Enzyme-Loaded Mesoporous Silica Particles with Tuning Wettability as a Pickering Catalyst for Enhancing Biocatalysis
Abstract
:1. Introduction
2. Results and Discussion
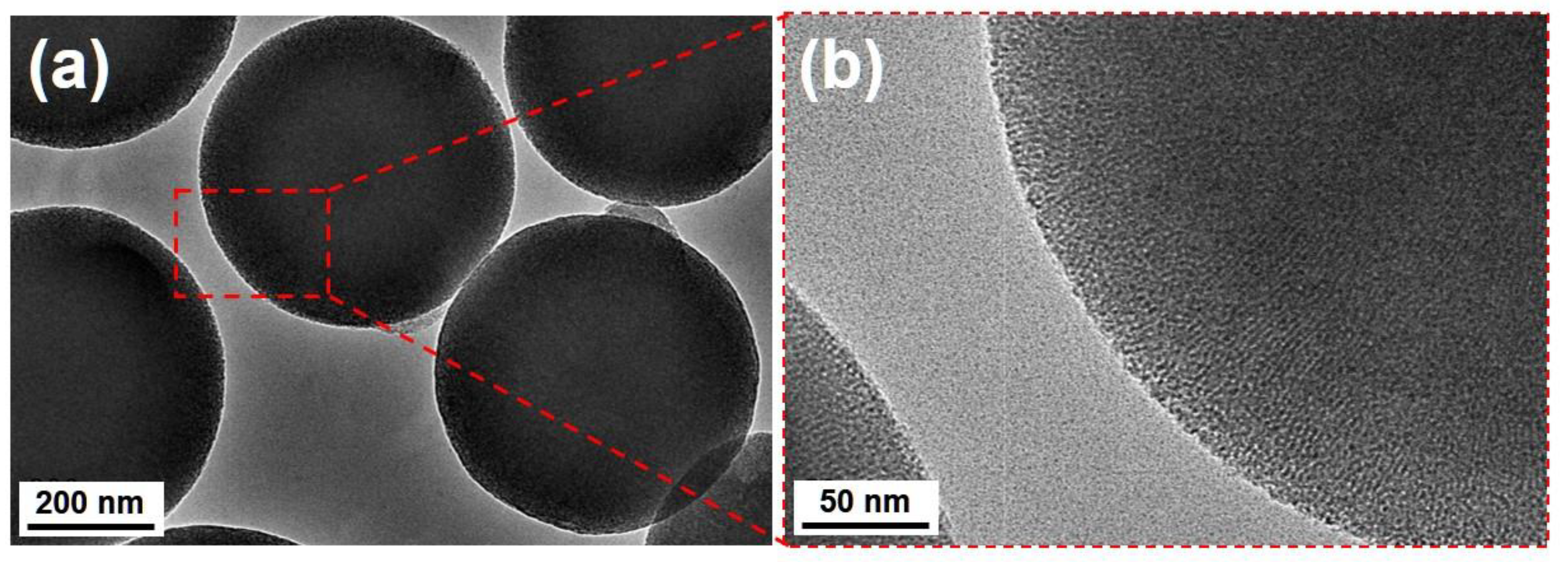
2.1. Characterization of MSPs
2.2. Characterization of Encapsulation of PCL into MSPs
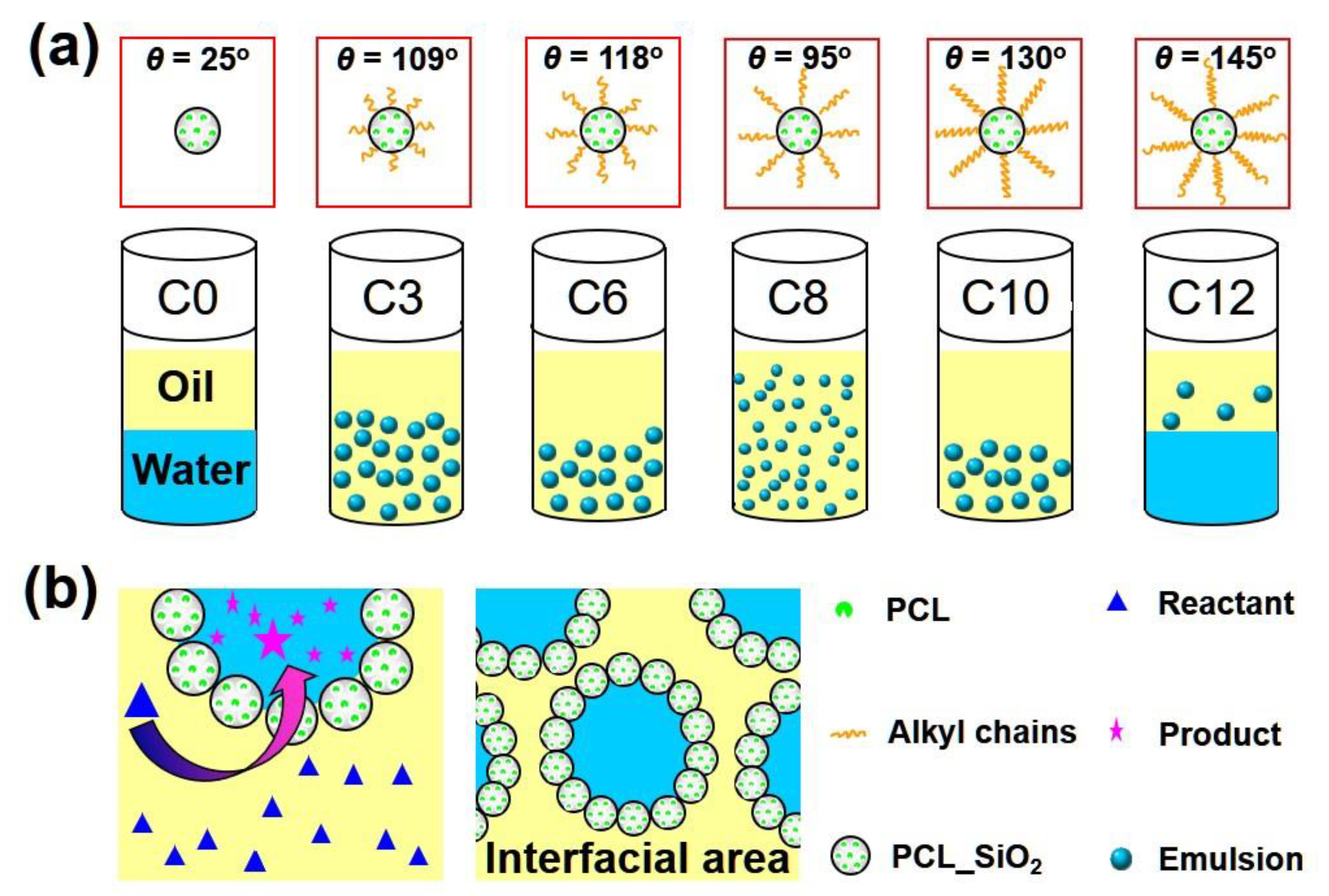
2.3. Characterization of Pickering Emulsions Stabilized by Different Silylating Agents Modified LMSPs
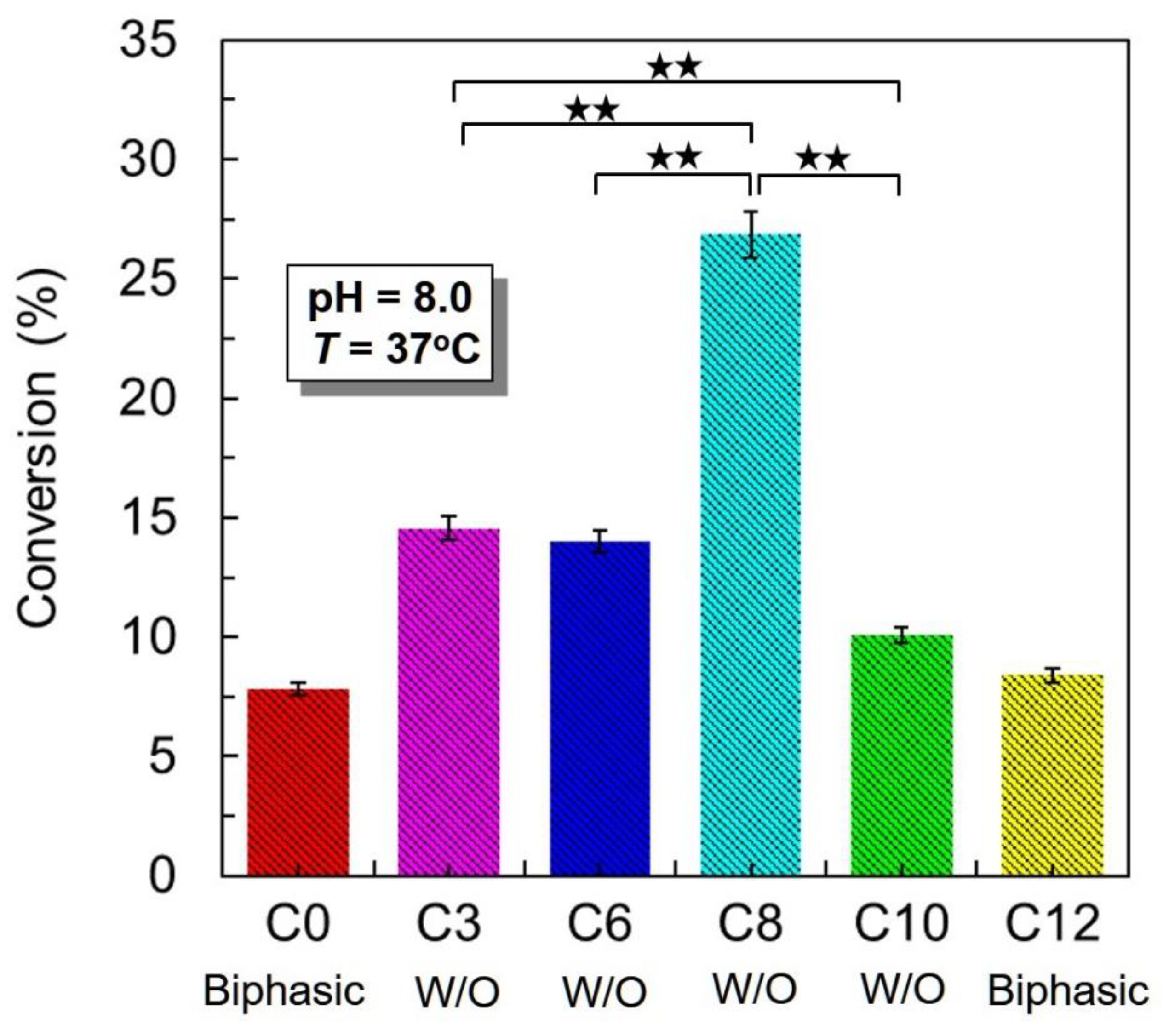
2.4. Conversion of Tributyrin Hydrolysis Reaction with the Catalysis of LMSPs_C8 in Pickering Emulsion or Biphasic System
2.5. Conversion of Tributyrin Hydrolysis Reaction in Different Silylating Agents Modified LMSPs Stabilized Pickering Emulsion
2.6. Effect of the Cycles of Reaction on relative activity
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of MSPs
3.3. Encapsulation of PCL into the MSPs
3.4. Evaluation of Different Silylating Agents Modified LMSPs Surface Wettability
3.5. Preparation of Pickering Emulsion Stabilized by Different Silylating Agents Modified LMSPs
3.6. The Effect of the Substrate Concentration on the Conversion with Biphasic Condition
3.7. The effect of Wettability of LMSPs on the Conversion with Biphasic Condition
3.8. Assessments of the Catalytic Performance and Reusability of the Pickering Emulsion Stabilized by Modified LMSPs
3.9. Characterization Techniques
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MSPs | The mesoporous silica particles |
| LMSPs | Lipase-immobilized mesoporous silica particles |
| LMSPs_Cn | n carbon atoms silane grafted LMSPs |
| TCA | The three-phase contact angle |
| PCL | Lipase PS from Pseudomonas cepacia |
| W/O | Water-in-oil |
| V | The volume of the supernatant and washing solutions |
| CS | The molarity of tributyrin |
| C3 | Trimethylchlorosilane |
| C6 | Hexyltrichlorosilane |
| C8 | Octyltrichlorosilane |
