Synergistic Effect of New ZrNi5/Nb2O5 Catalytic Agent on Storage Behavior of Nanocrystalline MgH2 Powders
Abstract
:1. Introduction
2. Results and Discussion
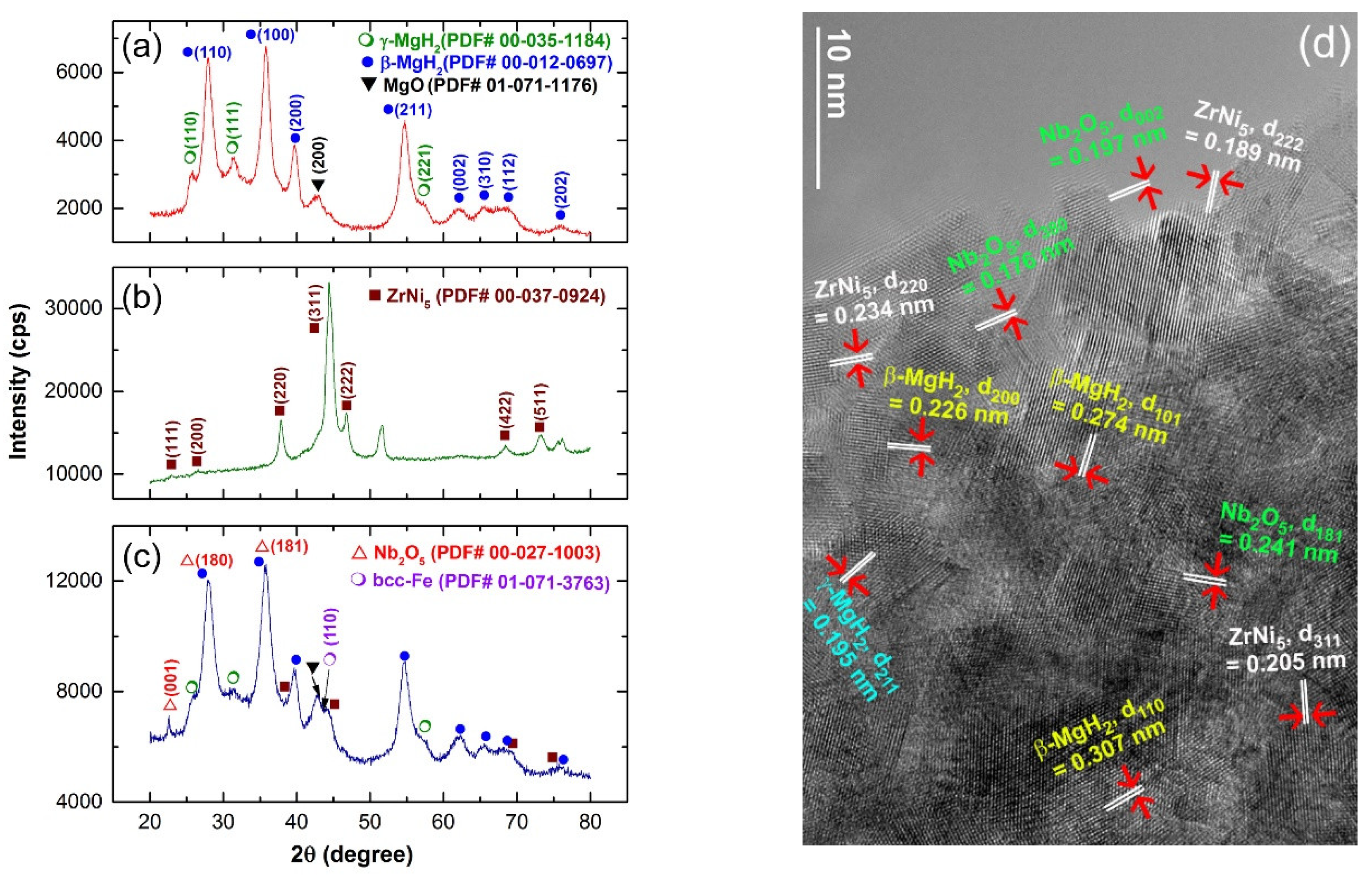
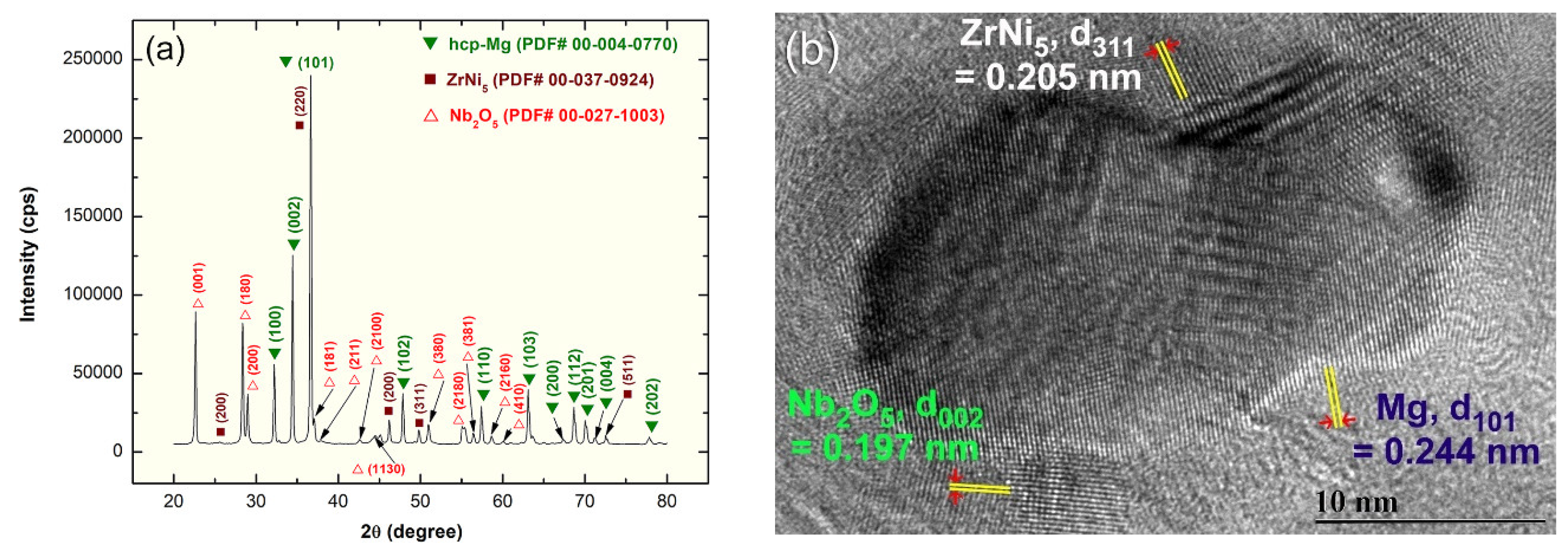
2.1. Crystal Structure
2.2. EDS Analysis
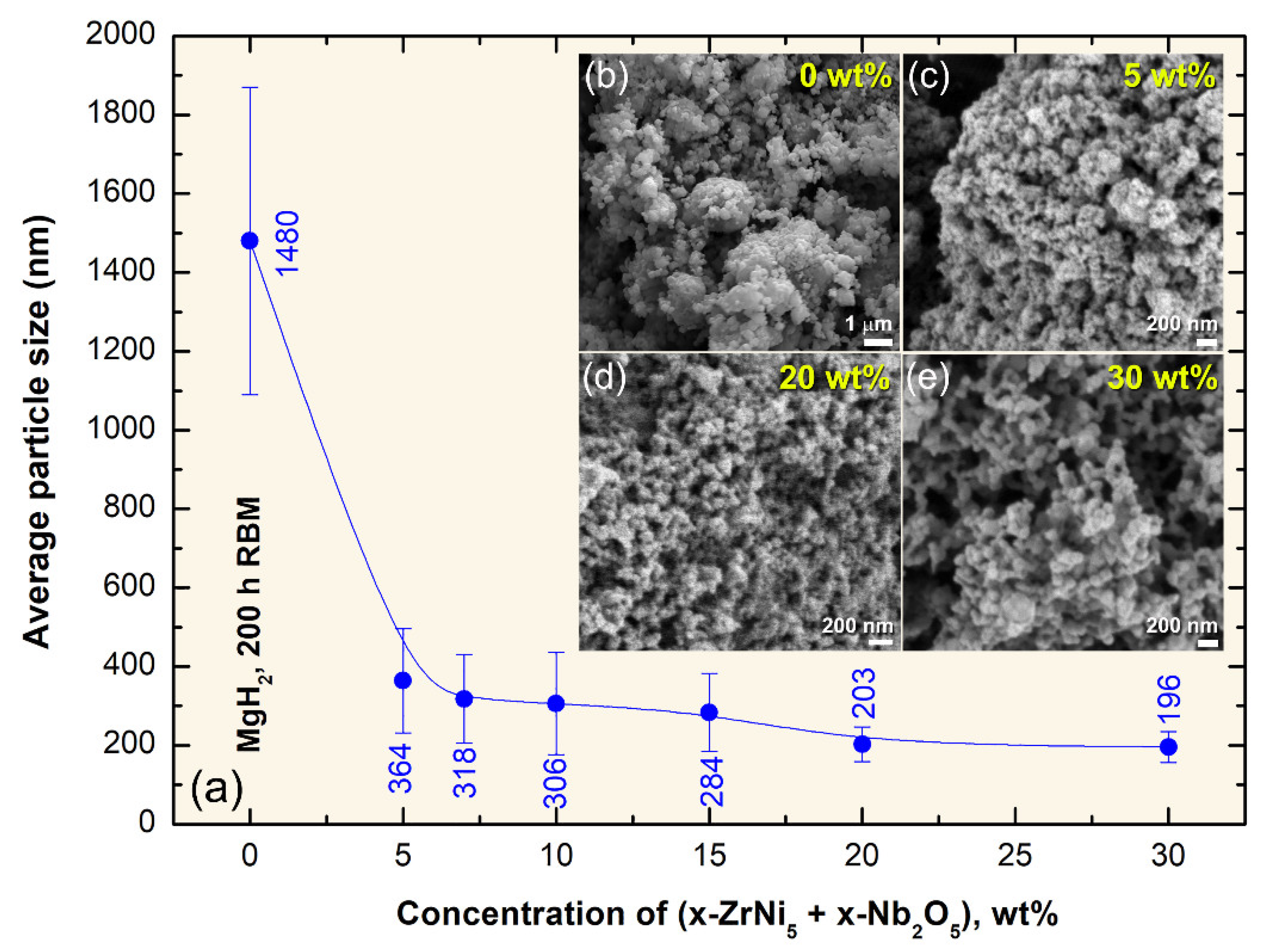
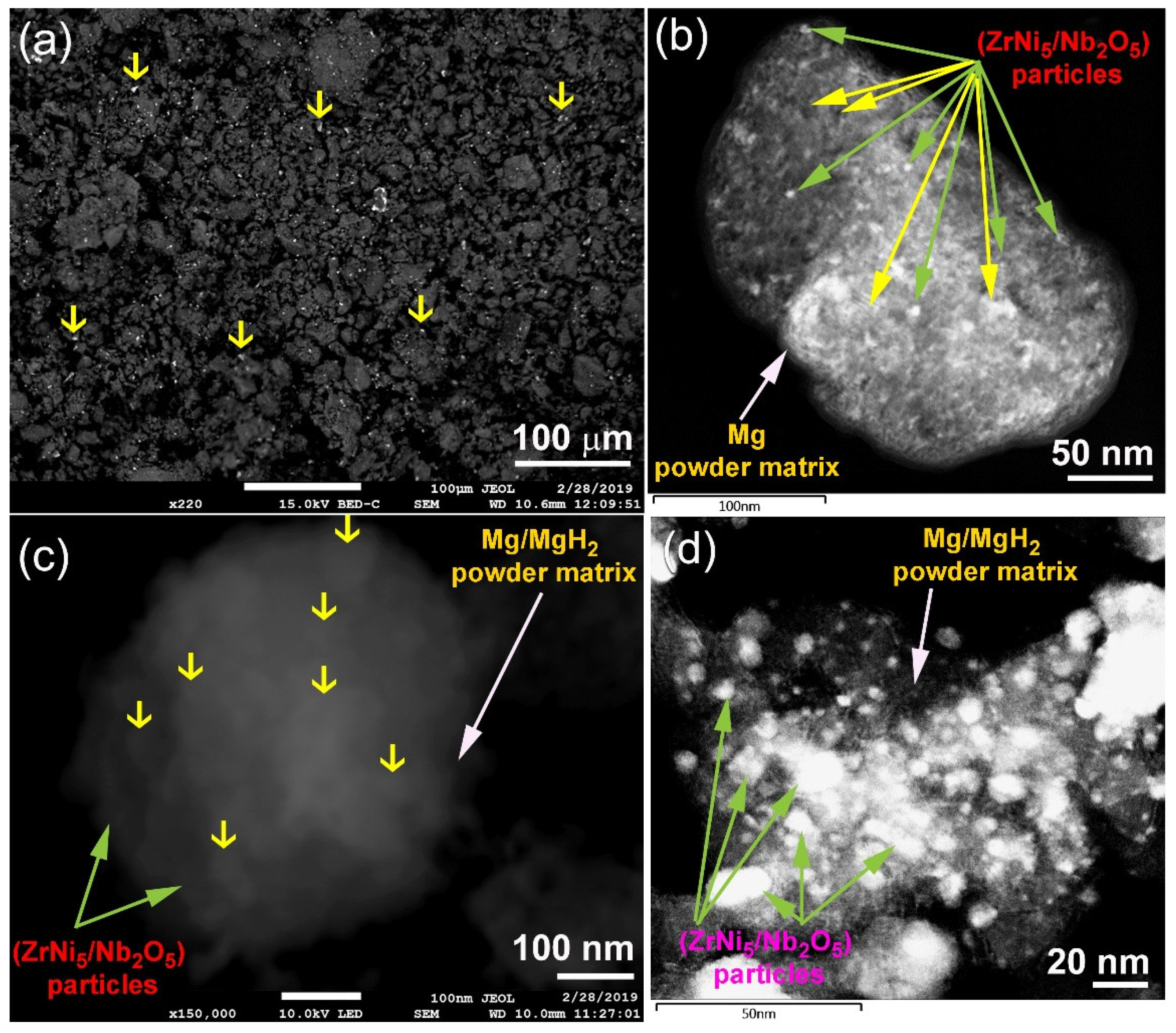
2.3. Morphology
2.4. Thermal Analysis
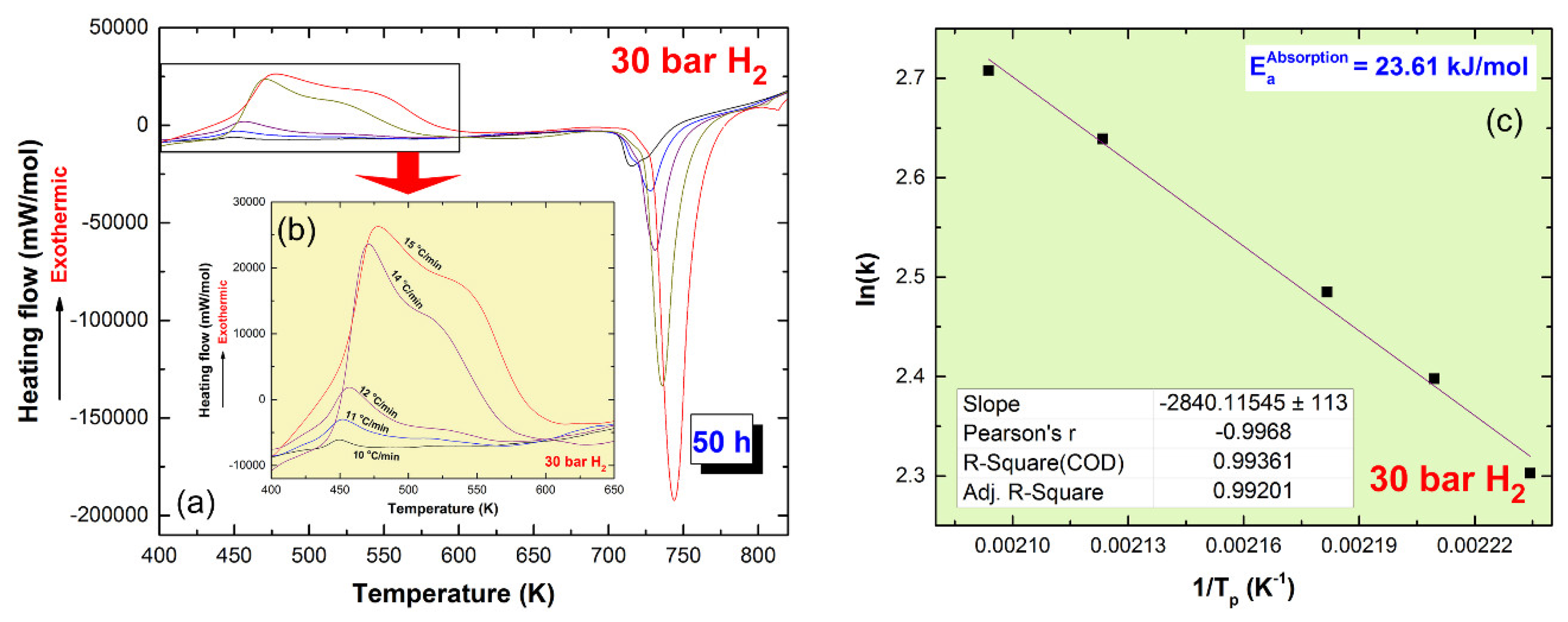
2.4.1. High-Pressure Differential Scanning Calorimetry
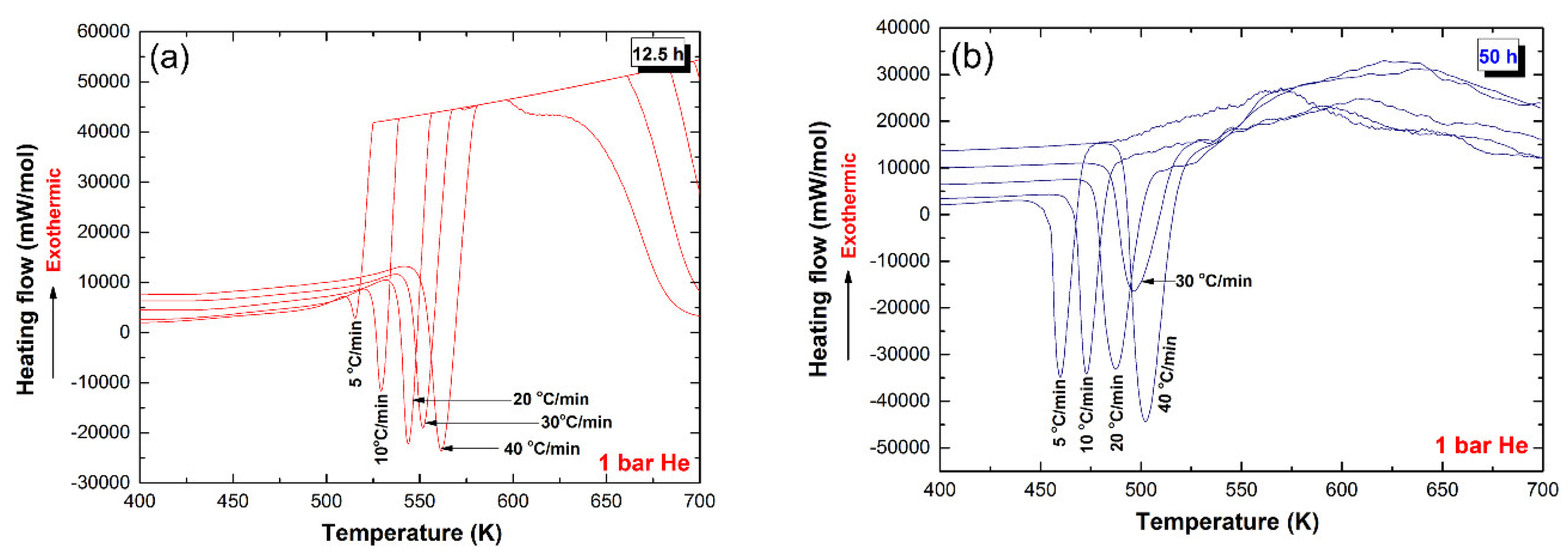
2.4.2. Normal-Pressure Differential Scanning Calorimetry
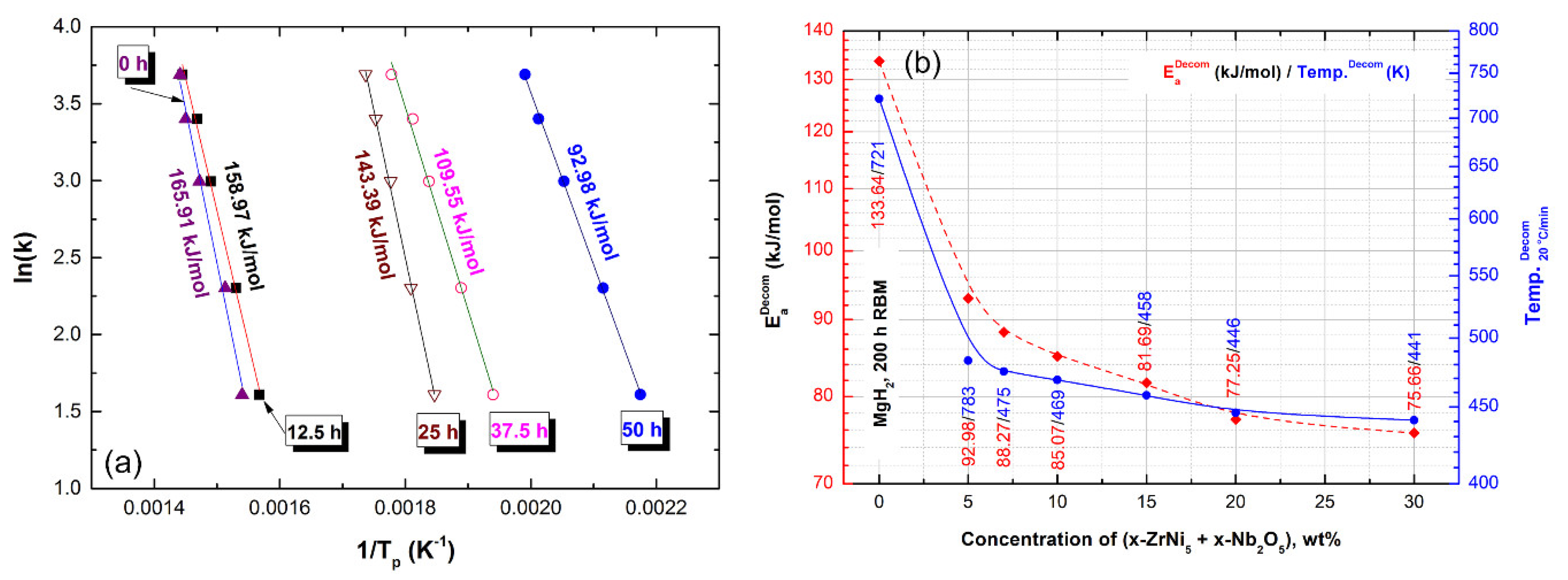
2.5. Hydrogenation/Dehydrogenation Kinetics
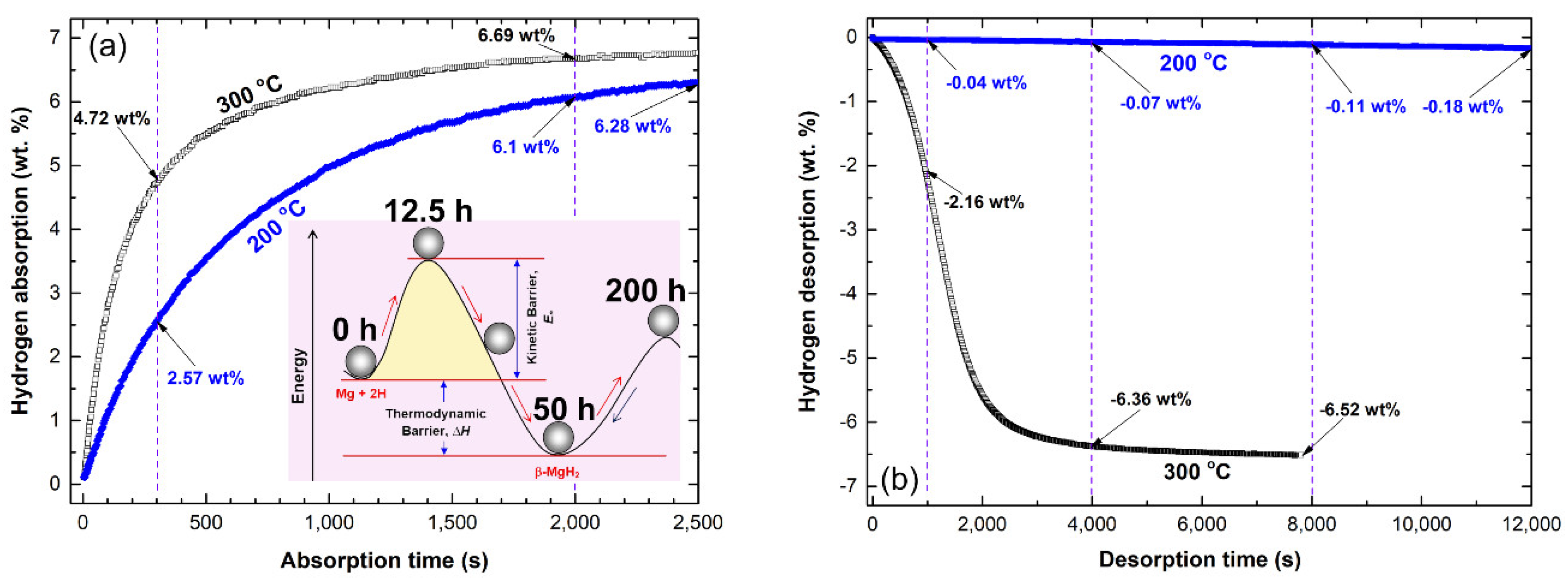
2.5.1. Pure MgH2 Nanocrystalline Powders
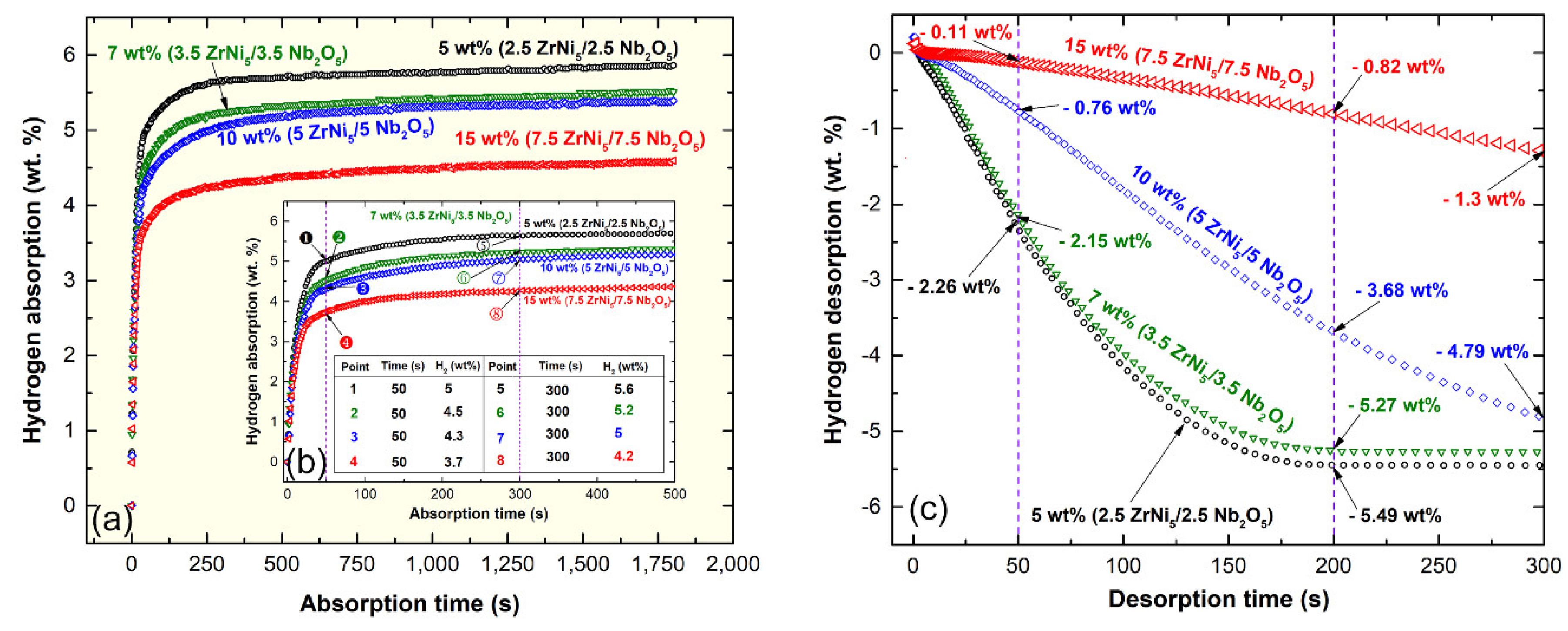
2.5.2. Nanocomposite MgH2/y-wt% (x-ZrNi5/x-Nb2O5) Powders
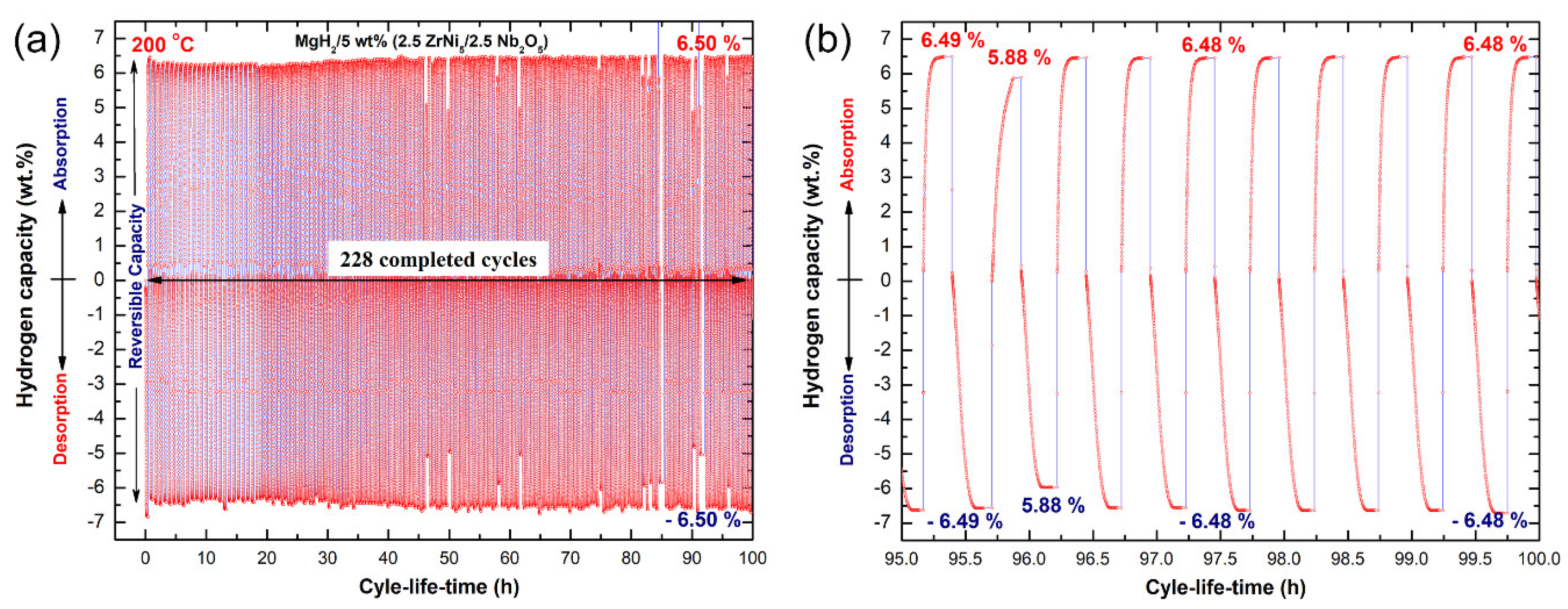
2.6. Hydrogenation/Dehydrogenation Cycle-Life-Time
3. Materials and Methods
3.1. Sample Preparations
3.1.1. MgH2 Nanocrystalline Powders
3.1.2. Catalysts
- ZrNi5 Intermetallic Compound
- Preparation of ZrNi5/Nb2O5 Catalysts
3.1.3. Preparation of MgH2/y-wt% (x-ZrNi5/x-Nb2O5) Nanocomposite Powders
3.2. Sample Characterizations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schlapbach, L.; Zuttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Jain, I.P.; Lal, C.; Jain, A. Hydrogen storage in Mg: A most promising material. Int. J. Hydrogen Energ. 2010, 35, 5133–5144. