On the Effects of Doping on the Catalytic Performance of (La,Sr)CoO3. A DFT Study of CO Oxidation
Abstract
1. Introduction
2. Results
2.1. LaCoO3 vs. (La,Sr)CoO3
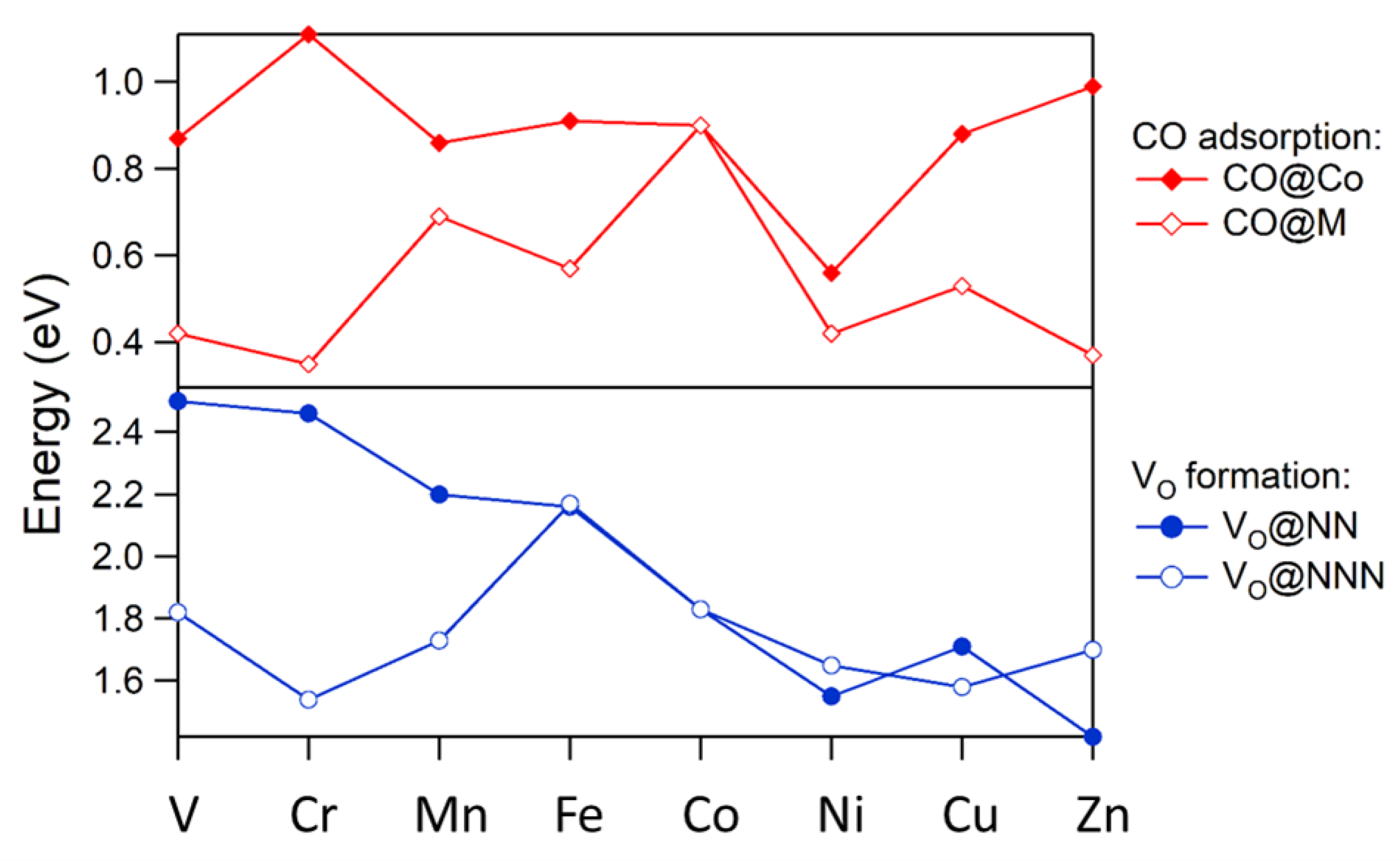
2.2. Doping LSCO at the Co Site
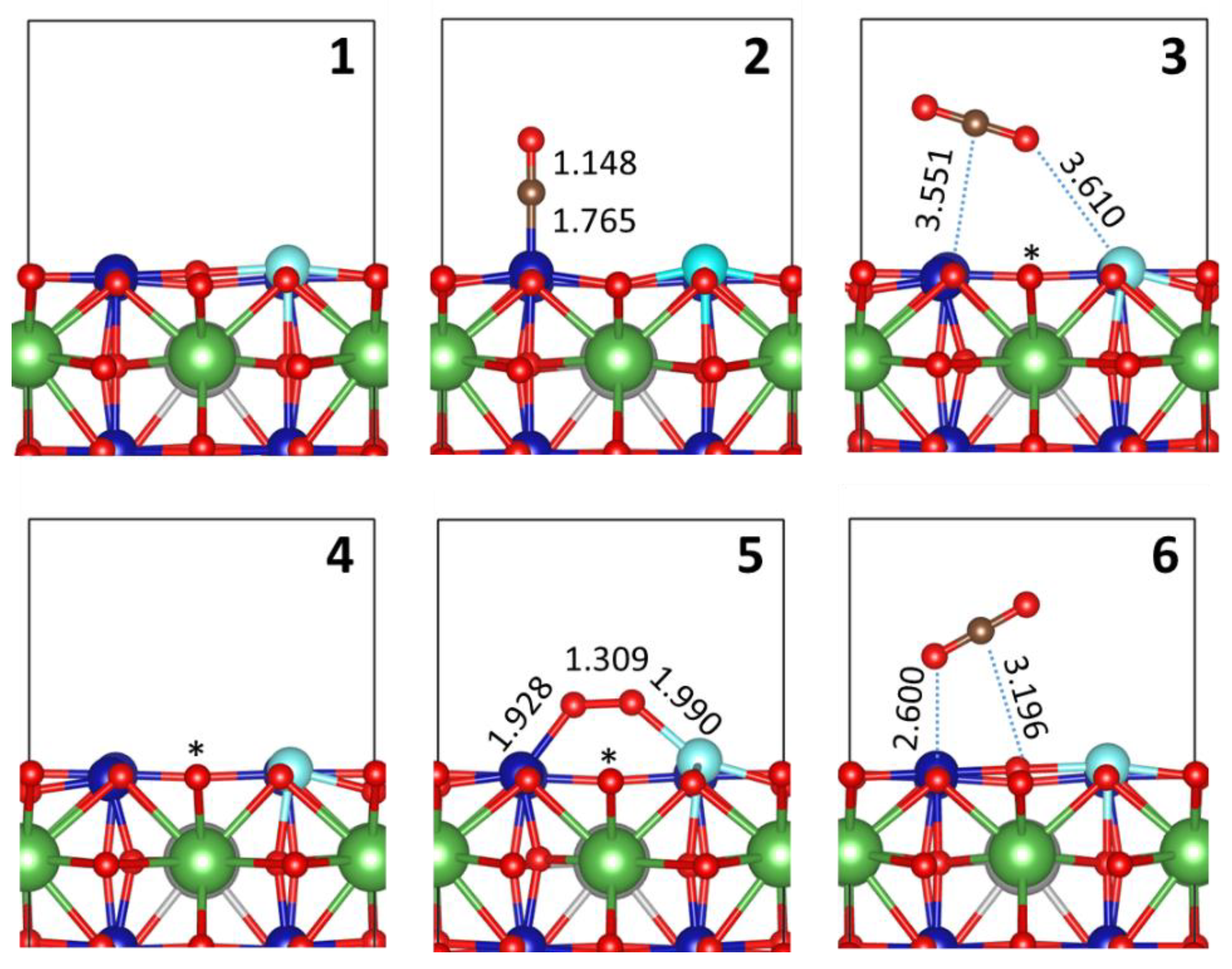
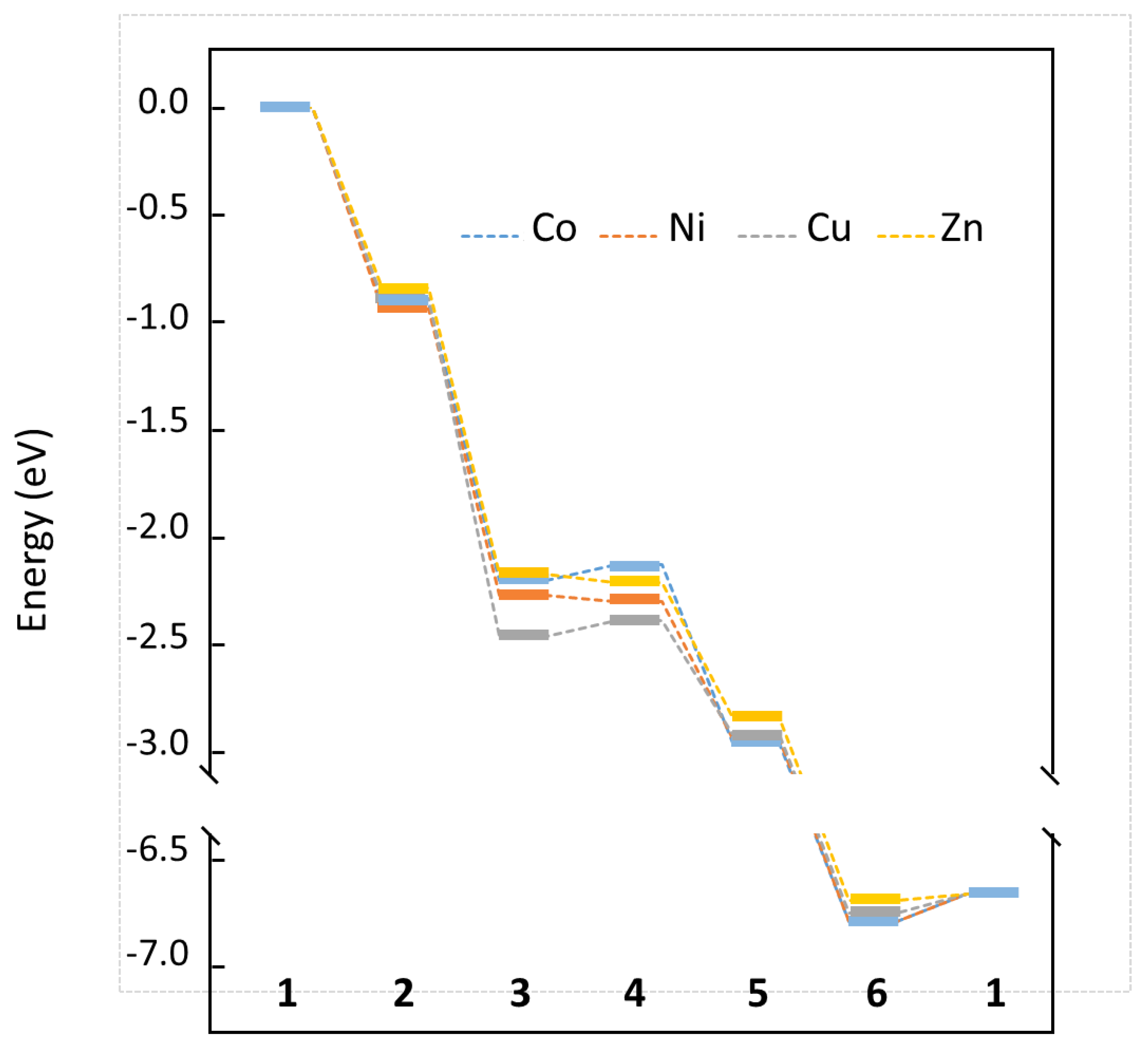
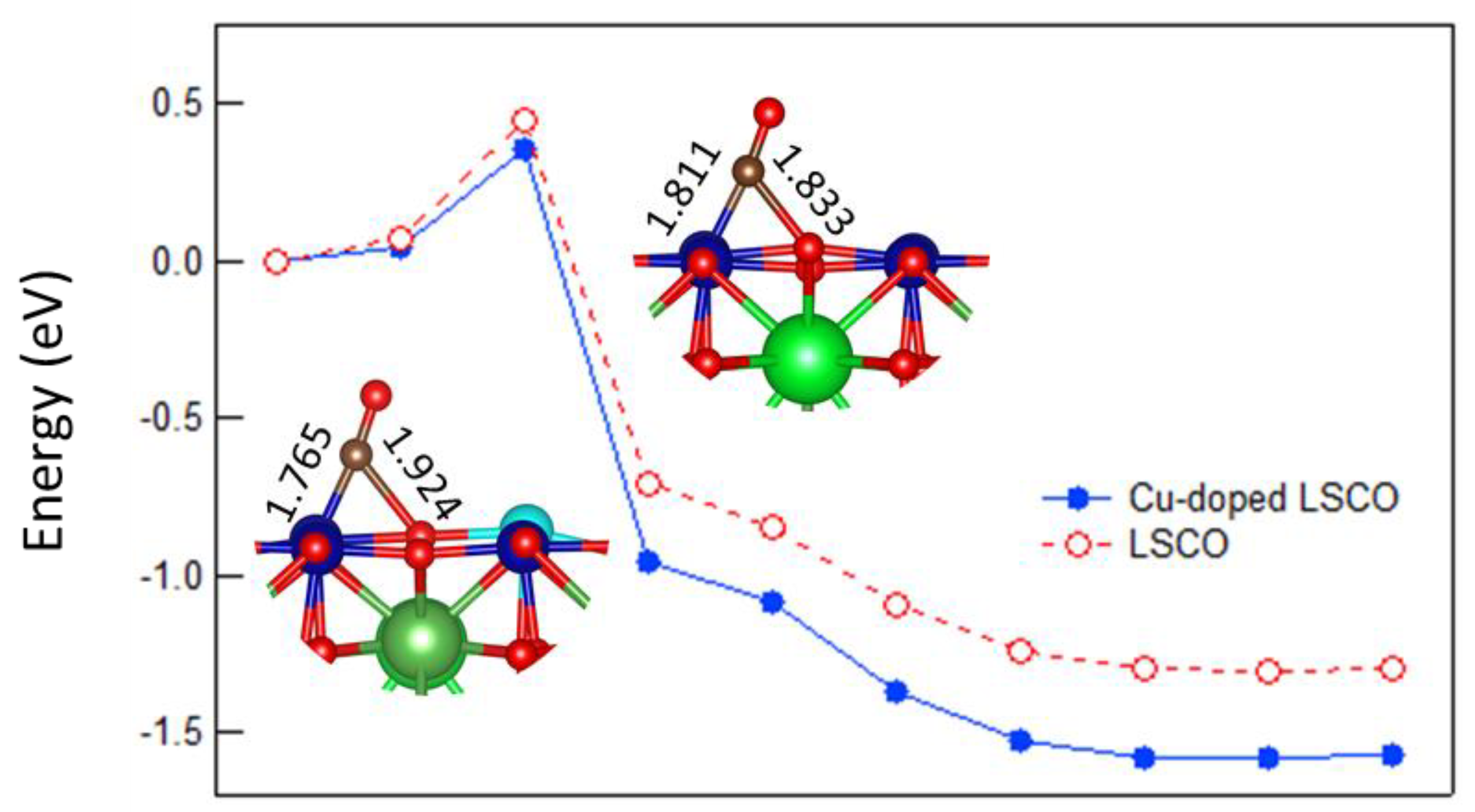
2.3. CO Oxidation at LSCO: Effects of Doping at the Co Site
3. Theoretical Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McFarland, E.W.; Metiu, H. Catalysis by Doped Oxides. Chem. Rev. 2013, 113, 4391–4427. [Google Scholar] [CrossRef]
- Carlotto, S.; Natile, M.M.; Glisenti, A.; Vittadini, A. Catalytic Mechanisms of NO Reduction in a CO-NO Atmosphere at Co- and Cu-Doped SrTiO3(100) Surfaces. J. Phys. Chem. C 2018, 122, 449–454. [Google Scholar] [CrossRef]
