Photocatalytic Degradation of Different VOCs in the Gas-Phase over TiO2 Thin Films Prepared by Ultrasonic Spray Pyrolysis
Abstract
1. Introduction
2. Results
2.1. Material Characterization
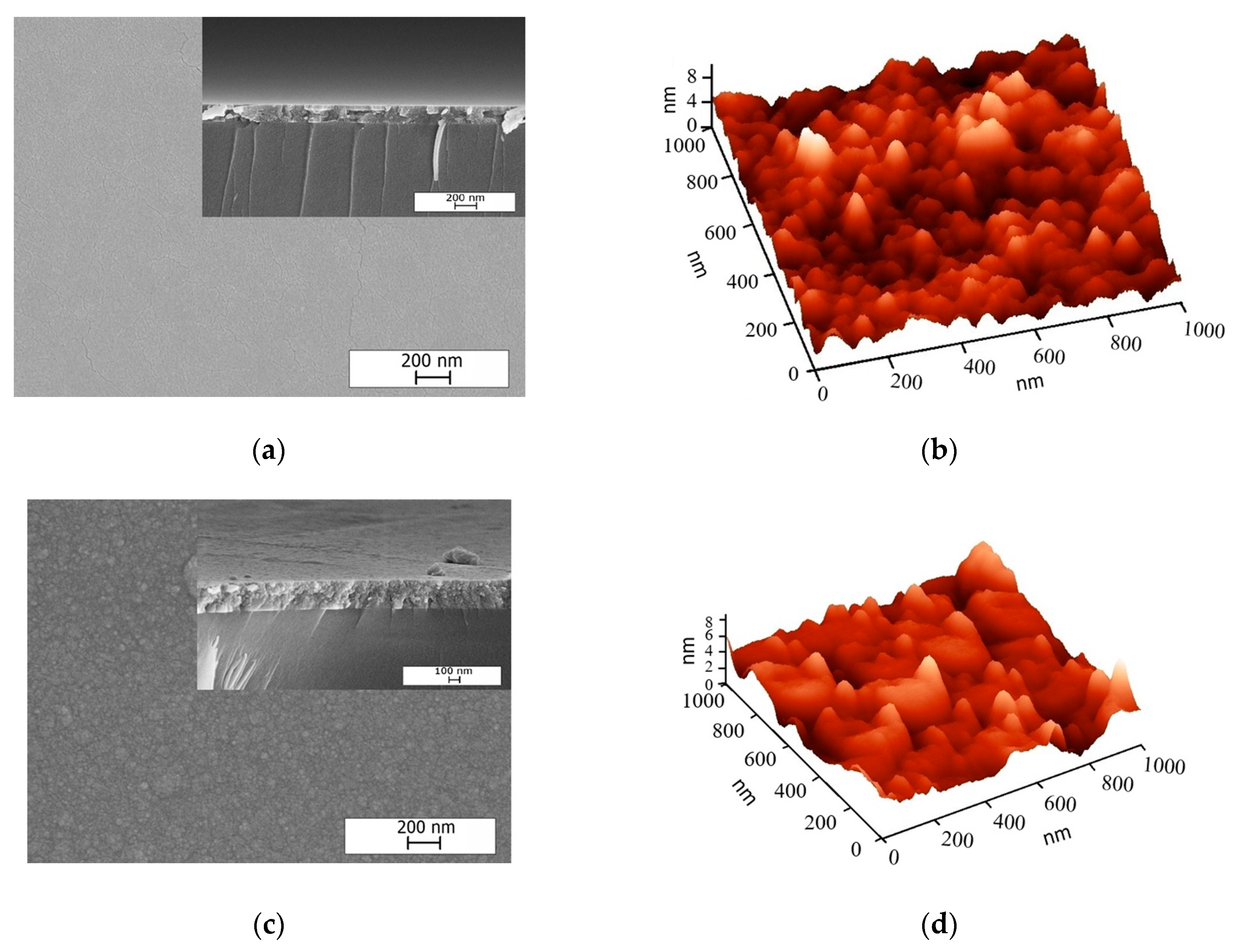
2.1.1. Surface Morphology
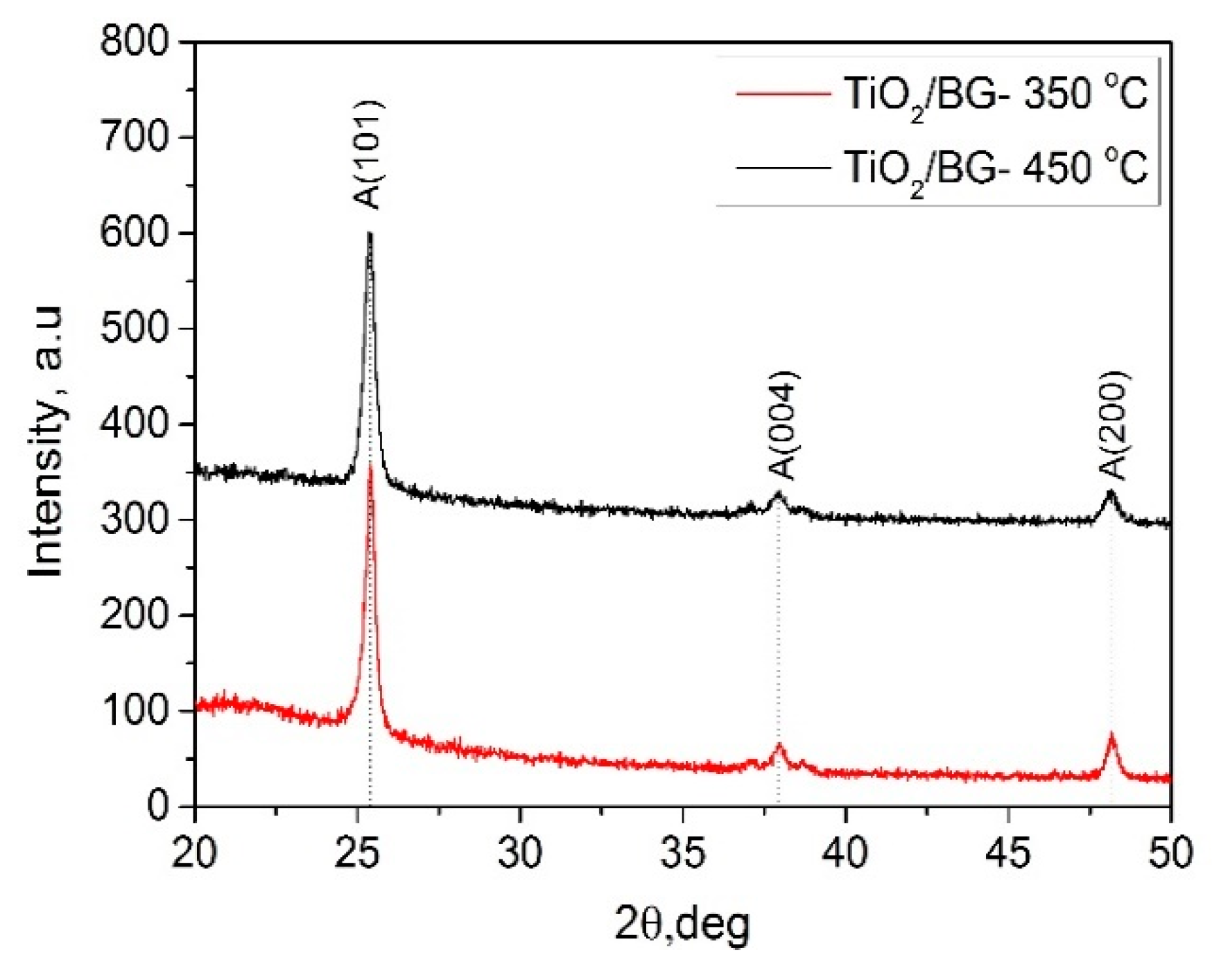
2.1.2. Structural Properties
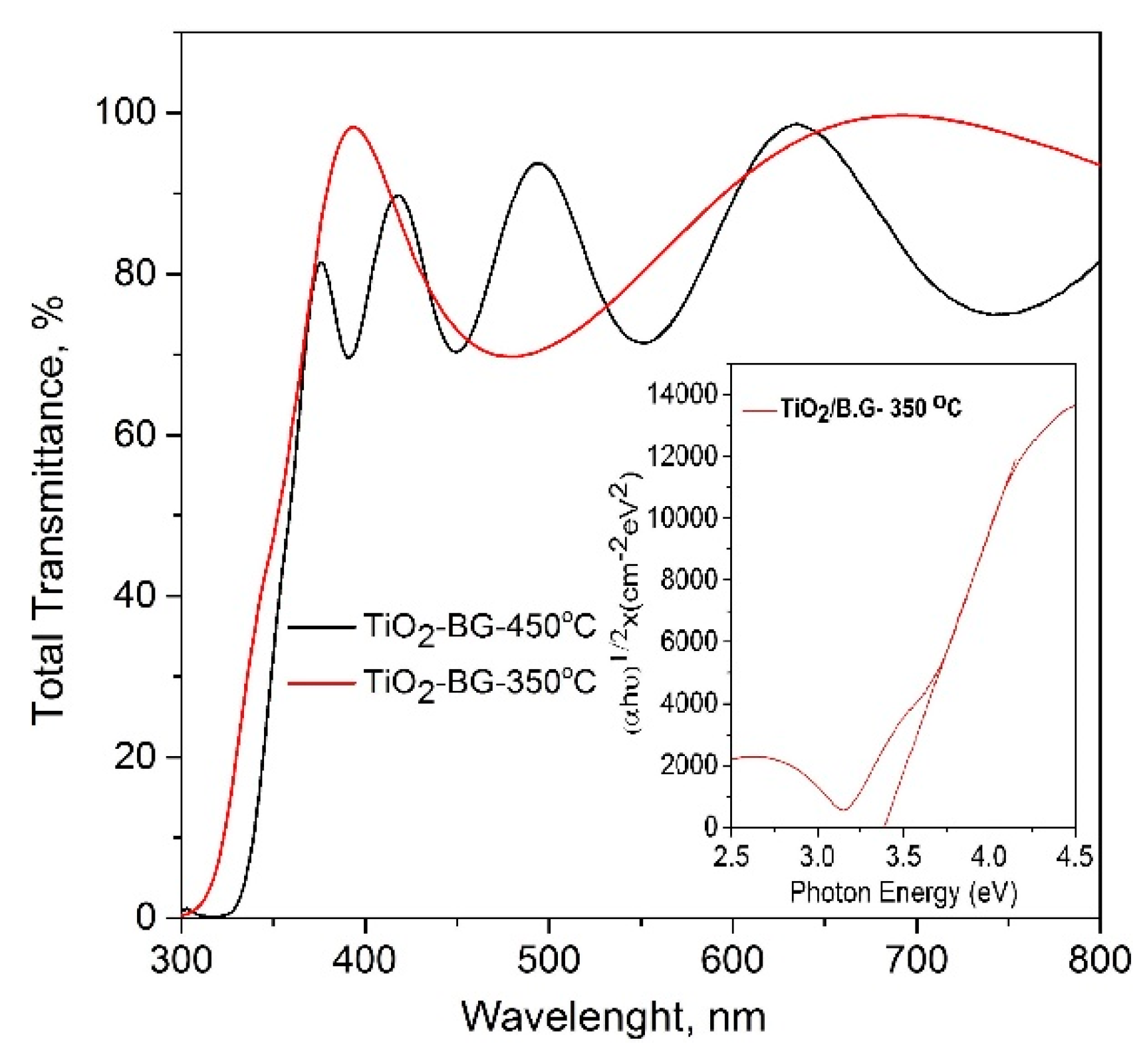
2.1.3. Optical Properties
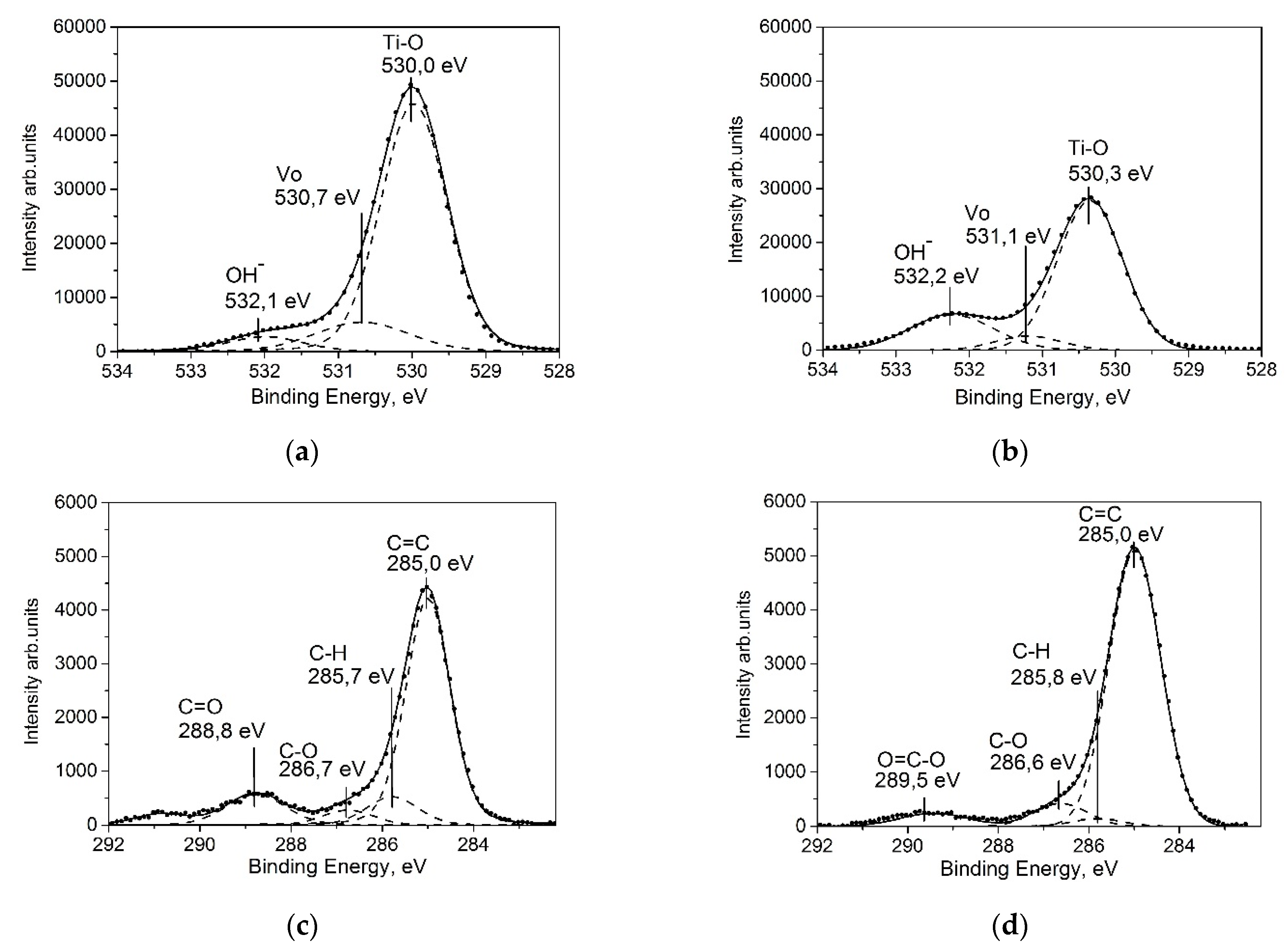
2.1.4. XPS Study
XPS Data Analysis of Aged-TiO2 Thin Films
XPS Data Analysis of Aged-TiO2 Thin Films after UV-Treatment
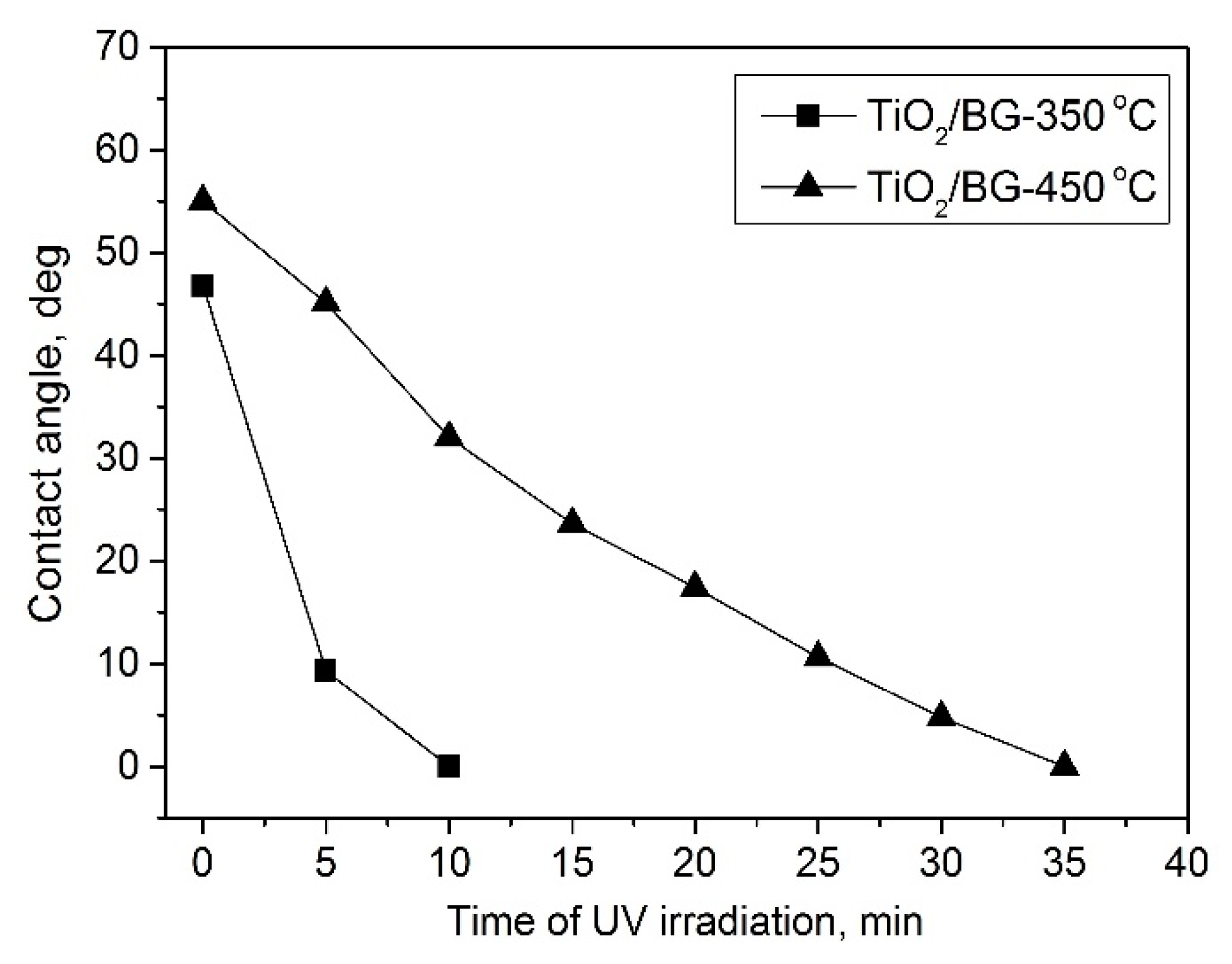
2.2. Wettability
2.3. Photocatalytic Activity
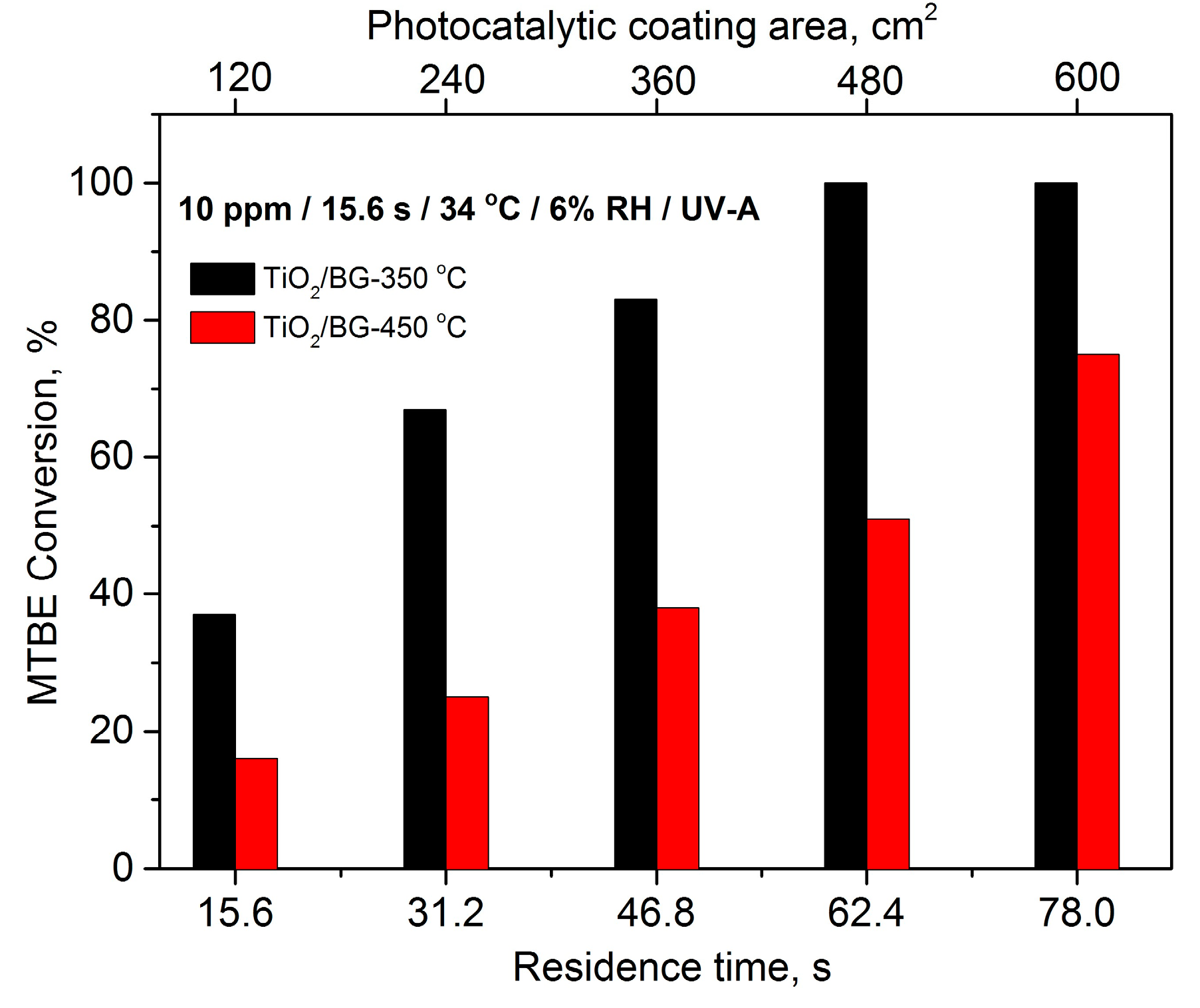
2.3.1. The Effect of Deposition Temperature on Photocatalytic Activity of TiO2 Thin Films
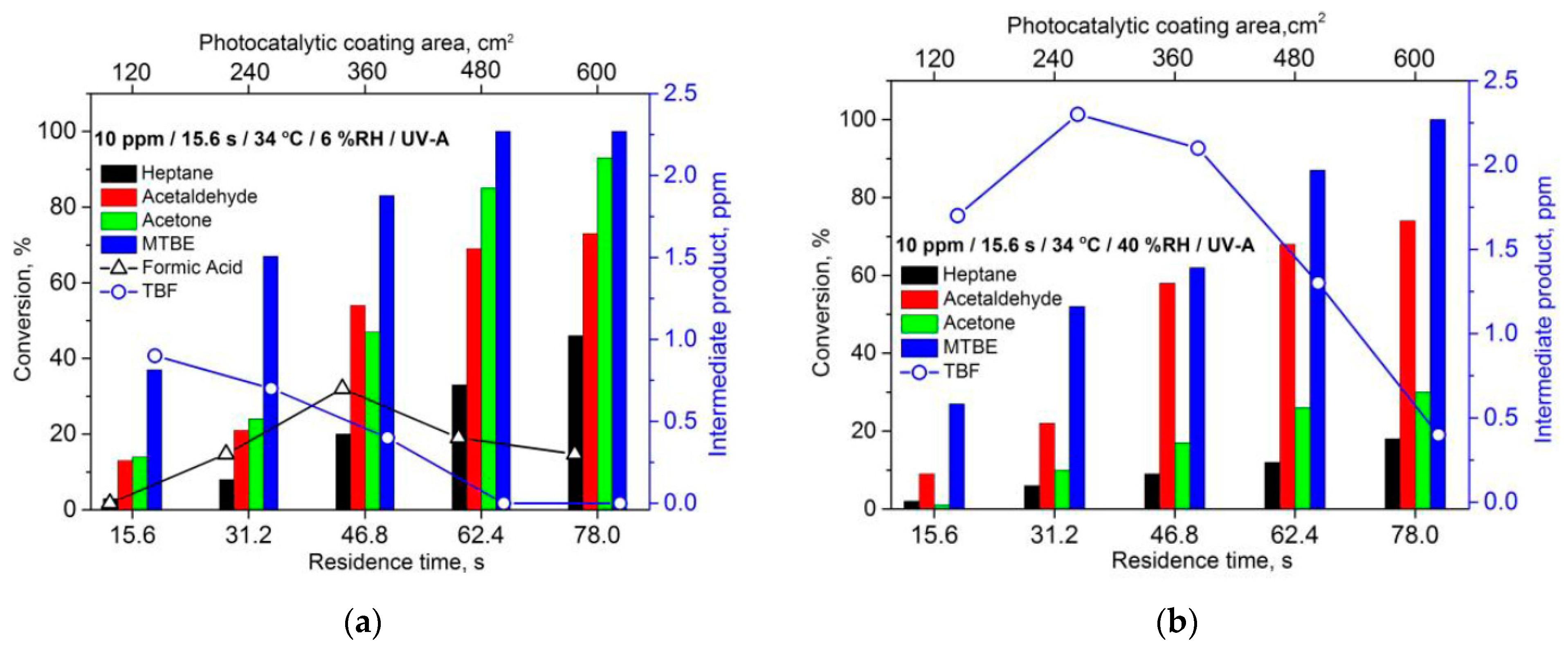
2.3.2. The Effect of Air Humidity on Photocatalytic Activity of TiO2 Thin Films
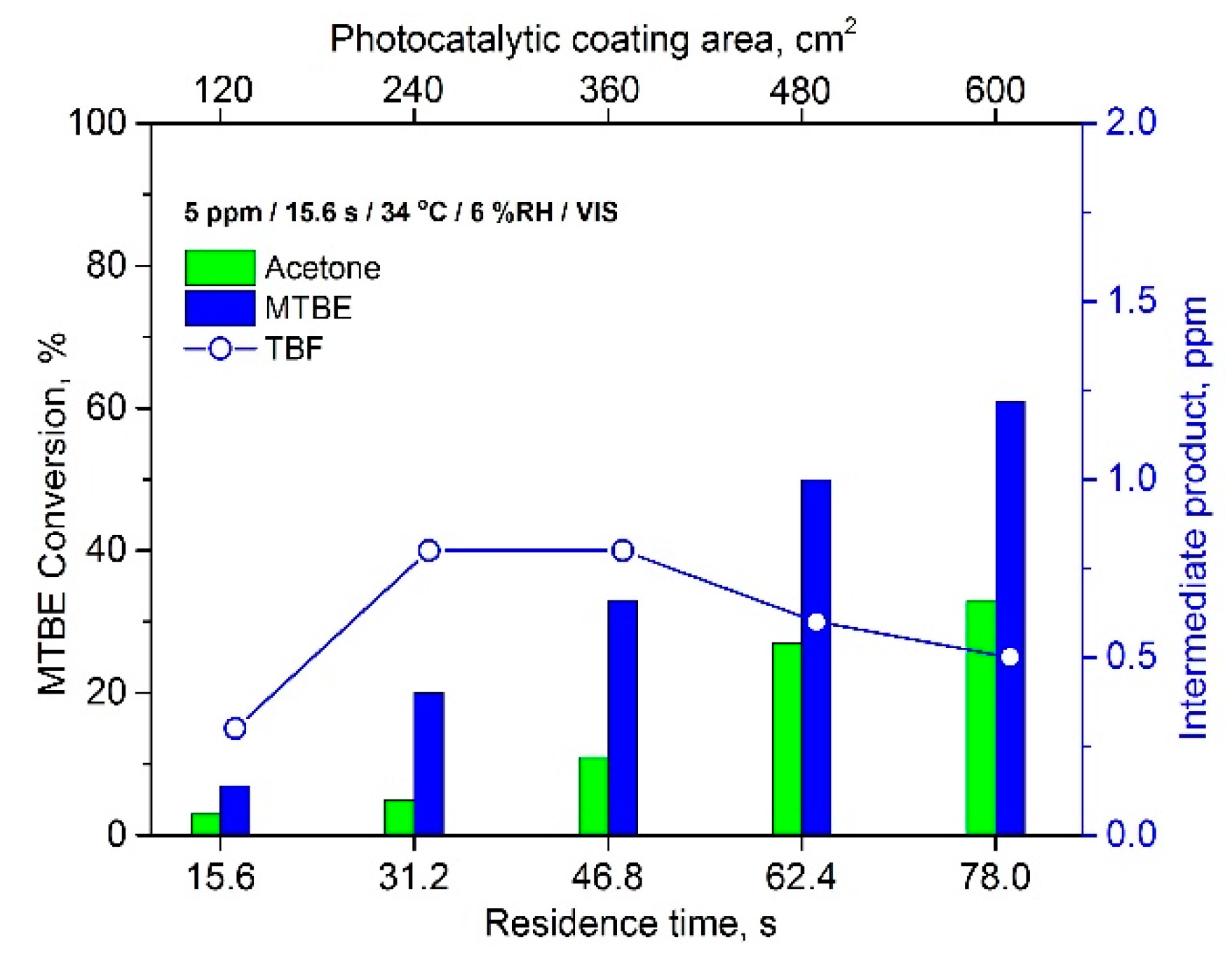
2.3.3. The Effect of Residence Time and Inlet Concentration of VOCs on Photocatalytic Activity of TiO2 Thin Films
2.3.4. The Effect of Irradiation Source on Photocatalytic Activity of TiO2 Thin Films
3. Materials and Methods
3.1. Thin Film Synthesis and Materials Characterization
3.2. Photocatalytic Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wallace, L.A. The Total Exposure Assessment Methodology (TEAM) Study Summary and Analysis; U.S. Environmental Protection Agency: Washington, DC, USA, 1987; pp. 59–73.
- Schmidt, M.C.; Buchbinder, A.M.; Weitz, E.; Geiger, M.F. Photochemistry of the indoor air pollutant acetone on Degussa P25 TiO2 studied by chemical ionization mass spectrometry. J. Phys. Chem. A 2007, 111, 13023–13031. [Google Scholar] [CrossRef]
