Developing a Novel Enzyme Immobilization Process by Activation of Epoxy Carriers with Glucosamine for Pharmaceutical and Food Applications
Abstract
1. Introduction
2. Results and Discussion
2.1. Immobilization of PGA
2.2. Immobilization of Protease N on Sepabeads EC-EP Glucosamine
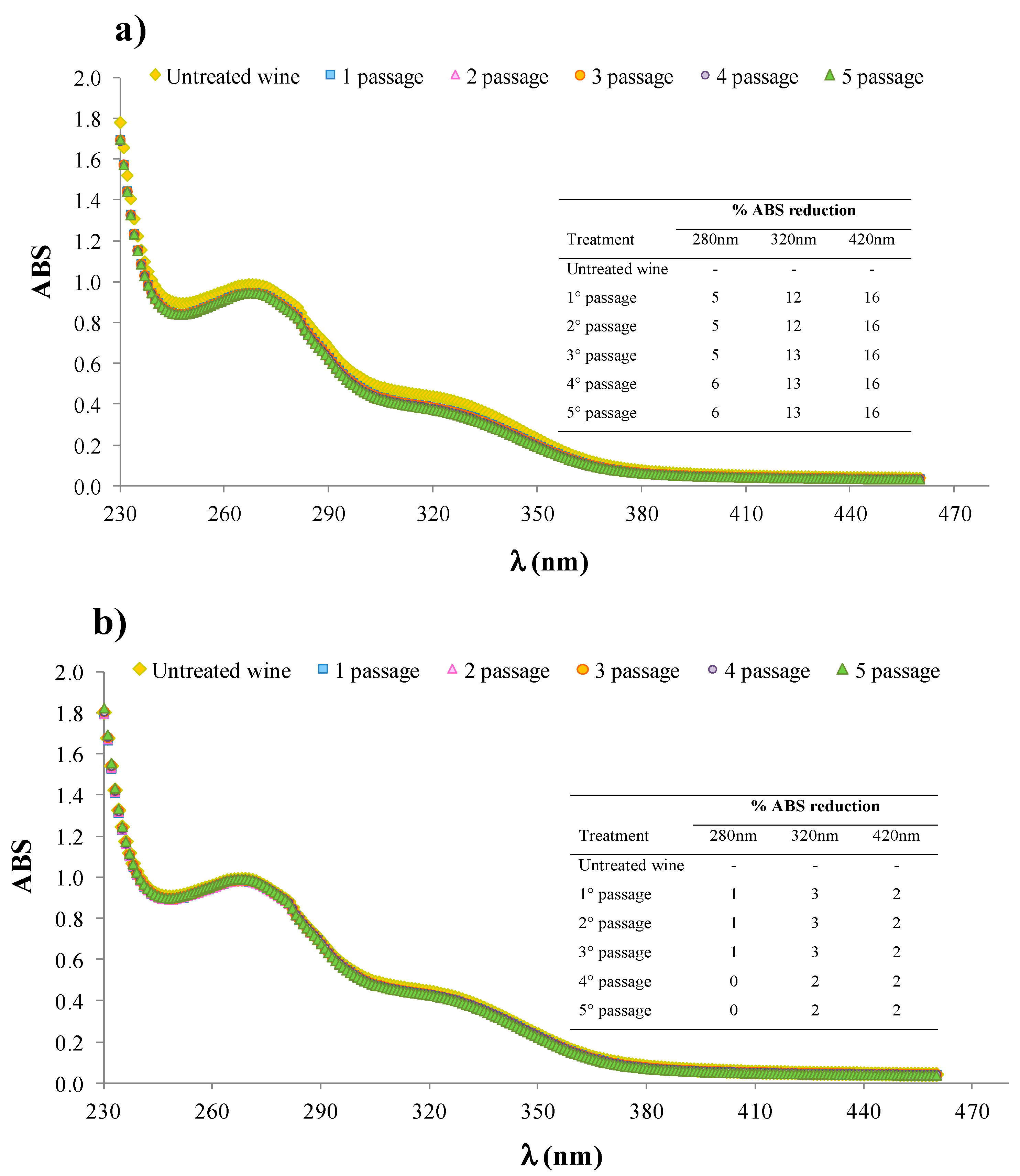
2.3. Immobilization of Bromelain (BR) on Relizyme EP403/S-Glucosamine
2.4. Immobilization γ-glutamyl Transpeptidase (GGT) on Relizyme EP403/S-Glucosamine
3. Materials and Methods
3.1. Enzyme Immobilization
3.1.1. Immobilization of PGA on Epoxy Acrylic Carriers
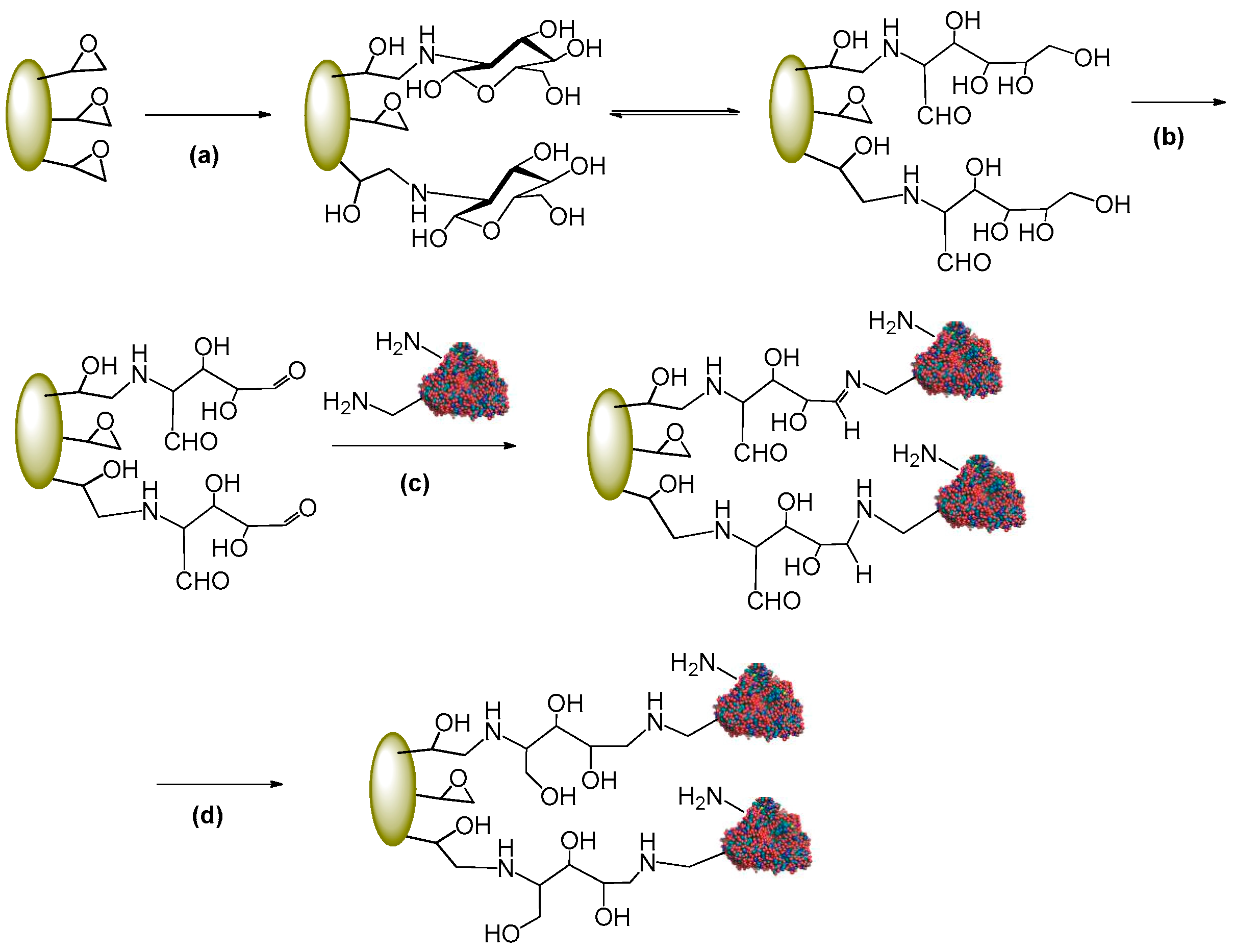
3.1.2. Activation of Epoxy Carriers with Glucosamine
3.1.3. General procedure for Enzyme Immobilization on Glucosamine Activated Carriers
3.1.4. Immobilization on Glutaraldehyde-Activated Resin
3.2. Enzyme Activity Assay
3.2.1. PGA Activity Assay
3.2.2. Protease N Activity Assay
3.2.3. Bromelain Activity Assay
3.2.4. γ-Glutamyl Transpeptidase Activity Assay
3.3. Enzymatic Reactions
3.3.1. Enzymatic Reactions Catalyzed by PGA
3.3.2. Enzymatic Reaction Catalyzed by Protease N
3.4. Enzymatic Reactions Catalyzed by Immobilized Bromelain
3.4.1. Wine Treatment in Packed-Bad Reactor
3.4.2. Heat Test
3.4.3. Effect on Phenolic and Chromatic Indexes of White Wine
3.5. General Procedure for Stability Assays
3.6. Enzyme Recycling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wells, A.S.; Finch, G.L.; Michels, P.C.; Wong, J.W. Use of enzymes in the manufacture of Active Pharmaceutical Ingredients—A science and safety-based approach to ensure patient safety and drug quality. Org. Process Res. Dev. 2012, 16, 1986–1993. [Google Scholar] [CrossRef]
- Tao, J.; Xu, J.-H. Biocatalysis in development of green pharmaceutical processes. Curr. Opin. Chem. Biol. 2009, 13, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Woodley, J.M. New opportunities for biocatalysis: Making pharmaceutical processes greener. Trends Biotechnol. 2008, 26, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, K.T.; Ingram, L.O. Engineering biocatalysts for production of commodity chemicals. J. Mol. Microbiol. Biotechnol. 2008, 15, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.N. Synthesis of chiral pharmaceutical intermediates by biocatalysis. Coord. Chem. Rev. 2008, 252, 659–701. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Tao, J. Biocatalysis for green chemistry and drug development. Prog. Chem. 2007, 19, 1919–1927. [Google Scholar]