| C10 | Decyltrichlorosilane |
| C12 | Dodecyltrichlorosilane |
References
- Klibanov, A.M. Improving enzymes by using them in organic solvents. Nature 2001, 409, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M. Biocatalysis and Biotransformations. Catalysts 2018, 8, 216. [Google Scholar] [CrossRef]
- Weiss, C.K.; Landfester, K. Enzymatic catalysis at interfaces-heterophase systems as substrates for enzymatic action. Catalysts 2013, 3, 401. [Google Scholar] [CrossRef]
- Wittenboer, A.; Niemeijer, B.; Karmee, S.; Ansorge-Schumacher, M. Systematic assessment of the stability of benzaldehyde lyase in aqueous-organic biphasic systems and its stabilization by modification with methoxy-poly(ethylene) glycol. J. Mol. Catal. B 2010, 67, 208–213. [Google Scholar] [CrossRef]
- Pera-Titus, M.; Leclercq, L.; Clacens, J.M.; DeCampo, F.; Nardello-Rataj, V. Pickering interfacial catalysis for biphasic systems: From emulsion design to green reactions. Angew. Chem. Int. Ed. 2015, 54, 2006–2021. [Google Scholar] [CrossRef]
- Zhang, W.J.; Fu, L.M.; Yang, H.Q. Micrometer-scale mixing with Pickering emulsions: Biphasic reactions without stirring. ChemSusChem 2014, 7, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.B.; Wang, J.L.; Chen, L.; Lu, M.Z.; Zheng, Z.; Jing, R.; Chen, H.L.; Shen, X.B. A green strategy to enhance a liquid-liquid heterogeneous reaction with a magnetic recyclable Pickering emulsion. ChemCatChem 2015, 7, 616–624. [Google Scholar] [CrossRef]
- Huang, J.P.; Yang, H.Q. A pH-switched Pickering emulsion catalytic system: High reaction efficiency and facile catalyst recycling. Chem. Commun. 2015, 51, 7333–7336. [Google Scholar] [CrossRef]
- Zhou, W.J.; Fang, L.; Fan, Z.Y.; Albela, B.; Bonneviot, L.; De Campo, F.; Pera-Titus, M.; Clacens, J.M. Tunable catalysts for solvent-free biphasic systems: Pickering interfacial catalysts over amphiphilic silica nanoparticles. J. Am. Chem. Soc. 2014, 136, 4869–4872. [Google Scholar] [CrossRef]