- Chang, C.P.; Chen, J.N.; Lu, M.C. Heterogeneous photocatalytic oxidation of acetone for air purification by near UV-irradiated titanium dioxide. J. Environ. Sci. Health A 2003, 38, 1131–1143. [Google Scholar] [CrossRef]
- FarLend, W.H.; United States Environmental Protection Agency. Health and Environmental Effects Document for N-Heptane: Final Draft; EPA: Washington, DC, USA, 1989; p. 5.
- Dundar, I.; Krichevskaya, M.; Katerski, A.; Oja Acik, I. TiO2 thin films by ultrasonic spray pyrolysis as photocatalytic material for air purification. R. Soc. Open Sci. 2019, 6, 181578. [Google Scholar] [CrossRef]
- Schiewecka, A.; Uhdea, E.; Salthammera, T.; Salthammer, L.C.; Morawska, L.; Mazaheric, M.; Kumar, P. Smart homes and the control of indoor air quality. Renew. Sustain. Energy Rev. 2018, 94, 705–718. [Google Scholar] [CrossRef]
- Degallaix, M.C. Air cleaning devices: A growing market polluted by confusing communication-certified performances for air cleaners. J. Rehva 2017, 1, 49–51. [Google Scholar]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.S. Photocatalytic oxidation technology for indoor environment air purification: The state-of-the-art. Appl. Catal. B Environ. 2017, 203, 247–269. [Google Scholar] [CrossRef]
- Alonso-Telleza, A.; Massona, R.; Robert, D.; Kellera, N.; Keller, V. Comparison of Hombikat UV100 and P25 TiO2 performance in gas-phase photocatalytic oxidation reactions. J. Photochem. Photobiol. A Chem. 2012, 250, 58–65. [Google Scholar] [CrossRef]