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Alkandary, A.; Aldakheel, F.; Al-Saidi, M.; Al-Ajmi, F.; Banyan, M. Performance and fuel cell applications of reacted ball-milled MgH2/5.3 wt% TiH2 nanocomposite powders. RSC Adv. 2018, 8, 38175–38185. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Shaban, E.; Aldakheel, F.; Alkandary, A.; Behbehani, M.; Al-Saidi, M. Synthetic nanocomposite MgH2/5wt. % TiMn2 powders for solid-hydrogen storage tank integrated with PEM fuel cell. Sci. Rep. 2018, 7, 13296. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, S.; Qu, H. Recent progress in magnesium hydride modified through catalysis and nanoconfinement. Int. J. Hydrogen Energy 2018, 43, 1545–1565. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Al-Nasrallah, E.; Banyan, M.; Al-Ajmi, F. Bulk nanocomposite MgH2/10 wt% (8 Nb2O5/2 Ni) solid-hydrogen storage system for fuel cell applications. Int. J. Hydrogen Energy 2018, 27, 23382–23396. [Google Scholar] [CrossRef]
- Yartys, V.A.; Lototskyy, M.V.; Akiba, E.; Albert, R.; Antonov, V.E.; Ares, J.R.; Baricco, M.; Bourgeois, N.; Buckley, C.E.; Bellosta von Colbe, J.M. Magnesium based materials for hydrogen based energy storage: Past, present and future. Int. J. Hydrogen Energy 2019, 44, 7809–7859. [Google Scholar] [CrossRef]
- Lototskyy, M.V.; Tolj, I.; Pickering, L.; Sita, C.; Barbir, F.; Yartys, V. The use of metal hydrides in fuel cell applications. Prog. Nat. Sci. Mater. Int. 2017, 27, 3–20. [Google Scholar] [CrossRef]
