Bio-based Catalysts from Biomass Issued after Decontamination of Effluents Rich in Copper—An Innovative Approach towards Greener Copper-based Catalysis
Abstract
:1. Introduction
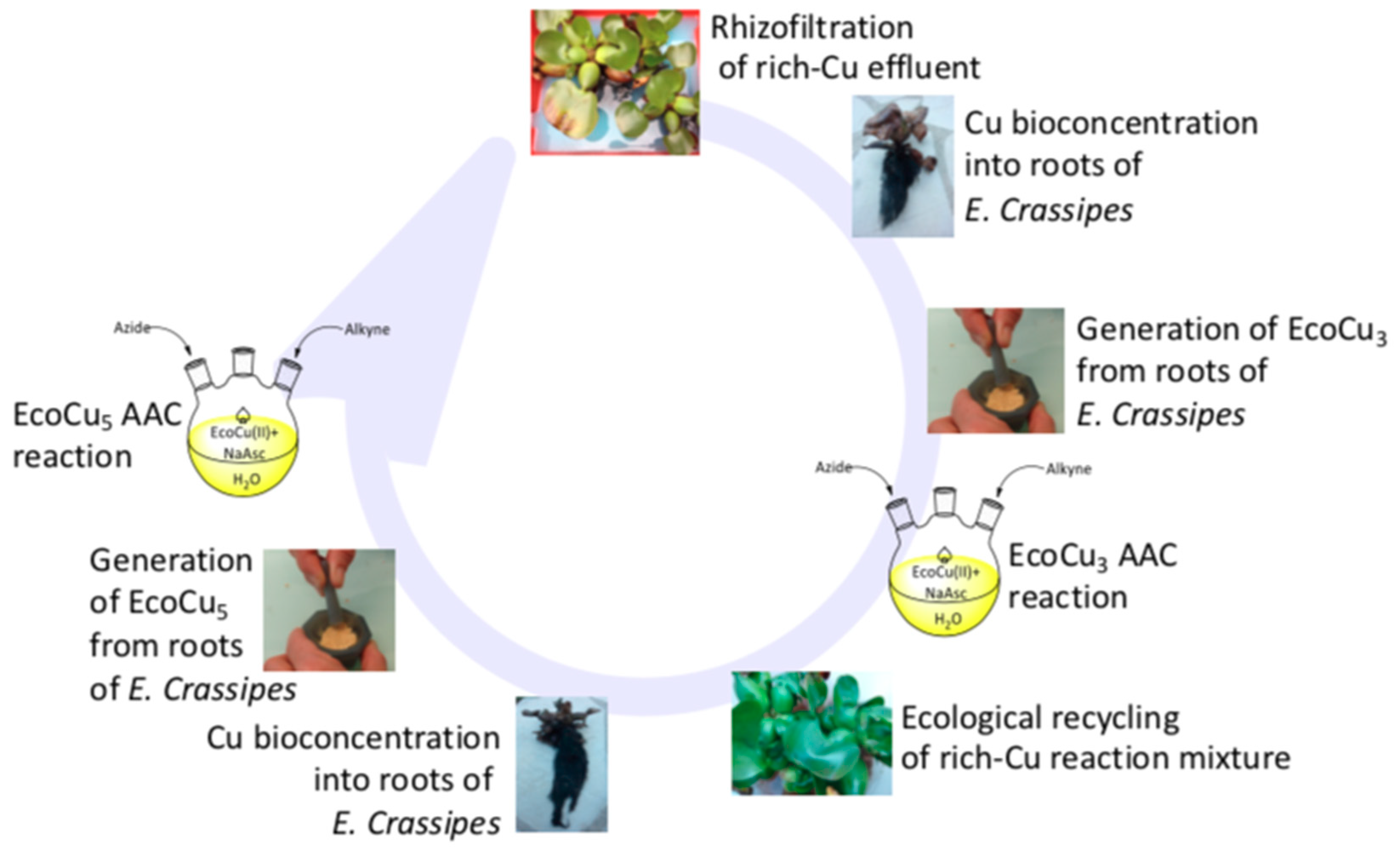
2. Phytoaccumulation of Copper
2.1. Preparation via Rhizofiltration
2.2. Preparation by Biosorption
3. Preparation and Characterisation of the Copper Ecocatalyst, Eco-Cu
3.1. Preparation via Rhizofiltration
3.2. Identification of the Degree of Oxidation
3.3. Direct-Injection Mass Spectrometric Analysis of Eco-Cu3
3.4. Morphology Study of Eco-Cu3 by BET Analyses
3.5. XRD Analysis of the Eco-Cu3 Catalyst
3.6. Analysis of the Acidic Properties of the Eco-Cu
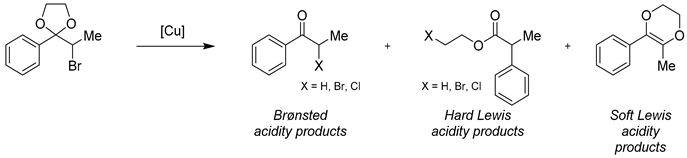
3.6.1. Lewis and Brønsted Acidic Character
3.6.2. Analysis of the Lewis and Brønsted Acid Properties by the Corma Method
4. Study of the Synthetic Potential of the Eco-Cu Catalysts
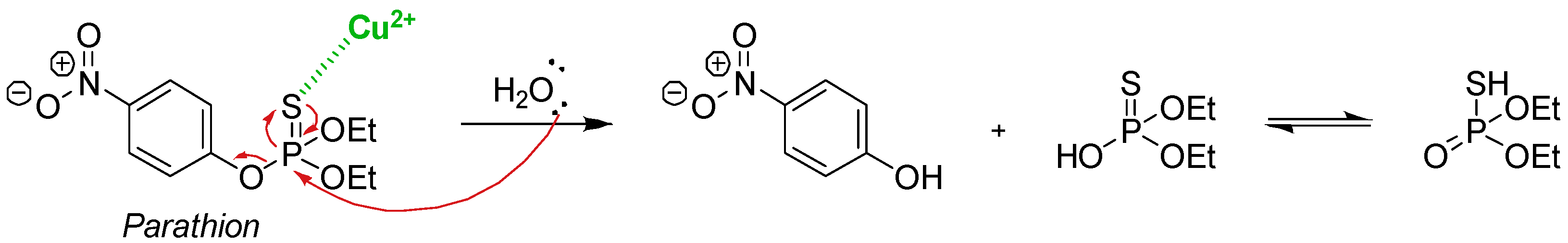
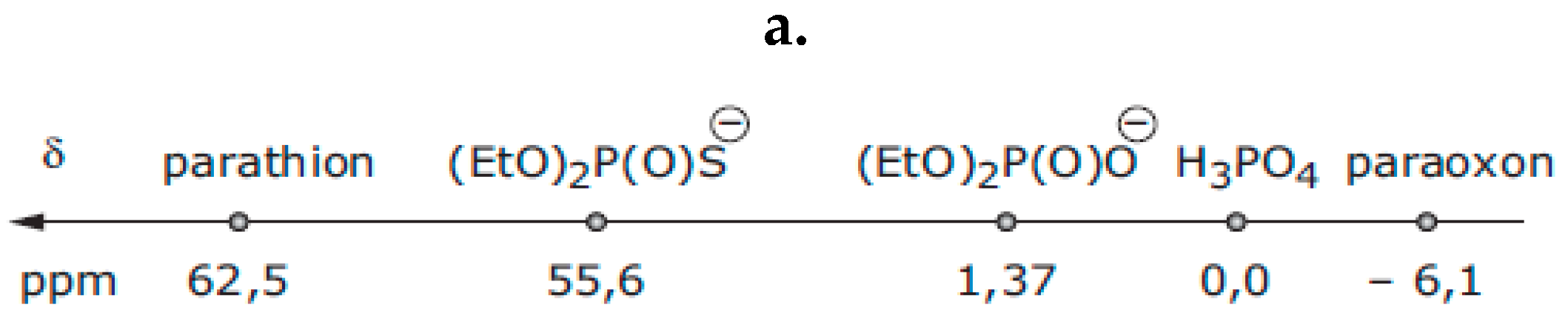
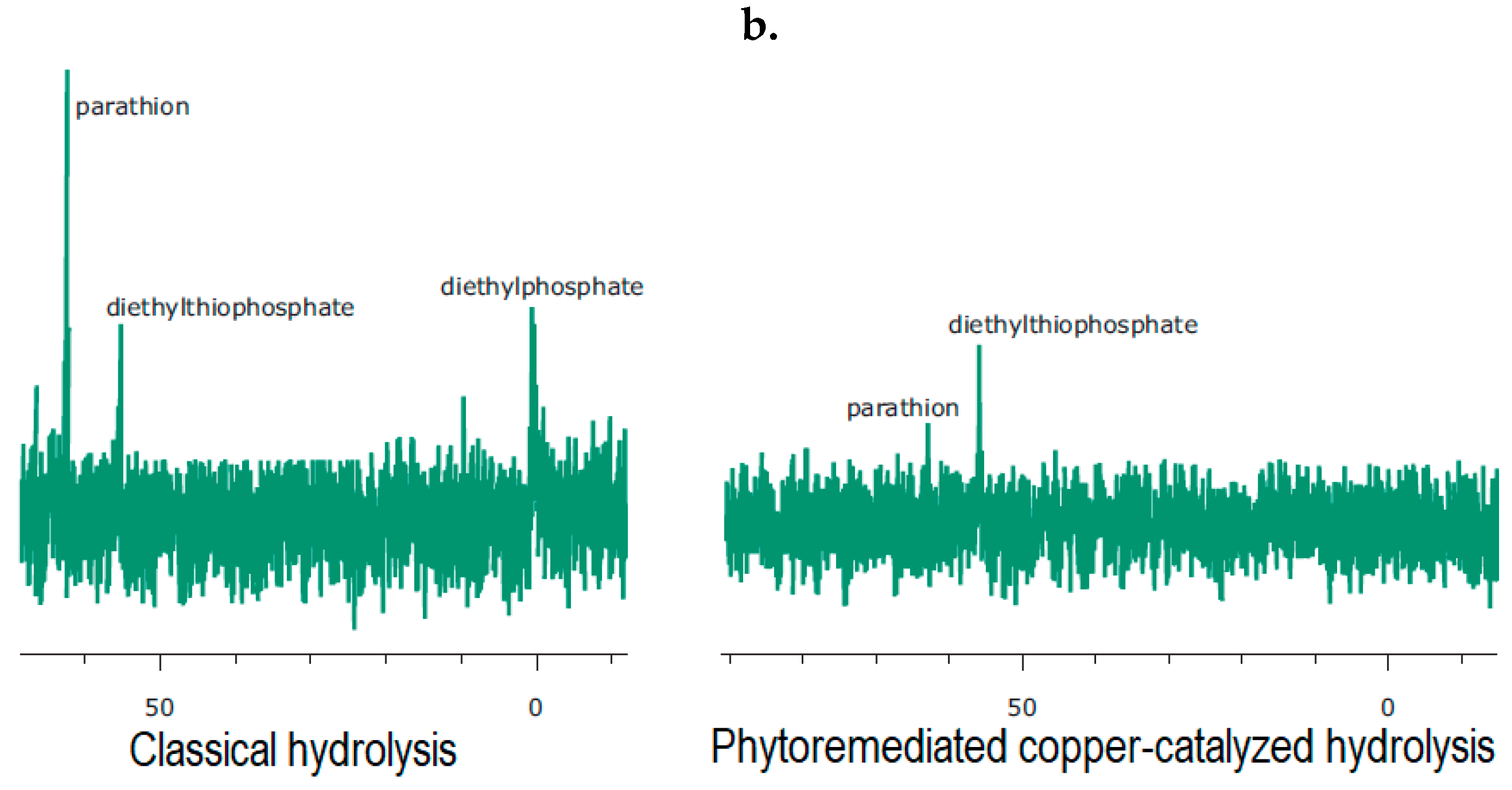
4.1. Cu-Catalysed Hydrolysis of Thiophosphates
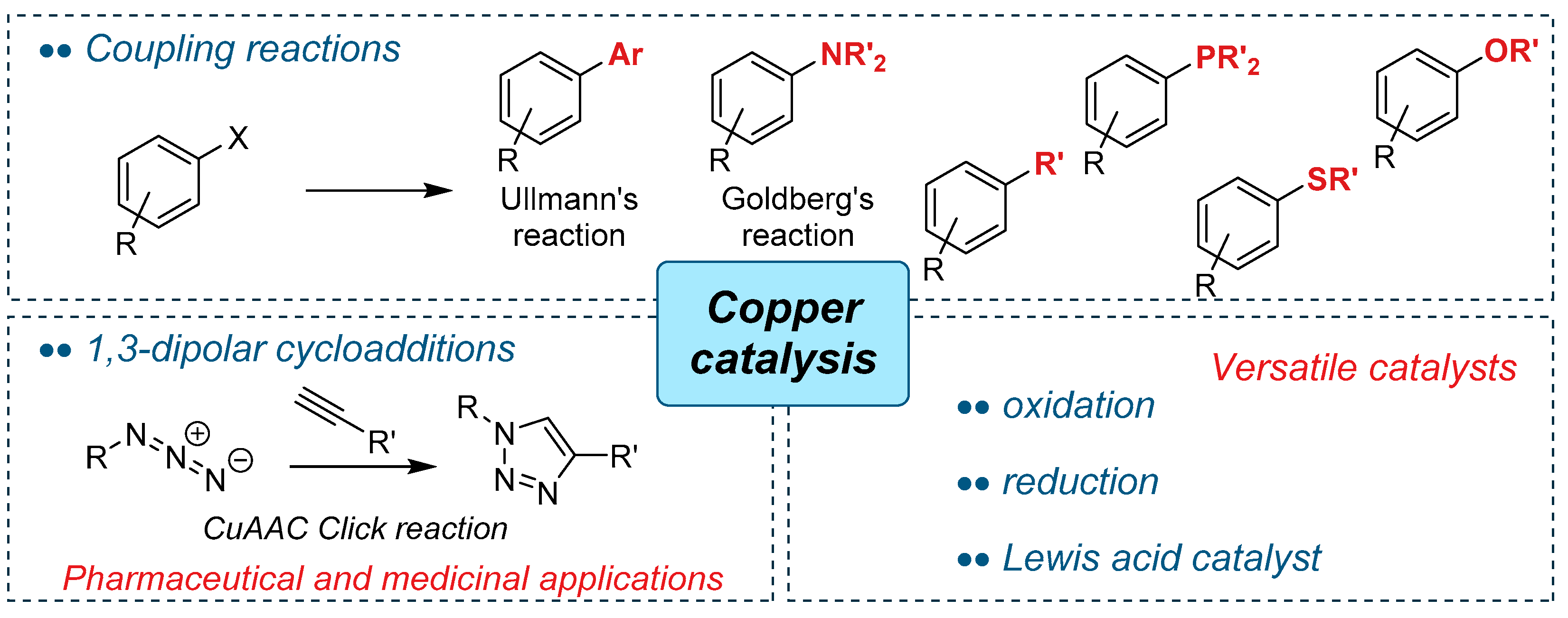
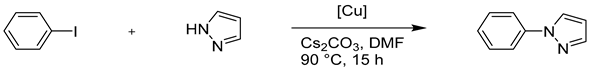
4.2. Copper-Catalysed Ullmann Coupling Reactions
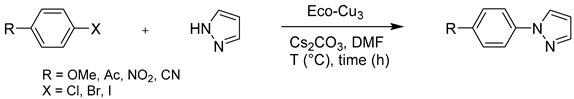
4.2.1. N-Arylation Ullmann-type Reaction
4.2.2. O-Arylation Ullmann-type Reaction
4.3. The Copper(I)-Catalysed Alkyne-Azide Cycloaddition (CuAAC) “Click” Reaction
4.3.1. Application of Eco-Cu in the CuAAC Reaction
4.3.2. Recycling and Reuse of the Ecocatalysts
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Brandsma, L.; Vasilevsky, S.F.; Verkruijsse, H.D. Application of transition metal catalysts in organic synthesis; Springer: Berlin, Germany; New York, NY, USA, 1999; ISBN 978-3-642-60328-0. [Google Scholar]
- Beller, M.; Bolm, C. (Eds.) Transition Metals for Organic Synthesis: Building Blocks and Fine Chemicals, 2nd rev. and enl. ed.; WILEY-VCH: Weinheim, Germany, 2004; ISBN 978-3-527-30613-8. [Google Scholar]
- Osborn, J.A.; Wilkinson, G.; Mrowca, J.J. Tris(triphenylphosphine)halorhodium(I). In Inorganic Syntheses; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; ISBN 978-0-470-13241-8. [Google Scholar]
- Perea-Buceta, J.E.; Fernández, I.; Heikkinen, S.; Axenov, K.; King, A.W.T.; Niemi, T.; Nieger, M.; Leskelä, M.; Repo, T. Diverting Hydrogenations with Wilkinson’s Catalyst towards Highly Reactive Rhodium(I) Species. Angew. Chem. Int. Ed. 2015, 54, 14321–14325. [Google Scholar] [CrossRef] [PubMed]
- Noyori, R.; Ohkuma, T.; Kitamura, M.; Takaya, H.; Sayo, N.; Kumobayashi, H.; Akutagawa, S. Asymmetric hydrogenation of .beta.-keto carboxylic esters. A practical, purely chemical access to .beta.-hydroxy esters in high enantiomeric purity. J. Am. Chem. Soc. 1987, 109, 5856–5858. [Google Scholar] [CrossRef]
- Zhou, Q.-L. Transition-Metal Catalysis and Organocatalysis: Where Can Progress Be Expected? Angew. Chem. Int. Ed. 2016, 55, 5352–5353. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, J.R.; Schindler, C.S. Catalyst: Sustainable Catalysis. Chem 2017, 2, 313–316. [Google Scholar] [CrossRef]
- Chemler, S.R. Copper catalysis in organic synthesis. Beilstein J. Org. Chem. 2015, 11, 2252–2253. [Google Scholar] [CrossRef] [PubMed]
- Evano, G.; Blanchard, N. (Eds.) Copper-Mediated Cross-Coupling Reactions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; ISBN 978-1-118-69065-9. [Google Scholar]
- Beletskaya, I.P.; Cheprakov, A.V. Copper in cross-coupling reactions: The post-Ullmann chemistry. Coord. Chem. Rev. 2004, 248, 2337–2364. [Google Scholar] [CrossRef]
- Ullmann, F.; Bielecki, J. Ueber Synthesen in der Biphenylreihe. Ber. Dtsch. Chem. Ges. 1901, 34, 2174–2185. [Google Scholar] [CrossRef]
- Ullmann, F. Ueber symmetrische Biphenylderivate. Justus Liebigs Ann. Chem. 1904, 332, 38–81. [Google Scholar] [CrossRef]