- Pollard, D.J.; Woodley, J.M. Biocatalysis for pharmaceutical intermediates: The future is now. Trends Biotechnol. 2007, 25, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Akoh, C.C.; Chang, S.-W.; Lee, G.-C.; Shaw, J.-F. Biocatalysis for the production of industrial products and functional foods from rice and other agricultural produce. J. Agric. Food Chem. 2008, 56, 10445–10451. [Google Scholar] [CrossRef]
- Aravindan, R.; Anbumathi, P.; Viruthagiri, T. Lipase applications in food industry. Indian J. Biotechnol. 2007, 6, 141–158. [Google Scholar]
- Longo, M.A.; Sanromán, M.A. Production of food aroma compounds: Microbial and enzymatic methodologies. Food Technol. Biotechnol. 2006, 44, 335–353. [Google Scholar]
- Greiner, R.; Konietzny, U. Phytase for food application. Food Technol. Biotechnol. 2006, 44, 125–140. [Google Scholar]
- Torres-Salas, P.; Pedrali, A.; Bavaro, T.; Ambrosini, S.; Marrubini, G.; Pappalardo, V.M.; Massolini, G.; Terreni, M.; Ubiali, D. Preparation of PUFA concentrates as acylglycerols via enzymatic hydrolysis of hempseed oil (Cannabis sativa L.) in a homogeneous low-water medium: PUFA-based glyceride concentrates from hempseed oil. Eur. J. Lipid Sci. Technol. 2014, 116, 1496–1504. [Google Scholar] [CrossRef]
- Bavaro, T.; Filice, M.; Temporini, C.; Tengattini, S.; Serra, I.; Morelli, C.F.; Massolini, G.; Terreni, M. Chemoenzymatic synthesis of neoglycoproteins driven by the assessment of protein surface reactivity. RSC Adv. 2014, 4, 56455–56465. [Google Scholar] [CrossRef]
- Sandoval, M.; Hoyos, P.; Cortés, A.; Bavaro, T.; Terreni, M.; Hernáiz, M.J. Development of regioselective deacylation of peracetylated β-d-monosaccharides using lipase from Pseudomonas stutzeri under sustainable conditions. RSC Adv. 2014, 4, 55495–55502. [Google Scholar] [CrossRef]
- Bavaro, T.; Torres-Salas, P.; Antonioli, N.; Morelli, C.F.; Speranza, G.; Terreni, M. Regioselective deacetylation of disaccharides via immobilized Aspergillus niger esterase(s)-catalyzed hydrolysis in aqueous and non-aqueous media. ChemCatChem 2013, 5, 2925–2931. [Google Scholar] [CrossRef]