- Bellosta von Colbe, J.M.; Ares, J.-R.; Barale, J.; Baricco, M.; Buckley, C.; Capurso, G.; Gallandat, N.; Grant, D.M.; Guzik, M.N.; Jacob, I. Application of hydrides in hydrogen storage and compression: Achievements, outlook and perspectives. Int. J. Hydrogen Energy 2019, 44, 7780–7808. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S. Recent developments on fabrication, characterization and implementation of MgH2-based solid-hydrogen materials in Kuwait Institute for Scientific Research. RSC Adv. 2019, in press. [Google Scholar]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Kinetic investigation of the effect of milling time on the hydrogen sorption reaction of magnesium catalyzed with different Nb2O5 contents. J. Alloys Compd. 2006, 407, 249–255. [Google Scholar] [CrossRef]
- Simchi, H.; Kaflou, A.; Simchi, A. Synergetic effect of Ni and Nb2O5 on dehydrogenation properties of nanostructured MgH2 synthesized by high-energy mechanical alloying. Int. J. Hydrogen Energy 2009, 34, 7724–7730. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Shaban, E.; Al-Shemmiri, A. Integrated Ni/Nb2O5 nanocatalytic agent dose for improving the hydrogenation/dehydrogenation kinetics of reacted ball milled MgH2 powders. Int. J. Hydrogen Energy 2014, 39, 21097–21106. [Google Scholar] [CrossRef]
- Hanada, N.; Ichikawa, T.; Hino, S.; Fujii, H. Remarkable improvement of hydrogen sorption kinetics in magnesium catalyzed with Nb2O5. J. Alloy. Compd. 2006, 420, 46–49. [Google Scholar] [CrossRef]
- Dehouche, Z.; Peretti, H.A.; Hamoudi, S.; Yoo, Y.; Belkacemi, K. Effect of activated alloys on hydrogen discharge kinetics of MgH2 nanocrystals. J. Alloys Compd. 2008, 432, 439. [Google Scholar]