- Carlotto, S.; Natile, M.M.; Glisenti, A.; Paul, J.-F.; Blanck, D.; Vittadini, A. Energetics of CO oxidation on lanthanide-free perovskite systems: The case of Co-doped SrTiO3. Phys. Chem. Chem. Phys. 2016, 18, 33282–33286. [Google Scholar] [CrossRef] [PubMed]
- Glisenti, A.; Natile, M.M.; Carlotto, S.; Vittadini, A. Co- and Cu-Doped Titanates: Toward a New Generation of Catalytic Converters. Catal. Lett. 2014, 144, 1466–1471. [Google Scholar] [CrossRef]
- Glisenti, A.; Pacella, M.; Guiotto, M.; Natile, M.M.; Canu, P. Largely Cu-doped LaCo1-xCuxO3 perovskites for TWC: Toward new PGM-free catalysts. Appl. Catal. B Environ. 2016, 180, 94–105. [Google Scholar] [CrossRef]
- Fuks, D.; Weizman, A.; Kotomin, E. Phase competition in (La1-c,Sr-c)CoO3 solid solutions: Ab initio thermodynamic study. Phys. Status Solidi B Basic Solid State Phys. 2013, 250, 864–869. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.O.; Penninger, M.; Kim, C.H.; Schneider, W.F.; Thompson, L.T. Experimental and Computational Investigation of Effect of Sr on NO Oxidation and Oxygen Exchange for La1-xSrxCoO3 Perovskite Catalysts. ACS Catal. 2013, 3, 2719–2728. [Google Scholar] [CrossRef]
- Penninger, M.W.; Kim, C.H.; Thompson, L.T.; Schneider, W.F. DFT Analysis of NO Oxidation Intermediates on Undoped and Doped LaCoO3 Perovskite. J. Phys. Chem. C 2015, 119, 20488–20494. [Google Scholar] [CrossRef]