- Bavaro, T.; Torres-Salas, P.; Ubiali, D.; Terreni, M. Regioselective enzymatic hydrolysis of hexa-O-acetyl-lactal in a green non-aqueous medium. RSC Adv. 2013, 3, 7355–7359. [Google Scholar] [CrossRef]
- Kallenberg, A.I.; van Rantwijk, F.; Sheldon, R.A. Immobilization of penicillin G acylase: The key to optimum performance. Adv. Synth. Catal. 2005, 347, 905–926. [Google Scholar] [CrossRef]
- Calleri, E.; Cattaneo, G.; Rabuffetti, M.; Serra, I.; Bavaro, T.; Massolini, G.; Speranza, G.; Ubiali, D. Flow-synthesis of nucleosides catalyzed by an immobilized purine nucleoside phosphorylase from Aeromonas hydrophila: Integrated systems of reaction control and product purification. Adv. Synth. Catal. 2015, 357, 2520–2528. [Google Scholar] [CrossRef]
- Bavaro, T.; Filice, M.; Bonomi, P.; Abu alassal, Q.; Speranza, G.; Guisán, J.M.; Terreni, M. Regioselective deprotection of peracetylated disaccharides at the primary position catalyzed by immobilized acetyl xylan esterase from Bacillus pumilus. Eur. J. Org. Chem. 2011, 2011, 6181–6185. [Google Scholar] [CrossRef]
- Cantone, S.; Ferrario, V.; Corici, L.; Ebert, C.; Fattor, D.; Spizzo, P.; Gardossi, L. Efficient immobilisation of industrial biocatalysts: Criteria and constraints for the selection of organic polymeric carriers and immobilisation methods. Chem. Soc. Rev. 2013, 42, 6262–6272. [Google Scholar] [CrossRef]
- DiCosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Kourkoutas, Y.; Bekatorou, A.; Banat, I.M.; Marchant, R.; Koutinas, A.A. Immobilization technologies and support materials suitable in alcohol beverages production: A review. Food Microbiol. 2004, 21, 377–397. [Google Scholar] [CrossRef]
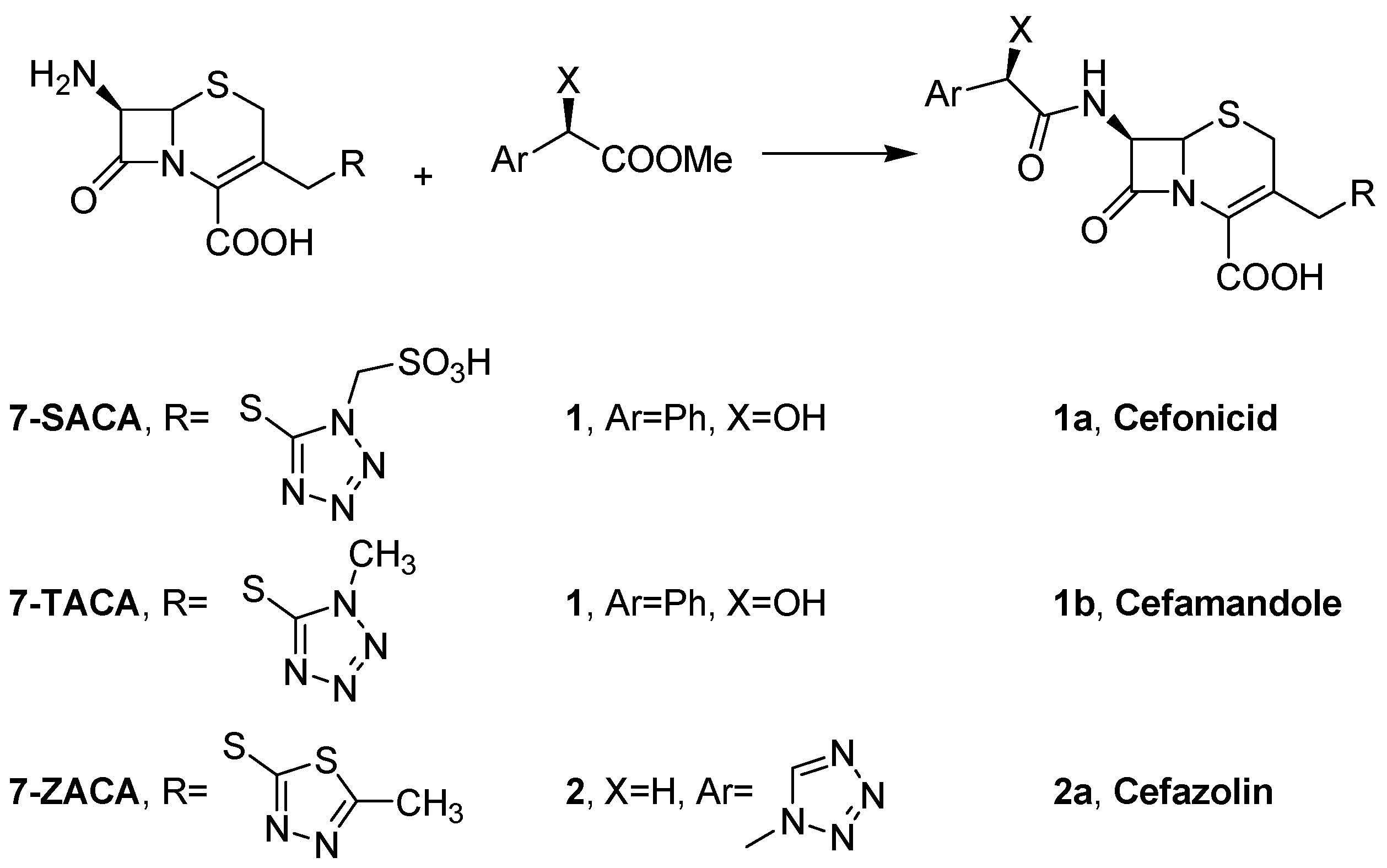
- Terreni, M.; Tchamkam, J.G.; Sarnataro, U.; Rocchietti, S.; Fernández-Lafuente, R.; Guisán, J.M. Influence of substrate structure on PGA-catalyzed acylations. Evaluation of different approaches for the enzymatic synthesis of Cefonicid. Adv. Synth. Catal. 2005, 347, 121–128. [Google Scholar] [CrossRef]
- Giesecke, U.E. An Enzymatic Process for Preparing β-Lactams. EP Patent 1394262A1, 3 March 2004. [Google Scholar]
