Plant-Mediated Enantioselective Transformation of Indan-1-One and Indan-1-ol
Abstract
1. Introduction
2. Results and Discussion
2.1. Reduction of Indan-1-One Using the Plant Enzymatic System
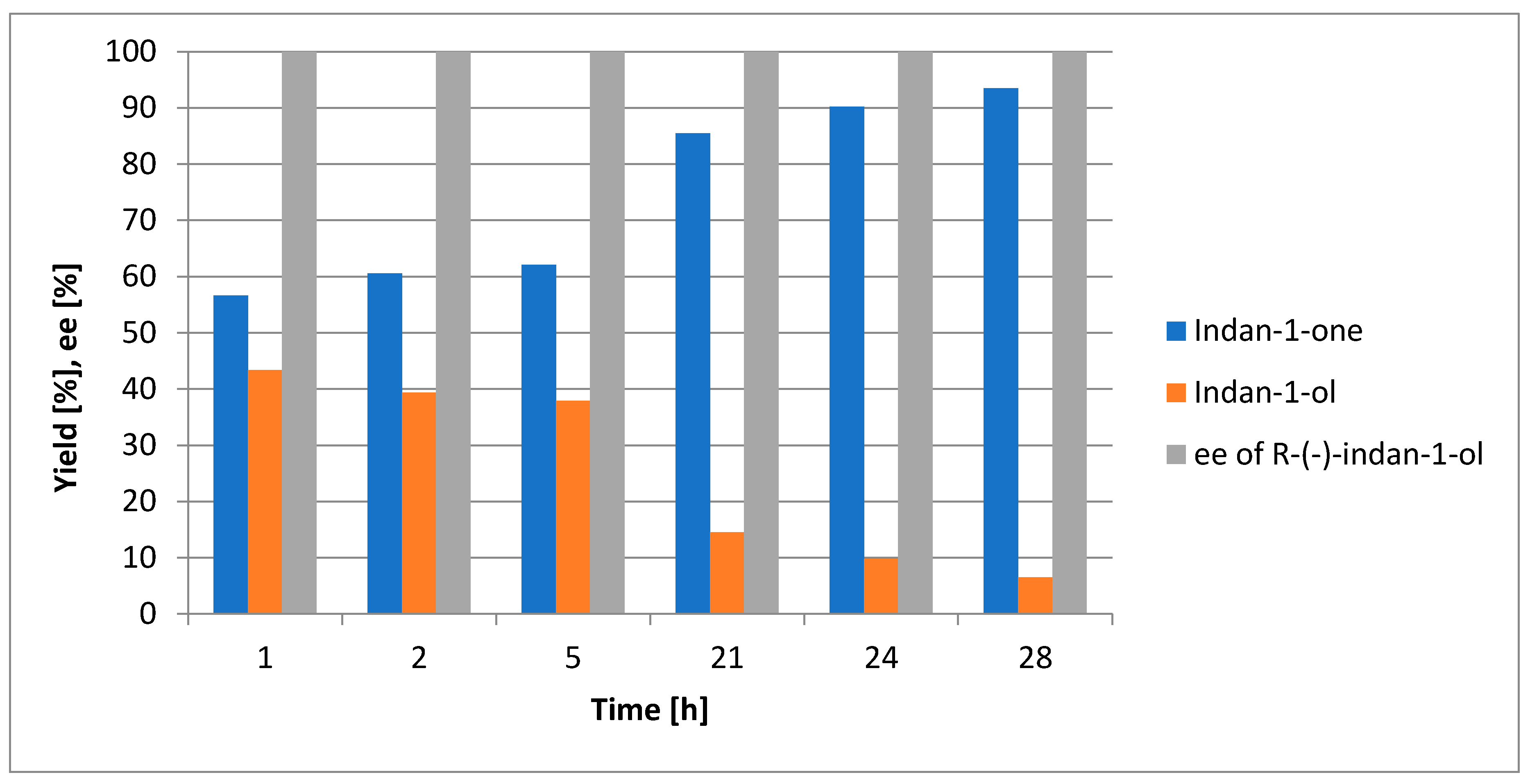
2.2. Oxidation of Indan-1-ol Using the Plant Enzyme System
3. Experimental
3.1. Biocatalysts
3.2. Duration of Biotransformation
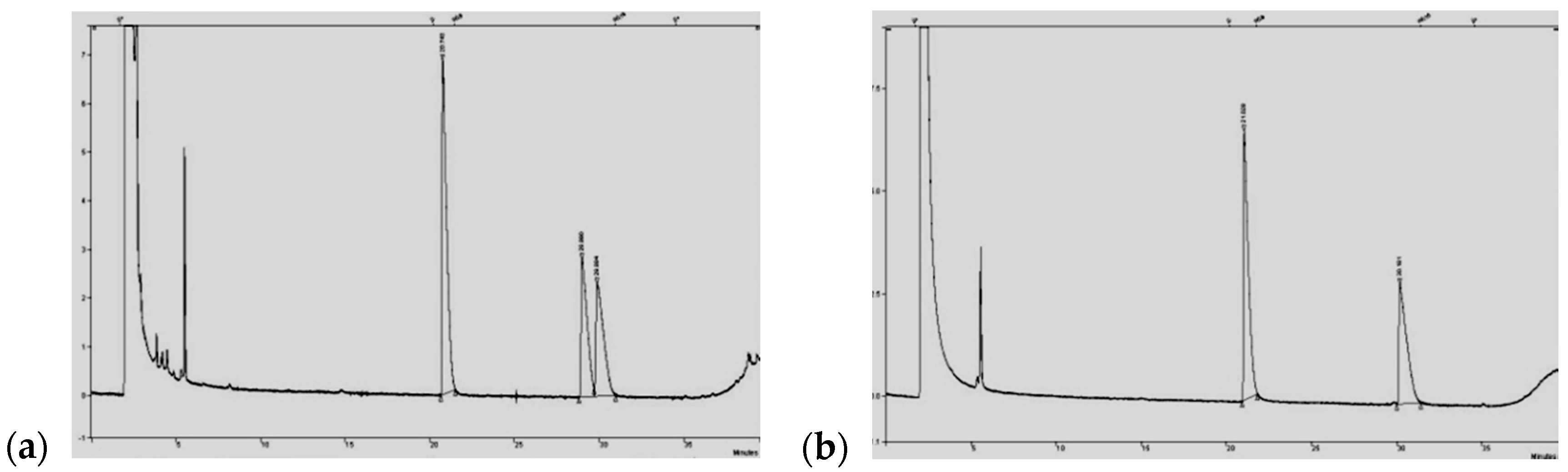
3.3. Screening Procedure
3.4. Procedure of Preparative Biotransformation
3.5. Investigation of Biotransformation over Time
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ballard, A.; Narduolo, S.; Ahmad, H.O.; Cosgrove, D.A.; Leach, A.G.; Buurma, N.J. The problem of racemization in drug discovery and tools to predict it. Expert Opin. Drug Discov. 2019. [Google Scholar] [CrossRef]
- Talsi, E.P.; Bryliakov, K.P. Autoamplification-enhanced oxidative kinetic resolution of sec-alcohols and alkyl mandelates, and its kinetic model. ChemCatChem 2018, 10, 2693–2699. [Google Scholar] [CrossRef]
- Hashimoto, T.; Shimazaki, Y.; Omatsu, Y.; Maruoka, K. Indanol-based chiral organoiodine catalysts for enantioselective hydrative dearomatization. Angew. Chem. Int. Ed. 2018, 57, 7200–7204. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, A.; Ahmed, M.; Ishii, H.; Ikegami, T. Immobilized β-cyclodextrin-based silica vs polymer monoliths for chiral nano liquid chromatographic separation of racemates. Talanta 2015, 132, 301–314. [Google Scholar] [CrossRef]
- Garbe, M.; Wei, Z.; Tannert, B.; Spannenberg, A.; Jiao, H.; Bachmann, S.; Scalone, M.; Junge, K.; Beller, M. Enantioselective hydrogenation of ketones using different metal complexes with a chiral PNP pincer ligand. Adv. Synth. Catal. 2019, 361, 1913–1920. [Google Scholar] [CrossRef]
- Kumar, R.; Banoth, L.; Banerjee, U.C.; Kaura, J. Enantiomeric separation of pharmaceutically important drug intermediates using a metagenomic lipase and optimization of its large scale production. Int. J. Biol. Macromol. 2017, 95, 995–1003. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, H.; Ang, E.L.; Zhao, H. Biocatalysis for the synthesis of pharmaceuticals and pharmaceutical intermediates. Bioorg. Med. Chem. Lett. 2018, 26, 1275–1284. [Google Scholar] [CrossRef]