- Fu, L.; Li, S.; Han, Z.; Liu, H.; Yang, H. Tuning the wettability of mesoporous silica for enhancing the catalysis efficiency of aqueous reactions. Chem. Commun. 2014, 50, 10045–10048. [Google Scholar] [CrossRef]
- Arditty, S.; Whitby, C.P.; Binks, B.P.; Schmitt, V.; Leal-Calderon, F. Some general features of limited coalescence in solid-stabilized emulsions. Eur. Phys. J. E 2003, 12, 273–281. [Google Scholar] [CrossRef]
- Zhang, M.; Wei, L.; Chen, H.; Du, Z.; Binks, B.P.; Yang, H. Compartmentalized droplets for continuous flow liquid-liquid interface catalysis. J. Am. Chem. Soc. 2016, 138, 10173–10183. [Google Scholar] [CrossRef]
- Wiese, S.; Spiess, A.C.; Richtering, W. Microgel-Stabilized smart emulsions for biocatalysis. Angew. Chem. Int. Ed. 2013, 52, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Bai, S.; Ansorge-Schumacher, M.B.; Wang, D. Nanoparticle cages for enzyme catalysis in organic media. Adv. Mater. 2011, 23, 5694–5699. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Zhang, M.; Zhang, X.; Xin, H.; Yang, H. Pickering Emulsion as an Efficient Platform for Enzymatic Reactions Without Stirring. ACS Sustain. Chem. Eng. 2016, 4, 6838–6843. [Google Scholar] [CrossRef]
- Cao, W.; Huang, R.; Qi, W.; Su, R.; He, Z. Self-Assembly of amphiphilic Janus particles into monolayer capsules for enhanced enzyme catalysis in organic media. ACS Appl. Mater. Interfaces 2015, 7, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, Y.X.; Hong, L.Z.; Ngai, T. Submicron inverse Pickering emulsions for highly efficient and recyclable enzymatic catalysis. Chem. Asian J. 2018, 13, 3533–3539. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; van Oers, M.C.; Rutjes, F.P.; Van Hest, J.C. Polymersome colloidosomes for enzyme catalysis in a biphasic system. Angew. Chem. Int. Ed. 2012, 51, 10746–10750. [Google Scholar] [CrossRef]