- Bianchia, C.L.; Gatto, S.; Pirola, C.; Naldoni, A.; Michele Di, A.; Cerrato, G.; Crocellàd, V.; Capucci, V. Photocatalytic degradation of acetone, acetaldehyde and toluene in gas-phase: Comparison between nano and micro-sized TiO2. Appl. Catal. B Environ. 2014, 146, 123–130. [Google Scholar] [CrossRef]
- Šuligoja, A.; Urška Lavrenčič Štangara, U.L.; Ristić, A.; Mazaj, M.; Verhovšekc, D.; Tušar, N.N. TiO2–SiO2 films from organic-free colloidal TiO2 anatase nanoparticles as photocatalyst for removal of volatile organic compounds from indoor air. Appl. Catal. B Environ. 2016, 184, 119–131. [Google Scholar] [CrossRef]
- Shayegan, Z.; Lee, C.S.; Haghighat, F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase—A review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef]
- Verbruggen, S.W.; Deng, S.; Kurttepeli, M.; Cott, D.J.; Vereecken, P.M.; Bals, S.; Detavernier, C.; Lenaerts, S. Photocatalytic acetaldehyde oxidation in air using spacious TiO2 films prepared by atomic layer deposition on supported carbonaceous sacrificial templates. Appl. Catal. B Environ. 2014, 160–161, 204–210. [Google Scholar] [CrossRef]
- Arabatzis, I.M.; Antonaraki, S.; Stergiopoulos, T.; Hiskia, A.; Papaconstantinou, E.; Bernard, M.C.; Falaras, P. Preparation, characterization and photocatalytic activity of nanocrystalline thin film TiO2 catalysts towards 3,5-dichlorophenol degradation. J. Photochem. Photobiol. A 2012, 149, 237–245. [Google Scholar] [CrossRef]
- Buchalska, M.; Surowka, M.; Hamalainen, J.; Livonen, T.; Leskela, M.; Macyk, W. Photocatalytic activity of TiO2 films on Si support prepared by atomic layer deposition. Catal. Today 2015, 252, 14–19. [Google Scholar] [CrossRef]