- Sambiagio, C.; Marsden, S.P.; Blacker, A.J.; McGowan, P.C. Copper catalysed Ullmann type chemistry: from mechanistic aspects to modern development. Chem. Soc. Rev. 2014, 43, 3525–3550. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S. Recent advancement of Ullmann-type coupling reactions in the formation of C–C bond. ChemTexts 2016, 2, 17. [Google Scholar] [CrossRef]
- Antilla, J.C.; Buchwald, S.L. Copper-Catalyzed Coupling of Arylboronic Acids and Amines. Org. Lett. 2001, 3, 2077–2079. [Google Scholar] [CrossRef] [PubMed]
- Kwong, F.Y.; Klapars, A.; Buchwald, S.L. Copper-Catalyzed Coupling of Alkylamines and Aryl Iodides: An Efficient System Even in an Air Atmosphere. Org. Lett. 2002, 4, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Kwong, F.Y.; Buchwald, S.L. Mild and Efficient Copper-Catalyzed Amination of Aryl Bromides with Primary Alkylamines. Org. Lett. 2003, 5, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Antilla, J.C.; Klapars, A.; Buchwald, S.L. The Copper-Catalyzed N-Arylation of Indoles. J. Am. Chem. Soc. 2002, 124, 11684–11688. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, I. Ueber Phenylirungen bei Gegenwart von Kupfer als Katalysator. Ber. Dtsch. Chem. Ges. 1906, 39, 1691–1692. [Google Scholar] [CrossRef]
- Strieter, E.R.; Bhayana, B.; Buchwald, S.L. Mechanistic Studies on the Copper-Catalyzed N-Arylation of Amides. J. Am. Chem. Soc. 2009, 131, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Jiao, N. Copper-Catalyzed C–H Azidation of Anilines under Mild Conditions. J. Am. Chem. Soc. 2012, 134, 18924–18927. [Google Scholar] [CrossRef] [PubMed]
- Marcoux, J.-F.; Doye, S.; Buchwald, S.L. A General Copper-Catalyzed Synthesis of Diaryl Ethers. J. Am. Chem. Soc. 1997, 119, 10539–10540. [Google Scholar] [CrossRef]
- Wan, Z.; Jones, C.D.; Koenig, T.M.; Pu, Y.J.; Mitchell, D. Vinyl aryl ethers from copper-catalyzed coupling of vinyl halides and phenols. Tetrahedron Lett. 2003, 44, 8257–8259. [Google Scholar] [CrossRef]
- Ma, D.; Cai, Q.; Xie, X. CuI/N,N-Dimethylglycine-Catalyzed Cross-Coupling Reaction of Vinyl Halides with Phenols and its Application to the Assembly of Substituted Benzofurans. Synlett 2005, 11, 1767–1770. [Google Scholar] [CrossRef]
- Wolter, M.; Nordmann, G.; Job, G.E.; Buchwald, S.L. Copper-Catalyzed Coupling of Aryl Iodides with Aliphatic Alcohols. Org. Lett. 2002, 4, 973–976. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Li, D.; Zhang, Z. Novel Synthesis of 2-Oxo-3-butynoates by Copper-Catalyzed Cross-Coupling Reaction of Terminal Alkynes and Monooxalyl Chloride. J. Org. Chem. 2003, 68, 10172–10174. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-Y.; Wang, Y.-J.; Cheng, J.-H.; Lee, C.-F. Copper-Catalyzed Coupling of Alkynes with Alkenyl Halides. Synlett 2012, 23, 930–934. [Google Scholar] [CrossRef]
- Liwosz, T.W.; Chemler, S.R. Copper-Catalyzed Oxidative Heck Reactions between Alkyltrifluoroborates and Vinyl Arenes. Org. Lett. 2013, 15, 3034–3037. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-K.; Yoon, S.-K.; Kim, Y.-M. Copper-Catalyzed Coupling Reaction of Terminal Alkynes with Aryl- and Alkenyliodonium Salts. Org. Lett. 2001, 3, 2697–2699. [Google Scholar] [CrossRef] [PubMed]
- Okuro, K.; Furuune, M.; Miura, M.; Nomura, M. Copper-catalyzed coupling reaction of aryl and vinyl halides with terminal alkynes. Tetrahedron Lett. 1992, 33, 5363–5364. [Google Scholar] [CrossRef]
- Okamoto, K.; Watanabe, M.; Sakata, N.; Murai, M.; Ohe, K. Copper-Catalyzed C–H Cyanation of Terminal Alkynes with Cyanogen Iodide. Org. Lett. 2013, 15, 5810–5813. [Google Scholar] [CrossRef] [PubMed]