- Mączka, W.K.; Mironowicz, A. Enantioselective hydrolysis of 1-aryl ethyl acetates and reduction of aryl methyl ketones using carrot, celeriac and horseradish enzyme systems. Tetrahedron Asymmetry 2002, 13, 2299–2302. [Google Scholar] [CrossRef]
- Mączka, W.K.; Mironowicz, A. Enantioselective reduction of bromo- and methoxy-acetophenone derivatives using carrot and celeriac enzymatic system. Tetrahedron Asymmetry 2004, 15, 1965–1967. [Google Scholar] [CrossRef]
- Meshram, S.H.; Ramesh, T.; Nanubolu, J.B.; Srivastava, A.K.; Adari, B.R.; Sahu, N. Green synthesis of enantiopure quinoxaline alcohols using Daucus carota. Chirality 2019, 31, 312–320. [Google Scholar] [CrossRef]
- Kazici, H.C.; Bayraktar, E.; Mehmetoglu, Ü. Production of precursors for anti-Alzheimer drugs: Asymmetric bioreduction in a packed-bed bioreactor using immobilized D. carota cells. Prep. Biochem. Biotechnol. 2017, 47, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Mironowicz, A. Biotransformations of racemic acetates by potato and topinambur tubers. Phytochemistry 1998, 47, 1531–1534. [Google Scholar] [CrossRef]
- Itoh, K.; Nakamura, K.; Utsukihara, T.; Sakamaki, H.; Horiuchi, A.C. Stereoselective oxidation of racemic 1-arylethanols by basil cultured cells of Ocimum basilicum cv. purpurascens. Biotechnol. Lett. 2008, 30, 951–954. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.H.; Utsunomiya, R.S.; Omori, A.T.; Porto, A.L.M.; Comasseto, J.V. Edible catalysts for clean chemical reactions: Bioreduction of aromatic ketones and biooxidation of secondary alcohols using plants. J. Mol. Catal. B Enzym. 2006, 38, 84–90. [Google Scholar] [CrossRef]
- Utsukihara, T.; Horiuchi, A.C. Production of chiral aromatic alcohol by acetophenone and 1-arylethanol derivatives using vegetables. Indian J. Chem. 2019, 58B, 69–74. [Google Scholar]
- Nasário, F.D.; Cazetta, T.; Moran, P.J.S.; Rodrigues, J.A.R. Deracemization of 1-phenylethanol via tandem biocatalytic oxidation and reduction. Tetrahedron Asymmetry 2016, 27, 404–409. [Google Scholar] [CrossRef]
- Liu, J.; Wu, S.; Li, Z. Recent advances in enzymatic oxidation of alcohols. Curr. Opin. Chem. Biol. 2018, 43, 77–86. [Google Scholar] [CrossRef]
- Carvalho da Silva, R.A.; de Mesquita, B.M.; de Farias, I.F.; Garcia do Nascimento, P.G.; Gomes de Lemos, T.L.; Queiroz Monte, F.J. Enzymatic chemical transformations of aldehydes, ketones, esters and alcohols using plant fragments as the only biocatalyst: Ximenia americana grains. Mol. Catal. 2018, 445, 187–194. [Google Scholar] [CrossRef]