- Stefanov, B.I.; Niklasson, G.A.; Granqvist, C.G.; Österlund, L. Gas-phase photocatalytic activity of sputter-deposited anatase TiO2 films: Effect of 001 preferential orientation, surface temperature and humidity. J. Catal. 2016, 335, 187–196. [Google Scholar] [CrossRef]
- Tryba, B.; Jafari, S.; Sillanpää, M.; Nitta, A.; Ohtanic, B.; Morawski, A.W. Influence of TiO2 structure on its photocatalytic activity towards acetaldehyde decomposition. Appl. Surf. Sci. 2019, 470, 376–385. [Google Scholar] [CrossRef]
- Seo, H.O.; Woo, T.G.; Park, E.J.; Cha, B.J.; Kim, I.H.; Wook, H.S.; Kim, Y.D. Enhanced photocatalytic activity of TiO2 films by removal of surface carbon impurities; the role of water vapor. Appl. Surf. Sci. 2017, 420, 808–816. [Google Scholar] [CrossRef]
- Blount, C.M.; Kim, D.H.; Falconer, J.L. Transparent thin-film TiO2 photocatalysts with high activity. Environ. Sci. Technol. 2001, 35, 2988–2994. [Google Scholar] [CrossRef]
- Negishi, N.; Matsuzawa, S.; Takeuchi, K.; Pichat, P. Transparent Micrometer-Thick TiO2 films on SiO2-coated glass prepared by repeated dip-coating/calcination: Characteristics and photocatalytic activities for removing acetaldehyde or toluene in air. Chem. Mater. 2007, 19, 3808–3814. [Google Scholar] [CrossRef]
- Watanabe, T.; Fukayama, S.; Masahiro, M.; Fujishima, A.; Hashimoto, K. Photocatalytic activity and photo-induced wettability conversion of TiO2 thin film prepared by sol-gel process on a soda-lime glass. J. Sol-Gel Sci. Technol. 2000, 19, 71–76. [Google Scholar] [CrossRef]