- Liu, J.; Lan, G.; Peng, J.; Li, Y.; Li, C.; Yang, Q. Enzyme confined in silica-based nanocages for biocatalysis in a Pickering emulsion. Chem. Commun. 2013, 49, 9558–9560. [Google Scholar] [CrossRef]
- Gao, J.; Li, D.; Jiang, Y.; Ma, L.; Zhou, L.; He, Y. Pickering emulsion stabilized by immobilized bienzyme nanoparticles: A novel and robust system for enzymatic purification of isomaltooligosaccharide. Ind. Eng. Chem. Res. 2014, 53, 18163–18169. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, X.; Chen, Y.; Zhou, L.; He, Y.; Ma, L.; Gao, J. Pickering emulsion stabilized by lipase-containing periodic mesoporous organosilica particles: A robust biocatalyst system for biodiesel production. Bioresour. Technol. 2014, 153, 278–283. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, L.L.; Xie, X.M.; Yang, S. Amphiphilic carbonaceous microsphere-stabilized oil-in-water Pickering emulsions and their applications in enzyme catalysis. ChemPlusChem 2016, 81, 629–636. [Google Scholar] [CrossRef]
- Shi, J.; Wang, X.; Zhang, S.; Tang, L.; Jiang, Z. Enzyme-Conjugated ZIF-8 particles as efficient and stable Pickering interfacial biocatalysts for biphasic biocatalysis. J. Mater. Chem. B 2016, 4, 2654–2661. [Google Scholar] [CrossRef]
- Wang, J.; Huang, R.; Qi, W.; Su, R.; He, Z. Oriented enzyme immobilization at the oil/water interface enhances catalytic activity and recyclability in a Pickering emulsion. Langmuir 2017, 33, 12317–12325. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, X.; Jiang, Y.; Zhou, Y.; Ma, L.; He, Y.; Gao, J. Biocatalytic Pickering emulsions stabilized by lipase-immobilized carbon nanotubes for biodiesel production. Catalysts 2018, 8, 587. [Google Scholar] [CrossRef]
