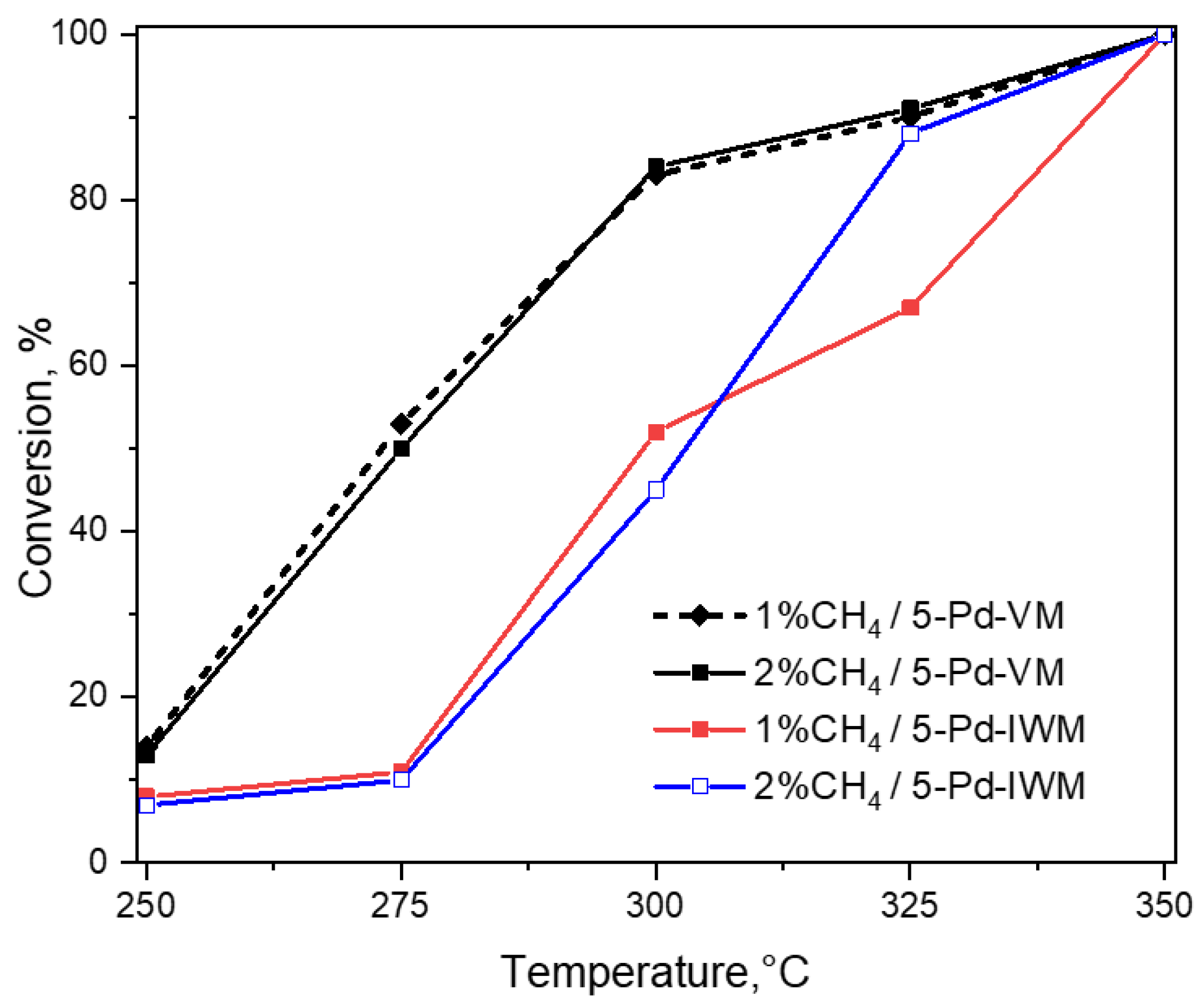
2.3.1. Conversion of Methane in 1–2% Methane, 4% Oxygen, Balance Helium Gas Feed
- (i)
- The catalyst 5-Pd-VM showed higher activity at 275 °C compared to the catalyst 5-Pd-IWM which gave very low activity at this temperature with both 1% and 2% methane in the feed; the trends were similar at 300 °C, with 85% conversion by 5-Pd-VM and about 50% by 5-Pd-IWM.