- Uyeda, C.; Tan, Y.; Fu, G.C.; Peters, J.C. A New Family of Nucleophiles for Photoinduced, Copper-Catalyzed Cross-Couplings via Single-Electron Transfer: Reactions of Thiols with Aryl Halides Under Mild Conditions (O °C). J. Am. Chem. Soc. 2013, 135, 9548–9552. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-J.; Zhao, Y.-Q.; Feng, T.; Feng, Y.-S. Chan–Lam-Type S-Arylation of Thiols with Boronic Acids at Room Temperature. J. Org. Chem. 2012, 77, 2878–2884. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Ma, D. Synthesis of Aryl Sulfones via l-Proline-Promoted CuI-Catalyzed Coupling Reaction of Aryl Halides with Sulfinic Acid Salts. J. Org. Chem. 2005, 70, 2696–2700. [Google Scholar] [CrossRef] [PubMed]
- Gelman, D.; Jiang, L.; Buchwald, S.L. Copper-Catalyzed C−P Bond Construction via Direct Coupling of Secondary Phosphines and Phosphites with Aryl and Vinyl Halides. Org. Lett. 2003, 5, 2315–2318. [Google Scholar] [CrossRef] [PubMed]
- Huisgen, R. 1,3-Dipolar Cycloadditions. Past and Future. Angew. Chem. Int. Ed. 1963, 2, 565–598. [Google Scholar] [CrossRef]
- Stanley, L.M.; Sibi, M.P. Enantioselective Copper-Catalyzed 1,3-Dipolar Cycloadditions. Chem. Rev. 2008, 108, 2887–2902. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Sharpless, K.B. The growing impact of click chemistry on drug discovery. Drug Discov. Today 2003, 8, 1128–1137. [Google Scholar] [CrossRef]
- Lutz, J.-F.; Zarafshani, Z. Efficient construction of therapeutics, bioconjugates, biomaterials and bioactive surfaces using azide–alkyne “click” chemistry. Adv. Drug Deliv. Rev. 2008, 60, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Lipshutz, B.H.; Lower, A.; Noson, K. Copper(I) Hydride-Catalyzed Asymmetric Hydrosilylation of Heteroaromatic Ketones. Org. Lett. 2002, 4, 4045–4048. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Igarashi, T.; Yanagisawa, Y.; Kawauchi, N.; Hashimoto, H.; Yoshimura, J. Oxidative Cleavage of 4,6-O-Benzylidene Ring with t-Butyl Hydroperoxide and Copper(II) Chloride. Preparation of Methyl 4-O- and 6-O-Benzoylhexopyranoside Derivatives. Chem. Lett. 1988, 17, 1699–1702. [Google Scholar] [CrossRef]
- Curtis, E.J.C.; Jones, J.K.N. SOME OPEN-CHAIN DERIVATIVES OF GLUCOSE AND MANNOSE. Can. J. Chem. 1960, 38, 890–895. [Google Scholar] [CrossRef]
- Kocieński, P.J. Protecting Groups, 3rd ed.; Georg Thieme Verlag: Stuttgart, Germany; New York, NY, USA, 2005; ISBN 978-3-13-135603-1. [Google Scholar]
- Lipshutz, B.H.; Unger, J.B.; Taft, B.R. Copper-in-charcoal (Cu/C) promoted diaryl ether formation. Org. Lett. 2007, 9, 1089–1092. [Google Scholar] [CrossRef] [PubMed]
- Buckley, B.R.; Butterworth, R.; Dann, S.E.; Heaney, H.; Stubbs, E.C. Copper-in-Charcoal Revisited: Delineating the Nature of the Copper Species and Its Role in Catalysis. ACS Catal. 2015, 5, 793–796. [Google Scholar] [CrossRef]
- Jiao, J.; Zhang, X.-R.; Chang, N.-H.; Wang, J.; Wei, J.-F.; Shi, X.- Y.; Chen, Z.-G. A facile and practical copper powder-catalyzed, organic solvent-and ligand-free Ullmann amination of aryl halides. J. Org. Chem. 2011, 76, 1180–1183. [Google Scholar] [CrossRef] [PubMed]
- Shelke, S.N.; Bankar, S.R.; Mhaske, G.R.; Kadam, S.S.; Murade, D.K.; Bhorkade, S.B.; Rathi, A.K.; Bundaleski, N.; Teodoro, O.M.; Zboril, R. Iron oxide-supported copper oxide nanoparticles (Nanocat-Fe-CuO): magnetically recyclable catalysts for the synthesis of pyrazole derivatives, 4-methoxyaniline, and Ullmann-type condensation reactions. ACS Sustainable Chem. Eng. 2014, 2, 1699–1706. [Google Scholar] [CrossRef]
- Benyahya, S.; Monnier, F.; Taillefer, M.; Man, M.W.C.; Bied, C.; Ouazzani, F. Efficient and Versatile Sol-Gel Immobilized Copper Catalyst for Ullmann Arylation of Phenols. Adv. Synth. Catal. 2008, 350, 2205–2208. [Google Scholar] [CrossRef]
- Wang, M.; Yuan, B.; Ma, T.; Jiang, H.; Li, Y. Ligand-free coupling of phenols and alcohols with aryl halides by a recyclable heterogeneous copper catalyst. RSC Adv. 2012, 2, 5528–5530. [Google Scholar] [CrossRef]
- Ling, P.; Li, D.; Wang, X. Supported CuO/γ-Al2O3 as heterogeneous catalyst for synthesis of diaryl ether under ligand-free conditions. J. Mol. Catal. A: Chem. 2012, 357, 112–116. [Google Scholar] [CrossRef]