- Yao, N.; Yeung, K.L. Investigation of the performance of TiO2 photocatalytic coatings. Chem. Eng. J. 2011, 167, 13–21. [Google Scholar] [CrossRef]
- Kluson, P.; Luskova, H.; Cajthaml, T.; Solcova, O. Non thermal preparation of photoactive titanium (IV) oxide thin layers. Thin Solid Films 2006, 495, 18–23. [Google Scholar] [CrossRef]
- Yu, J.C.; Yu, J.; Zhao, J. Enhanced photocatalytic activity of mesoporous and ordinary TiO2 thin films by sulfuric acid treatment. Appl. Catal. B Environ. 2002, 36, 31–43. [Google Scholar] [CrossRef]
- Yu, C.J.; Yu, J.; Ho, W.; Zhao, J. Light-induced super-hydrophilicity and photocatalytic activity of mesoporous TiO2 thin films. J. Photochem. Photobiol. A Chem. 2002, 148, 331–339. [Google Scholar] [CrossRef]
- Coronado, J.M.; Zorn, M.E.; Tejedor-Tejedor, I.; Anderson, M.A. Photocatalytic oxidation of ketones in the gas phase over TiO2 thin films: A kinetic study on the influence of water vapor. Appl. Catal. B Environ. 2003, 43, 329–344. [Google Scholar] [CrossRef]
- Maudhuit, A.; Raillard, C.; Héquet, V.; Le Coq, L.; Sablayrolles, J.; Molins, L. Adsorption phenomena in photocatalytic reactions: The case of toluene, acetone and heptane. Chem. Eng. J. 2011, 170, 464–470. [Google Scholar] [CrossRef]
- Shang, J.; Du, Y.; Xu, Z. Photocatalytic oxidation of heptane in the gas-phase over TiO2. Chemosphere 2002, 46, 93–99. [Google Scholar] [CrossRef]
- Galanos, E.; Poulopoulos, S.; Philippopoulos, C. Photocatalytic destruction of methyl tert butyl ether in the gas phase using titanium dioxide. J. Environ. Sci. Health A 2002, 9, 1665–1675. [Google Scholar] [CrossRef]