- (ii)
- Catalyst 5-Pd-VM produced a high conversion of 90% methane at 325 °C for both 1% and 2% methane in the gas feed, while the conversions at this temperature for 5-Pd-IWM were much lower (67%) with 1% methane and about 88% with the 2% methane in the gas feed.
- (iii)
- Both catalysts converted 100% methane at 350 °C under both lean and stoichiometric conditions.
2.3.2. Conversion of Methane in 1% Methane, 4% Oxygen, and Balance Nitrogen Gas Feed
2.4. Long-Term Stability Test of the Catalysts
2.5. Effect of Steam
2.6. Active Phases and Tentative Mechanism
- (i)
- The catalyst 5-Pd-VM contains more smaller particles 3–5 nm than 5-Pd-IWM (Figure 5).
- (ii)
- The particle size distribution was narrower (2–14 nm) in 5-Pd-VM than 5-Pd-IWM (2–18 nm) as shown in (Figure 5).
- (iii)
- PdO/PdOx in 5-Pd-VM tends to be reduced at lower temperatures indicating the interaction between PdO species and the support was comparatively weaker (TPR results in Figure 7).
3. Materials and Methods
3.1. Catalyst Synthesis
3.2. Catalyst Characterization
3.2.1. ICP-AES
3.2.2. XPS
3.2.3. Chemisorption and Physisorption
3.2.4. STEM
3.2.5. XRD
3.3. Activity Measurements
3.4. Effect of Steam
3.5. Stability Test of the Catalysts
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Arandiyan, H.; Gao, X.; Li, J. Recent Advances in Catalysts for Methane Combustion. Catal. Surv. Asia 2015, 19, 140–171. [Google Scholar] [CrossRef]
- Gélin, P.; Primet, M. Complete oxidation of methane at low temperature over noble metal based catalysts: A review. Appl. Catal. B Environ. 2002, 39, 1–37. [Google Scholar] [CrossRef]
- Monai, M.; Montini, T.; Gorte, R.J.; Fornasiero, P. Catalytic Oxidation of Methane: Pd and Beyond. Eur. J. Inorg. Chem. 2018, 2018, 2884–2893. [Google Scholar] [CrossRef]
- Banerjee, A.; McGuire, J.; Lawnick, O.; Bozack, M. Low-Temperature Activity and PdO-PdOx Transition in Methane Combustion by a PdO-PdOx/γ-Al2O3 Catalyst. Catalysts 2018, 8, 266. [Google Scholar] [CrossRef]
- Jiang, L.; Zheng, Y.; Chen, X.; Xiao, Y.; Cai, G.; Zheng, Y.; Zhang, Y.; Huang, F. Catalytic Activity and Stability over Nanorod-Like Ordered Mesoporous Phosphorus-Doped Alumina Supported Palladium Catalysts for Methane Combustion. ACS Catal. 2018, 8, 11016–11028. [Google Scholar]
- Gorte, R.J.; Arroyo-Ramirez, L.; Chung, Y.-C.; Zhang, S.; Graham, G.W.; Onn, T.M.; Pan, X. Improved Thermal Stability and Methane-Oxidation Activity of Pd/Al2O3 Catalysts by Atomic Layer Deposition of ZrO2. ACS Catal. 2015, 5, 5696–5701. [Google Scholar]
- Goodman, E.D.; Dai, S.; Yang, A.C.; Wrasman, C.J.; Gallo, A.; Bare, S.R.; Hoffman, A.S.; Jaramillo, T.F.; Graham, G.W.; Pan, X.; et al. Uniform Pt/Pd Bimetallic Nanocrystals Demonstrate Platinum Effect on Palladium Methane Combustion Activity and Stability. ACS Catal. 2017, 7, 4372–4380. [Google Scholar] [CrossRef]
- Simplício, L.M.T.; Brandão, S.T.; Sales, E.A.; Lietti, L.; Bozon-Verduraz, F. Methane combustion over PdO-alumina catalysts: The effect of palladium precursors. Appl. Catal. B Environ. 2006, 63, 9–14. [Google Scholar] [CrossRef]
- Yoshida, H.; Nakajima, T.; Yazawa, Y.; Hattori, T. Support effect on methane combustion over palladium catalysts. Appl. Catal. B Environ. 2007, 71, 70–79. [Google Scholar] [CrossRef]