- Mullick, K.; Biswas, S.; Kim, C.; Ramprasad, R.; Angeles-Boza, A.M.; Suib, S.L. Ullmann Reaction Catalyzed by Heterogeneous Mesoporous Copper/Manganese Oxide: A Kinetic and Mechanistic Analysis. Inorg. Chem. 2017, 56, 10290–10297. [Google Scholar] [CrossRef] [PubMed]
- Udayakumar, V.; Gowsika, J.; Pandurangan, A.; Sabarathinam, S.; Lu, F. An efficient copper catalyzed 3D mesoporous aluminosilicate for the synthesis of dibenzodiazonines in the Ullmann cross-coupling reaction. New J. Chem. 2018, 42, 13065–13073. [Google Scholar]
- Benyahya, S.; Monnier, F.; Wong Chi Man, M.; Bied, C.; Ouazzan, F.; Taillefer, M. Sol–gel immobilized and reusable copper catalyst for arylation of phenols from aryl bromides. Green Chem. 2009, 11, 1121–1123. [Google Scholar]
- Bhadra, S.; Sreedhar, B.; Ranu, B.C. Recyclable Heterogeneous Supported Copper-Catalyzed Coupling of Thiols with Aryl Halides: Base-Controlled Differential Arylthiolation of Bromoiodobenzenes. Adv. Synth. Catal. 2009, 351, 2369–2378. [Google Scholar] [CrossRef]
- Bodhak, C.; Kundu, A.; Pramanik, A. An efficient and recyclable chitosan supported copper(II) heterogeneous catalyst for C–N cross coupling between aryl halides and aliphatic diamines. Tet. Lett. 2015, 56, 419–424. [Google Scholar] [CrossRef]
- Garnier, T.; Danel, M.; Magné, V.; Pujol, A.; Bénéteau, V.; Pale, P.; Chassaing, S. Copper(I)–USY as a Ligand-Free and Recyclable Catalyst for Ullmann-Type O-, N-, S-, and C-Arylation Reactions: Scope and Application to Total Synthesis. J. Org. Chem. 2018, 83, 6408–6422. [Google Scholar] [CrossRef] [PubMed]
- Evano, G.; Blanchard, N.; Toumi, M. Copper-Mediated Coupling Reactions and Their Applications in Natural Products and Designed Biomolecules Synthesis. Chem. Rev. 2008, 108, 3054–3131. [Google Scholar] [CrossRef] [PubMed]
- Evano, G.; Theunissen, C.; Pradal, A. Impact of copper-catalyzed cross-coupling reactions in natural product synthesis: the emergence of new retrosynthetic paradigms. Nat. Prod. Rep. 2013, 30, 1467. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Z. Development and Applications of the Copper-Catalyzed Azide-Alkyne Cycloaddition (CuAAC) as a Bioorthogonal Reaction. Molecules 2016, 21, 1393. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Fu, H. Copper-Catalyzed Cascade Synthesis of Alkyl 6-Aminobenzimidazo[2,1-a]isoquinoline-5-carboxylates. J. Org. Chem. 2011, 76, 4600–4605. [Google Scholar] [CrossRef] [PubMed]
- Murru, S.; Mondal, P.; Yella, R.; Patel, B.K. Copper(I)-Catalyzed Cascade Synthesis of 2-Substituted 1,3-Benzothiazoles: Direct Access to Benzothiazolones. Eur. J. Org. Chem. 2009, 31, 5406–5413. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Jamir, L.; Guin, S.; Patel, B.K. Copper(I)-Catalyzed Cascade Synthesis of 2-Arylsulfanyl- arylcyanamides. Adv. Synth. Catal. 2010, 352, 2538–2548. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, J.; Liu, M.; Ding, J.; Gao, W.; Huang, X.; Wu, H. Unexpected Copper-Catalyzed Cascade Synthesis of Quinazoline Derivatives. J. Org. Chem. 2013, 78, 11342–11348. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Fu, Y.; Fu, H.; Jiang, Y.; Zhao, Y. Highly efficient copper-catalyzed cascade synthesis of quinazoline and quinazolinone derivatives. Chem. Commun. 2008, 47, 6333–6335. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Yuan, Q.; Ma, D. Synthesis of 1,2-Disubstituted Benzimidazoles by a Cu-Catalyzed Cascade Aryl Amination/Condensation Process. Angew. Chem. Int. Ed. 2007, 46, 2598–2601. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Bao, W. Copper-Catalyzed Cascade Addition/Cyclization: An Efficient and Versatile Synthesis of N-Substituted 2-Heterobenzimidazoles. J. Org. Chem. 2009, 74, 5618–5621. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Fu, H.; Hu, L.; Jiang, Y.; Zhao, Y. Copper-Catalyzed Synthesis of Benzimidazoles via Cascade Reactions of o-Haloacetanilide Derivatives with Amidine Hydrochlorides. J. Org. Chem. 2008, 73, 7841–7844. [Google Scholar] [CrossRef] [PubMed]