- Wegman, M.A.; Janssen, M.H.A.; van Rantwijk, F.; Sheldon, R.A. Towards biocatalytic synthesis of β-lactam antibiotics. Adv. Synth. Catal. 2001, 343, 559–576. [Google Scholar] [CrossRef]
- Estruch, I.; Tagliani, A.R.; Guisán, J.M.; Fernández-Lafuente, R.; Alcántara, A.R.; Toma, L.; Terreni, M. Immobilization of the acylase from Escherichia coli on glyoxyl-agarose gives efficient catalyst for the synthesis of cephalosporins. Enzym. Microb. Technol. 2008, 42, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Terreni, M.; Ubiali, D.; Bavaro, T.; Pregnolato, M.; Fernández-Lafuente, R.; Guisán, J.M. Enzymatic synthesis of cephalosporins. The immobilized acylase from Arthrobacter viscosus: A new useful biocatalyst. Appl. Microbiol. Biotechnol. 2007, 77, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Temporini, C.; Bonomi, P.; Serra, I.; Tagliani, A.; Bavaro, T.; Ubiali, D.; Massolini, G.; Terreni, M. Characterization and study of the orientation of immobilized enzymes by tryptic digestion and HPLC-MS: Design of an efficient catalyst for the synthesis of cephalosporins. Biomacromolecules 2010, 11, 1623–1632. [Google Scholar] [CrossRef] [PubMed]
- Serra, I.; Ubiali, D.; Cecchini, D.A.; Calleri, E.; Albertini, A.M.; Terreni, M.; Temporini, C. Assessment of immobilized PGA orientation via the LC-MS analysis of tryptic digests of the wild type and its 3K-PGA mutant assists in the rational design of a high-performance biocatalyst. Anal. Bioanal. Chem. 2013, 405, 745–753. [Google Scholar] [CrossRef]
- Petenzi, M.; Bavaro, T.; Cornaggia, C.; Ubiali, D.; Pregnolato, M.; Pasini, D. Synthesis, post-modification and characterization of linear polystyrene-based supports for interaction with immobilized biocatalysts. Polym. Int. 2012, 61, 1611–1618. [Google Scholar] [CrossRef]
- Bonomi, P.; Bavaro, T.; Serra, I.; Tagliani, A.; Terreni, M.; Ubiali, D. Modulation of the microenvironment surrounding the active site of penicillin G acylase immobilized on acrylic carriers improves the enzymatic synthesis of cephalosporins. Molecules 2013, 18, 14349–14365. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, O.; Torres, R.; Ortiz, C.; Berenguer-Murcia, Á.; Rodrigues, R.C.; Fernandez-Lafuente, R. Heterofunctional supports in enzyme immobilization: From traditional immobilization protocols to opportunities in tuning enzyme properties. Biomacromolecules 2013, 14, 2433–2462. [Google Scholar] [CrossRef] [PubMed]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 2004, 37, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Mateo, C.; Godoy, C.; Pessela, B.C.C.; Rodrigues, D.S.; Giordano, R.L.C.; Fernandez-Lafuente, R.; Guisán, J.M. The co-operative effect of physical and covalent protein adsorption on heterofunctional supports. Process Biochem. 2009, 44, 757–763. [Google Scholar] [CrossRef]
- Adriano, W.S.; Filho, E.H.C.; Silva, J.A.; Giordano, R.L.C.; Gonçalves, L.R.B. Stabilization of penicillin G acylase by immobilization on glutaraldehyde-activated chitosan. Braz. J. Chem. Eng. 2005, 22, 529–538. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; van Langen, L.M.; van Rantwijk, F.; Sheldon, R.A. A new, mild cross-linking methodology to prepare cross-linked enzyme aggregates. Biotechnol. Bioeng. 2004, 86, 273–276. [Google Scholar] [CrossRef]
- Arsenault, A.; Cabana, H.; Jones, J.P. Laccase-based CLEAs: Chitosan as a novel cross-linking agent. Enzym. Res. 2011, 2011, 376015. [Google Scholar] [CrossRef]
- Sojitra, U.V.; Nadar, S.S.; Rathod, V.K. Immobilization of pectinase onto chitosan magnetic nanoparticles by macromolecular cross-linker. Carbohydr. Polym. 2017, 157, 677–685. [Google Scholar] [CrossRef]
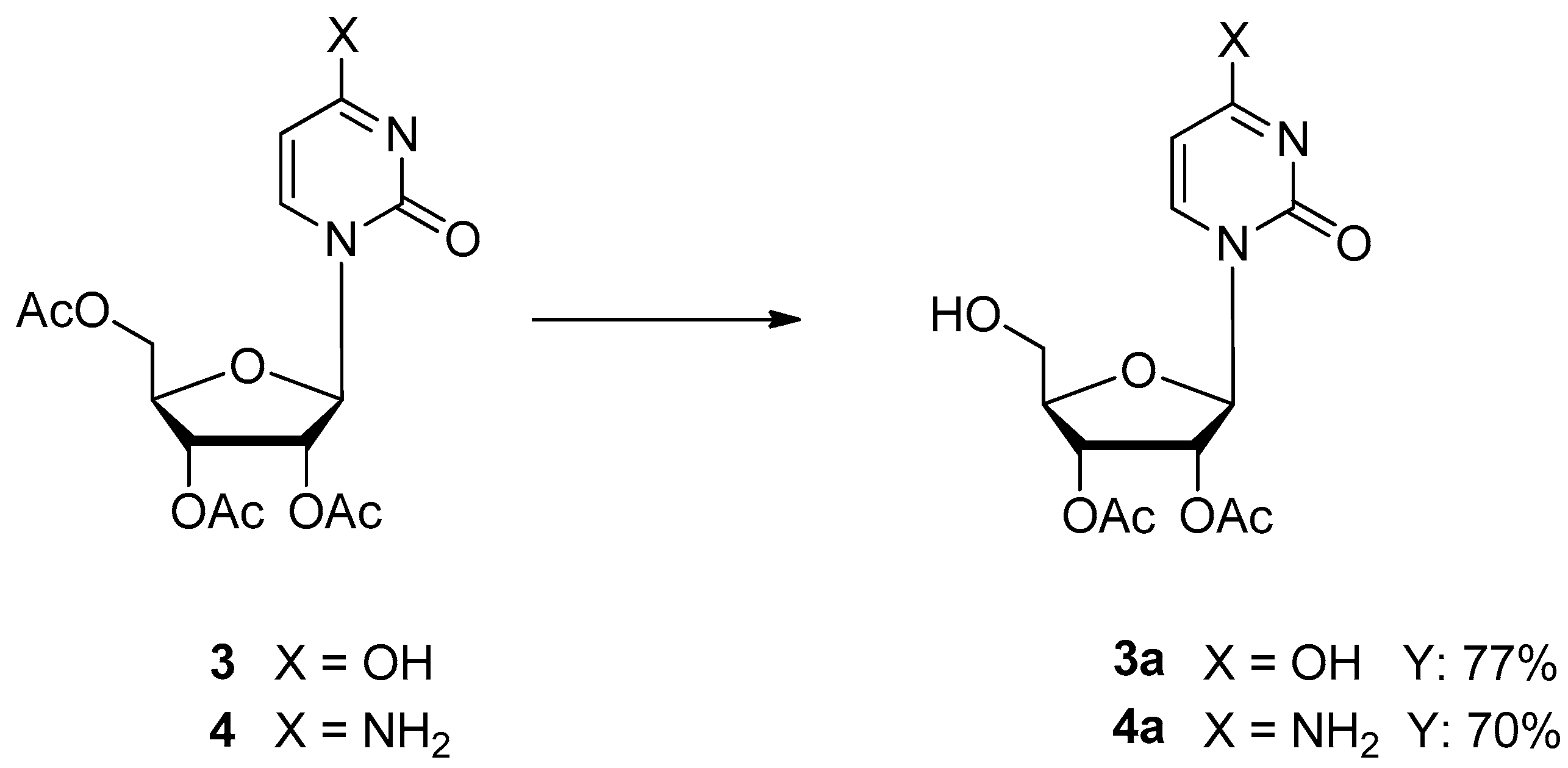
- Panero, J.; Trelles, J.; Rodano, V.; Montserrat, J.M.; Iglesias, L.E.; Lewkowicz, E.S.; Iribarren, A.M. Microbial hydrolysis of acetylated nucleosides. Biotechnol. Lett. 2006, 28, 1077–1081. [Google Scholar] [CrossRef]
- Bavaro, T.; Cattaneo, G.; Serra, I.; Benucci, I.; Pregnolato, M.; Terreni, M. Immobilization of neutral protease from Bacillus subtilis for regioselective hydrolysis of acetylated nucleosides: Application to Capecitabine synthesis. Molecules 2016, 21, 1621. [Google Scholar] [CrossRef] [PubMed]
- Bavaro, T.; Rocchietti, S.; Ubiali, D.; Filice, M.; Terreni, M.; Pregnolato, M. A versatile synthesis of 5′-functionalized nucleosides through regioselective enzymatic hydrolysis of their peracetylated precursors. Eur. J. Org. Chem. 2009, 2009, 1967–1975. [Google Scholar] [CrossRef]