- Sun, Z.; Glebe, U.; Charan, H.; Boker, A.; Wu, C. Enzyme–Polymer conjugates as robust Pickering interfacial biocatalysts for efficient biotransformations and one-pot cascade reactions. Angew. Chem. Int. Ed. 2018, 57, 13810–13814. [Google Scholar] [CrossRef]
- Wan, Y.; Zhao, D.Y. On the controllable soft-templating approach to mesoporous silicates. Chem. Rev. 2007, 107, 2821–2860. [Google Scholar] [CrossRef]
- Liu, J.; Qiao, S.Z.; Hu, Q.H.; Lu, G.Q. Magnetic nanocomposites with mesoporous structures: Synthesis and applications. Small 2011, 7, 425–443. [Google Scholar] [CrossRef]
- Binks, B.P.; Lumsdon, S.O. Influence of particle wettability on the type and stability of surfactant-free emulsions. Langmuir 2000, 16, 8622–8631. [Google Scholar] [CrossRef]
- Stiller, S.; Gers-Barlag, H.; Lergenmueller, M.; Pflucker, F.; Schulz, J.; Wittern, K.P.; Daniels, R. Investigation of the stability in emulsions stabilized with different surface modified titanium dioxides. Colloids Surf. A 2004, 232, 261–267. [Google Scholar] [CrossRef]
- Yu, C.; Fan, L.M.; Yang, J.; Shan, Y.Y.; Qiu, J.S. Phase-Reversal emulsion catalysis with CNT-TiO2 nanohybrids for the selective oxidation of benzyl alcohol. Chem. Eur. J. 2013, 19, 16192–16195. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Kostrov, X. Immobilization of enzymes on porous silicas—Benefits and challenges. Chem. Soc. Rev. 2013, 42, 6277–6289. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; He, J.; Guo, X. Architecture and performance of mesoporous silica-lipase hybrids via non-covalent interfacial adsorption. AIChE J. 2010, 56, 506–514. [Google Scholar] [CrossRef]
- Hudson, S.; Cooney, J.; Magner, E. Proteins in mesoporous silicates. Angew. Chem. Int. Ed. 2008, 47, 8582–8594. [Google Scholar] [CrossRef]
- Lee, C.H.; Lin, T.S.; Mou, C.Y. Mesoporous materials for encapsulating enzymes. Nano Today 2009, 4, 165–179. [Google Scholar] [CrossRef]