- Singh, A.K.; Srivastava, O.N. On the synthesis of the Mg2Ni alloy by mechanical alloying. J. Alloys Compd. 1995, 227, 63–68. [Google Scholar] [CrossRef]
- Valentoni, A.; Mulas, G.; Enzo, S.; Garroni, S. Remarkable hydrogen storage properties of MgH2 doped with VNbO5. Phys. Chem. Chem. Phys. 2018, 20, 4100–4108. [Google Scholar] [CrossRef] [PubMed]
- Milanese, C.; Girella, A.; Garroni, S.; Bruni, G.; Berbenni, V.; Matteazzi, P.; Marini, A. Synergetic effect of C (graphite) and Nb2O5 on the H2 sorption properties of the Mg-MgH2 system. Int. J. Hydrogen Energy 2010, 35, 9027–9037. [Google Scholar] [CrossRef]
- Idris, N.H.; Mustafa, N.S.; Ismail, M. MnFe2O4 nanopowder synthesized via a simple hydrothermal method for promoting hydrogen sorption from MgH2. Int. J. Hydrogen Energy 2017, 42, 21114–21120. [Google Scholar] [CrossRef]
- Juanhir, N.; Mustafa, N.S.; Sinin, A.M.; Ismail, M. Improved hydrogen storage properties of MgH2 by addition of Co2NiO nanoparticles. RSC Adv. 2015, 5, 60983–60989. [Google Scholar] [CrossRef]
- Mustafa, N.S.; Ismail, M. Hydrogen sorption improvement of MgH2 catalyzed by CeO2 nanopowder. J. Alloys Compd. 2017, 695, 2532–2538. [Google Scholar] [CrossRef]
- Pukazhselvan, D.; Nasani, N.; Correia, P.; Carbo-Argibay, E.; Otero-Irurueta, G.; Stroppa, D.G.; Fagg, D.P. Evolution of reduced Ti containing phase (s) in MgH2/TiO2 system and its effect on the hydrogen storage behavior of MgH2. J. Power Sources 2017, 362, 174–183. [Google Scholar] [CrossRef]