- Losfeld, G.; Escande, V.; Mathieu, T.; Grison, C. Phytoextraction et biodégradation dynamisée: une approche interdisciplinaire inventive au service de l’environnement. Techniques de l’ingénieur Innovations en énergie et environnement 2011, IN 135, 1–8. [Google Scholar]
- Clavé, G.; Garel, C.; Poullain, C.; Renard, B.-L.; Olszewski, T.K.; Lange, B.; Shutcha, M.; Faucon, M.-P.; Grison, C. Ullmann reaction through ecocatalysis: insights from bioresource and synthetic potential. RSC Adv. 2016, 6, 59550–59564. [Google Scholar] [CrossRef]
- Clavé, G.; Garoux, L.; Boulanger, C.; Hesemann, P.; Grison, C. Ecological Recycling of a Bio-Based Catalyst for Cu Click Reaction: A New Strategy for a Greener Sustainable Catalysis. ChemistrySelect 2016, 1, 1410–1416. [Google Scholar] [CrossRef]
- Grison, C.; Carrasco, D.; Stanovych, A. Method for the Production of a Material of Plant Origin That Is Rich in Phenolic Acids, Comprising at Least One Metal, for Carrying Out Organic Synthesis Reactions. PCT Int. Appl. (2018) WO 2018178374 A1, 4 October 2018. [Google Scholar]
- Cerino-Córdova, F.J.; Díaz-Flores, P.E.; García-Reyes, R.B.; Soto-Regalado, E.; Gómez-González, R.; Garza-González, M.T.; Bustamante-Alcántara, E. Biosorption of Cu(II) and Pb(II) from aqueous solutions by chemically modified spent coffee grains. Int. J. Environ. Sci. Technol. 2013, 10, 611–622. [Google Scholar] [CrossRef]
- Poddar, S.N. Ortho-hydroxy acetophenone oxime as an analytical reagent. Part I. Z. Anal. Chem. 1957, 154, 254–259. [Google Scholar] [CrossRef]
- Mehlig, J. Colorimetric Determination of Copper with Ammonia. Ind. Eng. Chem. Anal. Ed. 1941, 13, 533–535. [Google Scholar] [CrossRef]
- Ndlela, S.C.; Shanks, B.H. Reduction Behavior of Potassium-Promoted Iron Oxide under Mixed Steam/Hydrogen Atmospheres. Ind. Eng. Chem. Res. 2006, 45, 7427–7434. [Google Scholar] [CrossRef]
- Parry, E.P. An infrared study of pyridine adsorbed on acidic solids. Characterization of surface acidity. J. Catal. 1963, 2, 371–379. [Google Scholar] [CrossRef]
- Zaki, M.I.; Hasan, M.A.; Al-Sagheer, F.A.; Pasupulety, L. In situ FTIR spectra of pyridine adsorbed on SiO2–Al2O3, TiO2, ZrO2 and CeO2: General considerations for the identification of acid sites on surfaces of finely divided metal oxides. Colloids Surf., A 2001, 190, 261–274. [Google Scholar] [CrossRef]
- Corma, A.; García, H.; Primo, A.; Domenech, A. A test reaction to assess the presence of Brönsted and the softness/hardness of Lewis acid sites in palladium supported catalysts. New J. Chem. 2004, 28, 361–365. [Google Scholar] [CrossRef]
- Chambers, H.W.; Meek, E.C.; Chambers, J.E. Chapter 64—Chemistry of Organophosphorus Insecticides. In Hayes’ Handbook of Pesticide Toxicology (Third Edition); Krieger, R., Ed.; Academic Press: New York, NY, USA, 2010; pp. 1395–1398. ISBN 978-0-12-374367-1. [Google Scholar]
- Wilson, B.W. Chapter 68—Cholinesterases. In Hayes’ Handbook of Pesticide Toxicology (Third Edition); Krieger, R., Ed.; Academic Press: New York, NY, USA, 2010; pp. 1457–1478. ISBN 978-0-12-374367-1. [Google Scholar]
- Wogram, J.; Sturm, A.; Segner, H.; Liess, M. Effects of parathion on acetylcholinesterase, butyrylcholinesterase, and carboxylesterase in three-spined stickleback (Gasterosteus aculeatus) following short-term exposure. Environ. Toxicol. Chem. 2001, 20, 1528–1531. [Google Scholar] [CrossRef]
- Jan, Y.-H.; Richardson, J.R.; Baker, A.A.; Mishin, V.; Heck, D.E.; Laskin, D.L.; Laskin, J.D. Novel approaches to mitigating parathion toxicity: targeting cytochrome P450-mediated metabolism with menadione: Redox cycling inhibits parathion metabolism. Ann. N. Y. Acad. Sci. 2016, 1378, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Breslow, R. On the Mechanism of Thiamine Action. IV.1 Evidence from Studies on Model Systems. J. Am. Chem. Soc. 1958, 80, 3719–3726. [Google Scholar] [CrossRef]