- Park, W.E.; Joo, H.; Kang, J.W. Photodegradation of methyl tertiary butyl ether vapor with immobilized titanium dioxide. Sol. Energy Mater. Sol. Cells 2003, 80, 73–84. [Google Scholar] [CrossRef]
- Preis, S.; Kachina, A.; Santiago, C.N.; Kallas, J. The dependence on temperature of gas-phase photocatalytic oxidation of methyl tert-butyl ether and tert-butyl alcohol. Catal. Today 2005, 101, 353–358. [Google Scholar] [CrossRef]
- Oja Acik, I.; Mere, A.; Krunks, M.; Solterbeck, C.H.; Es-Souini, M. Properties of TiO2 films prepared by the spray pyrolysis method. In Solid State Phenomena; Trans Tech Publications: Baech, Switzerland, 2004; Volume 99, pp. 259–264. [Google Scholar] [CrossRef]
- Polivtseva, S.; Oja Acik, I.; Katerski, A.; Mere, A.; Mikli, V.; Krunks, M. Spray Pyrolysis deposition of SnxSy thin films. Energy Procedia 2014, 60, 156–165. [Google Scholar] [CrossRef]
- Perednis, D.; Gauckler, L.J. Thin film deposition using spray pyrolysis. J. Electroceram. 2005, 14, 103–111. [Google Scholar] [CrossRef]
- Bang, J.H.; Didenko, Y.; Helmich, R.J.; Suslick, K.S. Nanostructured materials through ultrasonic spray pyrolysis. Mater. Matters 2012, 7, 15–20. [Google Scholar]
- Raut, N.C.; Mathews, T.; Chandramohan, P.; Srinivasan, M.P.; Dash, S.; Tyagi, A.K. Effect of temperature on the growth of TiO2 thin films synthesized by spray pyrolysis: Structural, compositional and optical properties. Mater. Res. Bull. 2011, 46, 2057–2063. [Google Scholar] [CrossRef]
- Powder Diffraction File (PDF), (PDF-2/Release 2013 RDB) (01-070-6826) (archived on 1 November 2019).