- Benucci, I.; Lombardelli, C.; Liburdi, K.; Acciaro, G.; Zappino, M.; Esti, M. Immobilised native plant cysteine proteases: Packed-bed reactor for white wine protein stabilisation. J. Food Sci. Technol. 2016, 53, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Toda, K. Preparation of immobilized papain covalently bound on natural cellulose for treatment of beer. Biotechnol. Lett. 1988, 10, 221–223. [Google Scholar] [CrossRef]
- Cappannella, E.; Benucci, I.; Lombardelli, C.; Liburdi, K.; Bavaro, T.; Esti, M. Immobilized lysozyme for the continuous lysis of lactic bacteria in wine: Bench-scale fluidized-bed reactor study. Food Chem. 2016, 210, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Speranza, G.; Morelli, C.F. γ-Glutamyl transpeptidase-catalyzed synthesis of naturally occurring flavor enhancers. J. Mol. Catal. B Enzym. 2012, 84, 65–71. [Google Scholar] [CrossRef]
- Scaramozzino, F.; Estruch, I.; Rossolillo, P.; Terreni, M.; Albertini, A.M. Improvement of catalytic properties of Escherichia coli penicillin G acylase immobilized on glyoxyl agarose by addition of a six-amino-acid tag. Appl. Environ. Microbiol. 2005, 71, 8937–8940. [Google Scholar] [CrossRef]
- Benucci, I.; Esti, M.; Liburdi, K.; Garzillo Anna, M.V. Pineapple stem bromelain immobilized on different supports: Catalytic properties in model wine. Biotechnol. Prog. 2012, 28, 1472–1477. [Google Scholar]
- Marangon, M.; Vincenzi, S.; Lucchetta, M.; Curioni, A. Heating and reduction affect the reaction with tannins of wine protein fractions differing in hydrophobicity. Anal. Chim. Acta 2010, 660, 110–118. [Google Scholar] [CrossRef]
- Benucci, I.; Lombardelli, C.; Cacciotti, I.; Liburdi, K.; Nanni, F.; Esti, M. Chitosan beads from microbial and animal sources as enzyme supports for wine application. Food Hydrocoll. 2016, 61, 191–200. [Google Scholar] [CrossRef]
- Morelli, C.F.; Calvio, C.; Biagiotti, M.; Speranza, G. pH-Dependent hydrolase, glutaminase, transpeptidase and autotranspeptidase activities of Bacillus subtilis γ-glutamyltransferase. FEBS J. 2014, 281, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Guisán, J.M. Aldehyde-agarose gels as activated supports for immobilization-stabilization of enzymes. Enzym. Microb. Technol. 1988, 10, 375–382. [Google Scholar] [CrossRef]