- Liu, D.; Seeburg, D.; Kreft, S.; Bindig, R.; Hartmann, I.; Schneider, D.; Enke, D.; Wohlrab, S. Rice Husk Derived Porous Silica as Support for Pd and CeO2 for Low Temperature Catalytic Methane Combustion. Catalysts 2019, 9, 26. [Google Scholar] [CrossRef]
- Li, Y.; Luo, C.; Liu, Z.; Lin, F. Experimental Study on Catalytic Combustion of Methane in a Microcombustor with Metal Foam Monolithic Catalyst. Catalysts 2018, 8, 536. [Google Scholar] [CrossRef]
- Cargnello, M.; Jaén, J.J.D.; Garrido, J.C.H.; Bakhmutsky, K.; Montini, T.; Gámez, J.J.C.; Gorte, R.J.; Fornasiero, P. Exceptional Activity for Methane Combustion over Modular Pd@CeO2 Subunits on Functionalized Al2O3. Science 2012, 337, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Khader, M.; Al-Marri, M.; Ali, S.; Abdelmoneim, A. Active and Stable Methane Oxidation Nano-Catalyst with Highly-Ionized Palladium Species Prepared by Solution Combustion Synthesis. Catalysts 2018, 8, 66. [Google Scholar] [CrossRef]
- Xiao, C.; Yang, Y.; Meng, D.; Dong, L.; Luo, L.; Tan, Z. Stable and active monolithic palladium catalyst for catalytic oxidation of methane using nanozeolite silicalite-1 coating on cordierite. Appl. Catal. A Gen. 2017, 531, 197–202. [Google Scholar] [CrossRef]
- Li, M.; Gui, P.; Zheng, L.; Li, J.; Xue, G.; Liang, J. Active Component Migration and Catalytic Properties of Nitrogen Modified Composite Catalytic Materials. Catalysts 2018, 8, 125. [Google Scholar] [CrossRef]
- Ercolino, G.; Stelmachowski, P.; Specchia, S. Catalytic Performance of Pd/Co3O4 on SiC and ZrO2 Open Cell Foams for Process Intensification of Methane Combustion in Lean Conditions. Ind. Eng. Chem. Res. 2017, 56, 6625–6636. [Google Scholar] [CrossRef]
- Zhang, X.; Long, E.; Li, Y.; Zhang, L.; Guo, J.; Gong, M.; Chen, Y. The effect of CeO2 and BaO on Pd catalysts used for lean-burn natural gas vehicles. J. Mol. Catal. A Chem. 2009, 308, 73–78. [Google Scholar] [CrossRef]
- Pantaleo, G.; Liotta, L.F.; Venezia, A.M.; Kantcheva, M.; Di Carlo, G. Effect of Ti(IV) loading on CH4 oxidation activity and SO2 tolerance of Pd catalysts supported on silica SBA-15 and HMS. Appl. Catal. B Environ. 2011, 106, 529–539. [Google Scholar]
- Liotta, L.F.; Di Carlo, G.; Pantaleo, G.; Garrido, J.C.H.; Venezia, A.M. Pd (1 wt%)/LaMn0.4Fe0.6O3 catalysts supported over silica SBA-15: Effect of perovskite loading and support morphology on methane oxidation activity and SO2 tolerance. Top. Catal. 2012, 55, 782–791. [Google Scholar] [CrossRef]
- Ma, J.; Lou, Y.; Cai, Y.; Zhao, Z.; Wang, L.; Zhan, W.; Guo, Y.; Guo, Y. The relationship between the chemical state of Pd species and the catalytic activity for methane combustion on Pd/CeO2. Catal. Sci. Technol. 2018, 8, 2567–2577. [Google Scholar] [CrossRef]
- Martin, A.; Liu, D.; Atia, H.; Schneider, M.; Wohlrab, S.; Pohl, M.-M.; Radnik, J.; Seeburg, D. Structural Changes of Highly Active Pd/MeOx (Me = Fe, Co, Ni) during Catalytic Methane Combustion. Catalysts 2018, 8, 42. [Google Scholar]
- Lundgren, E.; Nilsson, J.; Gustafson, J.; Grönbeck, H.; Skoglundh, M.; Newton, M.A.; Fouladvand, S.; Carlsson, P.-A.; Martin, N.M. Chemistry of Supported Palladium Nanoparticles during Methane Oxidation. ACS Catal. 2015, 5, 2481–2489. [Google Scholar]