- Mustafa, N.S.; Sulaiman, N.N.; Ismail, M. Effect of SrFe12O19 nanopowder on the hydrogen sorption properties of MgH2. RSC Adv. 2016, 6, 110004–110010. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, T.; Qin, C.; Zhu, M.; Li, X. Improved hydrogen storage properties of Mg–V nanoparticles prepared by hydrogen plasma–metal reaction. J. Power Sources 2011, 196, 9599–9604. [Google Scholar] [CrossRef]
- Ismail, M. Influence of different amounts of FeCl3 on decomposition and hydrogen sorption kinetics of MgH2. Int. J. Hydrogen Energy 2014, 39, 2567–2574. [Google Scholar] [CrossRef]
- Polanski, M.; Bystrzycki, J. Comparative studies of the influence of different nano-sized metal oxides on the hydrogen sorption properties of magnesium hydride. J. Alloys Compd. 2009, 486, 697–701. [Google Scholar] [CrossRef]
- Börrnert, C.; Carrillo-Cabrera, W.; Simon, P.; Langbein, H.J. V2.38Nb10.7O32.7: A V2O5–Nb2O5 mixed oxide tunnel structure related to the tetragonal tungsten bronzes. Solid State Chem. 2010, 183, 1038–1045. [Google Scholar]
- Zaluska, A.; Zaluski, L.; Ström–Olsen, J.O. Nanocrystalline magnesium for hydrogen storage. J. Alloys Compd. 1999, 288, 217–225. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Shaban, E.; Al-Halaili, B. Nanocrystalline β-γ-β cyclic phase transformation in reacted ball milled MgH2 powders. Int. J. Hydrogen Energy 2014, 39, 12727–12740. [Google Scholar] [CrossRef]