- Dupin, J.; Gonbeau, D.; Vinatier, P.; Levasseur, A. Systematic XPS studies of metal oxides, hydroxides and peroxide. Phys. Chem. Chem. Phys. 2000, 2, 1319–1324. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, S.; Lee, H.; Kumar, R. Formation of oxygen vacancies and Ti3+ state in TiO2 thin film and enhanced optical properties by air plasma treatment. Nat. Sci. Rep. 2016, 6, 32355. [Google Scholar] [CrossRef]
- Sarkar, A.; Khan, G. The formation and detection techniques of oxygen vacancies in titanium oxide-based nanostructures. Nanoscale 2019, 11, 3414–3444. [Google Scholar] [CrossRef]
- Huang, Y.; Tien, H.; Ma, C.; Yang, S.; Wu, S.; Liu, H.; Mai, Y. Effect of extended polymer chains on properties of transparent graphene nanosheets conductive film. J. Mater. Chem. 2011, 21, 18236–18241. [Google Scholar] [CrossRef]
- Amor, S.; Jacquet, M.; Fioux, P.; Nardin, M. XPS characterization of plasma treated and zinc oxide coated PET. Appl. Surf. Sci. 2009, 255, 5052–5061. [Google Scholar] [CrossRef]
- Gromyko, I.; Krunks, M.; Dedova, T.; Katerski, A.; Klauson, D.; Oja Acik, I. Surface properties of sprayed and electrodeposited ZnO rod layers. Appl. Surf. Sci. 2017, 405, 521–528. [Google Scholar] [CrossRef]
- Chen, W.; Koshy, P.; Christopher, S.C. Effects of film topology and contamination as a function of thickness on the photo-induced hydrophilicity of transparent TiO2 thin films deposited on glass substrates by spin coating. J. Mater. Sci. 2016, 51, 2465–2480. [Google Scholar] [CrossRef]
- Jimmy, C.; Yu, J.; Yuk, T.H.; Zhang, L. Effect of surface microstructure on the photoinduced hydrophilicity of porous TiO2 thin films. J. Mater. Chem. 2002, 12, 81–85. [Google Scholar] [CrossRef]
- McCafferty, E.; Wightman, J.P. Determination of the concentration of surface hydroxyl groups on metal oxide films by a quantitative XPS method. Surf. Interface Anal. 1998, 26, 549–564. [Google Scholar] [CrossRef]
- Li, J.; Tang, S.; Lu, L.; Zeng, H.C. Preparation of nanocomposites of metals, metal oxides, and carbon nanotubes via self-assembly. J. Am. Chem. Soc. 2007, 129, 9401–9409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.; Jiu, H.; Ni, C.; Zhang, X.; Xu, M. Graphene-based hollow TiO2 composites with enhanced photocatalytic activity for removal of pollutants. J. Phys. Chem. Solids 2015, 86, 82–89. [Google Scholar] [CrossRef]
- Oja, I.; Mere, A.; Krunks, M.; Nisumaa, R.; Solterbeck, C.; Es-Souni, M. Structural and electrical characterization of TiO2 films grown by spray pyrolysis. Thin Solid Films 2006, 515, 674–677. [Google Scholar] [CrossRef]
- Simonsen, M.E.; Li, Z.; Søgaard, E.G. Influence of the OH- groups on the photocatalytic activity and photoinduced hydrophilicity of microwave assisted sol–gel TiO2 film. Appl. Surf. Sci. 2009, 255, 8054–8062. [Google Scholar] [CrossRef]
- Ohtsu, N.; Masahashi, N.; Mizukoshi, Y.; Wagatsuma, K. Hydrocarbon decomposition on a hydrophilic TiO2 surface by UV-irradiation: Spectral and quantitative analysis using in-situ XPS technique. Langmuir 2009, 25, 11586–11591. [Google Scholar] [CrossRef]
- Holtzinger, C.; Rapenne, L.; Chaudouet, P.; Berthome, G.; Langlet, M. Thickness effects in naturally superhydrophilic TiO2–SiO2 nanocomposite films deposited via a multilayer sol–gel route. J. Sol-Gel Sci. Technol. 2012, 64, 465–479. [Google Scholar] [CrossRef]
- Simonsen, M.E.; Sørensen, M.B.; Søgaard, E.G. Comparison of methods for evaluation of activity of photocatalytic films. Environ. Sci. Pollut. Res. 2012, 19, 3772–3781. [Google Scholar] [CrossRef]
- Zheng, J.; Bao, S.; Guo, Y.; Jin, P. Natural hydrophobicity and reversible wettability conversion of flat anatase TiO2 thin film. ACS Appl. Mater. Interfaces 2014, 63, 1351–1355. [Google Scholar] [CrossRef]
- Chen, H.; Nanayakkara, E.C.; Grassian, V. Titanium dioxide photocatalysis in atmospheric chemistry. Chem. Rev. 2012, 112, 5919–5948. [Google Scholar] [CrossRef] [PubMed]
- Sakai, N.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Quantitative evaluation of the photoinduced hydrophilic conversion properties of TiO2 thin film surfaces by the reciprocal of contact angle. J. Phys. Chem. B 2003, 107, 1028–1035. [Google Scholar] [CrossRef]