- Miller, J.B.; Malatpure, M. Pd catalysts for total oxidation of methane: Support effects. Appl. Catal. A Gen. 2015, 495, 54–62. [Google Scholar] [CrossRef]
- Christ, B.V. Handbook of Monochromatic Xps Spectra. The Elements of Native Oxides; Wiley-VCH: Weinheim, Germany, 2000; p. 49265. ISBN 0-471-49265-5. [Google Scholar]
- Gil, S.; Retailleau, L.; Garcia-Vargas, J.; Ousmane, M.; Pantaleo, G.; Giroir-Fendler, A.; Liotta, L. Catalytic Oxidation of Propene over Pd Catalysts Supported on CeO2, TiO2, Al2O3 and M/Al2O3 Oxides (M = Ce, Ti, Fe, Mn). Catalysts 2015, 5, 671–689. [Google Scholar] [CrossRef]
- Sadokhina, N.; Ghasempour, F.; Auvray, X.; Smedler, G.; Nylén, U.; Olofsson, M.; Olsson, L. An Experimental and Kinetic Modelling Study for Methane Oxidation over Pd-based Catalyst: Inhibition by Water. Catal. Lett. 2017, 147, 2360–2371. [Google Scholar] [CrossRef]
- Bychkov, V.Y.; Tyulenin, Y.P.; Gorenberg, A.Y.; Sokolov, S.; Korchak, V.N. Evolution of Pd catalyst structure and activity during catalytic oxidation of methane and ethane. Appl. Catal. A Gen. 2014, 485, 1–9. [Google Scholar] [CrossRef]
- Xiong, H.; Wiebenga, M.H.; Carrillo, C.; Gaudet, J.R.; Pham, H.N.; Kunwar, D.; Oh, S.H.; Qi, G.; Kim, C.H.; Datye, A.K. Design considerations for low-temperature hydrocarbon oxidation reactions on Pd based catalysts. Appl. Catal. B Environ. 2018, 236, 436–444. [Google Scholar] [CrossRef]
- Schwartz, W.R.; Pfefferle, L.D. Combustion of methane over palladium-based catalysts: Support interactions. J. Phys. Chem. C 2012, 116, 8571–8578. [Google Scholar] [CrossRef]
- Di, L.; Xu, W.; Zhan, Z.; Zhang, X. Synthesis of alumina supported Pd-Cu alloy nanoparticles for CO oxidation via a fast and facile method. RSC Adv. 2015, 5, 71854–71858. [Google Scholar] [CrossRef]
- Ercolino, G.; Karimi, S.; Stelmachowski, P.; Specchia, S. Catalytic combustion of residual methane on alumina monoliths and open cell foams coated with Pd/Co3O4. Chem. Eng. J. 2017, 326, 339–349. [Google Scholar] [CrossRef]
- Van Giezen, J.C.; Van Den Berg, F.R.; Kleinen, J.L.; Van Dillen, A.J.; Geus, J.W. The effect of water on the activity of supported palladium catalysts in the catalytic combustion of methane. Catal. Today 1999, 47, 287–293. [Google Scholar] [CrossRef]
- Stefanov, P.; Todorova, S.; Naydenov, A.; Tzaneva, B.; Kolev, H.; Atanasova, G.; Stoyanova, D.; Karakirova, Y.; Aleksieva, K. On the development of active and stable Pd-Co/γ-Al2O3 catalyst for complete oxidation of methane. Chem. Eng. J. 2015, 266, 329–338. [Google Scholar] [CrossRef]
- Ercolino, G.; Stelmachowski, P.; Grzybek, G.; Kotarba, A.; Specchia, S. Optimization of Pd catalysts supported on Co3O4 for low-temperature lean combustion of residual methane. Appl. Catal. B Environ. 2017, 206, 712–725. [Google Scholar] [CrossRef]
- Burch, R.; Urbano, F.J.; Loader, P.K. Methane combustion over palladium catalysts: The effect of carbon dioxide and water on activity. Appl. Catal. A Gen. 1995, 123, 173–184. [Google Scholar] [CrossRef]
- Ciuparu, D.; Pfefferle, L. Support and water effects on palladium based methane combustion catalysts. Appl. Catal. A Gen. 2001, 209, 415–428. [Google Scholar] [CrossRef]