- Amira, S.; Huot, J. Effect of cold rolling on hydrogen sorption properties of die-cast and as-cast magnesium alloys. J. Alloy. Compd. 2012, 520, 287–294. [Google Scholar] [CrossRef]
- Jorge, A.M.; de Lima, G.F.; Triques, M.R.M.; Botta, W.J.; Kiminami, C.S.; Nogueira, R.P.; Yavari, A.R.; Langdon, T.G. Correlation between hydrogen storage properties and textures induced in magnesium through ECAP and cold rolling. Int. J. Hydrogen Energy 2014, 39, 3810–3821. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Banyan, M.; Al-Ajmi, F. Discovering a New MgH2 Metastable Phase. RSC Adv. 2018, 8, 32003–32008. [Google Scholar] [CrossRef]
- Shen, C.; Aguey-Zinsou, K.F. Can γ-MgH2 improve the hydrogen storage properties of magnesium? J. Mater. Chem. A 2017, 5, 8644–8652. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, Z.; Yarahmadi, S.S.; Gregory, D.H. Facile Preparation of β-/γ- MgH2 Nanocomposites under Mild Conditions and Pathways to Rapid Dehydrogenation. Phys. Chem. Chem. Phys. 2016, 18, 10492–10498. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Wang, Y.; Xu, H.; Li, X. Hydrogen storage properties of magnesium ultrafine particles prepared by hydrogen plasma-metal reaction. Mater. Sci. Eng. B-Solid. 2004, 110, 221–226. [Google Scholar] [CrossRef]
- Xia, G.; Tan, Y.; Wu, F.; Fang, F.; Sun, D.; Guo, Z.; Huang, Z.; Yu, X. Graphene-wrapped reversible reaction for advanced hydrogen storage. Nano Energy 2016, 26, 488–495. [Google Scholar] [CrossRef]
- Xia, G.; Tan, Y.; Chen, X.; Sun, D.; Guo, Z.; Liu, H.; Ouyang, L.; Zhu, M.; Yu, X. Monodisperse Magnesium Hydride Nanoparticles Uniformly Self-Assembled on Graphene. Adv. Mater. 2015, 27, 5981–5988. [Google Scholar] [CrossRef]