- Taillefer, M.; Xia, N.; Ouali, A. Efficient Iron/Copper Co-Catalyzed Arylation of Nitrogen Nucleophiles. Angew. Chem. Int. Ed. 2007, 46, 934–936. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.; Giacovazzi, R.; Ouali, A.; Taillefer, M.; Jutand, A. Activation of aryl halides by Cu0/1,10-phenanthroline: Cu0 as precursor of CuI catalyst in cross-coupling reactions. Chem. Commun. 2008, 45, 6051–6053. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Xing, L.; Wang, X.; Cheng, C.; Su, D.; Hu, Y. Highly Practical “Ligand-Free-Like” Copper-Catalyzed N-Arylation of Azoles in Lower Nitrile Solvents. Adv. Synth. Catal. 2008, 350, 1253–1257. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.; Zhang, B.; Zhang, M. Direct N-Arylation of Azaheterocycles with Aryl Halides under Ligand-free Condition. Chin. J. Chem. 2012, 30, 2389–2393. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.; Yang, L.; Zhang, M. N-Arylation of heterocycles promoted by tetraethylenepentamine in water. Tetrahedron 2013, 69, 6230–6233. [Google Scholar] [CrossRef]
- Kim, A.Y.; Lee, H.J.; Park, J.C.; Kang, H.; Yang, H.; Song, H.; Park, K.H. Highly Efficient and Reusable Copper-Catalyzed N-Arylation of Nitrogen-Containing Heterocycles with Aryl Halides. Molecules 2009, 14, 5169–5178. [Google Scholar] [CrossRef] [PubMed]
- Son, S.U.; Park, I.K.; Park, J.; Hyeon, T. Synthesis of Cu2O coated Cu nanoparticles and their successful applications to Ullmann-type amination coupling reactions of aryl chlorides. Chem. Commun. 2004, 7, 778–779. [Google Scholar] [CrossRef] [PubMed]
- Larsson, P.-F.; Correa, A.; Carril, M.; Norrby, P.-O.; Bolm, C. Copper-Catalyzed Cross-Couplings with Part-per-Million Catalyst Loadings. Angew. Chem. Int. Ed. 2009, 48, 5691–5693. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Song, J.; Liu, H.; Shi, J.; Ma, J.; Fan, H.; Wang, W.; Zhang, P.; Han, B. Acceleration of Suzuki coupling reactions by abundant and non-toxic salt particles. Green Chem. 2014, 16, 1198–1201. [Google Scholar] [CrossRef]
- Fan, G.; Zhang, H.; Cheng, S.; Ren, Z.; Hu, Z.; Wang, Z. Lewis Acid-Promoted Suzuki Reaction using Palladium Chloride Anchored on a Polymer as a Catalyst. Aust. J. Chem. 2008, 61, 610–614. [Google Scholar] [CrossRef]
- Sud, A.; Deshpande, R.M.; Chaudhari, R.V. Rate enhancement in palladium catalyzed Heck reactions by Lewis acid promoters. Catal. Commun. 2007, 8, 183–186. [Google Scholar] [CrossRef]
- Garel, C.; Renard, B.-L.; Escande, V.; Galtayries, A.; Hesemann, P.; Grison, C. C–C bond formation strategy through ecocatalysis: Insights from structural studies and synthetic potential. Appl. Catal. A-Gen. 2015, 504, 272–286. [Google Scholar] [CrossRef]
- Drapeau, M.P.; Ollevier, T.; Taillefer, M. On the Frontier Between Nucleophilic Aromatic Substitution and Catalysis. Chem. Eur. J. 2014, 20, 5231–5236. [Google Scholar]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Meldal, M.; Tornøe, C.W. Cu-Catalyzed Azide−Alkyne Cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef] [PubMed]
- Thirumurugan, P.; Matosiuk, D.; Jozwiak, K. Click Chemistry for Drug Development and Diverse Chemical–Biology Applications. Chem. Rev. 2013, 113, 4905–4979. [Google Scholar] [CrossRef] [PubMed]
- Agalave, S.G.; Maujan, S.R.; Pore, V.S. Click Chemistry: 1,2,3-Triazoles as Pharmacophores. Chem. Asian J. 2011, 6, 2696–2718. [Google Scholar] [CrossRef] [PubMed]
- McKay, C.S.; Finn, M.G. Click Chemistry in Complex Mixtures: Bioorthogonal Bioconjugation. Chem. Biol. 2014, 21, 1075–1101. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Astruc, D. The copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) “click” reaction and its applications. An overview. Coord. Chem. Rev. 2011, 255, 2933–2945. [Google Scholar] [CrossRef]