- Aldabalde, V.; Arcia, P.; Gonzalez, A.; Gonzalez, D. Enzymatic synthesis of chiral heteroaryl alcohols using plant fragments as the only biocatalyst and reducing agent. Green Chem. Lett. Rev. 2007, 1, 25–30. [Google Scholar] [CrossRef]
- Bennamane, M.; Zeror, S.; Aribi-Zouioueche, L. Asymmetric Reduction of Ketones by biocatalysis using clementine mandarin (Citrus reticulata) fruit grown in Annaba or by ruthenium catalysis for access to both enantiomers. Chirality 2015, 27, 205–210. [Google Scholar] [CrossRef]
- Bennamane, M.; Razi, S.; Zeror, S.; Aribi-Zouioueche, L. Preparation of chiral phenylethanols using various vegetables grown in Algeria. Biocatal. Agric. Biotechnol. 2018, 14, 52–56. [Google Scholar] [CrossRef]
- Cordell, G.A.; Lemos, T.L.G.; Monte, F.J.Q.; de Mattos, M.C. Vegetables as chemical reagents. J. Nat. Prod. 2007, 70, 478–492. [Google Scholar] [CrossRef] [PubMed]
- Machado, L.L.; Monte, F.J.Q.; de Oliveira, M.C.F.; de Mattos, M.C.; Lemos, T.L.G.; Gotor-Fernández, V.; de Gonzalo, G.; Gotor, V. Bioreduction of aromatic aldehydes and ketones by fruits’barks of Passiflora edulis. J. Mol. Catal. B Enzym. 2008, 55, 130–133. [Google Scholar] [CrossRef]
- Yadav, J.S.; Nanda, S.; Thirupathi Reddy, P.; Bhaskar Rao, A. Efficient enantioselective reduction of ketones with Daucus carota root. J. Org. Chem. 2002, 67, 3900–3903. [Google Scholar] [CrossRef] [PubMed]
- Pavokovic, D.; Buda, R.; Andrašec, F.; Roje, M.; Cvjetko Bubalo, M.; Radojcic Redovnikovic, I. Plant-mediated asymmetric reduction of 1-(3,4-dimethylphenyl)ethanone. Tetrahedron Asymmetry 2017, 28, 730–733. [Google Scholar] [CrossRef]
- Catallo, W.J.; Shupe, T.F.; Eberhardt, T.L. Hydrothermal processing of biomass from invasive aquatic plants. Biomass Bioenergy 2008, 32, 140–145. [Google Scholar] [CrossRef]
- Lawton, R.O.; Alexander, L.D.; Setzer, W.N.; Byler, K.G. Floral essential oil of Guettarda poasana inhibits yeast growth. Biotropica 1993, 25, 483–486. [Google Scholar] [CrossRef]
- Fushimi, K.; Horikawa, M.; Suzuki, K.; Sekiya, A.; Kanno, S.; Shimura, S.; Kawagishi, H. Applanatines A to E from the culture broth of Ganoderma applanatum. Tetrahedron 2010, 66, 9332–9335. [Google Scholar] [CrossRef]