- Yiu, H.H.P.; Wright, P.A. Enzymes supported on ordered mesoporous solids: A special case of an inorganic-organic hybrid. J. Mater. Chem. 2005, 15, 3690–3700. [Google Scholar] [CrossRef]
- Xie, C.Y.; Meng, S.X.; Xue, L.H.; Bai, R.X.; Yang, X.; Wang, Y.L.; Qiu, Z.P.; Binks, B.P.; Guo, T.; Meng, T. Light and magnetic dual-responsive Pickering emulsion micro-reactors. Langmuir 2017, 33, 14139–14148. [Google Scholar] [CrossRef]
- Bai, R.X.; Xu, L.H.; Dou, R.K.; Meng, S.X.; Xie, C.Y.; Zhang, Q.; Guo, T.; Meng, T. Light-Triggered release from Pickering emulsions stabilized by TiO2 nanoparticles with tailored wettability. Langmuir 2016, 32, 9254–9264. [Google Scholar] [CrossRef]
- Kostakis, T.; Ettelaie, R.; Murray, B.S. Effect of high salt concentrations on the stabilization of bubbles by silica particles. Langmuir 2006, 22, 1273–1280. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, T.; Bai, R.; Wang, W.; Yang, X.; Guo, T.; Wang, Y. Enzyme-Loaded Mesoporous Silica Particles with Tuning Wettability as a Pickering Catalyst for Enhancing Biocatalysis. Catalysts 2019, 9, 78. https://doi.org/10.3390/catal9010078
Meng T, Bai R, Wang W, Yang X, Guo T, Wang Y. Enzyme-Loaded Mesoporous Silica Particles with Tuning Wettability as a Pickering Catalyst for Enhancing Biocatalysis. Catalysts. 2019; 9(1):78. https://doi.org/10.3390/catal9010078
Chicago/Turabian StyleMeng, Tao, Ruixue Bai, Weihao Wang, Xin Yang, Ting Guo, and Yaolei Wang. 2019. "Enzyme-Loaded Mesoporous Silica Particles with Tuning Wettability as a Pickering Catalyst for Enhancing Biocatalysis" Catalysts 9, no. 1: 78. https://doi.org/10.3390/catal9010078
APA StyleMeng, T., Bai, R., Wang, W., Yang, X., Guo, T., & Wang, Y. (2019). Enzyme-Loaded Mesoporous Silica Particles with Tuning Wettability as a Pickering Catalyst for Enhancing Biocatalysis. Catalysts, 9(1), 78. https://doi.org/10.3390/catal9010078