- Garrity, K.F.; Bennett, J.W.; Rabe, K.M.; Vanderbilt, D. Pseudopotentials for high-throughput DFT calculations. Comput. Mater. Sci. 2014, 81, 446–452. [Google Scholar] [CrossRef]
- Wang, L.; Maxisch, T.; Ceder, G. Oxidation energies of transition metal oxides within the GGA+U framework. Phys. Rev. B 2006, 73, 195107. [Google Scholar] [CrossRef]

| Compound | 1 VO/cell | 2 VO/cell | CO Adsorption | NO Adsorption |
|---|---|---|---|---|
| LCO | 2.12 | 5.15 | 0.98 | 1.10 |
| LSCO | 1.83 | 4.37 | 0.90 | 1.16 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glisenti, A.; Vittadini, A. On the Effects of Doping on the Catalytic Performance of (La,Sr)CoO3. A DFT Study of CO Oxidation. Catalysts 2019, 9, 312. https://doi.org/10.3390/catal9040312
Glisenti A, Vittadini A. On the Effects of Doping on the Catalytic Performance of (La,Sr)CoO3. A DFT Study of CO Oxidation. Catalysts. 2019; 9(4):312. https://doi.org/10.3390/catal9040312
Chicago/Turabian StyleGlisenti, Antonella, and Andrea Vittadini. 2019. "On the Effects of Doping on the Catalytic Performance of (La,Sr)CoO3. A DFT Study of CO Oxidation" Catalysts 9, no. 4: 312. https://doi.org/10.3390/catal9040312
APA StyleGlisenti, A., & Vittadini, A. (2019). On the Effects of Doping on the Catalytic Performance of (La,Sr)CoO3. A DFT Study of CO Oxidation. Catalysts, 9(4), 312. https://doi.org/10.3390/catal9040312