- Lallana, E.; Fernandez-Trillo, F.; Sousa-Herves, A.; Riguera, R.; Fernandez-Megia, E. Click Chemistry with Polymers, Dendrimers, and Hydrogels for Drug Delivery. Pharm. Res. 2012, 29, 902–921. [Google Scholar] [CrossRef] [PubMed]
- Aragão-Leoneti, V.; Campo, V.L.; Gomes, A.S.; Field, R.A.; Carvalho, I. Application of copper(I)-catalysed azide/alkyne cycloaddition (CuAAC) ‘click chemistry’ in carbohydrate drug and neoglycopolymer synthesis. Tetrahedron 2010, 66, 9475–9492. [Google Scholar] [CrossRef]
- Kele, P.; Li, X.; Link, M.; Nagy, K.; Herner, A.; Lőrincz, K.; Béni, S.; Wolfbeis, O.S. Clickable fluorophores for biological labeling—with or without copper. Org. Biomol. Chem. 2009, 7, 3486–3490. [Google Scholar] [CrossRef] [PubMed]
- Grammel, M.; Hang, H.C. Chemical reporters for biological discovery. Nat. Chem. Biol. 2013, 9, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Hein, J.E.; Fokin, V.V. Copper-catalyzed azide–alkyne cycloaddition (CuAAC) and beyond: new reactivity of copper(I) acetylides. Chem. Soc. Rev. 2010, 39, 1302–1315. [Google Scholar] [CrossRef] [PubMed]
- Luz, I.; Llabrés i Xamena, F.X.; Corma, A. Bridging homogeneous and heterogeneous catalysis with MOFs: “Click” reactions with Cu-MOF catalysts. J. Catal. 2010, 276, 134–140. [Google Scholar] [CrossRef]
- Taskin, O.S.; Dadashi-Silab, S.; Kiskan, B.; Weber, J.; Yagci, Y. Highly Efficient and Reusable Microporous Schiff Base Network Polymer as a Heterogeneous Catalyst for CuAAC Click Reaction. Macromol. Chem. Phys. 2015, 216, 1746–1753. [Google Scholar] [CrossRef]
- Díez-González, S.; Nolan, S.P. [(NHC)2Cu]X Complexes as Efficient Catalysts for Azide–Alkyne Click Chemistry at Low Catalyst Loadings. Angew. Chem. Int. Ed. 2008, 47, 8881–8884. [Google Scholar] [CrossRef] [PubMed]
- Vidal, C.; García-Álvarez, J. Glycerol: a biorenewable solvent for base-free Cu(I)-catalyzed 1,3-dipolar cycloaddition of azides with terminal and 1-iodoalkynes. Highly efficient transformations and catalyst recycling. Green Chem. 2014, 16, 3515–3521. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
| Plant | Cu Concentration in Effluent (mg/L) | Roots (wt. % ± SD) | BCF 4 (in Roots) |
|---|---|---|---|
| Bacopa monnieri1 | 10.5 | 1.34 ± 0.011 | 1279 |
| Lolium multiflorum2 | 10.6 | 0.71 ± 0.0036 | 666 |
| Eichhornia crassipes3 | 10.5 | 2.55 ± 0.027 | 2430 |
| Quantity of Coffee Grounds (g) | Initial Concentration of Cu (mg/L) | Final Concentration of Cu (mg/L) |
|---|---|---|
| 1 | 15 | 0 |
| 1 | 101 | 54 |
| 2.5 | 315 | 186 |
| Eco-Cu | Plant | Mineral Composition (%wt. ± %RSD) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Na | Mg | Al | K | Ca | Fe | Zn | Cu | ||
| Eco-Cu1 | Bacopa monierri | 5.29 | 1.86 | 1.93 | 3.48 | 4.91 | 1.21 | 0.04 | 4.75 |
| ±0.18 | ±0.53 | ±1.14 | ±0.29 | ±0.77 | ±0.34 | ±0.77 | ±0.45 | ||
| Eco-Cu2 | Lolium multiflorum | 0.50 | 0.36 | 0.00 | 0.72 | 22.18 | 0.02 | 0.05 | 2.02 |
| ±0.69 | ±1.48 | ±6.00 | ±0.71 | ±0.59 | ±1.86 | ±0.72 | ±1.05 | ||
| Eco-Cu3 | Eichhornia crassipes | 0.84 | 0.26 | 0.05 | 0.14 | 0.61 | 0.81 | 0.03 | 10.37 |
| ±0.57 | ±0.95 | ±1.82 | ±0.34 | ±6.97 | ±0.21 | ±5.86 | ±0.23 | ||
| Eco-Cu4 | Functionalized coffee grounds | 0.46 | 0.62 | 0.06 | 0.10 | 8.81 | 0.08 | 0.17 | 52.51 |
| ±0.74 | ±0.63 | ±5.30 | ±1.04 | ±0.08 | ±2.31 | ±5.81 | ±2.16 | ||
| Catalyst | Lewis Acidity (1445–1460 cm−1) | Lewis Acidity (1600–1640 cm−1) | Brønsted Acidity (1500–1540 cm−1) |
|---|---|---|---|
| CuCl2 | 1450 | 1606 | - |
| CuCl2·2H2O | 1449 | 1605, 1635 | - |
| Eco-Cu1 | 1449 | 1602, 1633 | 1529 |
| Eco-Cu2 | 1447 | 1607 | - |
| Eco-Cu3 | 1449 | 1606, 1645 | 1530 |
| Catalysts | Conversion Rate (%) 1 | Brønsted Acidity Products (%) 1 | Hard Lewis Acidity Products (%) 1 | Soft Lewis Acidity Products (%) 1 |
|---|---|---|---|---|
| Anhydrous CuCl2 | 49 | 52 | 41 | 7 |
| CuCl2.2H2O | 65 | 39 | 58 | 3 |
| Eco-Cu3 | 100 | 64 | 36 | 0 |
| Entry 1 | [Cu] Catalyst | wt. % of Eco-Cu | [Cu] Quantity (mol. %) | Yields 2 |