- Xia, G.; Zhang, L.; Chen, X.; Huang, Y.; Sun, D.; Fang, F.; Guo, Z.; Yu, X. Carbon hollow nanobubbles on porous carbon nanofibers: An ideal host for high-performance sodium-sulfur batteries and hydrogen storage. Energy Storage Mater. 2018, 14, 314–323. [Google Scholar] [CrossRef]


| Zone | Content (wt%) | ||
|---|---|---|---|
| Mg | ZrNi5 | Nb2O5 | |
| 1 | 94.80 | 2.53 | 2.49 |
| 2 | 95.02 | 2.52 | 2.46 |
| 3 | 94.95 | 2.54 | 2.51 |
| 4 | 94.99 | 2.53 | 2.48 |
| 5 | 94.99 | 2.49 | 2.52 |
| 6 | 94.02 | 2.51 | 2.47 |
| 7 | 95.01 | 2.48 | 2.51 |
| 8 | 95.02 | 2.49 | 2.49 |
| 9 | 95.01 | 2.47 | 2.52 |
| 10 | 94.95 | 2.52 | 2.53 |
| 11 | 95.04 | 2.48 | 2.48 |
| 12 | 95.01 | 2.52 | 2.47 |
| 13 | 95.0 | 2.47 | 2.53 |
| 14 | 94.94 | 2.56 | 2.50 |
| Catalytic Agents | Ea (kJ/mol) | References |
|---|---|---|
| Present study Pure MgH2, 200 h of RBM | 113.67 | - |
| Present study 5 wt% (2.5 ZrNi5 + 2.5 Nb2O5) | 92.98 | - |
| VnbO5 | 99 | [17] |
| Nb2O5 | 102 | [18] |
| MnFe2O4 | 108 | [19] |
| Co2NiO | 118 | [20] |
| CeO2 | 109 | [21] |
| TiO2 | 111 | [22] |
| SrFe12O19 | 114 | [23] |
| V | 119 | [24] |
| FeCl3 | 130 | [25] |
| Cr2O3 | 86 | [26] |
| Material Type | Way of Preparations | H2 Storage Capacity (wt%) | Hydrogenation/Dehydrogenation Kinetics | Ref. |
|---|---|---|---|---|
| MgH2 of the present work | Reactive ball milling of pure Mg powders under 50 H2 bar, 200 h | 6.69 −6.52 | Absorption: 300 °C/10 bar/2000 s Desorption: 300 °C/0.2 bar/8000 s | |