- Jing, L.Q.; Qu, Y.C.; Wang, B.Q.; Li, S.D.; Jiang, B.J.; Yang, L.B.; Fu, W.; Fu, H.G.; Sun, J.Z. Review of photoluminescence performance of nano-sized semiconductor materials and its relationships with photocatalytic activity. Sol. Energy Mater. Sol. Cells 2006, 90, 1773–1787. [Google Scholar] [CrossRef]
- Akbarzadeh, R.; Umbarkar, B.S.; Sonawane, S.R.; Takle, S.; Dongare, K.M. Vanadia–titania thin films for photocatalytic degradation of formaldehyde in sunlight. Appl. Catal. A 2010, 374, 103–109. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M. Hierarchical photocatalysts. Chem. Soc. Rev. 2016, 45, 2603–2636. [Google Scholar] [CrossRef]
- Rehman, S.; Ullah, R.; Butt, A.M.; Gohar, N.D. Strategies of making TiO2 and ZnO visible light active. J. Hazard. Mater. 2009, 170, 560–569. [Google Scholar] [CrossRef]
- Neti, N.R.; Parmar, G.R.; Bakardjieva, S.; Subrt, J. Thick film titania on glass supports for vapour phase photocatalytic degradation of toluene, acetone, and ethanol. Chem. Eng. J. 2010, 163, 219–229. [Google Scholar] [CrossRef]
- Demeestere, K.; Dewulf, J.; Van Langenhove, H.; Sercu, B. Gas–solid adsorption of selected volatile organic compounds on titanium dioxide Degussa P25. Chem. Eng. Sci. 2003, 58, 2255–2267. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C. Photocatalytic degradation of VOCs on various commercial titanium dioxides: Impact of operating parameters on removal efficiency and by products generation. Build. Environ. 2018, 138, 275–282. [Google Scholar] [CrossRef]
- Ibrahim, H.; de Lasa, H. Kinetic Modeling of the Photocatalytic Degradation of Air-Borne Pollutants. AIChE J. 2004, 50, 1017–1027. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.; Byrne, J.A.; O’Sheaf, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Valeeva, A.A.; Kozlova, E.A.; Vokhmintsev, A.S.; Kamalov, R.V.; Dorosheva, I.B.; Saraev, A.A.; Weinstein, I.A.; Rempel, A.A. Nonstoichiometric titanium dioxide nanotubes with enhanced catalytical activity under visible light. Sci. Rep. 2018, 8, 9607. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Maa, T.; Liu, S. Enhanced photocatalytic activity of mesoporous TiO2 aggregates by embedding carbon nanotubes as electron-transfer channel. Phys. Chem. Chem. Phys. 2011, 13, 3491–3501. [Google Scholar] [CrossRef] [PubMed]
- Krysa, J.; Novotna, P.; Kment, S.; Mills, A. Effect of glass substrate and deposition technique on the properties of sol-gel TiO2 thin films. J. Photochem. Photobiol. A 2011, 222, 81–86. [Google Scholar] [CrossRef]
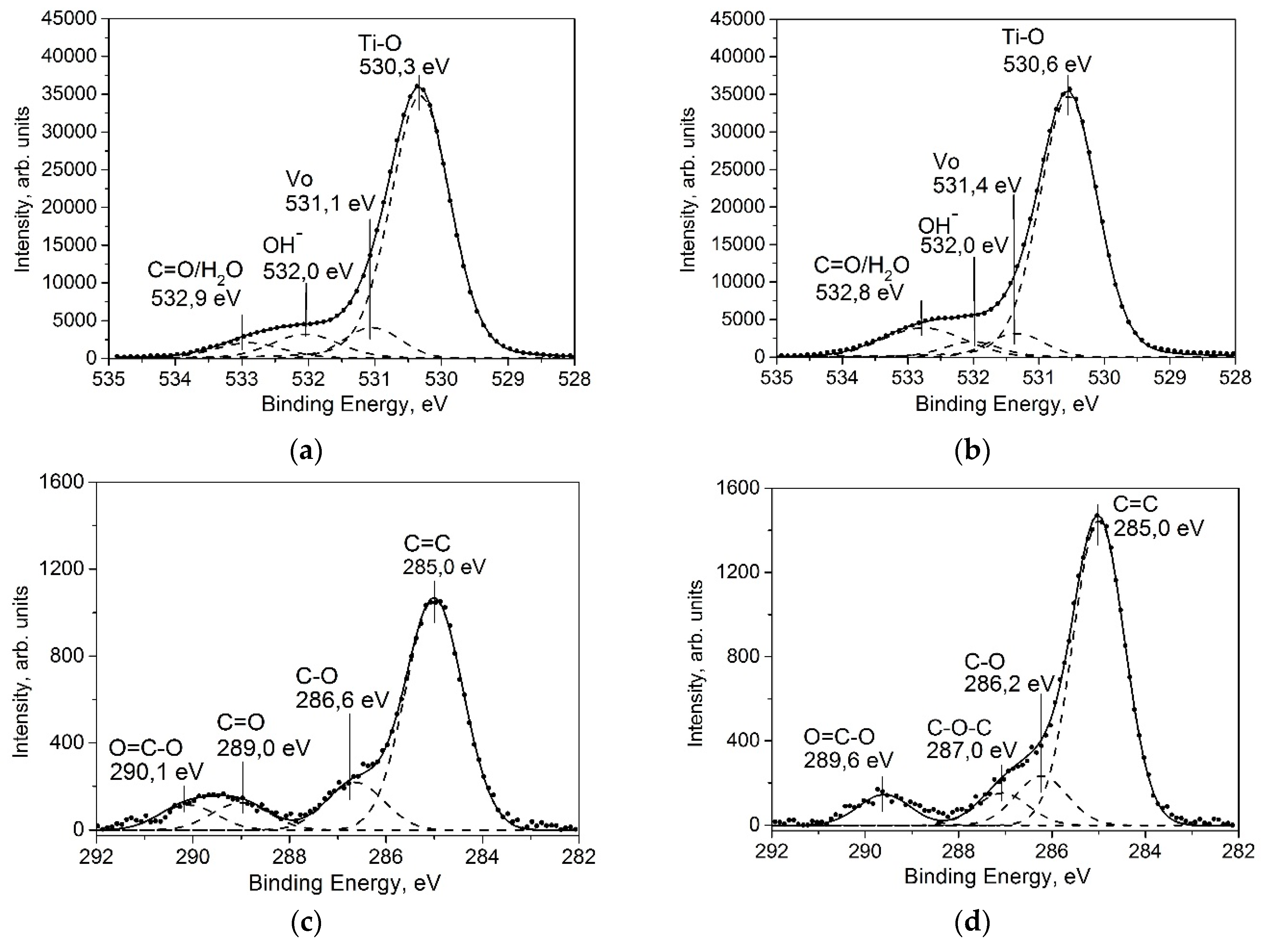

| Deposition Temperature (°C) | Aged-TiO2 Samples (before UV-Treatment) | After UV-Treatment | ||
|---|---|---|---|---|
| (Vo)/(Ti–O) (at%/at%) | (OH−)/(Ti–O) (at%/at%) | (Vo)/(Ti–O) (at%/at%) | (OH−)/(Ti–O) (at%/at%) | |
| 350 | 0.17 | 0.08 | 0.12 | 0.11 |
| 450 | 0.09 | 0.30 | 0.09 | 0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dundar, I.; Krichevskaya, M.; Katerski, A.; Krunks, M.; Oja Acik, I. Photocatalytic Degradation of Different VOCs in the Gas-Phase over TiO2 Thin Films Prepared by Ultrasonic Spray Pyrolysis. Catalysts 2019, 9, 915. https://doi.org/10.3390/catal9110915
Dundar I, Krichevskaya M, Katerski A, Krunks M, Oja Acik I. Photocatalytic Degradation of Different VOCs in the Gas-Phase over TiO2 Thin Films Prepared by Ultrasonic Spray Pyrolysis. Catalysts. 2019; 9(11):915. https://doi.org/10.3390/catal9110915
Chicago/Turabian StyleDundar, Ibrahim, Marina Krichevskaya, Atanas Katerski, Malle Krunks, and Ilona Oja Acik. 2019. "Photocatalytic Degradation of Different VOCs in the Gas-Phase over TiO2 Thin Films Prepared by Ultrasonic Spray Pyrolysis" Catalysts 9, no. 11: 915. https://doi.org/10.3390/catal9110915
APA StyleDundar, I., Krichevskaya, M., Katerski, A., Krunks, M., & Oja Acik, I. (2019). Photocatalytic Degradation of Different VOCs in the Gas-Phase over TiO2 Thin Films Prepared by Ultrasonic Spray Pyrolysis. Catalysts, 9(11), 915. https://doi.org/10.3390/catal9110915