- Serra, I.; Cecchini, D.A.; Ubiali, D.; Manazza, E.M.; Albertini, A.M.; Terreni, M. Coupling of site-directed mutagenesis and immobilization for the rational design of more efficient biocatalysts: The case of immobilized 3G3K PGA from E. coli. Eur. J. Org. Chem. 2009, 2009, 1384–1389. [Google Scholar] [CrossRef]
- Hale, L.P.; Greer, P.K.; Trinh, C.T.; James, C.L. Proteinase activity and stability of natural bromelain preparations. Int. Immunopharmacol. 2005, 5, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Tate, S.S.; Meister, A. γ-Glutamyl transpeptidase from kidney. Methods Enzymol. 1985, 113, 400–419. [Google Scholar] [PubMed]
- Bavaro, T.; Ubiali, D.; Brocca, S.; Rocchietti, S.; Nieto, I.; Pregnolato, M.; Lotti, M.; Terreni, M. Recombinant lipase from Candida rugosa for regioselective hydrolysis of peracetylated nucleosides. A comparison with commercial non-recombinant lipases. Biocatal. Biotransform. 2010, 28, 108–116. [Google Scholar] [CrossRef]
- Pregnolato, M.; Terreni, M.; Ubiali, D.; Bavaro, T. 2′,3′-Di-O-acyl-5-Fluoronucleosides. EP Patent 2176278A2, 21 April 2010. [Google Scholar]
- Vincenzi, S.; Marangon, M.; Tolin, S.; Curioni, A. Protein evolution during the early stages of white winemaking and its relations with wine stability: Protein evolution in white wine during winemaking. Aust. J. Grape Wine Res. 2011, 17, 20–27. [Google Scholar] [CrossRef]
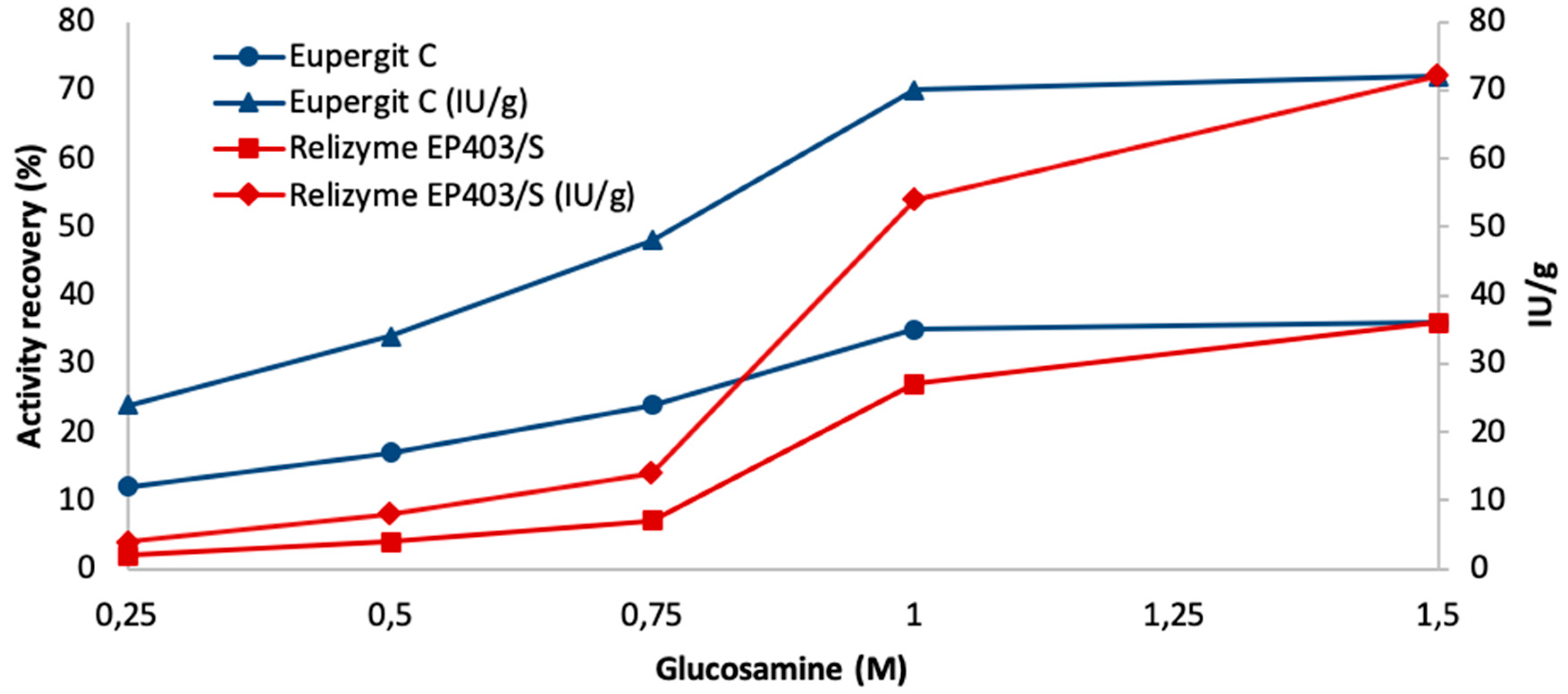
| Carrier | Glucosamine (M) | Immobilization a (%) | Activity Recovery (%) b (IU/g) |
|---|---|---|---|
| Eupergit C | 1 | 97 | 35 (70) |
| Eupergit C250L | 1 | 80 | 23 (46) |
| Sepabeads EC-EP | 1 | 95 | 34 (68) |
| Relizyme EP403/S | 1 | 92 | 27 (54) |
| 1.5 | 96 | 36 (72) |
| Activated Carrier | Product | Conversion (%) a |
|---|---|---|
| Eupergit C-epoxy | 1a | 71 (±0.7) b |
| Eupergit C-1 M glucosamine | 1a | 77 (±0.5) |
| Eupergit C250L-epoxy | 1a | 72 (±0.8) |
| Eupergit C250L-1 M glucosamine | 1a | 80 (±0.4) |
| Relizyme EP403/S-epoxy | 1a | 70 (±1.1) |
| Relizyme EP403/S-1.5 M glucosamine | 1a | 73 (±0.8) |