|---|---|---|---|---|
| 1 | Eco-Cu1 | 4.8 | 1 | 84 |
| 2 | Eco-Cu2 | 2.0 | 1 | 77 |
| 3 | Eco-Cu3 | 10 | 1 | 85 |
| 4 | Eco-Cu3 | 10 | 0.25 | 57 |
| 5 | CuCl2 | - | 1 | 48 3 |
| 6 | CuCl2 | - | 3 | 79 3 |

| Entry | Nitrogen Nucleophile | Yields (%) 1,2 |
|---|---|---|
| 1 | Pyrazole | 85 |
| 2 | Imidazole | 54 |
| 3 | 2-Pyrrolidinone | 31 3 |
| 4 | Aniline | 0 3 |
| 5 | Pyrrolidine | 6 3 |
| 6 | Morpholine | 5 3 |
| Entry | Aryl Halide | T (°C) | Time (h) | Yield (%) 1,2 |
|---|---|---|---|---|
| 1 | R = H; X = I | 90 | 15 | 85 |
| 2 | R = OMe; X = I | 90 | 15 | 63 |
| 3 | R = OMe; X = I | 110 | 15 | 93 |
| 4 | R = COMe; X = I | 90 | 4 | >98 |
| 5 | R = COMe; X = Br | 90 | 15 | 31 |
| 6 | R = NO2; X = Br | 90 | 4 | >98 |
| 7 | R = NO2; X = Cl | 90 | 4 | >98 |
| 8 | R = CN; X = Cl | 90 | 15 | 73 |
| 9 | R = CN; X = Cl | 110 | 15 | 89 |
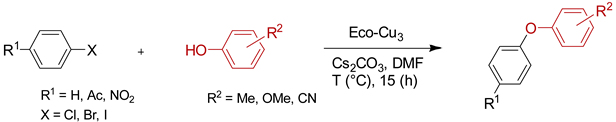
| Entry | Aryl Halide | T (°C) | Product | Yield (%) 1,2 |
|---|---|---|---|---|
| 1 | R 1 = H; X = I | 110 | 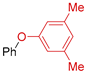 | 66 3 |
| 2 | R 1 = H; X = I | 110 | 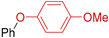 | 64 3 |
| 3 | R 1 = RCOMe; X = I | 110 |  | >98 |
| 4 | R 1 = H; X = I | 130 | 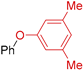 | >98 |
| 5 | R 1 = H; X = I | 130 |  | >98 |
| 6 | R 1 = H; X = Br | 130 | 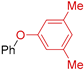 | 84 |
| 7 | R 1 = H; X = Br | 130 | 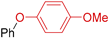 | 82 |
| 8 | R 1 = H; X = Br | 130 | 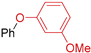 | 92 |
| 9 | R 1 = H; X = Br | 130 | 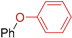 | 51 3 |
| 10 | R 1 = NO2; X = Br | 110 | 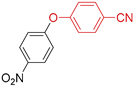 | >98 |
| 11 | R 1 = NO2; X = Cl | 110 | 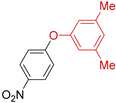 | >98 |
| 12 | R 1 = NO2; X = Cl | 110 | 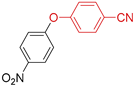 | >98 |

| Entry | Solvent | Conversion (%) 1,2 |
|---|---|---|
| 1 | H2O | >99 |
| 2 | EtOH | >99 |
| 3 | i-PrOH | >99 |
| 4 | 2-Me-THF | >99 |
| 5 | DMF | >99 |
| 6 | H2O 3 | 0 |
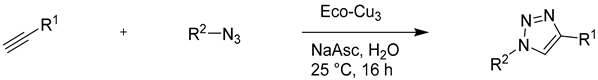
| Entry | Azide | Alkyne | Yield (%) 1,2 (with Eco-Cu3) | Yield (%) (other [Cu]) |
|---|---|---|---|---|
| 1 | 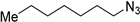 |  | >99 (92)3 | >99 [110] |
| 2 | 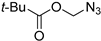 | 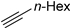 | >99 | - |
| 3 |  |  | >99 (78)3 | >99 [110] |
| 4 |  | 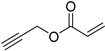 | 98 | 98 [109] |
| 5 |  |  | >99 | 61 [109] |
| 6 | 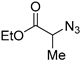 |  | >99 | 85 [109] |
| 7 | 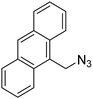 |  | >99 | 93 [109] |
| 8 |  |  | >99 | 79 [110] |
| 9 |  | 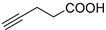 | >99 | >99 [111] |
| 10 |  | 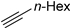 | >99 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olszewski, T.K.; Adler, P.; Grison, C. Bio-based Catalysts from Biomass Issued after Decontamination of Effluents Rich in Copper—An Innovative Approach towards Greener Copper-based Catalysis. Catalysts 2019, 9, 214. https://doi.org/10.3390/catal9030214
Olszewski TK, Adler P, Grison C. Bio-based Catalysts from Biomass Issued after Decontamination of Effluents Rich in Copper—An Innovative Approach towards Greener Copper-based Catalysis. Catalysts. 2019; 9(3):214. https://doi.org/10.3390/catal9030214
Chicago/Turabian StyleOlszewski, Tomasz K., Pauline Adler, and Claude Grison. 2019. "Bio-based Catalysts from Biomass Issued after Decontamination of Effluents Rich in Copper—An Innovative Approach towards Greener Copper-based Catalysis" Catalysts 9, no. 3: 214. https://doi.org/10.3390/catal9030214
APA StyleOlszewski, T. K., Adler, P., & Grison, C. (2019). Bio-based Catalysts from Biomass Issued after Decontamination of Effluents Rich in Copper—An Innovative Approach towards Greener Copper-based Catalysis. Catalysts, 9(3), 214. https://doi.org/10.3390/catal9030214