- Persson, K.; Pfefferle, L.D.; Schwartz, W.; Ersson, A.; Järås, S.G. Stability of palladium-based catalysts during catalytic combustion of methane: The influence of water. Appl. Catal. B Environ. 2007, 74, 242–250. [Google Scholar] [CrossRef]
- Huang, F.; Chen, J.; Hu, W.; Li, G.; Wu, Y.; Yuan, S.; Zhong, L.; Chen, Y. Pd or PdO: Catalytic active site of methane oxidation operated close to stoichiometric air-to-fuel for natural gas vehicles. Appl. Catal. B Environ. 2017, 219, 73–81. [Google Scholar] [CrossRef]
- Forzatti, P. Status and perspectives of catalytic combustion for gas turbines. Catal. Today 2003, 83, 3–18. [Google Scholar] [CrossRef]
- Gholami, R.; Smith, K.J. Activity of PdO/SiO2 catalysts for CH4 oxidation following thermal treatments. Appl. Catal. B Environ. 2015, 168–169, 156–163. [Google Scholar] [CrossRef]
- Sulmonetti, T.P.; Pang, S.H.; Claure, M.T.; Lee, S.; Cullen, D.A.; Agrawal, P.K.; Jones, C.W. Vapor phase hydrogenation of furfural over nickel mixed metal oxide catalysts derived from layered double hydroxides. Appl. Catal. A Gen. 2016, 517, 187–195. [Google Scholar] [CrossRef]
- Walton, K.S.; Snurr, R.Q. Applicability of the BET method for determining surface areas of microporous metal-organic frameworks. J. Am. Chem. Soc. 2007, 129, 8552–8556. [Google Scholar] [CrossRef]

| Properties | Values | |
|---|---|---|
| 5-Pd-VM | 5-Pd-IWM | |
| Targeted Wt% Pd | 5.0% | 5.0% |
| Pd Wt% [ICP-AES] | 4.6% | 4.1% |
| Surface Area * CO uptake in chemisorption | 127 m2/g 54.4 μmol/g | 129 m2/g 49.9 μmol/g |
| Dispersion | 12.6% | 13.1% |
| Metal Surface Area | 2.6 m2/g | 2.4 m2/g |
| Nanoparticle diameter (hemisphere) | 8.9 nm | 8.6 nm |
| Sample | PdOx (Atomic %) | PdO (Atomic %) |
|---|---|---|
| 5-Pd-VM | 23 | 77 |
| 5-Pd-VM Post | 24 | 76 |
| 5-Pd-IWM | 16 | 84 |
| 5-Pd-IWM Post | 11 | 89 |
| Temperature, °C | Methane Conversion, % | |
|---|---|---|
| 5-Pd-VM | 5-Pd-IWM | |
| 250 | 61 | 18 |
| 275 | 100 | 46 |
| 300 | 100 | 100 |
| Reaction Period, h | Methane Conversion, % | |||
|---|---|---|---|---|
| 5-Pd-VM | 5-Pd-IWM | |||
| T25 | T100 | T25 | T100 | |
| 24 | 25 | 100 | 18 | 76 |
| 48 | 24 | 100 | 15 | 75 |
| 72 | 23 | 100 | 10 | 67 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banerjee, A.C.; Golub, K.W.; Hakim, M.A.; Billor, M.Z. Comparative Study of the Characteristics and Activities of Pd/γ-Al2O3 Catalysts Prepared by Vortex and Incipient Wetness Methods. Catalysts 2019, 9, 336. https://doi.org/10.3390/catal9040336
Banerjee AC, Golub KW, Hakim MA, Billor MZ. Comparative Study of the Characteristics and Activities of Pd/γ-Al2O3 Catalysts Prepared by Vortex and Incipient Wetness Methods. Catalysts. 2019; 9(4):336. https://doi.org/10.3390/catal9040336
Chicago/Turabian StyleBanerjee, Anil C., Kristina W. Golub, Md. Abdul Hakim, and Mehmet Z. Billor. 2019. "Comparative Study of the Characteristics and Activities of Pd/γ-Al2O3 Catalysts Prepared by Vortex and Incipient Wetness Methods" Catalysts 9, no. 4: 336. https://doi.org/10.3390/catal9040336
APA StyleBanerjee, A. C., Golub, K. W., Hakim, M. A., & Billor, M. Z. (2019). Comparative Study of the Characteristics and Activities of Pd/γ-Al2O3 Catalysts Prepared by Vortex and Incipient Wetness Methods. Catalysts, 9(4), 336. https://doi.org/10.3390/catal9040336