| Eupergit C-epoxy | 1b | 72 (±0.5) b |
| Eupergit C-1 M glucosamine | 1b | 78 (±0.7) |
| Eupergit C250L-epoxy | 1b | 72 (±1) |
| Eupergit C250L-1 M glucosamine | 1b | 80 (±0.5) |
| Eupergit C-epoxy | 2a | 79 (±1.2) b |
| Eupergit C-1 M glucosamine | 2a | 90 (±0.5) |
| Eupergit C250L-epoxy | 2a | 76 (±2) |
| Eupergit C250L-1 M glucosamine | 2a | 83 (±1.7) |
| Relizyme EP403/S-epoxy | 2a | 88 (±1.2) |
| Relizyme EP403/S-1.5 M glucosamine | 2a | 92 (±0.8) |
| Treatment | Net Haze after Heat Test (ΔNTU) | % Haze Potential Reduction/IU | ||
|---|---|---|---|---|
| R-GDH | R-Glu | R-GDH | R-Glu | |
| Untreated wine | 204 | 204 | - | - |
| 1° passage | 191 | 173 | 6 | 15 |
| 2° passage | 187 | 151 | 8 | 26 |
| 3° passage | 189 | 142 | 8 | 30 |
| 4° passage | 183 | 94 | 10 | 54 |
| 5° passage | 183 | 95 | 10 | 53 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra, I.; Benucci, I.; Robescu, M.S.; Lombardelli, C.; Esti, M.; Calvio, C.; Pregnolato, M.; Terreni, M.; Bavaro, T. Developing a Novel Enzyme Immobilization Process by Activation of Epoxy Carriers with Glucosamine for Pharmaceutical and Food Applications. Catalysts 2019, 9, 843. https://doi.org/10.3390/catal9100843
Serra I, Benucci I, Robescu MS, Lombardelli C, Esti M, Calvio C, Pregnolato M, Terreni M, Bavaro T. Developing a Novel Enzyme Immobilization Process by Activation of Epoxy Carriers with Glucosamine for Pharmaceutical and Food Applications. Catalysts. 2019; 9(10):843. https://doi.org/10.3390/catal9100843
Chicago/Turabian StyleSerra, Immacolata, Ilaria Benucci, Marina Simona Robescu, Claudio Lombardelli, Marco Esti, Cinzia Calvio, Massimo Pregnolato, Marco Terreni, and Teodora Bavaro. 2019. "Developing a Novel Enzyme Immobilization Process by Activation of Epoxy Carriers with Glucosamine for Pharmaceutical and Food Applications" Catalysts 9, no. 10: 843. https://doi.org/10.3390/catal9100843
APA StyleSerra, I., Benucci, I., Robescu, M. S., Lombardelli, C., Esti, M., Calvio, C., Pregnolato, M., Terreni, M., & Bavaro, T. (2019). Developing a Novel Enzyme Immobilization Process by Activation of Epoxy Carriers with Glucosamine for Pharmaceutical and Food Applications. Catalysts, 9(10), 843. https://doi.org/10.3390/catal9100843