- Gallou, I.; Senanayake, C.H. cis-1-Amino-2-indanol in drug design and applications to asymmetric processes. Chem. Rev. 2006, 106, 2843–2874. [Google Scholar] [CrossRef]
- Calitz, C.; Gouws, C.; Viljoen, J.; Steenekamp, J.; Wiesner, L.; Abay, E.; Hamman, J. Herb-drug pharmacokinetic interactions: Transport and metabolism of Indinavir in the presence of selected herbal products. Molecules 2015, 20, 22113–22127. [Google Scholar] [CrossRef]
- Lourenco, N.M.T.; Barreiros, S.; Afonso, C.A.M. Enzymatic resolution of Indinavir precursor in ionic liquids with reuse of biocatalyst and media by product sublimation. Green Chem. 2007, 9, 734–736. [Google Scholar] [CrossRef]
- Kameyama, M.; Siqueira, F.A.; Garcia-Mijares, M.; Silva, L.F., Jr.; Silva, M.T.A. Indatraline: Synthesis and effect on the motor activity of Wistar rats. Molecules 2011, 16, 9421–9438. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Wang, K.; Rizzi, J.P.; Huang, H.; Grina, J.A.; Schlachter, S.T.; Wang, B.; Wehn, P.M.; Yang, H.; Dixon, D.D.; et al. 3-[(1S,2S,3R)-2,3-Difluoro-1-hydroxy-7-methylsulfonylindan-4-yl]oxy-5-fluorobenzonitrile (PT2977), a hypoxia-inducible factor 2α (HIF-2α) inhibitor for the treatment of clear cell Renal cell carcinoma. J. Med. Chem. 2019, 62, 6876–6893. [Google Scholar] [CrossRef] [PubMed]
- Nagaki, M.; Soma, N.; Ono, K.; Yamanouchi, K.; Tsujiguchi, T.; Kawakami, J.; Chounan, Y. Biotransformation of indanol, fluorenol and their analogs using tissue-cultured cells and their antimicrobial activity. Trans. Mater. Res. Soc. Jpn. 2019, 44, 29–33. [Google Scholar] [CrossRef]
- Uzura, A.; Katsuragi, T.; Tani, Y. Conversion of various aromatic compounds by resting cells of Fusarium moniliforme strain MS31. J. Biosci. Bioeng. 2001, 92, 381–384. [Google Scholar] [CrossRef]
- Stampfer, W.; Kosjek, B.; Faber, K.; Kroutil, W. Biocatalytic asymmetric hydrogen transfer employing Rhodococcus ruber DSM 44541. J. Org. Chem. 2003, 68, 402–406. [Google Scholar] [CrossRef]
- Bouzemi, N.; Aribi-Zouioueche, L.; Fiaud, J.C. Combined lipase-catalyzed resolution/Mitsunobu esterification for the production of enantiomerically enriched arylalkyl carbinols. Tetrahedron Asymm 2006, 17, 797–800. [Google Scholar] [CrossRef]