| Commercial Mg powders | As-received | 0 1.4 | Absorption: 300 °C/10 bar/120 h 400 °C/10 bar/120 min | [28] |
| Nanocrystalline MgH2 | Ball milling under Ar gas for 20 h | 6.0 | Absorption: 300 °C/120 min | [28] |
| Ultrafine Mg nanoparticles | Hydrogen-plasms-metal reaction under a mixture of 70% Ar + 30% H2 at 25 V/300A. | 7.5 | Absorption: 300 °C/40 bar/30 min | [35] |
| Sample | Concentrations (wt%) | ||
|---|---|---|---|
| MgH2 | ZrNi5 | Nb2O5 | |
| 1 | 95 | 2.5 | 2.5 |
| 2 | 93 | 3.5 | 3.5 |
| 3 | 90 | 5 | 5 |
| 4 | 85 | 7.5 | 7.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Eskandarany, M.S.; Banyan, M.; Al-Ajmi, F. Synergistic Effect of New ZrNi5/Nb2O5 Catalytic Agent on Storage Behavior of Nanocrystalline MgH2 Powders. Catalysts 2019, 9, 306. https://doi.org/10.3390/catal9040306
El-Eskandarany MS, Banyan M, Al-Ajmi F. Synergistic Effect of New ZrNi5/Nb2O5 Catalytic Agent on Storage Behavior of Nanocrystalline MgH2 Powders. Catalysts. 2019; 9(4):306. https://doi.org/10.3390/catal9040306
Chicago/Turabian StyleEl-Eskandarany, M. Sherif, Mohammad Banyan, and Fahad Al-Ajmi. 2019. "Synergistic Effect of New ZrNi5/Nb2O5 Catalytic Agent on Storage Behavior of Nanocrystalline MgH2 Powders" Catalysts 9, no. 4: 306. https://doi.org/10.3390/catal9040306
APA StyleEl-Eskandarany, M. S., Banyan, M., & Al-Ajmi, F. (2019). Synergistic Effect of New ZrNi5/Nb2O5 Catalytic Agent on Storage Behavior of Nanocrystalline MgH2 Powders. Catalysts, 9(4), 306. https://doi.org/10.3390/catal9040306