- Olejniczak, T.; Mironowicz, A.; Wawrzeńczyk, C. Lactones 12. Enzymatic lactonization of γ,δ-epoxy esters by the apple fruit and Jerusalem artichoke bulb. Bioorg. Chem. 2003, 31, 199–205. [Google Scholar] [CrossRef]
- Xie, B.; Yang, J.; Yang, Q.; Yuan, W. Enantioselective reduction of fluorenones in surfactant-aqueous solution by fruits and vegetables. J. Mol. Catal. B Enzym. 2009, 61, 284–288. [Google Scholar] [CrossRef]
- Bártíková, H.; Skálová, L.; Stuchlíková, L.; Vokřál, I.; Vaněk, T.; Podlipná, R. Xenobiotic-metabolizing enzymes in plants and their role in uptake and biotransformation of veterinary drugs in the environment. Drug Metab. Rev. 2015, 47, 374–387. [Google Scholar]
- Robineau, T.; Batard, Y.; Nedelkina, S.; Cabello-Hurtado, F.; LeRet, M.; Sorokine, O.; Didierjean, L.; Werck-Reichhart, D. The chemically inducible plant cytochromeP450 CYP76B1 actively metabolizes phenylureas and other xeno-biotics. Plant Physiol. 1998, 118, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, C.; Pallotta, M.L. Mitochondria-localized NAD biosynthesis by nicotinamide mononucleotide adenylyltransferase in Jerusalem artichoke (Helianthus tuberosus L.) heterotrophic tissues. Planta 2011, 234, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Mironowicz, A. Wykorzystanie komórek roślinnych do biotransformacji ksenobiotycznych, strukturalnie zróżnicowanych związków chemicznych. In Zeszyty Naukowe Akademii Rolniczej we Wrocławiu Nr 354 Rozprawy CL XXII; Wydawnictwo Akademii Rolniczej we Wrocławiu: Wrocław, Poland, 1999. [Google Scholar]
- Mączka, W.; Sołtysik, D.; Wińska, K.; Grabarczyk, M.; Szumny, A. Plant-mediated biotransformations of S(+)- and R(−)-carvones. Appl. Sci. 2018, 8, 2605. [Google Scholar]
- Rebelo, S.L.H.; Simoes, M.M.Q.; Neves, M.G.P.M.S.; Silva, A.M.S.; Tagliatesta, P.; Cavaleiro, J.A.S. Oxidation of bicyclic arenes with hydrogen peroxide catalysed by Mn(III) porphyrins. J. Mol. Catal. A Chem. 2003, 232, 135–142. [Google Scholar] [CrossRef]
- Lie, F.; Chen, Y.; Wang, Z.; Li, Z. Enantioselective benzylic hydroxylation of indan and tetralin with Pseudomonas monteilii TA-5. Tetrahedron Asymmetry 2009, 20, 1206–1211. [Google Scholar] [CrossRef]
| Biocatalyst | % of Alcohol | ee [%] |
|---|---|---|
| A. ampeloprasum L. (leek) | 0 | 0 |
| A. graveolens L. (celeriac) | 8.4 | 99 R-(−) |
| B. vulgaris L. (beet) | 0 | 0 |
| D. carota L. (carrot) | 8.5 | 99 S-(+) |
| H. tuberosus L. (Jerusalem artichoke) | 3.6 | 99 S-(+) |
| P. sativa L. (parsnip) | 7.4 | 99 S-(+) |
| P. crispum L. (parsley) | 8.4 | 99 S-(+) |
| R. sativus L. (radish) | 0 | 0 |
| S. tuberosum L. (potato) | 0 | 0 |
| M. pumila L. (apple “Gloster”) | 3.1 | 99 R-(−) |
| C. oblonga Mill. (quince) | 0 | 0 |
| Biocatalyst | Ketone [%] | The Unreacted Alcohol | |
|---|---|---|---|
| % | ee [%] | ||
| A. ampeloprasum L. (leek) | 2.5 | 97.5 | 1.2 S-(+) |
| A. graveolens L. (celeriac) | 44.4 | 55.6 | 4.4 S-(+) |
| B. vulgaris L. (beet) | 30.8 | 69.2 | 12.8 R-(−) |
| D. carota L. (carrot) | 16.1 | 83.9 | 12.8 S-(+) |
| H. tuberosus L. (Jerusalem artichoke) | 99.5 | 0.5 | 99 R-(−) |
| P. sativa L. (parsnip) | 28.9 | 71.2 | 7.3 S-(+) |
| P. crispum L. (parsley) | 31.2 | 68.8 | 8.4 S-(+) |
| R. sativus L. (radish) | 3.4 | 96.6 | 1.3 S-(+) |
| S. tuberosum L. (potato) | 17.8 | 82.2 | 12.4 S-(+) |
| M. pumila L. (apple “Gloster”) | 6.2 | 93.8 | 3.6 S-(+) |
| C. oblonga Mill. (quince) | 4.7 | 95.3 | 2.2 R-(−) |
| Biocatalyst | pH |
|---|---|
| A. ampeloprasum L. (leek) | 6.0 |
| A. graveolens L. var. rapaceum (celeriac) | 6.2 |
| B. vulgaris L. (beet) | 5.9 |
| D. carota L. (carrot), | 6.5 |
| H. tuberosus L. (Jerusalem artichoke) | 6.9 |
| P. sativa L. (parsnip) | 6.5 |
| P. sativum Hoffm: (parsley), | 6.5 |
| R. sativus L. (radish) | 5.9 |
| S. tuberosum L. (potato) | 5.9 |
| M. pumila L.(apple) | 4.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mączka, W.; Wińska, K.; Grabarczyk, M.; Galek, R. Plant-Mediated Enantioselective Transformation of Indan-1-One and Indan-1-ol. Catalysts 2019, 9, 844. https://doi.org/10.3390/catal9100844
Mączka W, Wińska K, Grabarczyk M, Galek R. Plant-Mediated Enantioselective Transformation of Indan-1-One and Indan-1-ol. Catalysts. 2019; 9(10):844. https://doi.org/10.3390/catal9100844
Chicago/Turabian StyleMączka, Wanda, Katarzyna Wińska, Małgorzata Grabarczyk, and Renata Galek. 2019. "Plant-Mediated Enantioselective Transformation of Indan-1-One and Indan-1-ol" Catalysts 9, no. 10: 844. https://doi.org/10.3390/catal9100844
APA StyleMączka, W., Wińska, K., Grabarczyk, M., & Galek, R. (2019). Plant-Mediated Enantioselective Transformation of Indan-1-One and Indan-1-ol. Catalysts, 9(10), 844. https://doi.org/10.3390/catal9100844






